Abstract
Background
One of the main factors that reduce fruit quality and lead to economically important losses is oversoftening. Textural changes during fruit ripening are mainly due to the dissolution of the middle lamella, the reduction of cell-to-cell adhesion and the weakening of parenchyma cell walls as a result of the action of cell wall modifying enzymes. Pectins, major components of fruit cell walls, are extensively modified during ripening. These changes include solubilization, depolymerization and the loss of neutral side chains. Recent evidence in strawberry and apple, fruits with a soft or crisp texture at ripening, suggests that pectin disassembly is a key factor in textural changes. In both these fruits, softening was reduced as result of antisense downregulation of polygalacturonase genes. Changes in pectic polymer size, composition and structure have traditionally been studied by conventional techniques, most of them relying on bulk analysis of a population of polysaccharides, and studies focusing on modifications at the nanostructural level are scarce. Atomic force microscopy (AFM) allows the study of individual polymers at high magnification and with minimal sample preparation; however, AFM has rarely been employed to analyse pectin disassembly during fruit ripening.
Scope
In this review, the main features of the pectin disassembly process during fruit ripening are first discussed, and then the nanostructural characterization of fruit pectins by AFM and its relationship with texture and postharvest fruit shelf life is reviewed. In general, fruit pectins are visualized under AFM as linear chains, a few of which show long branches, and aggregates. Number- and weight-average values obtained from these images are in good agreement with chromatographic analyses. Most AFM studies indicate reductions in the length of individual pectin chains and the frequency of aggregates as the fruits ripen. Pectins extracted with sodium carbonate, supposedly located within the primary cell wall, are the most affected.
Keywords: Atomic force microscopy, AFM, plant cell wall, fruit softening, fruit ripening, homogalacturonan, pectins, rhamnogalacturonan, nanostructure, postharvest physiology
INTRODUCTION
Ripening of fleshy fruit is one of the last developmental phases of fruit ontogeny and involves many genetic, biochemical and physiological modifications. These changes include the accumulation of pigments and sugars, the production of aromatic compounds and flesh softening (Gapper et al., 2013). All these changes have evolved to make edible fruits more attractive to seed dispersal organisms. Ripening also enhances fruit organoleptic properties, making them adequate for human consumption. However, fruit quality diminishes as ripening reaches an advanced stage, mainly due to fruit oversoftening, increased pathogen susceptibility and the development of undesirable flavour and skin colour, leading to important economic losses during postharvest fruit management (Bapat et al., 2010). Thus, the rate of fruit softening determines not only the postharvest shelf life but also other economically important aspects, such as the frequency of harvesting, the handling procedures and the distance that the fruits can be transported. Therefore, delaying fruit softening is one of the major targets of breeding programmes for most commodities. Based on the degree of softening during ripening, fleshy fruits are classified into two categories (Bourne, 1979): those that soften greatly as they ripen, acquiring a melting texture and usually having a short postharvest life (e.g. strawberry, peach, apricot, tomato); and those that soften moderately as they ripen, retain a crisp texture and have a long storage life (e.g. apple, pear, watermelon).
Firmness and juiciness are the most important textural components in the case of fleshy fruits (Toivonen and Brummell, 2008). Both features are largely determined by the characteristics of parenchyma cells (shape and size, cell wall thickness and strength, cell turgor) and the extent and strength of adhesion areas between adjacent cells (Harker et al., 1997). During ripening, parenchyma cell walls are extensively modified, altering their mechanical properties, and cell adhesion is significantly reduced as a result of middle lamella dissolution. Cell wall and middle lamella modifications leading to fruit softening result from the action of cell wall modifying enzymes (e.g. polygalacturonase, pectin methylesterase, pectate lyase, β-galactosidase, cellulase), generally encoded by ripening-related genes (Brummell and Harpster, 2001; Goulao and Oliveira, 2008; Mercado et al., 2011). Other cell wall proteins, with no hydrolytic enzymatic activity, such as expansins, also have a role in softening (Brummell et al., 1999). In general, the cell wall disassembly process responsible for softening involves the depolymerization of matrix glycans, the solubilization and depolymerization of pectins and the loss of neutral sugars from pectin side chains (Brummell, 2006; Goulao and Oliveira, 2008). The extension of these changes varies greatly among different species (Mercado et al., 2011). Recently, it has been suggested that the structural integrity of the xyloglucan network maintained by xyloglucosyltransferase/endohydrolase (XTH) could be important during fruit softening. This activity is generally higher during fruit expansion and then declines or remains constant during ripening (Goulao et al., 2007; Mercado et al., 2011). Miedes and Lorences (2009) suggested that XTH genes could be involved in the maintenance of cell wall structure rather than cell wall disassembly, and therefore the decrease in XTH gene expression and activity could contribute to softening. Supporting this hypothesis, overexpression of the SlXTH1 gene in tomato reduced fruit softening (Miedes et al., 2010). On the other hand, although less studied than cell wall disassembly, cellular turgor also plays a role in fruit softening (Harker et al., 1997). A loss of turgor pressure is generally observed during fruit ripening due to regulated accumulation of apoplastic solutes (Wada et al., 2009). Transpirational water loss through the cuticle could also be involved, especially in fruits with thick and well developed cuticles, such as tomato (Saladié et al., 2007; Lara et al., 2014). Cell turgor may also be influenced by cell wall modifications taking place during fruit softening (Thomas et al., 2008), and it is therefore difficult to separate active turgor changes from effects due to passive water loss and modification of cell wall mechanical properties.
Pectin is the most abundant polymer in the middle lamella, and it regulates intercellular adhesion (Willats et al., 2001), but primary fruit cell walls are also rich in polyuronides, accounting for up to 60 % of cell wall mass in many fruits (Redgwell et al., 1997a). Probably, pectins are the cell wall components that change most during fruit softening, but their role in fruit firmness and softening is considered controversial. This could be due to the results obtained with tomato fruit, which has been used extensively as a model to study fruit ripening. Ripening of this fruit is characterized by large increases in polygalacturonase mRNA levels and protein and enzymatic activities, which correlate with the rate of softening (Brummell and Harpster, 2001). However, the downregulation of a polygalacturonase gene to reach a residual gene expression of 1 % when compared with wild-type fruits did not reduce softening, although transgenic overripe fruits showed an improved storage life (Smith et al., 1990; Kramer et al., 1992; Brummell and Labavitich, 1997). Moreover, although depolymerization of solubilized pectins was suppressed in transgenic tomatoes, the solubility of pectins remained at wild-type levels (Smith et al., 1990). These early findings led to the hypothesis that polygalacturonase-mediated pectin disassembly is neither necessary nor sufficient to induce fruit softening, especially at early stages of the process (Hadfield and Bennett, 1998). This hypothesis was later supported by studies performed in the tomato mutant rin, showing altered ripening behaviour (Giovannoni et al., 1989). However, more recent evidence in different species suggests an important role of pectin modifications in fruit softening. Thus, the downregulation of a pectate lyase or polygalacturonase gene in strawberry significantly reduced fruit softening (Jiménez-Bermúdez et al., 2002; Quesada et al., 2009). Similarly, silencing of the PG1 gene in apple, a crisp fruit with textural features completely different from strawberry, also diminished softening (Atkinson et al., 2012).
Pectin is one the most complex natural plant biopolymers (Vincken et al., 2003; Voragen et al., 2009). This complexity makes it difficult to characterize polyuronides and to infer their role in the cell wall when using conventional analysis techniques, most of which rely on measuring the colligative properties and characteristics of a population of polysaccharides (Round et al., 2001). Atomic force microscopy (AFM) makes it possible to analyse individual polymer chains and to study the degree of heterogeneity within a sample with minimal sample preparation (Morris et al., 2010; Liu and Cheng, 2011). This technique has been used to determine the structure and functionality of polysaccharides from different sources, mainly focusing on aspects related to food characteristics (Kirby et al., 1995a; Morris et al., 2001). However, AFM has been little used to study pectin modifications during fruit softening. The aim of this paper is to review the potential of AFM as a tool to gain insight into the pectin disassembly process during fruit ripening.
PECTIN DISASSEMBLY DURING FRUIT RIPENING
Traditional methodological approaches to the study of cell wall disassembly during ripening involve the purification of the cell wall and the isolation of different cell wall fractions based on their solubility in different solvents (Brett and Waldron, 1996). In this approach, pectins are extracted from cell wall material after sequential treatments with water or phenol:acetic acid:water (PAW), chelating agents such as trans-1,2-diaminocyclohexane-N,N,N′N′-tetraacetic acid (CDTA), which solubilize pectins ionically bound to the cell wall, and sodium carbonate, which releases pectins covalently bound to the wall by ester linkages (Brummell, 2006). Despite the arbitrary nature of this sequential pectin extraction procedure, it has been suggested that fractions represent different polymer moieties in muro. Thus, water extraction yields mainly pectins [water-soluble pectins (WSPs)] freely soluble in the apoplast and already solubilized by processes occurring in vivo (Redgwell et al., 1992); chelated soluble pectins (CSPs) present in the CDTA fraction are enriched in homogalacturonan from the middle lamella; and sodium carbonate-soluble pectins (SSPs) show characteristically high ratios of neutral sugars to uronic acid, suggesting enrichment in rhamnogalactorunan I (RG-I) from the primary wall (Brummell, 2006). Significant amounts of polyuronides, usually enriched in neutral sugars, remain in the cell wall after this sequential fractionation (Redgwell et al., 1997b; Brummell et al., 2004).
Pectin solubilization is a common feature of fleshy fruit ripening. This process comprises an increase in the content of pectin loosely bound to the cell wall, mainly WSP but in some cases also CSPs, that occurs in parallel with a decrease in the amount of covalently bound pectins. It is thought that pectin solubilization occurs at the expense of polyuronides tightly bound to the wall (Wakabayashi, 2000; Brummell, 2006; Mercado et al., 2011). Pectin solubilization varies greatly among species. This process is very evident in fruits such as avocado (Wakabayashi et al., 2000), kiwifruit, tomato, persimmon (Redgwell et al., 1997a), strawberry (Figueroa et al., 2010), melon (Rose et al., 1998) and peach (Brummell et al., 2004), but in general it is less important in fruit with a crisp texture at ripening, such as apple, watermelon and nashi pear (Redgwell et al., 1997a; Hiwasa et al., 2004). Several processes have been proposed as responsible for pectin solubilization. The loss of arabinan and galactan side chains from RG-I could contribute to solubilization because neutral chains might anchor pectins to the wall either through binding to matrix glycans or cellulose (Popper and Fry, 2005; Zykwinska et al., 2005) or by physical entanglement with other wall polymers. Supporting this hypothesis, many fruits show a marked loss of neutral residues, mainly arabinose and galactose, during ripening (Gross and Sams, 1984; Redgwell et al., 1997b). However, neutral sugar loss and pectin solubilization are not correlated in some fruits; for example, plum shows extensive solubilization with no loss of arabinose or galactose, while apple and nashi pear show extensive loss of galactose but very slight pectin solubilization (Redgwell et al., 1997b). According to Redgwell et al. (1997b), pectin solubilization and loss of galactose take place mostly in different cell wall polysaccharides. The loss of neutral sugars could also induce pectin solubilization indirectly, by increasing wall porosity and thus allowing access of other hydrolase enzymes to their substrate. This hypothesis was suggested by Smith et al. (2002) to explain the increase in firmness in transgenic tomato fruit with a β-galactosidase gene silenced.
An alternative or complementary cause of solubilization would be the depolymerization of chelated and/or covalently bound pectins as a result of the action of pectinase enzymes, such as polygalacturonase. Some fruits display a substantial loss of high molecular weight polymers coupled with an increase in intermediate and short pectins, e.g. avocado, tomato, peach, kiwifruit (Redgwell et al., 1992, 1997a; Huber and O'Donoghue, 1993; Brummell et al., 2004). By contrast, other fruits show low to moderate depolymerization of pectins, e.g. strawberry, blueberry, melon and apple (Redgwell et al., 1997a; Rose et al., 1998; Vicente et al., 2007). Although depolymerization is not as general as polyuronide solubilization, recent evidence obtained in fruits with crisp and soft texture when ripened supports a key role of pectinases in softening. In strawberry, a soft fruit, Jiménez-Bermúdez et al. (2002) showed that the silencing of a pectate lyase, an enzyme that catalyses the cleavage of glycosidic bonds of de-esterified pectins by a β-elimination reaction, in contrast to the hydrolytic mechanism of polygalacturonase, resulted in fruits firmer than the control at the ripening stage, without affecting other fruit quality traits, such as soluble solids, colour and anthocyanin content. Cell wall analyses of these fruits indicated less depolymerization of CSPs and SSPs, as well as a lower degree of pectin solubilization (Santiago-Doménech et al., 2008). More recently, Quesada et al. (2009) obtained strawberry plants with a polygalacturonase gene downregulated. These plants displayed a significant increase in fruit firmness at ripening, to a degree even higher than that seen in plants obtained by the silencing of the pectate lyase gene (García-Gago et al., 2009). Cell wall modifications detected in these fruits were similar to those seen in antisense pectate lyase plants, i.e. a 42 % decrease in polyuronide solubilization concurrently with slightly lower depolymerization of chelated and covalently bound pectins, decreased cell wall swelling and an increase in cell-to-cell adhesion (Posé et al., 2013). The silencing of a polygalacturonase gene in apple, a fruit with a crisp texture, caused cell wall changes similar to those described for strawberry and also improved fruit firmness and textural characteristics (Atkinson et al., 2012). This set of results challenges the previous notion that pectin depolymerization alone is not the determinant of softening, an idea mainly supported by the minor effect of polygalacturonase silencing on tomato fruit firmness observed in earlier work (Hadfield and Bennett, 1998).
Progressive demethylesterification of homogalacturonan by the action of pectin methylesterase is common to the ripening of many fruits, creating large regions of negatively charged side groups (Prasanna et al., 2007; Goulao and Oliveira, 2008). In the absence of Ca2+, this cell wall modification would contribute to the loosening of pectins by electrostatic repulsion (Brummell, 2006). However, the Ca2+ concentration in the apoplast is high enough to allow cross-linking of unesterified homogalacturonan chains by ionic interactions (Almeida and Huber, 1999). Furthermore, functional studies of pectin methylesterase genes in tomato (Tieman and Handa, 1994) and wild strawberry (Fragaria vesca) (Osorio et al., 2008) failed to find an effect of this gene on fruit firmness. However, the degree of pectin esterification could be related to tissue integrity and cell adhesion. Thus, transgenic tomato fruits with the pectin methylesterase gene silenced showed a reduced shelf life as a result of an almost complete loss of tissue integrity in senescent fruits (Tieman and Handa, 1994). An altered pattern of methyl esterification has also been observed in the Cnr (Colourless non-ripening) tomato mutant, which fails to soften but displays a severe reduction in pericarp cell adhesion (Orfila et al., 2002). Alternative hypotheses to explain the solubilization of pectins during fruit ripening are the synthesis of more freely soluble forms of pectin as the fruit ripens (Huber, 1984) and the degradation of the RG-I backbone by rhamnogalacturonases (Molina-Hidalgo et al., 2013). These last authors found that transient silencing of a gene encoding a rhamnogalacturonate lyase prevented the dissolution of the middle lamella during strawberry fruit ripening, suggesting a key role of RG-I in fruit softening.
In conclusion, the processes that lead to solubilization of polyuronides in the wall during fruit softening are diverse and recent evidence supports a role for polyuronide depolymerization as one of the main mechanism involved, in spite of its initial rejection as a possible cause. Considering the evidence together, the involvement of different mechanisms depending on the species is possible. As suggested by Brummell (2006), a better understanding of how homogalacturonan, RG-I and RG-II are related to each other and to other polymers in the wall would help to explain this issue. The nanostructural characterization by AFM of the pectic chains present in these fractions and their structural modifications during ripening would shed light on how chain processing occurs during softening. The potential of this approach to provide better knowledge of this process is discussed in the next sections.
AFM ANALYSIS OF FRUIT PECTINS
AFM fundamentals
The atomic force microscope belongs to a family of instruments known as scanning probe microscopes, which generate images by ‘feeling’ rather than ‘looking at’ the specimen, allowing magnification higher than that of light microscopy and comparable to that of electronic microscopy, but obtainable under the more natural conditions used for light microscopy (Morris et al., 2010). In brief, a sharp tip is scanned relative to the sample surface and the changes in the magnitude of the force between tip and sample are measured and used to produce a three-dimensional image (profile) of the surface topography of the sample with nanometer resolution. The sample is mounted in a liquid cell on top of a piezoelectric transducer controlling the motion of the sample in the three spatial dimensions at high resolution. The tip is positioned on a flexible cantilever allowing the tip to move up and down as it tracks across the sample. The deflection of the cantilever, and hence the displacement of the tip, is monitored using a laser beam focused onto the end of the cantilever and then reflected onto a four-quadrant photodiode detector. As the tip moves in response to the topography of the sample, the angle of the reflected laser beam changes and the movement of the reflected spot on the surface of the photodetector is recorded as an electrical potential difference (error signal). This error signal can be used in a number of ways to generate a variety of images of the sample surface or of objects deposited on this surface. A full description of this system and the different operation modes is described elsewhere (Kirby et al., 1995a; Morris et al., 2010). In addition to the molecular/submolecular resolution of this microscope for biological systems, AFM allows the visualization of samples with minimal preparation under natural or physiological conditions, in air or aqueous environments (Morris et al., 2010; Liu and Cheng, 2011).
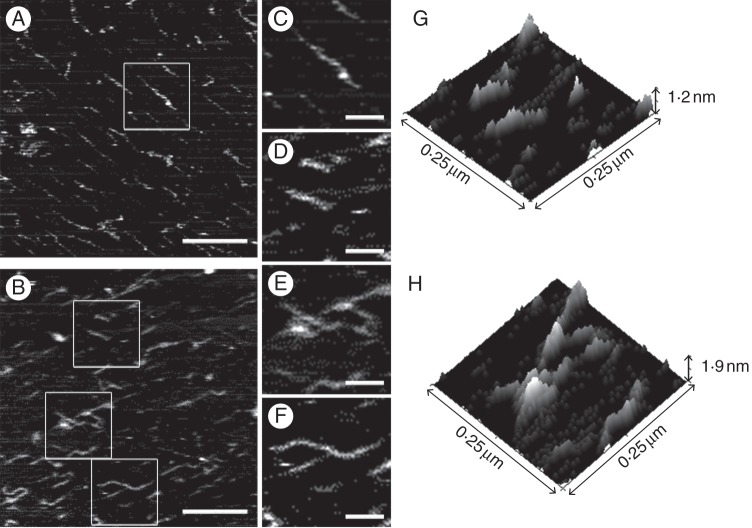
Each type of sample requires selection of the appropriate imaging mode. Among the materials that can be used as the solid phase, freshly cleaved mica is the most common because it is atomically flat, easy to handle and cost-effective (Kirby et al., 1995a). The polymers must be evenly spread over the surface in order to be visualized individually, by drop deposition or with sprays. Two main methods are used for imaging polysaccharides (Kirby et al., 1995a; Morris et al., 2010). In the first method, polymers are imaged in contact mode (DC mode) under alcohols with the AFM tip in direct contact with the sample. Imaging is usually done under a liquid (propanol or butanol), which allows greater precision in the control of the applied forces (Morris et al., 2010). In this mode, as the probe is permanently in contact with the sample, high-contrast images are obtained; however, use of this mode can generate a shear force that damages the probe and can also damage the sample if it is very soft. In the second method, samples are imaged in non-contact mode (AC mode) under air (McIntire and Brandt, 1997). Briefly, in this mode the cantilever is oscillated at a constant frequency above the sample and the weak attractive forces between tip and sample are monitored. As the sample is being viewed from a greater effective distance, there is a loss of resolution compared with conventional AFM. When the same samples have been studied with both techniques, similar results have been obtained (Morris et al., 2010). A variant of the non-contact mode is known as tapping mode. In this mode, during oscillation the probe contacts the surface of the sample intermittently at the lowest point, increasing the contrast of images compared with normal AC mode, reducing lateral forces during imaging and minimizing sample damage (Morris et al., 2010; Liu and Cheng, 2011). The three AFM techniques described above have been used to study the nanostructure of pectins isolated from fruits (Table 1). A typical image of pectins isolated from cultivated strawberry (Fragaria × ananassa) fruit and visualized under butanol in contact mode is shown in Fig. 1. This image clearly shows the complexity of extracted pectin structures, which might reflect the intricacy of the original pectin networks in muro.
Table 1.
Summary of AFM nanostructural characterization of pectins isolated from different fleshy fruit
| Fruit | Cultivar | Stage | Pectin fraction | Pectin length (nm) | Pectin width* (nm) | Image condition | Reference |
|---|---|---|---|---|---|---|---|
| Apricot (Prunus armeniaca) | Jinhong | ripe | CSP | 500–3000 | 23–234 | Air; TM | Chen et al. (2013) |
| Cherry (Prunus pseudocerasus) | Caode Bende | unripe | WSP | ND | 76–176 | Air; DC | Lai et al. (2013) |
| CSP | ND | 37–61 | |||||
| SSP | 448–749 | 37–140 | Zhang et al. (2008) | ||||
| ripe | WSP | ND | 37–82 | Lai et al. (2013) | |||
| CSP | ND | 17–55 | |||||
| SSP | 123–749 | 37–140 | Zhang et al. (2008) | ||||
| Jujube (Zizyphus jujuba) | Huanghua Zhanhua | unripe | CSP | 500, >3000 | 23–98 | Air; TM | Wang et al. (2012) |
| SSP | 500, >3000 | 35–156 | |||||
| ripe | CSP | <500, >3000 | 16–78 | ||||
| SSP | <500, 2500 | 16–78 | |||||
| Peach (Prunus persica) | Jinxiu Milu | ripe | WSP | 300–4200 | 20–100 | Air; TM | Yang et al. (2006a, b; 2009) |
| CSP | 100–3000 | ND | |||||
| SSP | 20–900 | 35–70 | |||||
| Cangfangzaosheng Songsenzaosheng | WSP | ND | 91–217 | Air; AC | Zhang et al. (2012) | ||
| CSP | ND | 91–181 | |||||
| Strawberry (Fragaria × ananassa) | Chandler | ripe | CSP | 20–500 | ND | Butanol; DC | Posé et al. (2012) |
| SSP | 20–320 | ND | |||||
| Shijixiang | WSP | 88–3043 | 23–78 | Air; TM | Chen et al. (2011) | ||
| CSP | ND | 23–78 | |||||
| SSP | 87–1152 | 23–59 | |||||
| Tomato (Solanum lycopersicum) | Rutger | unripe | CSP | 40–560 | ND | Butanol; DC | Kirby et al. (2008) |
| SSP | 20–400 | ND | Butanol; DC | Round et al. (2010) | |||
| Dongsheng Geruisi | turning | CSP | ND | 19–117 | Air; TM | Xin et al. (2010) | |
| ripe | CSP | ND | 15–117 |
*Width data correspond to non-corrected data and might be overestimated due to the probe broadening effect. See text for details.
ND, not described; DC, direct contact; AC, non-contact; TM, tapping mode.
Fig. 1.
AFM topographical images representative of cultivated strawberry (Fragaria × ananassa ‘Chandler’) CSPs at unripe (A) and ripe (B) developmental stages, drop-deposited onto mica and visualized in direct contact mode under butanol. (C) Enlarged view of boxed area in (A); (D–F) enlarged view of boxed areas in (B). The enlarged views show examples of (C, D) linear chains, (E) multichain aggregates and (F) branched chains. (G, H) Areas equivalent to those shown in (D) and (E), respectively, showing the topographical details of linear chains (G) and aggregates (H) in 3-D. Scale bars (A, B) = 250 nm; (C–F) = 75 nm.
Fruit pectin nanostructure
Table 1 summarizes the available AFM data about pectin fractions extracted from several fruits, including comparisons between different developmental stages and cultivars with different textural properties. Early AFM studies focused on the analysis of pectins from unripe tomatoes. Round et al. (1997) analysed pectin samples extracted with sodium carbonate. Images showed a mixed population of single polymers and aggregates. Interestingly, 20 % of single polymers showed long branches, ∼30 % of these having more than one branch. Lengths of the main backbone ranged between 30 and 390 nm and their size distribution fitted a log-normal curve. The range of length sizes for the branches was narrower (30–170 nm). Apparently, the removal of acetyl and methyl groups during alkaline extraction to obtain the SSP fraction does not modify pectic chain nanostructure, since tomato pectins extracted with CDTA and visualized by AFM were similar to those present in the SSP samples. However, the chains in the CDTA fraction were ∼3-fold longer than those extracted with sodium carbonate (Round et al., 2001; Kirby et al., 2008). Complementary analyses of neutral sugar composition and linkage suggested that these branches do not correspond to neutral sugars (Round et al., 2001), and it was postulated that the long branches observed in CSPs (enriched in homogalacturonan) and SSPs (enriched in RG-I) consist of polygalacturonic acid attached to the pectin backbone, with the neutral sugars present as short branches undetected by AFM (Round et al., 2001). This result has been supported by subsequent AFM experiments on pectins from apple (Zareie et al., 2003), peach (Yang et al., 2009) and red ripe strawberry fruits (Posé et al., 2012). In this last species, both CSPs and SSPs showed a similar percentage of branched polymers (8–9 %), but SSPs showed smaller but more branches per backbone (Posé et al., 2012). The lower size of strawberry SSPs correlated with the comparative profiles of both fractions obtained by size-exclusion chromatography (Posé et al., 2012).
The polygalacturonic acid nature of the pectin branches observed by AFM has been strongly supported by experiments evaluating the effect of mild acid hydrolysis on SSPs from unripe tomato (Round et al., 2010). This treatment releases different sugar residues present in polyuronides at different rates, with galactose and arabinose linkages the most labile and the galacturonic acid the most resistant (Thibault et al., 1993). Round et al. (2010) observed that almost complete hydrolysis of galactose, arabinose and rhamnose had no significant effect on backbone and branch length distributions in individual pectins visualized by AFM. This result suggests that the observed single polymers were mainly formed by homogalacturonan and contained either no rhamnose or only small amounts located at the ends of these chains. Besides isolated chains, complexes were also observed in these pectin samples, often possessing emerging strands with dimensions similar to those of isolated single chains. The size of the complexes observed in SSP samples by AFM decreased upon acid hydrolysis in parallel with the loss of neutral sugars. As the remnants of these complexes persisted after prolonged hydrolysis, Round et al. (2010) suggested that they may contain RG-I linked to irreducible homogalacturonan. Therefore, AFM results offer a new image of SSP chain composition, consisting of linear and sparsely branched homogalacturonan and aggregated complexes containing RG-I, irreducible aggregates of homogalacturonan and homogalacturonan polysaccharide chains (Round et al., 2010). The individual homogalacturonan chains observed either would be not linked to the RG-I or would be linked by bonds that are broken during cell wall fractionation. In this picture, neutral sugars would be coiled or aligned along and around the rhamnogalacturonan regions. Pectin aggregates of similar size and shape have been described in pectins from strawberry fruits, as can be observed in Fig. 1.
In most AFM studies, pectin length varied from 20 to 1000 nm, with pectin present in CSPs in general longer than those observed in SSPs (Table 1). However, pectin lengths several times higher than the range mentioned above have been reported for some fruits, e.g. peach (Yang et al., 2009) and unripe jujube (Wang et al., 2012). In SSPs from ripe strawberry fruit, Posé et al. (2012) also described the presence of large fibres within the background of individual molecules and aggregates, but these fibres disappeared when the samples were heated at 80 °C. Whether these large fibres represent novel supramolecular pectin structures or whether they are artefacts formed during the extraction and/or deposition of the samples on the mica substrate needs to be addressed. Besides pectin length, AFM allows the tridimensional measurement of pectin chains. Most reports indicate a height of the pectin chain in the range 0·5–2 nm. These measurements are in accordance with a helix structure of chains with different aggregation status (Yang et al., 2009), since the vertical height of single-stranded polysaccharides is ∼0·5 nm (McIntire and Brant, 1999). Contrary to height measurements, there is great variation in pectin chain widths amongst the different reports. Yang et al. (2009) observed that widths of pectins from peaches varied between 20 and 100 nm, and did not observe significant differences among either the pectin fractions analysed (WSP, CSP and SSP) or peach genotypes. By contrast, in strawberry fruit the width of SSP chains was smaller than those of WSP or CSP chains, ranging between 15 and 78 nm (Chen et al., 2011). Xin et al. (2010) reported a mean width of CSP chains of 35 nm in unripe tomato fruit, which decreased to 23 nm in turning fruit. Numerical regularities of the different pectin chain widths estimated in a sample have also been reported for some fruits. For example, Zhang et al. (2012) reported that chain width of the WSP and CSP fractions from peach followed three basic values – 54, 72 and 91 nm – and the widths of other pectin chains could be obtained by summing these basic units. In the same fruit, Yang et al. (2005) observed four basic width units for WSP but these units were smaller than those reported by Zhang et al. (2012) (11·7, 15·6, 19·5 and 35·1 nm). A pattern of pectin chain width distribution has also been observed in SSP chains from cherries, with four basic units (37, 47, 55 and 61 nm) (Zhang et al., 2008). These results suggest a natural structural conformation of pectins formed by intertwists between the putative basic units. However, pectin width data should be considered with extreme caution and only used for qualitative comparison of objects within a particular sample. This is because the molecular width measured by AFM is subject to ‘probe broadening’; because the radius of the AFM tip is large relative to the diameter of the molecules being measured, the image of the molecule being analysed is convoluted with the tip profile and the measured dimension is considerably overestimated (Morris et al., 2010). Kirby et al. (1995b) observed that the thickness of individual strands of the bacterial polysaccharide acetan was 5-fold greater than the value obtained when the measurements were performed in aggregated regions where the molecular chains had become aligned and the broadening effect was not present. McMaster et al. (1999) observed the width of a cylindrical object as ∼3-fold greater than the actual value. Morris et al. (1997) and Fishman et al. (2007) described a geometrical correction to estimate the tip broadening effect. Probe broadening effects will vary with the actual radius of the tips used and can vary considerably from image to image. This might explain why reported chain width values are usually almost an order of magnitude higher than the observed pectin heights, and hence the lack of correlation between pectin chain height and width and the contradictory results obtained in different fruits and studies.
In general, the nanostructural features of isolated pectins deduced from AFM images are in good agreement with data obtained using other approaches. Thibault et al. (1993), using high-performance size exclusion chromatography (HPSEC), reported that homogalacturonan pectins from different fruit sources hydrolysed in 0·1 m HCl contained ∼72–100 galacturonic acid residues. A similar degree of polymerization was found by Yapo et al. (2007) in HCl-hydrolysed citrus pectins using chromatographic techniques. According to Carpita and Gibeaut (1993), homogalacturonan, which is about 100 nm long, contains up to 200 galacturonic acid units. As discussed above, most AFM studies of isolated pectin chains reported a molecular length in the range 75–150 nm, which is in accordance with these data. The number of carbohydrate residues, as well as molecular weight, in individual pectins visualized by AFM can be estimated from contour length parameters, considering that extended chains adopt a 31 helical structure with a pitch of 1·34 nm, as deduced by Walkinshaw and Arnott (1981) based on X-ray diffraction data. Using this approach, Round et al. (2010) found that the minimum native size of linear chains in SSPs from unripe tomato could be ∼320 residues, slightly higher than the polymerization degree of homogalacturonan purified by mild acid hydrolysis (Thibault et al., 1993; Yapo et al., 2007). However, it should be taken into account that pectin length can be greatly influenced by the ripening stage of the fruit source, as discussed latter. As regards RG-I, the length of this polysaccharide is unknown (Carpita and McCann, 2000). McNeil et al. (1980) found that purified RG-I from sycamore cell walls treated with endopolygalacturonase had a molecular weight of ∼200 kDa, being the backbone formed by as many as 500 glycosyl residues. Interestingly, complexes found in AFM images of unripe tomato SSPs, which have been proposed to be composed mainly of RG-I but also contain homogalacturonan chains, have a slightly higher molecular weight of around 400 kDa (Round et al., 2010).
In summary, most nanostructural data on fruit pectins obtained by AFM analysis are in agreement with our previous knowledge on these polysaccharides, supporting the reliability of this approach in the study of pectin disassembly during fruit softening. The analysis of published AFM data differentiates two groups of reports: a first group describes pectin lengths <1000 nm and widths between 10 and 50 nm, including data on tomato (Round et al., 1997, 2010), strawberry (Posé et al., 2012) and cherry (Zhang et al., 2008; Lai et al., 2013), and a second group describes longer (>1000 nm) and wider (>50 nm) pectic chains, such as those observed in peach (Yang et al., 2009), jujube (Wang et al., 2012) and apricot (Chen et al., 2013). Applying the width correction described by Morris et al. (1997), the first group of pectins would correspond mainly to individual pectin chains while the large fibres would be highly packed pectins of several hundreds of laterally arranged chains. Whether or not this high level of packaging exists in muro needs to be addressed in the future. Similarly, the physicochemical nature of micellar aggregates and its relationship with isolated pectin chains should be further investigated.
AFM analysis of pectins during fruit ripening and storage
Fruit texture is closely related to the structural integrity of fruit tissues and pectins play a key role in cell-to-cell adhesion and cell wall stiffness. Several enzymes acting on pectins have been related to the changes that undergo cell walls during softening, such as polygalacturonase, pectate lyase, pectin methylesterase, pectin acetylesterase, β-galactosidase, β-xylosidase and α-arabinofuranosidase (Brummell and Harpster, 2001; Goulao and Oliveira, 2008; Mercado et al., 2011). Although there are numerous reports about biochemical changes in cell walls and pectins during fruit ripening and postharvest, few studies have analysed these processes at the nanostructural scale. Zhang et al. (2008) used AFM to study CSPs isolated from two Chinese cherry cultivars with crisp and soft textures. Two ripening stages were also compared: commercial ripe fruit and unripe fruit harvested 7 days before ripening. As expected, the firmer unripe fruits from both cultivars contained wider and longer chains than ripe fruits, as well as more polymers that were entangled together. However, comparison between cultivars did not support the same correlation, since firmness of the crisp cultivar was higher at both ripening stages, but the SSP fraction was richer in wider and shorter chains than in the same fraction of soft fruits at both ripening stages. A similar study was performed by Xin et al. (2010) in CSPs from tomato. In this case, turning stage fruits contained wider chains than light-red fruits and linear single and short chains were more frequently observed in light-red tomatoes. Yang et al. (2009) analysed three kinds of pectins (WSP, CSP and SSP) in two peach cultivars with contrasting texture – soft and crisp. The most significant difference between the two cultivars was the length of the SSPs, the SSP chains of the crisp fruit being longer than those of the soft cultivar (average length 249 and 57 nm, respectively). Additionally, a small number of CSP and SSP chains of the crisp genotype had branches, whilst few or no branched polymers appeared in the soft fruit pectin fractions. These authors suggested that differences in texture in peach could be mainly related to the neutral-sugar-rich pectin fraction from the primary cell wall. In Chinese jujube fruits, pectin length also decreased during ripening (Wang et al., 2012).
Representative topographical AFM images of CSPs from cultivated strawberry fruits at two ripening stages are shown in Fig. 1 to illustrate the pectin modifications that frequently occur during fruit ripening. Qualitative analysis of these images showed that CSP samples from unripe fruits (Fig. 1A) were enriched in long chains while shorter chains were more frequently observed in red fruits (Fig. 1B). Branched polymers (Fig. 1F) could be observed in both unripe and ripe samples, but the frequency of branching decreased in ripe samples. Some aggregates with emergent polymer strands could also be observed in these samples (Fig. 1E).
The nanostructural changes in pectins during fruit storage have been analysed in a few species. In peach fruit stored at different temperatures (2, 8 and 15 °C), Zhang et al. (2010) observed an increase in the content of WSP and CSP and a decrease in SSP during storage in parallel with a decrease in fruit firmness, which was higher at higher temperatures. However, fruit firmness was only correlated with the content of SSP. An AFM study of this fraction showed that most SSPs formed large aggregates in fresh fruits that were reduced during the storage period, along with the increase in storage temperature. Furthermore, the length and width of the pectin chains declined with increasing storage time and temperature. A similar result was observed when WSPs and CSPs were analysed, although CSPs in fruits at harvest were visualized mainly as single linear chains with a small number of aggregates (Zhang et al., 2012). The beneficial effects of controlled atmospheres of 2 % O2 and 5–10 % CO2 on the quality of peaches stored at low temperature have also been related to lower degradation of pectins at the nanostructural level (Yang et al., 2005, 2006a, b). Interestingly, a decrease in the number of branched polymers was observed with storage time and this was concomitant with a reduction in pectin size and number of aggregates (Yang et al., 2005, 2006a, b). In CSPs from apricots stored at 0 °C, Liu et al. (2009) also observed a decrease in the percentage of branched polymers with storage time. Furthermore, the frequency of short CSP chains increased with time, and the application of CaCl2, a treatment that extends postharvest life, supposedly due to the formation of calcium bridges between homogalacturonan chains, reduced the degradation of pectins (Liu et al., 2009). The lengths of WSPs and SSPs from strawberries decreased by 30–39 % during storage of the fruit at 4 °C for 15 days (Chen et al., 2011). As previously observed in apricot, CaCl2 treatment reduced the depolymerization of strawberry SSP polyuronides (Chen et al., 2011).
Besides being useful in the study of individual pectin polymers, AFM provides a useful means of studying their aggregation and the effect of factors affecting the formation of gels, e.g. acid-induced and calcium-induced gelation and pectin concentration (Fishman et al., 2007; Morris et al., 2010, 2011). Most of these studies were performed with fruit pectins extracted with chelating agents but were not related to the process of fruit softening. The structural information derived from gelation studies could be useful for understanding the self-assembly process in the middle lamella (Morris et al., 2011), a region that is substantially modified during fruit ripening. AFM could therefore provide a new tool to unravel the role of pectin aggregation in the process of middle lamella dissolution during fruit ripening.
CONCLUSIONS
The nanostructural characterization of pectins by AFM in fleshy fruits has revealed significant modifications of the pectic chains and aggregates present in pectin samples during fruit ripening and postharvest storage. In general, these changes involve a decrease in the number of pectin aggregates and reductions in the lengths of individual pectin chains and the widths of highly packed pectins, especially those extracted with sodium carbonate, supposedly located within the primary cell wall. Although additional work is needed to identify the physicochemical bases of the structures visualized by AFM, this technique could be a powerful means of gaining insight into the role of pectin disassembly mechanisms during fruit softening. It has already provided consistent evidence of differences in pectic chains and aggregates correlated with ripening stages and storage treatments.
ACKNOWLEDGEMENTS
This work was supported by the Ministerio de Educación y Ciencia of Spain and Feder EU Funds (grant reference AGL2011-24814). C.P. was supported by an FPI fellowship from the Spanish Government (grant reference BES-2009-027985). The research at the Institute of Food Research was supported by the BBSRC core grant to the institute.
LITERATURE CITED
- Almeida DPF, Huber DJ. Apoplastic pH and inorganic ion levels in tomato fruit: a potential means for regulation of cell wall metabolism during ripening. Physiologia Plantarum. 1999;105:506–512. [Google Scholar]
- Atkinson RG, Sutherland PW, Johnston SL, et al. Down-regulation of POLYGALACTURONASE1 alters firmness, tensile strength and water loss in apple (Malus × domestica) fruit. BMC Plant Biology. 2012;12:129–142. doi: 10.1186/1471-2229-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapat VA, Trivedi PK, Ghosh A, Sane VA, Ganapathi TR, Nath P. Ripening of fleshy fruit: molecular insight and the role of ethylene. Biotechnology Advances. 2010;28:94–107. doi: 10.1016/j.biotechadv.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Bourne MC. Texture of temperate fruits. Journal of Texture Studies. 1979;10:25–44. [Google Scholar]
- Brett C, Waldron K. Physiology and biochemistry of plant cell walls. Chapman & Hall; 1996. [Google Scholar]
- Brummell DA. Cell wall disassembly in ripening fruit. Functional Plant Biology. 2006;33:103–119. doi: 10.1071/FP05234. [DOI] [PubMed] [Google Scholar]
- Brummell DA, Harpster MH. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Molecular Biology. 2001;47:311–340. [PubMed] [Google Scholar]
- Brummell DA, Labavitch JM. Effect of antisense suppression of endopolygalacturonase activity on polyuronide molecular weight in ripening tomato fruit and in fruit homogenates. Plant Physiology. 1997;115:717–725. doi: 10.1104/pp.115.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummell DA, Harpster MH, Civello PM, Palys JM, Bennett AB, Dunsmuir P. Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. Plant Cell. 1999;11:2203–2216. doi: 10.1105/tpc.11.11.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummell DA, Del Cin V, Crisosto CH, Labavitch JM. Cell wall metabolism during maturation, ripening and senescence of peach fruit. Journal of Experimental Botany. 2004;55:2029–2039. doi: 10.1093/jxb/erh227. [DOI] [PubMed] [Google Scholar]
- Carpita N, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant Journal. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Carpita N, McCann M. The cell wall. In: Buchanan B, Gruissem W, Jones R, editors. Biochemistry and molecular biology of plants. Rockville, IL: American Society of Plant Physiologists; 2000. pp. 52–108. [Google Scholar]
- Chen F, Liu H, Yang H, et al. Quality attributes and cell wall properties of strawberries (Fragaria ananassa Duch.) under calcium chloride treatment. Food Chemistry. 2011;126:450–459. [Google Scholar]
- Chen Y, Chen F, Lai S, et al. In vitro study of the interaction between pectinase and chelate-soluble pectin in postharvest apricot fruits. European Food Research and Technology. 2013;237:987–993. [Google Scholar]
- Figueroa CR, Rosli HG, Civello PM, Martínez GA, Herrera R, Moya-León MA. Changes in cell wall polysaccharides and cell wall degrading enzymes during ripening of Fragaria chiloensis and Fragaria × ananassa fruits. Scientia Horticulturae. 2010;124:454–462. [Google Scholar]
- Fishman ML, Cooke PH, Chau HK, Coffin DR, Hotchkiss AT. Global structures of high methoxyl pectin from solution and in gels. Bio-macromolecules. 2007;8:573–578. doi: 10.1021/bm0607729. [DOI] [PubMed] [Google Scholar]
- Gapper NE, McQuinn RP, Giovannoni JJ. Molecular and genetic regulation of fruit ripening. Plant Molecular Biology. 2013;82:575–591. doi: 10.1007/s11103-013-0050-3. [DOI] [PubMed] [Google Scholar]
- García-Gago JA, López-Aranda JM, Muñoz-Blanco J, et al. Postharvest behaviour of transgenic strawberry with polygalacturonase or pectate lyase genes silenced. Acta Horticulturae. 2009;842:573–576. [Google Scholar]
- Giovannoni JJ, DellaPenna D, Bennett AB, Fischer RL. Expression of a chimeric polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. Plant Cell. 1989;1:53–63. doi: 10.1105/tpc.1.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulao LF, Oliveira CM. Cell wall modification during fruit ripening: when a fruit is not the fruit. Trends in Food Science & Technology. 2008;19:4–25. [Google Scholar]
- Goulao LF, Santos J, de Sousa I, Oliveira CM. Patterns of enzymatic activity of cell wall-modifying enzymes during growth and ripening of apples. Postharvest Biology and Technology. 2007;43:307–318. [Google Scholar]
- Gross KC, Sams CE. Changes in cell wall neutral sugar composition during fruit ripening: a species survey. Phytochemistry. 1984;23:2457–2461. [Google Scholar]
- Hadfield KA, Bennett AB. Polygalacturonases: many genes in search of a function. Plant Physiology. 1998;117:337–343. doi: 10.1104/pp.117.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker FR, Redgwell RJ, Hallet IC, Murray SH. Texture of fresh fruit. Horticultural Reviews. 1997;20:121–224. [Google Scholar]
- Hiwasa K, Nakano R, Hashimoto A, et al. European, Chinese and Japanese pear fruits exhibit differential softening characteristics during ripening. Journal of Experimental Botany. 2004;55:2281–2290. doi: 10.1093/jxb/erh250. [DOI] [PubMed] [Google Scholar]
- Huber DJ. Strawberry fruit softening - the potential roles of polyuronides and hemicelluloses. Journal of Food Science. 1984;49:1310–1315. [Google Scholar]
- Huber DJ, O'Donoghue EM. Polyuronides in avocado (Persea americana) and tomato (Lycopersicon esculentum) fruits exhibit markedly different patterns of molecular weight downshifts during ripening. Plant Physiology. 1993;102:473–480. doi: 10.1104/pp.102.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Bermúdez S, Redondo-Nevado J, Muñoz-Blanco J, et al. Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene. Plant Physiology. 2002;128:751–759. doi: 10.1104/pp.010671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby AR, Gunning AP, Morris VJ, Ridout MJ. Atomic force microscopy in food research: a new technique comes of age. Trends in Food Science & Technology. 1995a;6:359–365. [Google Scholar]
- Kirby AR, Gunning AP, Morris VJ, Ridout MJ. Observation of the helical structure of the bacterial polysaccharide acetan by atomic force microscopy. Biophysical Journal. 1995b;68:360–363. doi: 10.1016/S0006-3495(95)80195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby AR, MacDougall AJ, Morris VJ. Atomic force microscopy of tomato and sugar beet pectin molecules. Carbohydrate Polymers. 2008;71:640–647. [Google Scholar]
- Kramer M, Sanders R, Bolkan H, Waters C, Sheehy RE, Hiatt WR. Postharvest evaluation of transgenic tomatoes with reduced levels of polygalacturonase: processing, firmness and disease resistance. Postharvest Biology and Technology. 1992;1:241–255. [Google Scholar]
- Lai S, Chen F, Zhang L, Yang H, Deng Y, Yang B. Nanostructural difference of water-soluble pectin and chelate-soluble pectin among ripening stages and cultivars of Chinese cherry. Natural Product Research. 2013;27:379–385. doi: 10.1080/14786419.2012.696259. [DOI] [PubMed] [Google Scholar]
- Lara I, Belge B, Goulao LF. The fruit cuticle as a modulator of postharvest quality. Postharvest Biology and Technology. 2014;87:103–112. [Google Scholar]
- Liu D, Cheng F. Advances in research on structural characterisation of agricultural products using atomic force microscopy. Journal of the Science of Food and Agriculture. 2011;91:783–788. doi: 10.1002/jsfa.4284. [DOI] [PubMed] [Google Scholar]
- Liu H, Chen F, Yang H, et al. Effect of calcium treatment on nanostructure of chelate-soluble pectin and physicochemical and textural properties of apricot fruits. Food Research International. 2009;42:1131–1140. [Google Scholar]
- McIntire TM, Brant DA. Imaging of individual biopolymers and supramolecular assemblies using noncontact atomic force microscopy. Biopolymers. 1997;42:133–146. doi: 10.1002/(SICI)1097-0282(199708)42:2<133::AID-BIP3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- McIntire TM, Brant DA. Imaging of carrageenan macrocycles and amylose using noncontact atomic force microscopy. International Journal of Biological Macromolecules. 1999;26:303–310. doi: 10.1016/s0141-8130(99)00097-5. [DOI] [PubMed] [Google Scholar]
- McMaster TJ, Miles MJ, Kasarda DD, Shewry PR, Tatham AS. Atomic force microscopy of A-gliadin fibrils and in situ degradation. Journal of Cereal Science. 1999;31:281–286. [Google Scholar]
- McNeil M, Darvill AG, Albersheim P. Structure of plant cell walls. X. Rhamnogalacturonan I, a structurally complex pectic polysaccharide in the walls of suspension-cultured sycamore cells. Plant Physiology. 1980;66:1128–1134. doi: 10.1104/pp.66.6.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado JA, Pliego-Alfaro F, Quesada MA. Fruit shelf life and potential for its genetic improvement. In: Jenks MA, Bebeli PJ, editors. Breeding for fruit quality. Oxford: John Wiley & Sons; 2011. pp. 81–104. [Google Scholar]
- Miedes E, Lorences EP. Xyloglucan endotransglucosylase/hydrolases (XTHs) during tomato fruit growth and ripening. Journal of Plant Physiology. 2009;166:489–498. doi: 10.1016/j.jplph.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Miedes E, Herbers K, Sonnewald U, Lorences Overexpression of a cell wall enzyme reduces xyloglucan depolymerization and softening of transgenic tomato fruits. Journal of Agricultural Food Chemistry. 2010;58:5708–2713. doi: 10.1021/jf100242z. [DOI] [PubMed] [Google Scholar]
- Molina-Hidalgo FJ, Franco AR, Villatoro C, et al. The strawberry (Fragaria × ananassa) fruit-specific rhamnogalacturonate lyase 1 (FaRGLyase1) gene encodes an enzyme involved in the degradation of cell-wall middle lamellae. Journal of Experimental Botany. 2013;64:1471–1483. doi: 10.1093/jxb/ers386. [DOI] [PubMed] [Google Scholar]
- Morris VJ, Gunning AP, Kirby AR, Round A, Waldron K. Atomic force microscopy of plant cell wall, plant cell wall polysaccharides and gels. International Journal of Biological Macromolecules. 1997;21:61–66. doi: 10.1016/s0141-8130(97)00042-1. [DOI] [PubMed] [Google Scholar]
- Morris VJ, Mackie AR, Wilde PJ, Kirby AR, Mills EC, Gunning AP. Atomic force microscopy as a tool for interpreting the rheology of food biopolymers at the molecular level. Food Science and Technology. 2001;34:3–10. [Google Scholar]
- Morris VJ, Kirby AR, Gunning AP. Atomic force microscopy for biologists. London: Imperial College Press; 2010. [Google Scholar]
- Morris VJ, Gromer A, Kirby AR, Bongaerts RJM, Gunning AP. Using AFM and force spectroscopy to determine pectin structure and (bio) functionality. Food Hydrocolloids. 2011;25:230–237. [Google Scholar]
- Orfila C, Huisman MMH, Willats WGT, et al. Altered cell wall disassembly during ripening of Cnr tomato fruit: implications for cell adhesion and fruit softening. Planta. 2002;215:440–447. doi: 10.1007/s00425-002-0753-1. [DOI] [PubMed] [Google Scholar]
- Osorio S, Castillejo C, Quesada MA, et al. Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defence responses in wild strawberry (Fragaria vesca) Plant Journal. 2008;54:43–55. doi: 10.1111/j.1365-313X.2007.03398.x. [DOI] [PubMed] [Google Scholar]
- Popper ZA, Fry SC. Widespread occurrence of a covalent linkage between xyloglucan and acidic polysaccharides in suspension-cultured angiosperm cells. Annals of Botany. 2005;96:91–99. doi: 10.1093/aob/mci153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posé S, Kirby AR, Mercado JA, Morris VJ, Quesada MA. Structural characterization of cell wall pectin fractions in ripe strawberry fruits using AFM. Carbohydrate Polymers. 2012;88:882–890. [Google Scholar]
- Posé S, Paniagua C, Cifuentes M, Blanco-Portales R, Quesada MA, Mercado JA. Insights into the effects of polygalacturonase FaPG1 gene silencing on pectin matrix disassembly, enhanced tissue integrity, and firmness in ripe strawberry fruits. Journal of Experimental Botany. 2013;64:3803–3815. doi: 10.1093/jxb/ert210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanna V, Prabha TN, Tharanathan RN. Fruit ripening phenomena – an overview. Critical Reviews in Food Science and Nutrition. 2007;47:1–19. doi: 10.1080/10408390600976841. [DOI] [PubMed] [Google Scholar]
- Quesada MA, Blanco-Portales R, Posé S, et al. Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening. Plant Physiology. 2009;150:1022–1032. doi: 10.1104/pp.109.138297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgwell RJ, Melton LD, Brasch DJ. Cell wall dissolution in ripening kiwifruit (Actinidia deliciosa): solubilisation of the pectic polymers. Plant Physiology. 1992;98:71–81. doi: 10.1104/pp.98.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgwell RJ, Macrae E, Hallett I, Fischer M, Perry J, Harker R. In vivo and in vitro swelling of cell walls during fruit ripening. Planta. 1997a;203:162–173. [Google Scholar]
- Redgwell RJ, Fischer M, Kendall E, MacRae EA, Perry J, Harker R. Galactose loss and fruit ripening: high-molecular-weight arabinogalactans in the pectic polysaccharides of fruit cell walls. Planta. 1997b;203:174–181. [Google Scholar]
- Rose JKC, Hadfield KA, Labavitch JM, Bennett AB. Temporal sequence of cell wall disassembly in rapidly ripening melon fruit. Plant Physiology. 1998;117:345–361. doi: 10.1104/pp.117.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round AN, MacDougall AJ, Ring SG, Morris VJ. Unexpected branching in pectin observed by atomic force microscopy. Carbohydrate Research. 1997;303:251–253. doi: 10.1016/s0008-6215(01)00039-8. [DOI] [PubMed] [Google Scholar]
- Round AN, Rigby NM, MacDougall AJ, Ring SG, Morris VJ. Investigating the nature of branching in pectin by atomic force microscopy and carbohydrate analysis. Carbohydrate Research. 2001;331:337–342. doi: 10.1016/s0008-6215(01)00039-8. [DOI] [PubMed] [Google Scholar]
- Round AN, Rigby NM, MacDougall AJ, Morris VJ. A new view of pectin structure revealed by acid hydrolysis and atomic force microscopy. Carbohydrate Research. 2010;345:487–497. doi: 10.1016/j.carres.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Saladié M, Matas AJ, Isaacson T, et al. A reevaluation of the key factors that influence tomato fruit softening and integrity. Plant Physiology. 2007;144:1012–1028. doi: 10.1104/pp.107.097477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Doménech N, Jiménez-Bermúdez S, Matas AJ, et al. Antisense inhibition of a pectate lyase gene supports a role for pectin depolymerization in strawberry fruit softening. Journal of Experimental Botany. 2008;59:2769–2779. doi: 10.1093/jxb/ern142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJS, Watson CF, Morris PC, et al. Inheritance and effect on ripening of antisense polygalacturonase genes in transgenic tomatoes. Plant Molecular Biology. 1990;14:369–379. doi: 10.1007/BF00028773. [DOI] [PubMed] [Google Scholar]
- Smith DL, Abbott JA, Gross KC. Down-regulation of tomato β-galactosidase 4 results in decreased fruit softening. Plant Physiology. 2002;129:1755–1762. doi: 10.1104/pp.011025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault J-F, Renard CMGC, Axelos MAV, Roger P, Crépeau M-J. Studies of the length of homogalacturonic regions in pectins by acid hydrolysis. Carbohydrate Research. 1993;238:271–286. [Google Scholar]
- Thomas TR, Shackel KA, Matthews MA. Mesocarp cell turgor in Vitis vinifera L. berries throughout development and its relation to firmness, growth, and the onset of ripening. Planta. 2008;228:1067–1076. doi: 10.1007/s00425-008-0808-z. [DOI] [PubMed] [Google Scholar]
- Tieman DM, Handa AK. Reduction in pectin methylesterase activity modifies tissue integrity and cation levels in ripening tomato (Lycopersicon esculentum Mill.) fruits. Plant Physiology. 1994;106:429–436. doi: 10.1104/pp.106.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivonen PM, Brummell DA. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biology and Technology. 2008;48:1–14. [Google Scholar]
- Vicente AR, Ortugno C, Powell ALT, Greve LC, Labavitch JM. Temporal sequence of cell wall disassembly events in developing fruits. 1. Analysis of raspberry (Rubus idaeus) Journal of Agricultural and Food Chemistry. 2007;55:4119–4124. doi: 10.1021/jf063547r. [DOI] [PubMed] [Google Scholar]
- Vincken JP, Schols HA, Oomen RJFJ, et al. If homogalacturonan were a side chain of rhamnogalacturonan I. Implications for cell wall architecture. Plant Physiology. 2003;132:1781–1789. doi: 10.1104/pp.103.022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voragen A, Coenen G, Verhoef R, Schols H. Pectin, a versatile polysaccharide present in plant cell walls. Structural Chemistry. 2009;20:263–275. [Google Scholar]
- Wada H, Matthews MA, Shackel KA. Seasonal pattern of apoplastic solute accumulation and loss of cell turgor during ripening of Vitis vinifera fruit under field conditions. Journal of Experimental Botany. 2009;60:1773–1781. doi: 10.1093/jxb/erp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K. Changes in cell wall polysaccharides during fruit ripening. Journal of Plant Research. 2000;113:231–237. [Google Scholar]
- Wakabayashi K, Chun J-P, Huber DJ. Extensive solubilization and depolymerization of cell wall polysaccharides during avocado (Persea americana) ripening involves concerted action of polygalacturonase and pectinmethylesterase. Physiologia Plantarum. 2000;108:345–352. [Google Scholar]
- Walkinshaw MD, Arnott S. Conformations and interactions of pectins. I. X-ray diffraction analyses of sodium pectate in neutral and acidified forms. Journal of Molecular Biology. 1981;153:1055–1073. doi: 10.1016/0022-2836(81)90467-8. [DOI] [PubMed] [Google Scholar]
- Wang H, Chen F, Yang H, Chen Y, Zhang L, An H. Effects of ripening stage and cultivar on physicochemical properties and pectin nanostructure of jujubes. Carbohydrate Polymers. 2012;89:1180–1188. doi: 10.1016/j.carbpol.2012.03.092. [DOI] [PubMed] [Google Scholar]
- Willats WG, McCartney L, Mackie W, Knox JP. Pectin: cell biology and prospects for functional analysis. Plant Molecular Biology. 2001;47:9–27. [PubMed] [Google Scholar]
- Xin Y, Chen F, Yang H, Zhang P, Deng Y, Yang B. Morphology, profile and role of chelate-soluble pectin on tomato properties during ripening. Food Chemistry. 2010;121:372–380. [Google Scholar]
- Yang H, An H, Feng G, Li Y, Lai S. Atomic force microscopy of the water-soluble pectin of peaches during storage. European Food Research and Technology. 2005;220:587–591. [Google Scholar]
- Yang H, Lai S, An H, Li Y. Atomic force microscopy study of the ultrastructural changes of chelate-soluble pectin in peaches under controlled atmosphere storage. Postharvest Biology and Technology. 2006a;39:75–83. [Google Scholar]
- Yang H-S, Feng G-P, An H-J, Li Y-F. Microstructure changes of sodium carbonate-soluble pectin of peach by AFM during controlled atmosphere storage. Food Chemistry. 2006b;94:179–192. [Google Scholar]
- Yang H, Chen F, An H, Lai S. Comparative studies on nanostructures of three kinds of pectins in two peach cultivars using atomic force microscopy. Postharvest Biology and Technology. 2009;51:391–398. [Google Scholar]
- Yapo BM, Lerouge P, Thibault J-F, Ralet M-C. Pectins from citrus peel cell walls contain homogalacturonans homogeneous with respect to molar mass, rhamnogalacturonan I and rhamnogalacturonan II. Carbohydrate Polymers. 2007;69:426–435. [Google Scholar]
- Zareie MH, Gokmen V, Javadipour I. Investigating network, branching, gelation and enzymatic degradation in pectin by atomic force microscopy. Journal of Food Science and Technology. 2003;40:169–172. [Google Scholar]
- Zhang L, Chen F, An H, et al. Physicochemical properties, firmness, and nanostructures of sodium carbonate-soluble pectin of 2 Chinese cherry cultivars at 2 ripening stages. Journal of Food Science. 2008;73:N17–N22. doi: 10.1111/j.1750-3841.2008.00799.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen F, Yang H, et al. Changes in firmness, pectin content and nanostructure of two crisp peach cultivars after storage. Food Science and Technology. 2010;43:26–32. [Google Scholar]
- Zhang L, Chen F, Yang H, et al. Effects of temperature and cultivar on nanostructural changes of water-soluble and chelate-soluble pectin in peaches. Carbohydrate Polymers. 2012;87:816–821. doi: 10.1016/j.carbpol.2011.08.074. [DOI] [PubMed] [Google Scholar]
- Zykwinska AW, Ralet MCJ, Garnier CD, Thibault JFJ. Evidence for in vitro binding of pectin side chains to cellulose. Plant Physiology. 2005;139:397–407. doi: 10.1104/pp.105.065912. [DOI] [PMC free article] [PubMed] [Google Scholar]