Abstract
Background and Aims
Pectin is a complex macromolecule, the fine structure of which is influenced by many factors. It is used as a gelling, thickening and emulsifying agent in a wide range of applications, from food to pharmaceutical products. Current industrial pectin extraction processes are based on fruit peel, a waste product from the juicing industry, in which thousands of tons of citrus are processed worldwide every year. This study examines how pectin components vary in relation to the plant source (orange, lemon, lime, grapefruit) and considers the influence of extraction conditions on the chemical and macromolecular characteristics of pectin samples.
Methods
Citrus peel (orange, lemon, lime and grapefruit) from a commercial supplier was used as raw material. Pectin samples were obtained on a bulk plant scale (kilograms; harsh nitric acid, mild nitric acid and harsh oxalic acid extraction) and on a laboratory scale (grams; mild oxalic acid extraction). Pectin composition (acidic and neutral sugars) and physicochemical properties (molar mass and intrinsic viscosity) were determined.
Key Results
Oxalic acid extraction allowed the recovery of pectin samples of high molecular weight. Mild oxalic acid-extracted pectins were rich in long homogalacturonan stretches and contained rhamnogalacturonan I stretches with conserved side chains. Nitric acid-extracted pectins exhibited lower molecular weights and contained rhamnogalacturonan I stretches encompassing few and/or short side chains. Grapefruit pectin was found to have short side chains compared with orange, lime and lemon. Orange and grapefruit pectin samples were both particularly rich in rhamnogalacturonan I backbones.
Conclusions
Structural, and hence macromolecular, variations within the different citrus pectin samples were mainly related to their rhamnogalacturonan I contents and integrity, and, to a lesser extent, to the length of their homogalacturonan domains.
Keywords: Pectin, plant cell wall polysaccharide, Citrus peel, Rutaceae, orange, lime, lemon, grapefruit, industrial extraction, homogalacturonan, HG, rhamnogalacturonan, RGI, RGII
INTRODUCTION
Pectin is an important cell wall polysaccharide of higher plants. Pectin exists particularly in the middle lamella and the primary cell walls of dicotyledonous plants, where it plays a fundamental role in cell growth (Palin and Geitmann, 2012), mechanical strength (Wolf et al., 2009) and defence mechanisms (Lionetti et al., 2012). In industrial extraction processes pectin is mainly derived from citrus peel. It is used as a gelling, thickening and emulsifying agent in a wide range of applications from food to pharmaceutical products.
Pectin is a structurally complex polymer with at least 17 different monosaccharides interconnected through more than 20 different linkages (Ridley et al., 2001). The pectin polymer comprises different structural domains that are covalently linked one to another (Harholt et al., 2010). Pectin comprises predominantly homogalacturonan (HG), consisting of a linear backbone of consecutive 1,4-linked-α-d-galacturonic acid (GalA) units. GalA residues are partly methylesterified at C-6 (Voragen et al., 1995) and sometimes acetylesterified at O-2 or O-3 (Ralet et al., 2005). The degree and pattern of methylesterification are closely related to the plant origin, to the tissue from which the pectin was extracted and, in the case of fruit tissue, to the stage of maturation. HG domains have been isolated by acid hydrolysis (72 h) from commercial citrus pectin samples (Thibault et al., 1993; Ralet et al., 2008) and from citrus pectin samples recovered after sequential extraction of dry peel (Yapo et al., 2007). Average degrees of polymerization (dp) of 71–117 consecutive GalA residues were constantly observed. Similarly, an average dp of 97–116 was observed for HG domains isolated from citrus pectin samples by enzymatic means (Hellin et al., 2005). However, Round et al. (2010) estimated a dp of ∼300 for HG domains isolated from green tomato chelating agent-extracted pectin samples using a shorter acid hydrolysis treatment (24 h).
Rhamnogalacturonan I (RGI) contains a backbone consisting of [4-α-d-GalpA-(1,2)-α-l-Rhap-(1] disaccharide repeating units. Between 20 and 80 % of the rhamnose (Rha) residues are substituted at C-4, depending on the plant source and the extraction conditions used (Vincken et al., 2003). RGI side chains contain α-l-arabinofuranosyl (Ara) and/or β-d-galactopyranosyl (Gal) residues as linear and/or branched oligosaccharides or polysaccharides (Albersheim et al., 1996). The neutral sugar type and distribution vary between pectic fractions according to the origin of the pectin, the extraction conditions and the stage of tissue development (Round et al., 2001). Substituted HGs such as apiogalacturonan, xylogalacturonan and rhamnogalacturonan II (RGII) are less frequent building blocks of pectin (Mohnen, 2008). The way in which the different domains are linked to one another to form the pectin macromolecule remains unclear. To date, two types of model have been proposed. In the first model the pectin backbone consists of alternating RGI and HG domains (de Vries, 1981; Schols and Voragen, 1996). In the second model the backbone comprises RGI only, HG domains being connected to it as side chains (Vincken et al., 2003). In addition, connecting points between xylogalacturonan (XGA)/HG and RGI were identified, favouring the ‘alternating model’. However, as fully discussed in Coenen et al. (2007), it cannot be ruled out that only some of the connecting points have been identified. A surplus of HG domains has indeed been quantified, with an average of 17 HG domains for one RGI domain in acid-extracted lemon pectins (Ralet and Thibault, 2009). The surplus of HG domains is likely to be explained by their allocation as side chains attached to RGI of an HG–RGI backbone (Ralet and Thibault, 2009; Yapo, 2011).
Current industrial pectin extraction processes are based on fruit peel, a waste product from the juicing industry, in which thousands of tons of citrus (orange, lemon and lime) are processed worldwide every year. As a pre-treatment before pectin extraction, washing and drying of the peel provides necessary preservation for storage and/or transport. Afterwards, commercial pectin is extracted at high temperature by acid hydrolysis. The final product has a wide range of applications related to its GalA and neutral sugar content, the degree and pattern of methylesterification, molecular weight and intrinsic viscosity. The length of HG and the proportions of HG, RGI and RGII in the molecule may also influence pectin properties (Bonnin et al., 2002). Thus, a thorough investigation of these characteristics could provide further information about pectin applicability and lead to a better understanding of pectin variability and functionality in muro. In the present study, different extraction conditions were applied to commercial peel from various citrus sources. Sugar composition and macromolecular characteristics of the extracted pectin samples and subsequently isolated HG domains were determined. The main objective of the study was to determine the effect of the extraction conditions on pectin characteristics and to understand possible structural and macromolecular variations related to citrus sources.
MATERIALS AND METHODS
Plant material
CP Kelco kindly provided commercially available dry peel of orange, lemon, lime, and grapefruit. Because of the source of supply, it is not possible to give precise names of species.
Pectin extraction
Pectin samples were obtained from dry citrus peel using four different extraction methods and named according to the type of acid, the pH and the duration of extraction: mild nitric acid extraction (MN), harsh nitric acid extraction (HN), mild oxalic acid extraction (MO) and harsh oxalic acid extraction (HO). The extraction conditions (scale, acid type, temperature, pH and duration of extraction) are provided in Table 1.
Table 1.
Extraction conditions
| Scale | Acid type | Temperature (°C) | pH | Extraction time (h) | |
|---|---|---|---|---|---|
| MN | Bulk plant (kg) | Nitric | 72 | 2·1 | 3 |
| HN | Bulk plant (kg) | Nitric | 70 | 1·6 | 7 |
| MO | Laboratory (g) | Oxalic | 85 | 4·6 | 0·5 × 3a |
| HO | Bulk plant (kg) | Oxalic | 72 | 3·5 | 2·5 |
MN, mild nitric acid extraction; HN, harsh nitric acid extraction; MO, mild oxalic acid extraction; HO, harsh oxalic acid extraction.
aSequential extraction.
Dry peel was subjected to oxalic acid extraction at laboratory scale (MO). Ten grams of dry citrus peel was mixed with 400 mL of 0·25 % (w/v) ammonium oxalate (pH was adjusted to 4·6 with 12 mL of 0·2 m oxalic acid). The mixture was incubated at 40 °C for 30 min and then at 85 °C for 30 min. The slurry was filtered through a G3 sintered glass filter. The insoluble residue was mixed with 300 mL of oxalate buffer and extracted at 85 °C for 30 min. The process was repeated twice. The solution was concentrated by evaporation at 40 °C, extensively dialysed against distilled water and freeze-dried.
Dry peel was subjected to nitric acid or oxalic acid extraction at bulk plant scale. One kilogram of dry peel was mixed with 30 L of hot ion-exchanged water and the extraction temperature was set at 72 °C. To achieve the specific extraction pH, the corresponding acid was added to the hot water solution (nitric acid extraction, pH 1·6 or 2·1; oxalic acid extraction, pH 3·5). When the extraction duration was complete, 400 g cellulose pulp was dispersed in 10 L of hot deionized water and the slurry was added to the peel. The mixture was thoroughly mixed before filtration. The filtered juice was then subjected to ion exchange by adding the ion-exchange resin Amberlite™ IR-120-H (50 mL/1 L filtrate). After 30 min, the resin was removed by filtration through a nylon cloth. Three volumes of isopropanol were added to the pectin containing filtrate. The precipitated pectin was squeezed in a nylon filter cloth. Ten litres of isopropanol/water (60/40, vol/vol) was added to the precipitate and the pH of the suspension was adjusted to 3·5 with 10 % nitric acid. The precipitated pectin was squeezed in a nylon cloth and placed in a drying cabinet (67 °C, maximum 24 h). Dry pectin was milled and sieved (250 μm) in a Retsch ZM 200 milling instrument. Bulk plant-scale extractions were performed in triplicate and the extraction yield was calculated as follows:
where Mf and Mi represent the final mass of dry pectin (g) obtained and the initial mass of peel (g) used for the extraction, respectively. As a correction factor, Vs (volume of extraction solvent, L) was divided by Vj (volume of extracted juice, L) to account for volume losses before precipitation in isopropanol, which could happen while handling large volumes during bulk plant-scale extractions.
Monosacchargide analysis of dry peel and pectin samples
The galacturonic acid content of the peel and pectin samples and the subsequent acid-soluble and acid-insoluble fractions were determined colorimetrically by the automated meta-hydroxydiphenyl method (Thibault, 1979). Total neutral sugar contents were determined by the automated orcinol method (Tollier and Robin, 1979).
Twenty-five milligrams of ultra-ground dry peel (< 50 μm) was pre-hydrolysed in 13 m H2SO4 for 30 min at 25 °C, prior to hydrolysis in 1 m H2SO4 for 3 h at 100 °C. Pectin samples were hydrolysed in 2 m trifluoroacetic acid for 2 h 30 min at 120 °C. Sugars were reduced to their corresponding alditols and acetylated as described by Blakeney et al. (1983). Individual neutral sugars were analysed by gas chromatography. Extractability of sugars from peel was calculated as:
Preparation of homogalacturonans
Pectin samples were de-esterified as previously described (Thibault et al., 1993). Five hundred and fifty milligrams of pectin was solubilized in 40 mL of ice-cold distilled water. Subsequently, pH was adjusted to 12 with 1 m NaOH at 4 °C. The pH was re-adjusted to 12 after 1 and 14 h. One hour after the final adjustment, pH was decreased to pH 5 with 1 m HCl. Samples were dialysed against distilled water and de-esterified pectin solutions were kept at –18 °C.
A volume of 13·5 mL of de-esterified pectin solution was vigorously mixed with 1·5 mL of 1 m HCl. Samples were incubated in an oil bath for 24 h at 80 °C (Round et al., 2010). Samples were then centrifuged and the precipitate was separated from the soluble fraction. The precipitate was washed twice with 3 mL of 0·1 m HCl, suspended in 10 mL of distilled water, solubilized by increasing the pH to 6 with 1 m LiOH, and dialysed against distilled water. Prior to analysis, the soluble fraction was neutralized with 1 m NaOH.
Determination of macromolecular features
Thirty milligrams of pectin powder was solubilized in 7 mL of distilled water by stirring for 4 h at room temperature. The pH was adjusted to 6 with 1 m LiOH and the final volume was adjusted to 10 mL with distilled water. Samples were filtered through a syringe-driven filter unit (pore size 0·45 μm) before injection.
Molecular weight and intrinsic viscosity were determined at room temperature after high-performance size-exclusion chromatography. Elution was performed on one Shodex OH SB-G pre-column followed by two columns in series (OH-Pack SB-805 HQ and Shodex OH-Pack SB-804 HQ) with 0·05 m NaNO3 buffer at a constant flow rate of 42 mL h–1. A differential refractometer, a light-scattering device (low-angle light scattering and right-angle light scattering) and a differential viscosimeter (Viscotek, Malvern Instruments, Malvern, UK) were used as detectors.
RESULTS
Dry peel monosaccharide composition and extraction yields
The monosaccharide composition of dry citrus peel samples is summarized in Table 2. The peel samples were rich in GalA, glucose (Glc), Ara and Gal. Small amounts of xylose (Xyl), mannose (Man), Rha and fucose (Fuc) were also detected. These findings are in general agreement with those of Koubala et al. (2008). The highest GalA content was found in lime peel and the highest Rha content in orange and grapefruit peel. Minor variations were observed for Ara between peel samples. In contrast, Gal content varied significantly, orange and lemon peel samples exhibiting the highest Gal content. Variation in monosaccharide composition between the four citrus sources indicated minor heterogeneity in pectin structure. As previously reported, citrus peel samples were particularly rich in GalA (Ros et al., 1996) but poor in Ara and Gal compared with the other plant sources. For instance, a higher Ara content was reported for sugar beet pulp (Guillon and Thibault, 1988) and a higher Gal content for soybean meal (Huisman et al., 1998) and potato tuber (Vincken, 2000).
Table 2.
Monosaccharide compositions of dry peel samples (weight, %) and extraction yields (mg extract g–1 dry peel)
| Orange | Lemon | Lime | Grapefruit | |
|---|---|---|---|---|
| Sugars | ||||
| GalA | 24·08 ± 0·40 | 24·33 ± 1·24 | 29·74 ± 0·58 | 25·91 ± 0·58 |
| Rha | 1·54 ± 0·03 | 1·19 ± 0·18 | 1·14 ± 0·03 | 1·31 ± 0·05 |
| Ara | 7·85 ± 0·06 | 8·58 ± 0·36 | 8·78 ± 0·97 | 6·66 ± 0·31 |
| Gal | 5·37 ± 0·32 | 5·39 ± 0·59 | 2·87 ± 0·33 | 3·67 ± 0·02 |
| Glc | 18·50 ± 0·39 | 20·21 ± 0·94 | 16·57 ± 1·08 | 18·66 ± 0·02 |
| Xyl | 2·66 ± 0·02 | 2·64 ± 0·10 | 2·91 ± 0·21 | 3·12 ± 0·11 |
| Man | 1·87 ± 0·08 | 2·70 ± 0·52 | 1·95 ± 0·27 | 2·08 ± 0·24 |
| Fuc | 0·36 ± 0·01 | 0·57 ± 0·81 | 0·34 ± 0·02 | 0·44 ± 0·02 |
| Extraction yields | ||||
| MN | 167 ± 6·7 | 209 ± 3·2 | 269 ± 7·2 | 234 ± 1·7 |
| HN | 248 ± 4·3 | 306 ± 51·3 | 336 ± 35·2 | 273 ± 3·8 |
| MO | 185 ± – | 226 ± – | 291 ± – | 216 ± – |
| HO | 167 ± 2·1 | 221 ± 3·2 | 297 ± 26·2 | 280 ± 8·9 |
Data (± s.d.) are average of duplicates for monosaccharide compositions and triplicates for yields.
–, no data.
MN, mild nitric acid extraction; HN, harsh nitric acid extraction; MO, mild oxalic acid extraction; HO, harsh oxalic acid extraction.
Extraction yields varied from 167 to 336 mg g–1 of dry peel depending on plant source and extraction conditions (Table 2). Whatever the extraction condition, the lowest yields were obtained with orange and the highest with lime. With respect to extraction conditions, the highest yields were obtained by the HN method. Yield decreased significantly using the same acid but at higher pH (MN). Except for grapefruit, yields obtained by the use of oxalic acid (HO and MO) were roughly similar to yields obtained by the use of MN, although the extraction pHs differed. Levigne et al. (2002), using hydrochloric acid on sugar beet pulp, showed that pH was the main parameter influencing pectin extraction yield.
In the present study, not only pH but also acid type were important parameters acting on extraction yield.
Monosaccharide composition of pectin samples and extractability of individual sugars
All pectin samples were rich in GalA. The major neutral sugars were Ara, Gal and Rha. Trace amounts of Glc, Xyl and Man were also detected (Supplementary Data Table S1).
Both the type of acid used for extraction and the plant source had an influence on the monosaccharide composition of the pectin samples. Regarding plant source, orange and grapefruit pectin samples displayed slightly increased content of Rha compared with lemon and lime. Moreover, orange pectin samples had the lowest GalA content and the highest Ara and Gal contents. With respect to extraction conditions, for both acid types, GalA content rose significantly (P = 0·05) with increasing extraction temperature and time. Moreover, GalA content was higher in nitric acid-extracted pectin samples compared with oxalic acid-extracts. Similar variations were observed with respect to Rha: low pH increased Rha content significantly (P < 0·05). Decreasing pH along with increasing extraction time led to significant degradation of Ara side chains (P < 0·05). Conversely, Gal content increased almost linearly with decreasing pH.
In order to provide an overview of monosaccharide variation with respect to plant source and extraction conditions, GalA/Rha and (Ara + Gal)/Rha ratios were calculated. Since Ara and Gal are the major sugars in side chains and Rha is the branching point, the molar ratio between the former and the latter can provide a global insight into RGI decoration (amount and/or length).
Whatever the extraction condition, grapefruit pectin samples exhibited particularly low (Ara + Gal)/Rha ratios compared with pectin samples extracted from the other citrus peel samples, which all behaved similarly (Fig. 1A). Grapefruit pectin samples exhibited high Rha and moderate Gal and Ara contents (Supplementary Table 1). Although orange, lemon and lime pectin samples exhibited very similar (Ara + Gal)/Rha ratios, their structure is likely to differ. Indeed, orange pectin samples exhibited high Rha, Gal and Ara contents (Supplementary Table 1). In contrast, lemon and lime pectin samples exhibited moderate Rha, Gal and Ara contents. With respect to extraction conditions, higher (Ara + Gal)/Rha ratios were observed for pectin samples extracted with oxalic acid (MO and HO), due to both high Ara contents and low Rha contents. Conversely, the very low (Ara + Gal)/Rha ratio observed for pectin samples extracted with the HN method was due to both high Rha content and low Ara content.
Fig. 1.
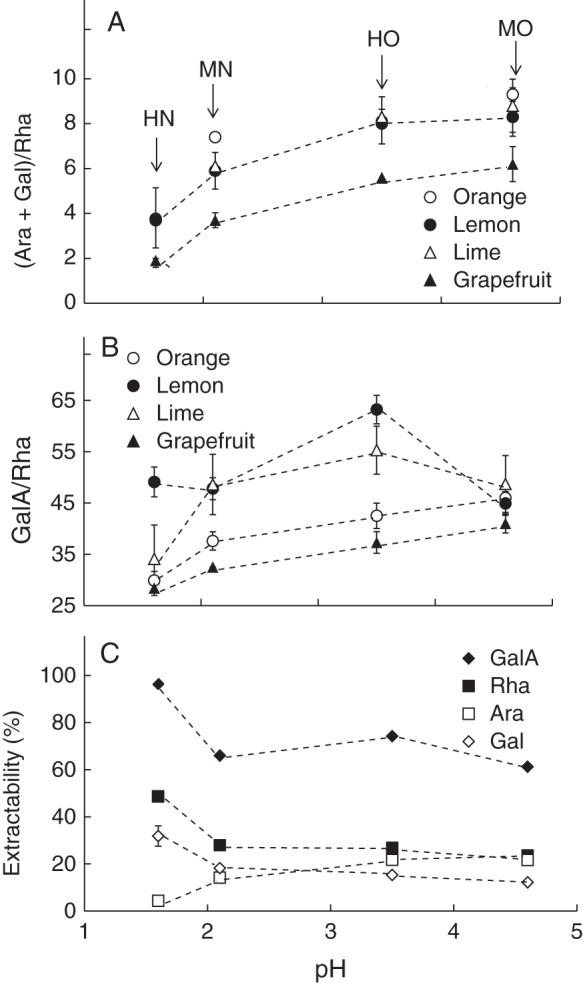
Monosaccharide composition of pectin samples with respect to plant source and extraction pH. HN, harsh nitric acid (pH 1·6); MN, mild nitric acid (pH 2·1); HO, harsh oxalic acid (pH 3·5); MO, mild nitric acid (pH 4·6). Standard deviations are indicated and refer to technical replicates. (A) (Ara + Gal)/Rha ratio of the different pectin samples. (B) GalA/Rha ratio of the different pectin samples. (C) Extractability [(sugar content in pectin sample × extraction yield)/sugar content in peel] of the four main sugars. Average values of the different pectin samples were used.
The ratio of GalA to Rha was calculated as a hypothetical representation of HG/RGI ratio within the pectin samples (Fig. 1B). The low GalA/Rha ratios observed for orange and grapefruit pectin samples were associated with high Rha contents (Supplementary Data Table S1). With respect to extraction conditions, the highest GalA/ Rha ratios were observed for oxalic acid extracts (HO for lemon and lime, MO for orange and grapefruit).
The extractability of each main individual monosaccharide was calculated into account taking the extraction yield and the monosaccharide composition of pectin samples and corresponding peels. Whatever the citrus source considered, the highest GalA, Rha and Gal extractability was observed for the HN method (Fig. 1C). GalA extractability was around 95 % while Rha extractability was only around 50 % and Gal around 30 %. The use of harsh extraction conditions allowed the solubilization of virtually all HG-rich pectin molecules. GalA, Rha and Gal extractability values were similar for MN, HO and MO, at ∼70, ∼30 and ∼15 %, respectively, highlighting a very modest solubilization of RGI-rich pectin under these extraction conditions. The amount of Ara detected increased with increasing pH of extraction.
All in all, the different types of acid and different pH levels implemented affected the extractability and degradability of pectin, leading to the recovery of various pectin populations, ranging from lowly branched, RGI-rich pectin together with HG-rich pectin when using nitric acid at low pH to highly branched-HG-rich pectin when using oxalic acid. Whatever the extraction condition, different pectin populations can be extracted depending on plant source. Longer and/or more numerous RGI stretches can be hypothesized for orange and grapefruit pectin samples compared with lime and lemon. Moreover, shorter and/or fewer side chains can be hypothesized for grapefruit.
Molecular weight and intrinsic viscosity of pectin samples
The extracted pectin samples were further characterized in terms of molecular weight and intrinsic viscosity (Supplementary Data Table S2; Fig. 2A, B). Molecular weight and intrinsic viscosity values were particularly low for pectin samples extracted at low pH and with a long extraction time (HN) (Table S2; Fig. 2A, B). Hydrolysis of neutral sugar side chains, particularly arabinan, and possible breakdown of the RGI backbone and eventually HG domains, can be hypothesized. In contrast, mild oxalic acid-extracted pectin samples exhibited particularly high molecular weight and intrinsic viscosity values. It is noteworthy that, irrespective of extraction conditions, orange and grapefruit pectin samples exhibited lower intrinsic viscosity values than lemon and lime pectin samples (Fig. 2B).
Fig. 2.
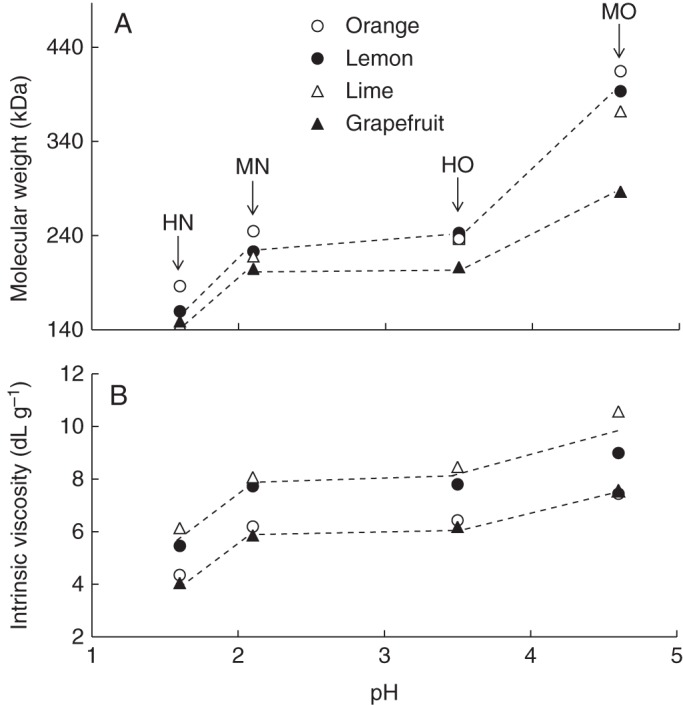
Macromolecular characteristics of pectin samples with respect to plant source and extraction pH. HN, harsh nitric acid (pH 1·6); MN, mild nitric acid (pH 2·1); HO, harsh oxalic acid (pH 3·5); MO, mild nitric acid (pH 4·6). (A) Weight-average molecular weight of the different pectin samples. (B) Intrinsic viscosity of the different pectin samples.
Macromolecular features of isolated homogalacturonans
The different pectin samples were de-esterified and subjected to mild acid hydrolysis under conditions allowing the partitioning of RGI degraded fragments, which are acid-soluble, and intact HG domains, which are acid-insoluble. Isolated homogalacturonans contained 98·5–99·3 % (mol % total sugars) GalA, indicating high purity of the isolated fractions (Supplementary Data Table S3). The amount did not vary significantly (P = 0·05) according to the pectin extraction conditions and citrus source. For a given extraction condition, isolated HG domains exhibited very similar macromolecular characteristics regardless of the pectin source (Supplementary Data Table S4). With respect to extraction conditions, HG domains isolated from HN-, MN-, and HO-extracted pectin samples exhibited similar macromolecular characteristics (Table S4). Significantly higher molecular weight values were observed for HGs isolated from MO-extracted pectin samples (Table S4).
Mark–Houwink plots and the HG/RGI relationship
The Mark–Houwink relationship between the intrinsic viscosity [η] and weight-average molecular weight (Mw):
gives information on polymer conformation, with a increasing as polymer stiffness increases.
The macromolecular characteristics of MN-extracted pectin samples and isolated HG domains were appraised using the Mark–Houwink representation in Fig. 4. For given molecular weight values, whole pectin samples exhibited lower intrinsic viscosity values than isolated HG domains. Moreover, among the three pectin samples, lime pectin exhibited the highest intrinsic viscosity throughout the molecular weight distribution. The measurable intrinsic viscosity difference between HG fragments and pectin samples increased with increasing molecular weight, suggesting that the number of RGI stretches and the (Ara + Gal)/Rha ratio increases as polymer size increases. The lime pectin curve had a high slope compared with orange and grapefruit curves, evidencing relatively higher stiffness for the former pectin sample. The pectin structure of lime resembles a stiff rod while orange and grapefruit pectins are more randomly coiled.
Fig. 4.
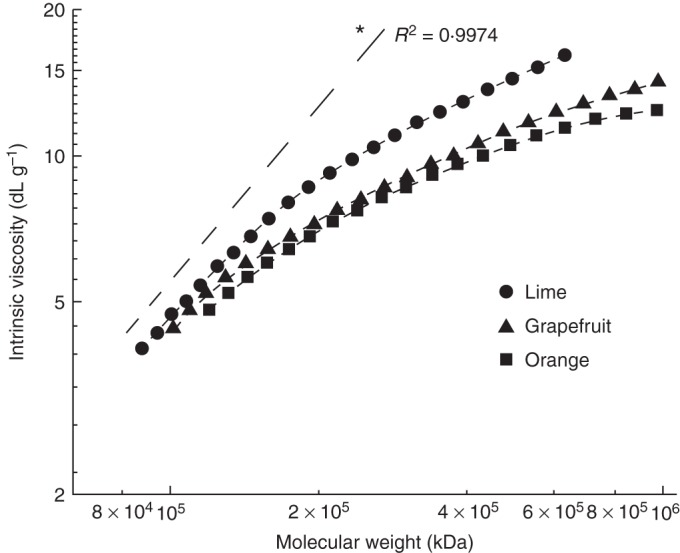
Mark–Houwink plot of log scale molecular weight vs log scale η of pectins extracted at pH 2·1 from lime pectin, grapefruit pectin and orange pectin. The dashed line was obtained by averaging nine curves from HN-, MN- and HO-extracted HGs. *P < 0·05).
DISCUSSION
Pectin structure depends on various extraction parameters, such as temperature, pH and duration (May, 1990; Kliemann et al., 2009; Methacanon et al., 2014). The present study demonstrates that both the type of acid and the pH value affected the characteristics of the extracted pectin samples. Pectin samples with different subdomain structure and diverse macromolecular characteristics were extracted.
Oxalic acid is reported to act as a chelating agent, deconstructing pectin networks by entrapping ions (mainly Ca2+) from egg-box junction zones (Aman and Westerlund, 1996). Thus, oxalic acid-based extractions are affected by how many ionic linkages the pectin has in the plant tissue (Ca2+ content and distribution of free acid groups in HG); thus the Ca2+ content of the plant tissue used and the corresponding molar ratio to oxalic acid during the extraction process must be considered. A molar excess of oxalic acid (oxalic acid/Ca2+ 1·2/1·0) is required in order to achieve optimal pectin extraction from citrus peel (Jensen et al., 2011). In the present study, MO extractions had largely sufficient amounts of oxalic acid to enable correct extraction of pectic material (Supplementary Data Table S5). The oxalic acid/Ca2+ molar ratio in lime and grapefruit HO extraction was <1·2, meaning that a higher yield may have been achieved. Nonetheless, oxalic acid allowed efficient extraction of very well preserved pectin macromolecules encompassing long and/or numerous RGI side chains together with long HG domains. Accordingly, oxalic acid-extracted pectin samples exhibited particularly high molecular weight and intrinsic viscosity values, in good agreement with previous findings on oxalic acid-extracted murta and mango pectin samples (Koubala et al., 2008) and banana pectin (Emaga et al., 2008).
Low extraction pH results in acidic hydrolysis of carbohydrates. Thibault et al. (1993) and Round et al. (2010) showed that Ara (pentose) is the most acid-labile sugar, followed by Gal and Rha, with GalA being the most resistant to acid hydrolysis. Moreover, linkages between two GalA residues are more stable than aldobiuronic (GalA-Rha) or pseudo-aldobiuronic (Rha-GalA) linkages (Ralet and Thibault, 2009). Pectin samples extracted by the HN method exhibited particularly low molecular weight and intrinsic viscosity values due to the degradation and loss of side chains, breakages in the RGI backbone and the hydrolysis of non-methylesterified segments of HG. Since the side chains only account for a maximum of 12 % of the weight of pectin, the major reason for the reduction is the breakage in the backbone.
The monosaccharide composition of the pectin samples in this work varied according to the extraction conditions applied. The observation that nitric acid-extracted pectins were richer in Rha and Gal suggests a different reaction mechanism (mainly dependent on pH) compared with oxalic acid extracts. In this respect, HO-, MN- and HN-extracted pectin samples can be defined as false negatives since degradation of arabinan lowered the Ara content. Different monosaccharide extractabilities also reflect a heterogeneous population of pectin or could represent differences in degradation of a homogeneous pool of pectin in citrus peel.
The use of different acids and pH levels affected both extractability and degradation of pectin, allowing the recovery of pectin samples with variable subdomain ratios and structure. It is, however, impossible to distinguish between the presence of different pools of pectin and variation in degradation. Oxalic acid enables dissolution of different pectin mixtures that are anchored by ionic interactions.
Subdomain structure and ratios were also shown to vary according to plant source. Grapefruit and orange pectin samples, both of which contained longer and/or more numerous RGI stretches than lime and lemon pectin samples, exhibited the lowest intrinsic viscosity values. The general stiffness of pectic molecules observed in the Mark–Houwink plots was found to correlate with their HG/RGI ratio, so that HG-rich pectin samples behaved as rather rigid macromolecules, while RGI-rich pectin samples were more flexible (Axelos and Thibault, 1991; Ralet et al., 2008). The fairly low HG/RGI ratios evidenced for orange and grapefruit pectin samples are likely to promote a rather flexible conformation, leading to decreased intrinsic viscosity values.
Additionally, the weight-average degrees of polymerization calculated for HG domains isolated from HN-, MN- and HO-extracted pectin samples (Fig. 3) were in good agreement with values reported by Thibault et al. (1993) and Hellin et al. (2005) for HG domains isolated from commercial citrus pectin. HG domains isolated from MO-extracted pectin samples exhibited a higher degree of polymerization, in agreement with the findings of Round et al. (2010) on HG domains isolated from cyclohexyldiaminetetraacetic acid-extracted green tomato pectin. These observations suggest that extractions with mild chelating agents are most likely to lead to better preservation of HG domain length.
Fig. 3.
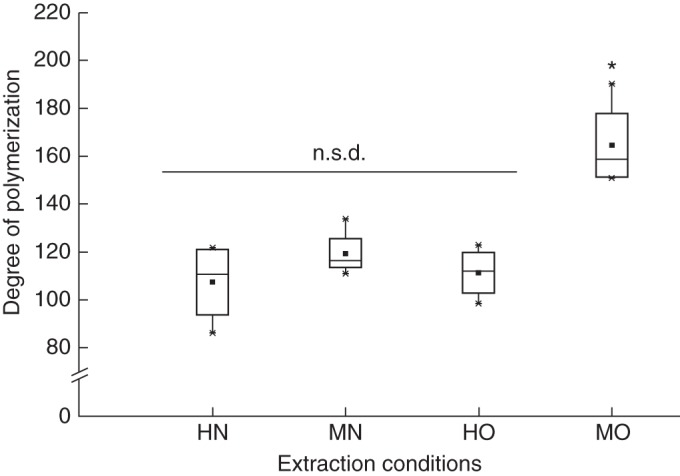
Box plot representation of the degree of polymerization of HG domains isolated from pectin samples extracted at different pH. HN, harsh nitric acid (pH 1·6); MN, mild nitric acid (pH 2·1); HO, harsh oxalic acid (pH 3·5); MO, mild nitric acid (pH 4·6). Average values of two extractions for each peel are denoted by the black squares. *P < 0·05; n.s.d., non-significant difference.
The monosaccharide composition and macromolecular differences evidenced are likely to be related to differences in the water retention capacity of the cell wall. The ratio of hydrophilic (RGI) to hydrophobic (HG) regions has been reported to contribute to the hydration of plant cell walls (Jones et al., 2003). Water binding and attachment properties of the pectin matrix have also been associated with different arabinan and arabinogalactan contents (Moore et al., 2008). It has been postulated that Myrothamnus flabellifolia plants are able to survive by developing a constrictively protective cell wall unusually rich in Ara polymers, most likely arabinan and arabinogalactan (Moore et al., 2008). Mosele et al. (2011) further provided evidence that arabinans are the main polymers making cell walls tolerant of desiccation in the bean(Tylosema esculentum. This is supported by the observation that cell wall arabinans play a crucial role in the response of guard cells to turgor pressure (Jones et al., 2003). Besides water retention, branched Ara and linear Gal side chains may be associated with physical stress distribution to load-bearing cellulose, as demonstrated by Ulvskov et al. (2005) in potato tubers. While these studies reinforce the importance of different RGI side chain compositions in muro, less is known in relation to their significance in gelling applications. Recently, Ngouémazong et al. (2012) reported decreases in stiffness and elasticity in calcium-sensitive gels after controlled removal of arabinans from carrot pectin. A similar study based on citrus pectin, focusing on the role of either arabinans or galactans in gelling applications, is ongoing.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was supported by the European Union Seventh Framework Programme FP7 2007–2013) under the WallTraC project (Grant Agreement number 263916). This article reflects the author's views only. The European Community is not liable for any use that may be made of the information contained herein.
LITERATURE CITED
- Albersheim P, Darvill AG, O'Neill MA, Schols HA, Voragen AGJ. A hypothesis: the same six polysaccharides are components of the primary cell wall of all higher plants. In: Visser J, Voragen AGJ, editors. Pectins and pectinases. Amsterdam: Elsevier Science; 1996. pp. 47–53. [Google Scholar]
- Aman P, Westerlund E. Cell wall polysaccharides: structural, chemical, and analytical aspects. In: Eliasson AC, editor. Carbohydrates in food. New York: Marcel Dekker; 1996. [Google Scholar]
- Axelos MAV, Thibault JF. Influence of the substituents of the carboxyl groups and of the rhamnose content on the solution properties and flexibility of pectin samples. International Journal of Biological Macromolecules. 1991;13:77–82. doi: 10.1016/0141-8130(91)90052-v. [DOI] [PubMed] [Google Scholar]
- Blakeney AB, Harris PJ, Henry RJ, Stone BA. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydrate Research. 1983;113:291–299. [Google Scholar]
- Bonnin E, Dolo E, Le Goff A, Thibault JF. Characterisation of pectin subunits released by an optimized combination of enzymes. Carbohydrate Research. 2002;337:1687–1696. doi: 10.1016/s0008-6215(02)00262-8. [DOI] [PubMed] [Google Scholar]
- Coenen GJ, Baks EJ, Verhoef RP, Schols HA, Voragen AGJ. Identification of the connecting linkage between homo- or xylogalacturonan and rhamnogalacturonan type I. Carbohydrate Polymers. 2007;70:224–235. [Google Scholar]
- Emaga TH, Ronkart SN, Robert C, Wathelet B, Paquot M. Characterisation of pectin samples extracted from banana peel (Musa AAA) under different conditions using an experimental design. Food Chemistry. 2008;108:463–471. doi: 10.1016/j.foodchem.2007.10.078. [DOI] [PubMed] [Google Scholar]
- Guillon F, Thibault JF. Further characterization of acid- and alkali-soluble pectins from sugar beet pulp. Food Science & Technology. 1988;21:198–205. [Google Scholar]
- Harholt J, Suttangkakul A, Scheller HV. Biosynthessis of pectin. Plant Physiology. 2010;153:384–395. doi: 10.1104/pp.110.156588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellin P, Ralet MC, Bonnin E, Thibault JF. Homogalacturonans from lime pectin samples exhibit homogenous charge density and molar mass distribution. Carbohydrate Polymers. 2005;60:307–317. [Google Scholar]
- Huisman MMH, Schols HA, Voragen AGJ. Cell wall polysaccharides from soybean (Glycine max) meal. Isolation and characterisation. Carbohydrate Polymers. 1998;37:87–95. [Google Scholar]
- Jensen SW, Sørensen SO, Rollin C. Process for extraction of pectin. United States: C. K. APS; 2011. [Google Scholar]
- Jones L, Milne JL, Ashford D, McQueen-Mason SJ. Cell wall arabinan is essential for guard cell function. Proceedings of the National Academy of Sciences of the USA. 2003;100:11783–11788. doi: 10.1073/pnas.1832434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliemann E, Nunes de Simas K, Amante ER, et al. Optimisation of pectin acid extraction from passion fruit peel (Passiflora edulis flavicarpa) using response surface methodology. International Journal of Food Science and Technology. 2009;44:476–483. [Google Scholar]
- Koubala BB, Kansci G, Mbome LI, Crepeau MJ, Thibault JF, Ralet MC. Effect of extraction conditions on some physicochemical characteristics of pectin samples from ‘Améliorée’ and ‘Mango’ mango peel. Food Hydrocolloids. 2008;22:1345–1351. [Google Scholar]
- Levigne S, Ralet MC, Thibault JF. Characterisation of pectin samples extracted from fresh sugar beet under different conditions using an experimental design. Carbohydrate Polymers. 2002;49:145–153. [Google Scholar]
- Lionetti V, Cervone F, Bellincampi D. Methyl esterification of pectin plays a role during plant-pathogen interactions and affects plant resistance to diseases. Journal of Plant Physiology. 2012;169:1623–1630. doi: 10.1016/j.jplph.2012.05.006. [DOI] [PubMed] [Google Scholar]
- May CD. Industrial pectin samples: sources, production and applications. Carbohydrate Polymers. 1990;12:79–99. [Google Scholar]
- Methacanon P, Krongsin J, Gamonpilas C. Pomelo (Citrus maxima) pectin: effects of extraction parameters and its properties. Food Hydrocolloids. 2014;35:383–391. [Google Scholar]
- Mohnen D. Pectin structure and biosynthesis. Current Opinion in Plant Biology. 2008;11:266–277. doi: 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Moore JP, Farrant JM, Driouich A. A role for pectin-associated arabinans in maintaining the flexibility of the plant cell wall during water deficit stress. Plant Signaling & Behavior. 2008;3:102–104. doi: 10.4161/psb.3.2.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosele MM, Hansen AS, Engelsen SB, et al. Characterisation of the arabinose-rich carbohydrate composition of immature and mature marama beans (Tylosema esculentum) Phytochemistry. 2011;72:1466–1472. doi: 10.1016/j.phytochem.2011.03.027. [DOI] [PubMed] [Google Scholar]
- Ngouémazong DE, Kabuye G, Fraeye I, et al. Effect of debranching on the rheological properties of Ca2+-pectin gels. Food Hydrocolloids. 2012;26:44–53. [Google Scholar]
- Palin R, Geitmann A. The role of pectin in plant morphogenesis. BioSystems. 2012;109:397–402. doi: 10.1016/j.biosystems.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Ralet MC, Cabrera JC, Bonnin E, Quéméner B, Hellin P, Thibault JF. Mapping sugar beet acetylation pattern. Phytochemistry. 2005;66:1832–1843. doi: 10.1016/j.phytochem.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Ralet MC, Crepeau MJ, Lefebvre J, Mouille G, Höfte H, Thibault JF. Reduced number of homogalacturonan domains in pectins of an arabidopsis mutant enhances the flexibility of the polymer. Biomacromolecules. 2008;9:1454–1460. doi: 10.1021/bm701321g. [DOI] [PubMed] [Google Scholar]
- Ralet MC, Thibault JF. Hydrodynamic properties of isolated pectin domains: a way to figure out pectin macromolecular structure? In: Schols HA, Visser RGF, Voragen AGJ, editors. Pectins and pectinases. Wageningen: Wageningen Academic Publishers; 2009. pp. 35–46. [Google Scholar]
- Ridley BL, O'Neil MA, Mohnen DA. Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry. 2001;57:929–967. doi: 10.1016/s0031-9422(01)00113-3. [DOI] [PubMed] [Google Scholar]
- Ros JM, Schols HA, Voragen AGJ. Extraction, characterisation, and enzymatic degradation of lemon peel pectins. Carbohydrate Research. 1996;282:271–284. [Google Scholar]
- Round AN, Rigby NM, MacDougall AJ, Ring SG, Morris VJ. Investigating the nature of branching in pectin by atomic force microscopy and carbohydrate analysis. Carbohydrate Research. 2001;331:337–342. doi: 10.1016/s0008-6215(01)00039-8. [DOI] [PubMed] [Google Scholar]
- Round AN, Rigby NM, MacDougall AJ, Morris VJ. A new view of pectin structure revealed by acid hydrolysis and atomic force microscopy. Carbohydrate Research. 2010;345:487–497. doi: 10.1016/j.carres.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Schols HA, Voragen AGJ. Complex pectins: structure elucidation using enzymes. In: Visser J, Voragen AGJ, editors. Pectins and pectinases. Amsterdam: Elsevier Science; 1996. pp. 3–19. [Google Scholar]
- Thibault JF. Automatisation du dosage des substances pectiques par la method au meta-hydroxydiphenyl. Food Science & Technology. 1979;12:247–251. [Google Scholar]
- Thibault JF, Renard CMGC, Axelos MAV, Roger P, Crépeau MJ. Studies of the length of homogalacturonic regions in pectin samples by acid-hydrolysis. Carbohydrate Research. 1993;238:271–286. [Google Scholar]
- Tollier MT, Robin JP. Adaptation of the orcinol-sulphuric acid method for the automatic titration of total neutral sugars-conditions of application to plant-extracts. Annales de Technologie Agricole. 1979;28:1–15. [Google Scholar]
- Ulvskov P, Wium H, Bruce D, et al. Biophysical consequences of remodeling the neutral side chains of rhamnogalacturonan I in tubers of transgenic potatoes. Planta. 2005;220:609–620. doi: 10.1007/s00425-004-1373-8. [DOI] [PubMed] [Google Scholar]
- Vincken JP. Remodeling pectin structure in potato. Developments in Plant Genetics and Breeding. 2000;6:245–256. [Google Scholar]
- Vincken JP, Schols H, Oomen RJFJ, McCann MC, Ulvskov P, Voragen AGJ. If homogalacturonan were a side chain of rhamnogalaturonan I. Implications for cell wall architecture. Plant Physiology. 2003;132:1781–1789. doi: 10.1104/pp.103.022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voragen AGJ, Pilnik W, Thibault JF, Axelos MAV, Renard CMGC. Pectin samples. In: Stephan AM, editor. Food polysaccharides and their applications. New York: Marcel Dekker; 1995. pp. 287–339. [Google Scholar]
- de Vries JA, Voragen AGJ, Rombouts FM, Pilnik W. Extraction and purification of pectin samples from alcohol insoluble solids from ripe and unripe apples. Carbohydrate Polymers. 1981;1:117–127. [Google Scholar]
- Wolf S, Mouille G, Pelloux J. Homogalacturonan methyl-esterification and plant development. Molecular Plant. 2009;2:851–860. doi: 10.1093/mp/ssp066. [DOI] [PubMed] [Google Scholar]
- Yapo BM. Pectic substances: from simple pectic polysaccharides to complex pectins. A new hypothetical model. Carbohydrate Polymers. 2011;86:373–385. [Google Scholar]
- Yapo BM, Lerouge P, Thibault JF, Ralet MC. Pectin samples from citrus peel cell walls contain homogalacturonans homogenous with respect to molar mass, rhamnogalacturonan I and rhamnogalacturonan II. Carbohydrate Polymers. 2007;69:426–435. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


