Abstract
Background
Arabinogalactan proteins (AGPs) are ubiquitous in green plants. AGPs comprise a widely varied group of hydroxyproline (Hyp)-rich cell surface glycoproteins (HRGPs). However, the more narrowly defined classical AGPs massively predominate and cover the plasma membrane. Extensive glycosylation by pendant polysaccharides O-linked to numerous Hyp residues like beads of a necklace creates a unique ionic compartment essential to a wide range of physiological processes including germination, cell extension and fertilization. The vital clue to a precise molecular function remained elusive until the recent isolation of small Hyp–arabinogalactan polysaccharide subunits; their structural elucidation by nuclear magentic resonance imaging, molecular simulations and direct experiment identified a 15-residue consensus subunit as a β-1,3-linked galactose trisaccharide with two short branched sidechains each with a single glucuronic acid residue that binds Ca2+ when paired with its adjacent sidechain.
Scope
AGPs bind Ca2+ (Kd ∼ 6 μm) at the plasma membrane (PM) at pH ∼5·5 but release it when auxin-dependent PM H+-ATPase generates a low periplasmic pH that dissociates AGP–Ca2+ carboxylates (pka ∼3); the consequential large increase in free Ca2+ drives entry into the cytosol via Ca2+ channels that may be voltage gated. AGPs are thus arguably the primary source of cytosolic oscillatory Ca2+ waves. This differs markedly from animals, in which cytosolic Ca2+ originates mostly from internal stores such as the sarcoplasmic reticulum. In contrast, we propose that external dynamic Ca2+ storage by a periplasmic AGP capacitor co-ordinates plant growth, typically involving exocytosis of AGPs and recycled Ca2+, hence an AGP–Ca2+ oscillator.
Conclusions
The novel concept of dynamic Ca2+ recycling by an AGP–Ca2+ oscillator solves the long-standing problem of a molecular-level function for classical AGPs and thus integrates three fields: AGPs, Ca2+ signalling and auxin. This accounts for the involvement of AGPs in plant morphogenesis, including tropic and nastic movements.
Keywords: Arabinogalactan proteins, plant cell wall protein, calcium signalling, hydroxyproline-rich glycoproteins, ion currents, AGP–Ca2+ flux capacitor
INTRODUCTION
Arabinogalactan proteins (AGPs) have been subjected to intensive study ever since their discovery more than 45 years ago as arabinogalactan (AG) ‘polysaccharides’ in the growth medium of cell cultures (Aspinall et al., 1969). It soon became clear that these classical AGPs (Table 1), initially defined by their composition, contained a small amount (5–10 %) of a hydroxyproline (Hyp)-rich protein component (Lamport, 1970) and 90–95 % AG. Since then their biological role has remained elusive and variously described as enigmatic and mysterious (Albersheim et al., 2011; Pickard, 2013). Many papers have suggested a general signalling function for AGPs while extensive reviews have provided much interesting background information (Fincher et al., 1983; Bacic et al., 1996; Du et al., 1996a; Kreuger and van Holst, 1996; Nothnagel, 1997; Serpe and Nothnagel, 1999; Stone and Valenta, 1999; Clarke et al., 2000; Jose-Estanyol and Puigdomenech, 2000; Majewska-Sawka and Nothnagel, 2000; Schultz et al., 2000, 2002; Gaspar et al., 2001; Showalter, 2001; Qin and Zhao, 2004; Knox, 2006; Pal and Das, 2006; Seifert and Roberts, 2007; Driouich and Baskin, 2008; Ellis et al., 2010; Nguema-Ona et al., 2012, 2013). While most reviews ascribe a signalling role to AGPs, here we present both direct and indirect evidence for a specific role of AGPs in Ca2+ signalling, a novel aspect not previously considered.
Table 1.
Classical AGPs: molecular properties
| Distribution: | ∼80 % periplasmic, ∼20 % cell wall; T = M + S + W |
| T = total AGPs; M = membrane-bound; S = soluble after cell breakage; M + S = periplasmic W = wall-bound. | |
| Quantification: e.g. BY-2 cells T = 600 μg AGPs g f. wt (Lamport et al., 2006) | |
| Molecular Size: | ∼120 kDa = ∼3 × 60 nm (Zhao et al., 2002) |
| Genes: | ∼19 in Arabidopsis (Schultz et al., 2000) |
| 13 in rice (Yang et al., 2007; Ma and Zhao, 2010; Showalter et al., 2010) | |
| At 17,18 & 19 (null; Coimbra et al., 2009) have a Lys-rich subdomain (Yang et al., 2007) | |
| eb1 is deficient in Gal synthesis (UDPGlc epimerase; Seifert et al., 2002) | |
| AtAGP17 (rat1) decreases Agrobacterium transformation (Gaspar et al., 2004) | |
| Polypeptide: | 87–739 aa residues in extended conformation (Showalter et al., 2010) |
| Hyp, Ala, Ser, dominate | |
| Lack Tyr, Phe, Trp and Cys | |
| Subdomain often a 12-r Lys-rich (Gao et al., 1999; Zhao et al., 2002; Yang et al., 2005, 2007) | |
| Glycosylation motifs: SP AP TP VP (Tan et al., 2003) | |
| Polysaccharide: | Arabinogalactan ‘beads’ or Hyp–AG glycomodules |
| Size: 15–150 sugar residues | |
| Backbone: β-1,3-linked galactose trisaccharides β-1,6-linked (Tan et al., 2004, 2010) | |
| Sidechains: bifurcated (Ara)3-Gal-(Rha-GlcU; Tan et al., 2010) | |
| Yariv reactivity: contentious; see text | |
| Post-translational modifications: | N-terminal signal peptide (Schultz et al., 2000) |
| C-terminal GPI lipid anchor (Oxley and Bacic, 1999; Svetek et al., 1999; Borner et al., 2003) | |
| Hydroxylation of peptidyl Pro via direct O2 fixation (Lamport, 1963a) | |
| O-Hyp glycosylation rules (no N-glycosylation) | |
| Non-contiguous Hyp–AG polysaccharides (Zhao et al., 2002) | |
| Contiguous Hyp short arabino-oligosaccharides | |
| 12 to 24 acidic Hyp–AGs (15–150 residues; Zhao et al., 2002) | |
| An Hyp–AG has 1 to 15 AG subunits | |
| AG subunit is a repetitive glycomotif of ∼15 sugar residues | |
| Glycomotif consensus: Ara6 Gal5 GlcA2 Rha2 | |
| AG bifurcated sidechain: Rha, GlcA, Ara3, Gal | |
| Lack fucose with exceptions (Wu et al., 2008, 2010) | |
| Glycomotif linkage analysis (mol %): | |
| Main chain: 3,6-Gal ×2 (13·3 %) 6-Gal ×1 (6·7 %)* | |
| Sidechain: 3,6-Gal ×2 4-GlcA ×2 | |
| 3,5-Ara ×2 t- Rha ×2 | |
| 3-Ara ×2 t- Ara ×2 | |
| Calcium binding: | GlcU/Ca2+ molar ratio 2:1 |
| ∼30 Ca2+-binding subunits/120 kDa AGP† |
* 6-linked Gal connects repetitive subunits (glycomotifs).
† AGPs approx. 120 kDa bind approx. 1 % Ca2+ w/w = 1·2 kDa Ca2+. Thus, moles bound Ca2+ = 1·2 kDa/40 Da.
The quest for AGP structure and function began with an approach based on 1,3,5-tris(4-β-d-glycopyranosyloxyphenylazo)-2,4,6-trihydroxybenzene as a specific precipitant of AGPs (Yariv et al., 1962); now known as the Yariv reagent, it has proved its versatility. Michael Jermyn exploited it beautifully to show that AGPs are ubiquitous in the plant kingdom (Jermyn and Yeow, 1975) and much subsequent progress has used the Yariv reagent to extend that pioneering work.
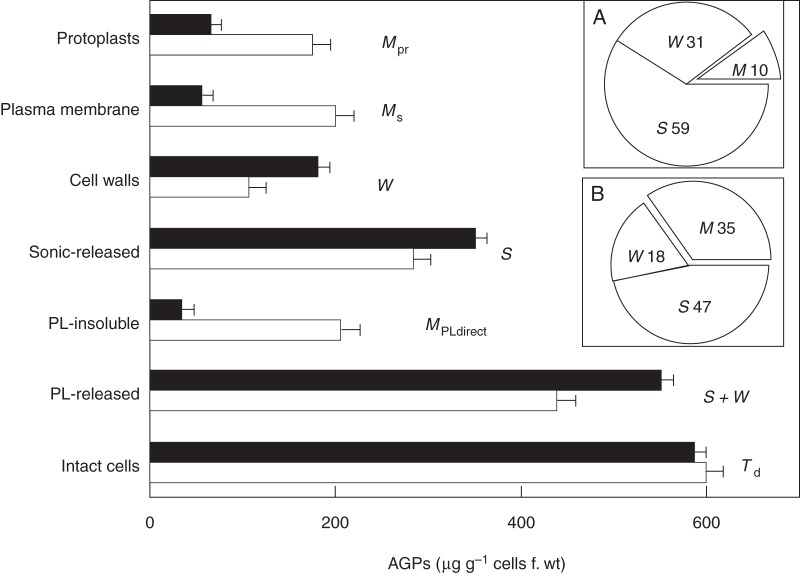
Yariv-agglutinated protoplasts isolated from various species were readily agglutinated by Yariv (Larkin, 1978); this gave the earliest indication of AGPs (then identified as β-lectins) primarily at the cell surface and was subsequently confirmed by their biochemical isolation from membrane preparations (Norman et al., 1990; Serpe and Nothnagel, 1996). Further use of Yariv as a histochemical reagent visualized AGPs at the cell surface of metabolically active tissues: styles (Gane et al., 1994), root cap and embryogenic cells (Samaj et al., 1999a; Thompson and Knox, 1998; Chapman et al., 2000), coleoptile epidermis (Schopfer, 1990), seedling roots and root epidermis (Willats and Knox, 1996; Lu et al., 2001), embryo (Tang et al., 2006) and cotyledons (Pal and Das, 2006). Yariv rapidly inhibited pollen tube growth (Roy et al., 1998); this suggested a direct involvement of AGPs in cell extension consistent with AGPs localized at the growing tips of pollen (Jauh and Lord, 1996; Coimbra et al., 2004; Castro et al., 2013). Most recently, Yariv assay adapted for whole cells (Lamport et al., 2006) enabled AGP distribution (Fig. 1) to be quantified in cell surface compartments: anchored to the plasma membrane; free in the periplasm; trapped in the cell wall matrix; and extruded into the growth medium where they provide a convenient source of mixed AGPs readily isolated by Yariv precipitation (Lamport, 2013a, b).
Fig. 1.
Yariv reagent assay of AGP distribution in tobacco BY-2 cells adapted to growth in 2 % NaCl versus non-adapted controls. Solid columns, salt-adapted; empty columns, non-adapted controls. Protoplasts (Mpr), AGPs remaining bound after approx. 2 h treatment with cellulase/pectolyase. Plasma membrane (Ms), PM-bound AGPs calculated from the relation M = Td − ( S + W). Cell walls (W), AGPs assayed in the isolated wall fraction. Sonic-released (S), soluble AGPs released by ultrasonic cell disruption. PL-insoluble, AGPs remaining bound to cells after treatment with pectolyase also reflect PM-bound AGPs, hence MPLdirect. PL-released, soluble AGPs released by pectolyase treatment of intact cells reflect soluble periplasmic AGPs plus AGPs in muro (S + W). Intact cells (Td), AGPs that remain bound to washed cells. Error bars, 1 s.e. Each data point represents a minimum of five separate experiments using 7-d cultures of salt-adapted cells and 7-d cultures for controls. Note similar values for AGPs in protoplasts, plasma membrane and the pectolyase-insoluble residue of non-adapted control cells, but significantly lower values for plasma membrane-associated AGPs in 2 % salt-adapted cells (340 mm NaCl). Insets: Ms, S and W as a percentage of Td: (A) salt-adapted; (B) control cells. [Reprinted from Lamport et al. (2006).]
These approaches implicated AGPs in a huge range of processes. A consensus emerged that AGPs were signalling molecules, an idea consistent with the assumed great heterogeneity of their polysaccharide substituents. Both the potential signalling role and possible polysaccharide heterogeneity are discussed here.
First, a signalling role, i.e. as signalling molecules per se, lacks direct evidence. The single possible exception of ‘xylogen’ (Motose et al., 2004) remains to be corroborated, nor is it a classical AGP (Table 1) defined here as an Hyp-rich polypeptide backbone with a:
N-terminal signal sequence for secretion;
C-terminal sequence for glycosylphosphatidylinositol (GPI) addition;
a classical AGP may contain as many as 24 O-Hyp-linked AG polysaccharides based on LeAGP-1 (Zhao et al., 2002) but the diversity of classical AGPs is well documented (Showalter et al., 2010);
stoichiometric Ca2+ binding by Hyp AGs: GlcA:Ca2+ 2:1.
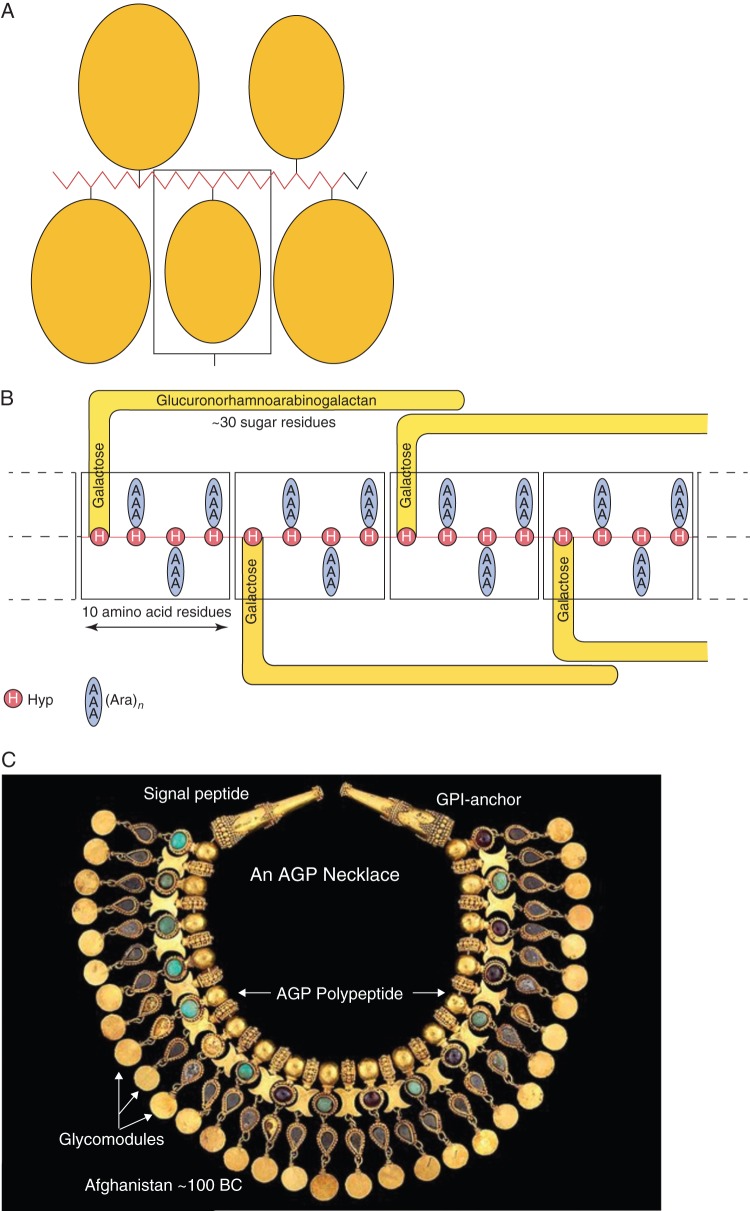
Classical AGPs have been variously modelled as a Wattle blossom, twisted hairy rope and most recently as a necklace (Fig. 2).
Fig. 2.
AGP models: wattle blossom, hairy rope, necklace. (A) The ‘wattle-blossom’ model. Each Hyp residue of about 24 is attached to an arabinogalactan chain that contains from one to 15 repeats of a β-(l-3)-linked galactose (Gal) oligosaccharide with a degree of polymerization (d.p.) of 12. The ‘wattle-blossom’ model depicts ‘the AGP as a whole is spheroidal’: GlcA, glucuronic acid; Rha, rhamnose; Gal, galactose; Ara, arabinose; p, pyranose; f, furanose. [Reprinted from Du et al. (1996a).] (B) The ‘twisted hairy rope’ model of an AGP. Hypothetical block size of 7 kDa contains 10 amino acid residues (1 kDa), 30 sugar residues (4·4 kDa) and 3 hydroxyproline (Hyp)-triarabinosides (1·32 kDa). The glucuronorhamnoarabinogalactan probably has a galactan backbone with glucuronic acid (GlcA), rhamnose (Rha) and arabinose (Ara) side chains. [Reprinted from Qi et al. (1991) as adapted by Du et al. (1996a).] (C) AGP modelled as a necklace. An elaborate robe decoration in the form of a necklace, (Tillya Tepe), 100 BC – 100 AD; National Museum of Afghanistan; Photograph Thierry Ollivier, Musée Guimet (reproduced with permission).
Secondly, AGP polysaccharide heterogeneity seems overemphasized; it does not include a wide variety of different sugars or glycosidic linkages (e.g. as in pectic RG-II) and is more accurately described as polydispersity due to the variable number of repetitive AG subunits (1–15). The AG consensus structure has the theoretical molar ratio: Gal5 Ara6 GlcA2 Rha2 (∼2246 Da; Tan et al., 2010), in agreement with an earlier conclusion of AG regularity (Churms et al., 1983; Gane et al., 1995b) although a somewhat larger subunit of ∼8 kDa.
A relative few classical AGPs comprise the bulk of cell surface AGPs based on amino acid analyses of HF-deglycosylated AGP polypeptides separated by reversed-phase liquid chromatography (Gao et al., 1999) and the narrow size distribution of AGPs separated by Superose-6 gel filtration (Lamport et al., 2006). Many other AGP-like molecules exist (Borner et al., 2002, 2003) but these make only a minor contribution to the total mass of AGPs; this includes the recently described classical AGP ‘APAP1’, At-AGP57C (a minor secreted component that crosslinks pectic RG-I in muro; Tan et al., 2013) and the non-classical AGP31 (Liu and Mehdy, 2007).
Structural elucidation exemplifies a biochemical approach. For AGPs this involves in particular the C-terminal GPI that anchors AGPs to the outer leaflet of the plasma membrane (Youl et al., 1998; Svetek et al., 1999; Borner et al., 2002) and the N-terminal signal sequence for secretion. However, the 90–95 % AG polysaccharide generally remained incompletely characterized due to its perceived overwhelming complexity. However, unlike most proteins polysaccharides derive their complexity from relatively simple repetitive subunits (Rees, 1977). Indeed, numerous earlier carbohydrate analyses clearly pointed to such an AG ground plan with small blocks of a β-1,3-linked galactan backbone separated by periodate-sensitive residues (Fincher et al., 1983). Size heterogeneity of Hyp-polysaccharides released by alkaline hydrolysis (Pope, 1977) initially deterred further analysis. However, designing (Hyp)-rich cell surface glycoproteins (HRGPs) as green fluorescent protein (GFP) fusion proteins (Shpak et al., 1999) solved the problem of purifying individual AGPs and novel AGP-like constructs (Xu et al., 2007). Thus, the tenacity of Li Tan with the combined forces of genetic engineering and state-of-the-art nuclear magnteic resonance imaging (NMR) characterized a range of small Hyp–AG polysaccharides that yielded evidence of a consensus 15-residue repetitive AG subunit (Fig. 3; Tan et al., 2004, 2010). Thus, variation in the number of repetitive AG subunits and minor variation in sugar composition may simply reflect AG polydispersity rather than true compositional AG heterogeneity.
Fig. 3.
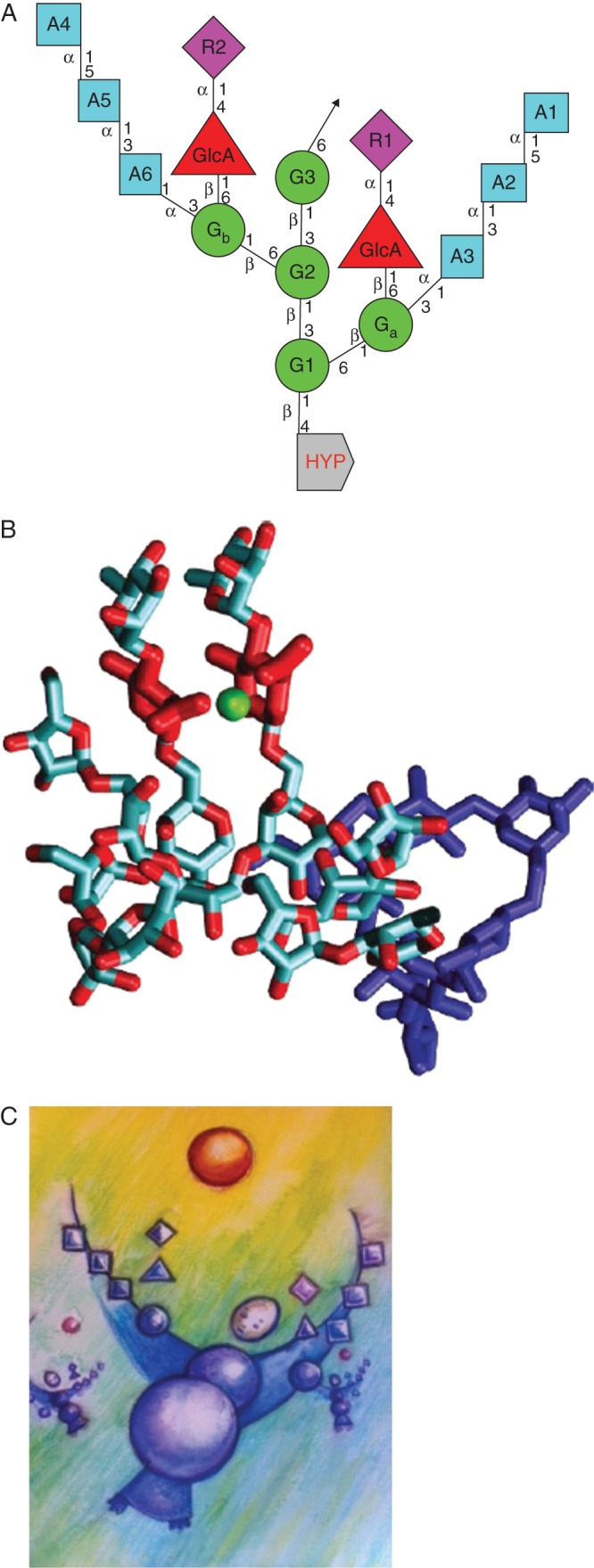
Hyp-arabinogalactan (Hyp–AG) subunits. (A) The linkage connectivity of sugars involved in the repetitive 15-residue consensus Hyp–AG, a conserved structure that accounts for Ca2+ binding by classical AGPs (Lamport and Varnai, 2013). A1–6 arabinose residues; G1–3 galactose mainchain residues; Ga and Gb, galactose sidedechain residues; R1 and R2, rhamnose sidechain residues; GlcA, glucuronic acid sidechain residues; Hyp, hydroxyproline. (B) Three-dimensional molecular model simulating an Hyp–AG with bound Ca2+. Hyp–AG interferon Hyp-polysaccharide-1 (IFNHP1) with Ca2+ ions (green) bound by two glucuronic acid (GlcA) sidechains (red); the galactan backbone is in dark blue and sidechains in light blue. [Reprinted from Lamport and Varnai (2013).] (C) ‘Molly Cool’ cartoon of an arabinogalactan subunit. Two hands are needed to catch a divalent Ca2+ ion. Credits: Amanda Dean and Freyja Dean.
Hyp–AG subunits (Fig. 3) have a relatively simple structure – a repetitive β-1,3-linked galactosyl trisaccharide backbone linked β-1,6 to successive galactosyl trisaccharides. Each repetitive galactosyl trisaccharide has two bifurcated sidechains: one branch an arabinofuranosyl trisaccharide, the other a rhamnosyl-glucuronic acid disaccharide. The five-residue sidechain structure is evidently widespread, first elucidated in gum arabic of Acacia senegal (Defaye and Wong, 1986). Such Hyp–AG conservation implies an essential role for AGP glucuronic acid residues, previously overlooked despite the ‘known’ approx. 1 % Ca2+ content of gum arabic (Anderson and Brown Douglas, 1988; Lamport and Varnai, 2013), and its cytochemical location as membrane-bound Ca2+ (Slocum and Roux, 1982). Significantly, gum arabic does not form a stable complex with Mg2+ (Kunkel et al., 1997).
3D computer models of the Hyp–AG subunit (Fig. 3b) showed a dramatically close approach of glucuronate carboxyls – a eureka moment that pointed to a specific biochemical role for the repeating Hyp–AG subunit in binding Ca2+. Subsequent experiments confirmed the tight binding constant (Kd ∼ 6·5 μm) together with the 2:1 GlcA:Ca2+ binding stoichiometry at pH 5 (Lamport and Varnai, 2013) and pH-dependent dissociation (Fig. 4). These data also corroborate the repetitive subunit structure of the Hyp–AG deduced from NMR experiments (Tan et al., 2010). Numerous methylation analyses of AGPs (Table 2) show approx. 7 % 1,6-linked Gal (i.e. one in every 15 residues of the consensus sequence) and thus further verify the consensus structure.
Fig. 4.
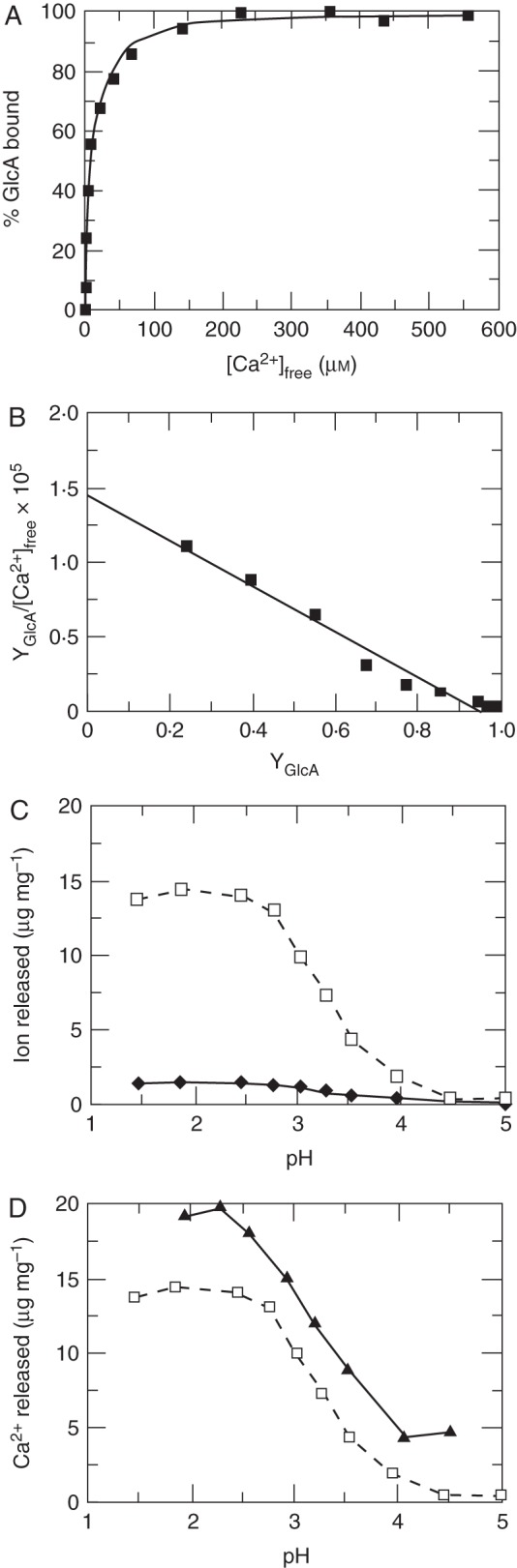
AGP Ca2+ binding: titration of Ca2+-depleted gum arabic with Ca2+ at pH 5. (A) Saturation binding data (black squares) with theoretical curve (line). (B) Scatchard analysis of binding data with best-fit line giving a Kd of 6·5 μm. YGlcA is the fraction of glucuronic acid bound. (C) pH-dependent cation release from Ca2+-saturated gum arabic: Ca2+ (dashed line) and Mg2+ (solid line). (D) Release of Ca2+ from gum arabic (dashed line) compared with pectin (solid line). [Reprinted from Lamport and Varnai (2013).]
Table 2.
Content (mol %) of 6-linked galactose in AGPs
| Species | Source | 6-Gal content (mol %)* | Reference(s) |
|---|---|---|---|
| Acer pseudoplatanus | Cultures | n.d. | Aspinall et al. (1969) |
| Acacia senegal | Gum exudate | 1·2 | Akiyama and Kato (1981) |
| Physcomitrella patens | Cultures | 2 | Lee et al. (2005) |
| Phleum pratense | Cultures | 5 | Sims et al. (2000) |
| Vitis vinifera | Grape juice | 6 | Saulnier et al. (1992) |
| Plantago major | Leaves | 7 | Samuelsen et al. (1998) |
| Rosa sp. | Cultures | 7 | Serpe and Nothnagel (1996) |
| Lolium multiflorum | Cultures | 8 | Bacic et al. (1987) |
| Nicotiana alata | Pistil (AGPNa3) | 8 | Du et al. (1996b) |
| Acacia erioloba | Gum exudate | 12 | Churms et al. (1986) |
| Raphanus sativus | Storage root | 14 | Kitazawa et al. (2013), Tsumuraya et al. (1988) |
| Nicotiana alata | Styles (GaRSGP) | 14 | Sommer-Knudsen et al. (1996) |
| N. alata | Stigma | 21 | Bacic et al. (1988) |
* Identified as 2,3,4-trimethyl galactose.
Significantly, AGPs bind Ca2+ more strongly than does pectin (Lamport and Varnai, 2013). Thus, the lower pKa and non-methyl esterification of glucuronic acid rationalizes Nature's choice of glucuronic acid for AGPs rather than the methyl esterified galacturonic acid that typifies pectin. Furthermore, these biochemical data imply a biological role for tightly bound AGP–Ca2+ (pH 5) by creating a periplasmic reservoir of Ca2+ that can be dissociated by activated H+-ATPase of the plasma membrane, thus feeding Ca+ channels (Wheeler and Brownlee, 2008; Verret et al., 2010) that supply cytosolic Ca2+ (Felle, 1988; Gehring et al., 1990a; Shishova and Lindberg, 2004). Hence the suggestion that AGP–Ca2+ at the periplasmic interface is the major source of cytosolic Ca2+ (Lamport and Varnai, 2013). In one fell swoop this scenario connects AGPs with Ca2+ signalling – a unifying hypothesis that differs from previous models (Trewavas, 2000; Dodd et al., 2010) but with considerable ramifications.
‘The subtlety of Nature far surpasseth the subtlety of Man's understanding’ Francis Bacon 1561–1621). Indeed, cell signalling molecules with their myriad interactions and interdependencies involving cross-talk, feedback, feed-forward and so on are of daunting complexity. A simplifying principle assumes that signalling networks do not operate independently but are integrated. Precisely how AGPs fit into this scheme we discuss below.
Ca2+ behaves as a universal signalling currency and acts as a ‘second messenger’ in plants (Hepler, 2005; Vanneste and Friml, 2013) and also in animals (Berridge, 1997) where it involves a huge range of processes most evident in muscle contraction (Ebashi and Endo, 1968) but also including cell migration (Tsai et al., 2014) and skin homeostasis (Vandenberghe et al., 2014). In plants, Ca2+ signalling involves an equally wide range of complex processes consistent with an earlier percipient comment that ‘Perhaps in the phloem there is an electrical control of Ca2+ flux reminiscent of the well-known control by the sarcoplasmic reticulum in striated muscle!’ (Pickard, 1973). However, the source of the Ca2+ signal involves dynamic Ca2+ storage by AGPs of the cell surface (Lamport et al., 2006; Lamport and Varnai, 2013) and thus differs radically from the classical internal endoplasmic reticulum storage of animals.
AGPs strongly associated with so many plant processes (Table 3) led to the idea of AGPs as signalling molecules per se. However, specific AGP receptors remain elusive most likely because they are non-existent. As an alternative we propose that the AGP–Ca2+ oscillator integrates most signalling pathways that are downstream from the early Ca2+ signal (Ma et al., 2013). This accounts for the ubiquity of AGPs where a trinity of primary messenger (e.g. auxin), secondary messenger (Ca2+) and AGPs comprise a global signalling paradigm, with the evidence summarized in the following six sections.
Table 3.
Processes that involve auxin, Ca2+ and AGPs
| Primary messenger auxin | Secondary messenger Ca2+ | AGP involvement | ||
|---|---|---|---|---|
| I. Auxin and extension growth | +++ | +++ | +++ | |
| II. Tropisms and mechanotransduction | Gravitropism | +++ | +++ | + |
| Thigmotropism | – | + + | + | |
| Pollen tube growth | – | +++ | +++ | |
| Stomatal movements | + | +++ | +++ | |
| Phototropism | +++ | +++ | ? | |
| III. Intracellular dynamics | +++ | +++ | + | |
| IV. Morphogenesis | Seeds | ? | ? | +++ |
| Germination | +++ | +++ | +++ | |
| Roots and lateral roots | +++ | + | +++ | |
| Shoots and branching | +++ | + + | +++ | |
| Leaves | +++ | ? | ? | |
| Flowering, fertilization and early embryogenesis | +++ | ? | +++ | |
| V. Stress, pathogenesis and symbiosis | Abiotic stress | ? | +++ | ? |
| Wound response | ? | + | +++ | |
| Salt stress | ? | +++ | +++ | |
| Pathogenesis and symbiosis | + | + + | +++ |
+, ++, and +++ indicate increasing evidence for involvement in a given process; see text for references.
THE AGP–Ca2+ OSCILLATOR: AUXIN AND EXTENSION GROWTH
AGPs and auxin are involved in most aspects of plant development; it is increasingly evident that auxin generates Ca2+ signals evidenced by increased cytosolic Ca2+ (Pickard, 1984; Gehring et al., 1990b; Tretyn et al., 1991; Irving et al., 1992; Ayling et al., 1994; Plieth and Trewavas, 2002; Shishova and Lindberg, 2010; Monshausen et al., 2011), including a particularly insightful recent review (Vanneste and Friml, 2013). We propose that the AGP–Ca2+ oscillator generates those signals and is thus an integral component of the following AGP–Ca2+–auxin signalling cascade (Fig. 5):
auxin-activated plasma membrane H+-ATPase releases protons at the surface;
decreases pH of AGPs strategically located at the plasma membrane;
low pH discharges AGP–Ca2+ (Fig. 5);
Ca2+ enters the cytosol via plasma membrane Ca2+ channels (Wheeler and Brownlee, 2008; Verret et al., 2010);
cytosolic Ca2+ increases;
activates Golgi vesicle exocytosis.
Fig. 5.
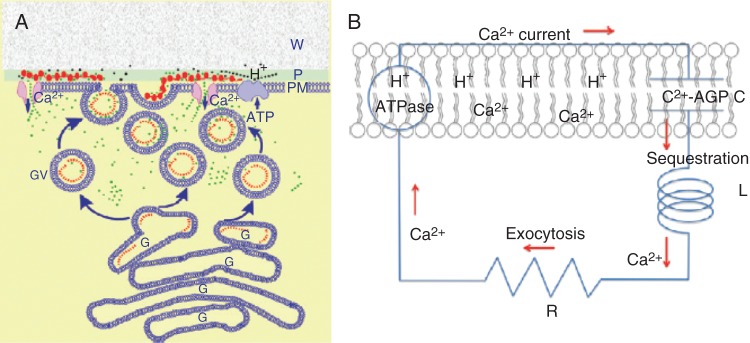
The AGP–calcium oscillator. (A) Ca2+ release from periplasmic AGPs. The oscillator generates pulses of Ca2+ (green dots) whose influx coordinates exocytosis and rapid tip growth. This involves a pulse of H+ (black dots) releasing Ca2+ from periplasmic AGPs (red beads) via stretch-activated Ca2+ channels into the cytosol, and then sequestration by exocytotic Golgi vesicles containing AGPs (red dots). Diffusion of the initial H+ pulse to the wall domain restores the periplasmic pH. Ca2+ is recycled by fusion of vesicles with the plasma membrane. Ca2+ bound to periplasmic AGPs is now ready for the next oscillation. W, wall; P, periplasm; PM, plasma membrane; G, Golgi; GV, Golgi exocytotic vesicles. (B) Ca2+ current as a molecular clock: analogous to an electronic series RLC circuit where R is resistance, L is inductance and C is capacitance, with membrane-bound AGPs as the capacitor, C; Ca2+ sequestration as the inductance, L; vesicle exocytosis as the resistor, R, which limits the recycling rate. Hypothetically, C and L largely determine the oscillator frequency and amplitude of the Ca2+ current, ICa. Note the reported high efflux of Cl– as a counterion may maintain electrical neutrality (Cosgrove and Hedrich, 1991; Zonia et al., 2002). [Reprinted from Lamport and Varnai (2013).]
While the above implies a role for AGPs in cell extension, there is also strong circumstantial evidence of AGP involvement in hypocotyl cell extension where a gibberellin-responsive gene (CsAGP1) encodes a classical AGP (Park et al., 2003).
The AGP–Ca2+ capacitor offers a new perspective on the source of cytosolic Ca2+ and its regulation
First, recycling Ca2+ via exocytosis of Golgi vesicles (Battey et al., 1999; Roy et al., 1999) recharges the AGP capacitor, and traps and conserves Ca2+, thus avoiding the uncertainties of an external apoplastic supply of free Ca2+.
Secondly, classical AGPs at the plasma membrane are by definition close to Ca2+ channels. This confers a huge kinetic advantage to Ca2+ ions for entry into the cytosol when low pH dissociates AGP–Ca2+.
Thirdly, plasma membrane-bound AGPs bind Ca2+. This drastically decreases the Ca2+ electrochemical gradient until low pH dissociates the AGP–Ca2+. [Note that pectic carboxyls will also bind free Ca2+.]
Fourthly, cells can adjust the precise size of the capacitor by controlling surface AGP levels (section IV) but also by the size of O-Hyp-linked polysaccharides – these can vary by a factor of ten or more (Lamport, 1977; Pope, 1977; Xu et al., 2008).
Fifthly, the Hyp–AG Ca2+-binding subunit is a consensus motif whose subtle variation presumably alters the Kd for Ca2+ and also the ability to discriminate against other divalent ions and monovalent ions, particularly Na+, that compete at high levels (Fig. 6). Replacing glucuronic with 4-O-methylglucuronic acid is probably the most frequent variation. Minor sugars such as 3-O-methylgalactose are present in lower plants (Popper et al., 2001) and include 3-O-methylrhamnose (acofriose) in AGPs isolated from the moss Physcomitrella (Popper et al., 2004; Fu et al., 2007). However, fucose (6-deoxy-l-galactose), frequently reported as a minor component of AGPs (Tryfona et al., 2012) and a likely conservative replacement for rhamnose (6-deoxy-l-mannose), has not been detected in any Hyp–AG, but we have yet to explore this planet's vast AGP resources!
Fig. 6.
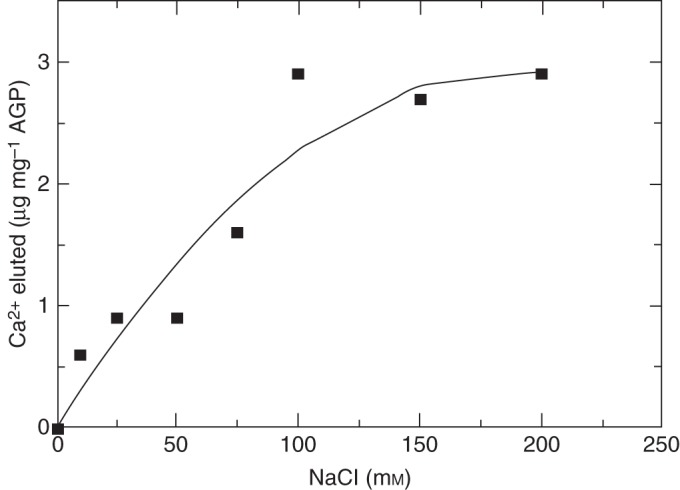
High levels of Na+ compete with AGP-bound Ca2+. An experiment illustrating that competition by 75 mm Na+ removed approx. 50 % of the Ca2+ from a carrot AGP–Ca2+ isolated from a carrot suspension culture. DTAL, unpublished data.
This review cannot do justice to all the ramifications of the AGP–Ca2+ capacitor. Here we discuss the correlation between AGPs, Ca2+ signalling and the primary messenger auxin with its classical effect on wall plasticity (Heyn, 1940). Increased wall plasticity or wall loosening, is pH-dependent (‘proton excretion’; Rayle and Cleland, 1980), but a biochemical basis for the firmly entrenched ‘acid growth’ hypothesis (Rayle and Cleland, 1992) remains recalcitrant; its major postulate that low pH in muro activates wall loosening enzymes per se lacks convincing evidence (Schopfer, 1993) despite the clear involvement of plasma membrane H+-ATPase in cell extension (Hager, 2003). However, the AGP–Ca2+ capacitor (Fig. 5) may resolve this problem as it identifies the source of cytosolic Ca2+ oscillations and shows how they are consistent ‘with the general concept of calcium acting as a second messenger in hormone action in plants’ (Felle, 1988; Hepler, 2005): low pH dissociates carboxylate-bound Ca2+ of AGPs at the plasma membrane (Fig. 5). Remarkably, however, AGPs may perform a dual function by acting as a pectic plasticizer after their release from the plasma membrane (Lamport, 2001).
A dual role of classical AGPs in cell extension is consistent with the following observations:
AGPs are strongly associated with rapid growth of cell suspension cultures and rapid cell extension during tip growth of moss protonema (Lee et al., 2005), pollen tubes (Jauh and Lord, 1996) root hairs (Samaj et al., 1999b) and coleoptile epidermal walls with AGPs suggested as ‘an epidermal wall-loosening factor in auxin mediated coleoptile growth’ (Schopfer, 1990).
Growth retardation in double null AGP mutant pollen tubes (Costa et al., 2013a), and in the protonema of Physcomitrella AGP1 knockouts (Lee et al., 2005) and in root hairs of the reb1 arabidopsis mutant (Ding and Zhu, 1997).
Tropisms and mechanosensory mechanisms (Toyota and Gilroy, 2013).
Auxin and Ca2+ signalling are connected (Vanneste and Friml, 2013).
AGP cell wall plasticizers may contribute to the resistance of resurrection plants to desiccation (Moore et al., 2006, 2013).
THE AGP–Ca2+ OSCILLATOR: TROPISMS AND MECHANOTRANSDUCTION
Gravitropism
Calcium signalling underlies all tropisms (Toyota and Gilroy, 2013), so the question devolves into the mechanism that elevates cytosolic Ca2+ (Toyota et al., 2008). Frequently this involves mechanotransduction exemplified by gravitropism where the biochemical mechanism now seems to involve the redistribution of auxin transporter activity and hence auxin itself (Baster et al., 2013). Furthermore, Ca2+ is well represented by stretch-activated Ca2+ channels in plants and algae (Ding et al., 1993; Verret et al., 2010) suggesting that the ultimate gravity sensor is the membrane and its stretch-sensitive receptors; this would include the calcium mechanosensitive receptors described by Pickard (Ding and Pickard, 1993) rather than starch grain ‘statoliths’ (Caspar and Pickard, 1989). Thus, ‘Although Ca2+ is usually discussed as a cytoplasmic regulator, apoplastic fluxes of this ion may also play a key role in gravitropism’ via stretch-sensitive Ca2+ channels that regulate Ca2+ flux (Toyota and Gilroy, 2013). Hence the term ‘flux capacitor’ borrowed from ‘Doc’ Brown immortalized by Christopher Lloyd in the Sci-Fi movie Back to the Future (Zemeckis, 1985) where Doc's invention of the aptly named capacitor was an integral component of the time machine powered by the continuous flux of a capacitor in an oscillating circuit; the allusion to time travel is a reminder that plant evolution is a form of time travel that depends on the AGP–Ca2+ and other biochemical oscillators!
Thigmotropism
The AGP–Ca2+ oscillator does not exclude a role for AGPs in mechanotransduction based on adhesion of plasma membrane to the cell wall by a ‘plasmalemmal reticulum’ involving AGPs and wall-associated kinases (WAKs; Gens et al., 2000; Pickard, 2007). Although precise details of such ‘tensegrity’ are lacking, stretch receptors with associated kinases and AGPs (Telewski, 2006) and more specifically mechanosensitive (MS) Ca2+ channels (Nakagawa et al., 2007; Swarbreck et al., 2013) are clearly involved in thigmotropism, which includes a wide range of processes ranging from the rapid movements of insectivorous plants and the tendrils of climbing plants to root growth (Weerasinghe et al., 2009) particularly root tips (Pickard, 2007).
Pollen tube growth
Ca2+ is essential to pollen tube growth (Mascarenhas, 1993; Chen et al., 2008; Chebli and Geitmann, 2012) and pollen tube directionality (Franklin-Tong, 1999); not surprisingly, AGPs are implicated as chemotropic agents (Cheung et al., 1995; Wu et al., 2000) although not corroborated by others (Sommer-Knudsen et al., 1998). This discrepancy may be resolved by considering the in vitro growth of millet pollen tubes that are directed by a polygalacturonic acid-calcium gel which forms a Ca2+ gradient (Reger et al., 1992). Thus, the signal guiding a pollen tube may be the Ca2+ gradient generated by AGP–Ca2+ dissociation in the transmitting tissue of female but not in male flowers lacking AGPs (Coimbra and Duarte, 2003) rather than AGPs themselves. Indeed, the apparent increase in AGP glycosylation from stigma to ovule (Wu et al., 1995) supports this interpretation and strongly hints at the ways in which AGPs may be involved in tip growth dependent on the essential ions: Ca2+, protons (H+) and borate (B(OH)4–; Holdaway-Clarke et al., 2003). One could, for example, view the tip as an exquisitely sensitive living Ca2+ electrode whose AGPs integrate local Ca2+ levels and thus enable directional growth by discriminating between small but highly localized changes in Ca2+. This is consistent with the early observation of a specfic chemotropic response to Ca2+ by growing pollen tubes (Mascarenhas and Machlis, 1962).
Stomatal movements
Stomata also exemplify the AGP–Ca2+ oscillator hypothesis. Not only are they replete with oscillator components but changes in cytosolic pH and calcium of guard cells precede stomatal movements (Irving et al., 1992; Kim et al., 2010). This is consistent with stretch-activated Ca2+ channels (Cosgrove and Hedrich, 1991) and the marked abundance of guard cell AGP epitopes (Majewska-Sawka et al., 2002) also demonstrated by cytochemical location of the ‘Lys-rich’ classical AGP AtAGP18 expressed as a GUS construct (fig. 3j in Yang and Showalter, 2007).
Phototropism
Finally, phototropism involves blue light receptors that initiate lateral auxin fluxes (Gehring et al., 1990b; Friml et al., 2002; Christie et al., 2011; Ding et al., 2013) and lead to increased cytosolic Ca2+ (Folta et al., 2003), indicating yet another possible role for the AGP–Ca2+ oscillator.
THE AGP–Ca2+ OSCILLATOR: INTRACELLULAR DYNAMICS
Early work with gibberellin-induced secretion of α-amylase first identified a specific Ca2+-dependent biochemical process (Chrispeels and Varner, 1967). Since then it has become clear that Ca2+ is directly involved in many processes: cell cycle regulation (Himanen et al., 2002; Vanneste et al., 2005), membrane trafficking including the transport of auxin efflux proteins (Baster et al., 2013), the balance between exo- and endocytosis (Paciorek et al., 2005; Robert et al., 2010; Vanneste and Friml, 2013), apoptosis (Levine et al., 1996) and programmed cell death (PCD; Jones, 2001; Chaves et al., 2002).
Most if not all of these processes involve the universal Ca2+ signal transducer calmodulin and calmodulin-like proteins (comprehensively reviewed by Bouche et al., 2005).
THE AGP–Ca2+ OSCILLATOR: MORPHOGENESIS
It is convenient to discuss morphogenesis beginning with the seed, as Jermyn first noted that AGPs were released from virtually all seeds by extraction of the seed meal with mild aqueous buffer (Jermyn and Yeow, 1975). This raises several questions: Where are these AGPs located in the seed? Are they identical to the classical AGPs of actively growing plant cells? Do they bind Ca2+? What is their functional significance in metabolically inactive seed tissues? And what role do they play during seed maturation and germination?
Seeds
Yariv as a cytochemical reagent identifies the cytochemical location of AGPs in seeds such as coffee (Sutherland et al., 2004) specifically in the thickened cell walls of the coffee endosperm where they are notably concentrated at the interface between the wall and the plasma membrane (fig. 2 in Redgwell et al., 2002; Redgwell et al., 2006). However in seeds of Jatropha curcas AGPs are particularly evident in vessels of the cotyledon and in the procambium ring of the embryo (Fig. 7.) but ‘no AGPs were detected in the endosperm’ (Sehlbach et al., 2013).
Fig. 7.
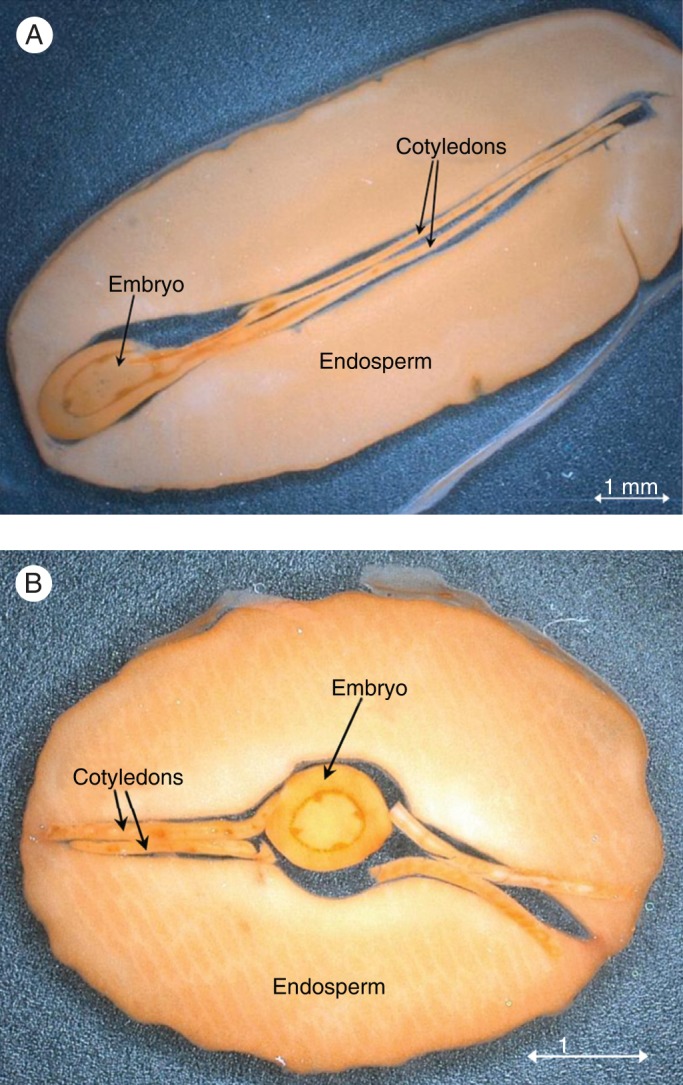
Jatropha curcas staining with Yariv. J. curcas seed sections after staining with β-d-glucosyl Yariv: (A) sagittal and (B) transverse section. [Reprinted from Sehlbach et al. (2013).]
Significant structural differences between the AGPs of seeds (Tryfona et al., 2010), storage roots (Tsumuraya et al., 1988; Table 2) and growing tissues (Tan et al., 2004, 2010) suggest different roles for AGPs in metabolically inactive versus metabolically active tissues. AGPs of resurrection plants reportedly contribute to the viability of their desiccated tissues (Moore et al., 2013); so by analogy, seed AGPs may also enhance the viability of dehydrated seed tissue, particularly as such AGPs (Jermyn and Yeow, 1975) may be surprisingly abundant and may include both classical AGPs and the much smaller AG peptides (Fincher et al., 1983; Fincher and Stone, 1974) that comprise, for example, >0·3 % d. wt of wheat flour (Loosveld et al., 1997; Tryfona et al., 2010) while classical AGPs account for approx. 0·8 % d. wt of tomato seeds (Lu et al., 2001) similar to BY-2 cells when adjusted to a fresh weight basis although there are clearly wide variations in different species (Boudjeko et al., 2009). Despite possible structural differences between classical and seed AGPs some evidently bind Ca2+ in germinating barley seeds, as described below.
Germination
Gibberellin (GA) induces Ca2+-dependent secretion of α-amylase by barley aleurone cells (Chrispeels and Varner, 1967) and also by their protoplasts (Suzuki et al., 2002). As the β-d-glucosyl Yariv reagent inhibits Ca2+-dependent amylase secretion by the protoplasts, a direct role for AGPs during germination is likely (Mashiguchi et al., 2008). Curiously, however, the Yariv reagent does not inhibit amylase secretion by intact aleurone cells or the germination of tomato seeds (Lu et al., 2001) possibly due to a permeability barrier as Yariv reagent markedly inhibits subsequent seedling growth (Lu et al., 2001). Indeed, judging from the role of auxin in root tip cell differentiation (Ding and Friml, 2010), lateral root initiation (Himanen et al., 2002; Lavenus et al., 2013), root hair formation (Jones et al., 2009; Ikeda et al., 2009) and root gravitropism (Toyota and Gilroy, 2013), the AGP–Ca2+ oscillator operates consistently during seed germination and seedling growth.
Roots and lateral roots
AGP epitopes that appear very early, within one or two cells of the apical initials, are notably associated with the determination of cell fate during root development. Thus, the AGP monoclonal JIM4-labelled developing pericycle cells in carrot root (Knox et al., 1989). A JIM13 AGP epitope confirmed differentiation at ‘the earliest stage of development’ (Dolan et al., 1995) in species-specific patterns (Casero et al., 1998) that were related to lateral root initiation (De Smet et al., 2006). Inhibition of auxin transport also inhibits lateral root initiation (Casimiro et al., 2001) and branching (Lavenus et al., 2013); thus, by increasing the size of their AGP–Ca2+ capacitor, pericycle cells may predestine their response to auxin during development. Interestingly, the reb1 mutant yields defective root epidermal cell walls with lower levels of AGPs (Ding and Zhu, 1997) although more recent work identified reb1 as a UDP–d-Glc epimerase defective mutant, and hence a galactose deficiency with concomitant pleiotropic effects on xyloglucan, pectin and AGP synthesis according to Nguema-Ona et al. (2006). Nevertheless, the location of AGPs precisely where Ca2+ signals will be needed seems entirely consistent with the AGP–Ca2+ oscillator paradigm.
Shoots and branching
Morphogenesis of the shoot, including its vascular system (Fukuda, 2004), branching (Shinohara et al., 2013) and leaf development (Scarpella et al., 2006), is more complex than the root, so the search for principal determinants has great appeal (Smolarkiewicz and Dhonukshe, 2013). However, such determinants depend greatly on the level studied: morphological, cytological, genetic, physiological and molecular.
AGPs provide a new perspective with their novel role as regulators of Ca2+ signalling and metabolism. The Ca2+ oscillator rationalizes this by relating the size of the AGP–Ca2+ capacitor to the amplitude of the Ca2+ signal, and hence cellular response. Significantly the size of the AGP–Ca2+ capacitor depends not only on AGP concentration at the protoplast surface but also on variation in the number of Hyp–AGs and their size in any given AGP (Lamport, 1977; Pope, 1977; Qi et al., 1991; Xu et al., 2008). Presumably, cells with an AGP deficit will not react to signals that require release of Ca2+. This may be relevant to the profound problem of branching where ‘apical dominance’ suppresses axillary buds but far less so in bushy growth. This involves complex interactions between auxin transport, and rapid redistribution of auxin efflux transporter PIN proteins by other growth substances, including cytokinins and the recently discovered branching repressors, strigolactones (Coombs, 2013; Jiang et al., 2013; Shinohara et al., 2013; Smith, 2013; Zhou et al., 2013). Intriguingly, over-expression of a single AGP gene dramatically altered the phenotype of tomato plants from tall to bushy (Sun et al., 2004). We tentatively suggest that overexpression of AGPs in axillary buds makes them more responsive to auxin signals by increasing the availability of Ca2+. There is a parallel here with phenotypic variation in Streptocarpus caused by the β-Yariv reagent and the conclusion that AGPs play a pivotal role in pattern formation during plant morphogenesis (Rauh and Basile, 2003). How far this simple scenario accounts for the activation of lateral buds after terminal shoot decapitation remains to be seen.
Leaves
Morphogenesis of the leaf is currently of great interest. In 1606 Adrian Spieghel wrote ‘But what is the leaf’ (Arber, 1950), answered by Nehemiah Grew (∼1666) co-founder of plant anatomy: ‘The skin of the leaf is only the amplification of that of a branch’ (Arber, 1950). Not surprisingly leaves also involve similar PIN-directed auxin gradients that mediate morphogenesis (Benkova et al., 2003; Scarpella et al., 2006; Barkoulas et al., 2008) by the formation of auxin maxima at sites of tissue outgrowth (Di Giacomo et al., 2013; cf. Fig. 3B). Again this implies involvement of the AGP–Ca2+ capacitor.
Flowering, fertilization and early embryogenesis
The transition from leaf morphogenesis to flowering (Taoka et al., 2013) and reproductive growth is essentially a study in leaf modification. Again AGPs are involved at every developmental stage evidenced by histochemical detection (Yariv) of AGPs in ovaries (Gane et al., 1995a), immunodetection of a non-classical AGP in styles (Sommer-Knudsen et al., 1996), direct isolation of classical AGPs from styles and stigmas (Gane et al., 1995b) and indirect evidence from auxin-dependent patterning of the ovule (Pagnussat et al., 2009). Indeed, ‘AGPs are essential for somatic embryogenesis’ (Kreuger and van Holst, 1993) although that idea was based on the assumption that AGPs are freely diffusible between cells.
AGP appearance may be dynamic or static. For example, AGP epitopes localized in unfertilized tobacco egg cells disappear rapidly after fertilization (Qin and Zhao, 2006) and the stylar transmitting tissue accumulated AGPs in response to pollination (Qin et al., 2007) while differential expression of AGPs during early embryogenesis (Costa et al., 2013b) suggests that asymmetric delivery of AGPs determines the fate of the basal cell (Souter and Lindsey, 2000). Indeed, specific AGPs associated with directional pollen tube tip growth are abundant along the pistil transmitting tissue pathway based on its reactivity with monoclonals MAC207 and JIM13 but not JIM8 (Coimbra and Duarte, 2003). In contrast, cells of the micropyllar nucellus pathway were JIM8- and MAC207-reactive (Coimbra and Salema, 1997). Finally, an arabidopsis double null mutant of two pollen-specific AGPs (agp6 agp11) decreased pollen tube growth with concomitant altered expression levels of calcium- and signalling-related genes. The suggested AGP calcium interaction via calmodulin (Costa et al., 2013a) is consistent with our proposal in section II that during pollen tube growth AGPs and AGP–Ca2+ dissociation may be a crucial determinant of Ca2+ gradients and pollen tube guidance.
THE AGP–Ca2+ OSCILLATOR: STRESS
The pervasive presence of Ca2+ oscillations and Ca2+ signalling in stress-related plant growth and development (Bose et al., 2011) is consistent with the major role of Ca2+ as a central node in the overall signalling web (Tuteja and Sopory, 2007) and, as inferred here, involvement of the AGP–Ca2+ oscillator as follows.
Abiotic stress and wound response
Abiotic stress such as drought, heat shock, cold shock, wound response and salinity increase cytosolic Ca2+ mostly due to influx from the apoplast (Knight et al., 1997; Neill et al., 2002; Lecourieux et al., 2006). Indirect evidence involves AGPs in these responses to stress: the Yariv reagent leads to abrupt cessation of pollen tube growth with massive accumulation of the Yariv–AGP complex at the tube tip (Roy et al., 1998). The Yariv reagent also triggers wound-like responses in cultured cells (Guan and Nothnagel, 2004) and PCD (Gao and Showalter, 1999; Chaves et al., 2002). PCD also involves Ca2+ influx (Groover and Jones, 1999). As the Yariv complex undoubtedly binds Ca2+, accumulation of a periplasmic AGP–Yariv complex represents a potentially larger pool of available Ca2+.
Acacia senegal exemplifies AGP upregulation in response to wounding even though the AGP (Qi et al., 1991; Goodrum et al., 2000) and AGP-like gum arabic polysaccharides (Siddig et al., 2005) function as a plastic wound sealant rather than an AGP–Ca2+ oscillator. Although known for many years, the significance of Ca2+ bound by the uronic acids of gum arabic only became clear when 3D molecular modelling of the Hyp–AG structure revealed the mechanism and rationale for specific Ca2+ binding by the Hyp–AG subunits of periplasmic AGPs (Lamport and Varnai, 2013).
Salt stress
Salt stress is of particular interest with huge economic and ecological significance. The salt overly-sensitive (SOS) signalling pathway (Zhu, 2001) enhances tolerance to saline conditions via SOS1 the Na+/H+ antiporter (Na+ efflux) activated via the SOS2 kinase in conjunction with the SOS3 Ca2+ sensor that detects elevated cytosolic levels of Ca2+ (Ishitani et al., 2000). An extracellular source of Ca2+ is critical (Tuteja and Sopory, 2007). However, high levels of Na+ compete with AGP–Ca2+ (Fig. 6) and may thus dissipate Ca2+ availability. Upregulation of AGP biosynthesis by salt-stressed tobacco cells (Lamport et al., 2006) may reflect a homeostatic adaptation for Ca2+ retention. Halophytes may have solved disruption to Ca2+ signalling by using AGPs that discriminate more effectively against Na+ by subtle variation of the Ca2+-binding subunit. This might account for the occurrence of 3-O-methylgalactose in desiccation-resistant lycophyte genera such as Lycopodium and Selaginella (Popper et al., 2001) and 3-O-methylrhamnose (acofriose) in AGPs isolated from the moss Physcomitrella (Popper et al., 2004; Fu et al., 2007) and Charophytes such as Chara and Coleochaete, but not observed (yet!?) in higher plants.
Pathogenesis and symbiosis
‘Hold your friends close but your enemies closer’ reflects the progress from parasite to symbiont. Both relationships involve calcium signalling (Lecourieux et al., 2006) with associated AGPs in root–microbe interactions comprehensively reviewed (Nguema-Ona et al., 2013). Frequently these AGPs appear as markers or predictors of potential cell fate that is presumably finally triggered by an auxin signal (Grunewald et al., 2009) or its repression (Navarro et al., 2006).
ONLY CONNECT … STRUCTURE WITH FUNCTION … BACK TO THE FUTURE OF AGPs
Hydroxyproline was first identified in plants in the late 1940s as a minor (secondary) amino acid (Joslyn and Stepka, 1949; Maehly and Paleus, 1950; Hunt, 1951; Steward et al., 1951). Later its main location bound to the primary cell wall (Dougall and Shimbayashi, 1960; Lamport and Northcote, 1960; Lamport, 1963b) suggested a structural protein by analogy with collagen, the major structural protein of animals. Subsequent work recognized two major families of cell surface HRGPs: the extensins crosslinked (Held et al., 2004) to the wall itself and classical AGPs primarily located at the surface of the plasma membrane. Both families are characteristically extended polypeptides rich in glycosylated Hyp. Despite this similarity in molecular design, differences in their glycosylation underpin quite different roles – extensins, self-assembling rod-like amphiphiles stabilized by short arabinooligosaccharides, are scaffolding proteins that template new cross-wall deposition (Cannon et al., 2008). By contrast, the exquisitely designed AG polysaccharides of classical AGPs possess repetitive subunits whose paired glucuronic acid residues bind substantial amounts of Ca2+ at the plasma membrane: hence, a Ca2+ signalling role. Nevertheless, this work only scratches the surface regarding our understanding of AGPs and their possible multifunctional role. There is much to do at all levels of AGP function from molecular to environment:
The challenge to dissect the molecular role of each AGP subdomain includes both protein and glycosubstituents each with their own fascinating problems.
The N-terminal signal sequence and C-terminal GPI-addition signal are well known. However, the 12-residue basic subdomain of LeAGP1, the most abundant and best known AGP of BY-2 cells, remains a mystery (Pogson and Davies, 1995; Li and Showalter, 1996). Such lysine-rich subdomains in other AGPs (Yang et al., 2005, 2007) may enable binding to phospholipid headgroups or pectate carboxylates and would contribute to orientation and cell surface ordering of AGPs (Gens et al., 2000; Pickard, 2007).
Molecular dissection of AGP glycosylation presents intriguing questions: the size and precise composition and spacing of Hyp–AGs along the AGP polypeptide as well as the total number of Hyp–AGs in an AGP are most likely determined or encoded by the primary amino acid sequence, especially the AP and SP motifs, whose numbers and clustering vary widely between different classical AGPs. Are Hyp–AGs ‘tuned’ to discriminate between Ca2+ and other cations such as Al3+ and high levels of Na+ (Fig. 6)? Besides the Ca2+-binding role of glucuronic acid residues (how ‘essential’ is the terminal rhamnose?) can we dissect the role of the β,1–3-linked AG backbone and its sidechain substituents? What does the triarabinosyl branch add? Some suggest that the arabinosyl sidechain is essential for binding of the Yariv reagent (Komalavilas et al., 1991; Serpe and Nothnagel, 1994; Classen et al., 2000).
This suggests a molecular role for the α-l-linked triarabinosyl sidechain in muro; based on the similar sterochemical configuration of α-l- and β-d-sugars, α-l-linked arabinosyl sidechains might dock with the terminal β-d-galacturonic acid of pectic RG-II sidechain-A; competitive disruption of the apiosyl borate crosslink (O'Neil et al., 2004) would thus plasticize the pectic network. However, others suggest that the Yariv reagent binds to the AG β-linked galactan backbone, a discrepancy that may reflect the different binding assays used (Kitazawa et al., 2013).
This raises further questions about Hyp–AG biosynthesis, which requires a minimum of eight or nine AGP glycosyltransferases to build a repetitive Hyp–AG; currently four have been identified: AtGALT2 (At4g21060), and Hyp-O-galactosyltransferase of the GT31 family (Basu et al., 2013); AtGALT31A (At1 g32930), a β-1,6-glactosyltransferase also in the GT31 family (Geshi et al., 2013); AtGlcAT14A (At5g39990), a β-glucuronosyltransferase of the GT14 family (Knoch et al., 2013); and AtFUT4 (At2g15390) and AtFUT6 (At1g14080), α-(1,2) fucosyltransferases of the GT37 family (Wu et al., 2010; Liang et al., 2013). The evolution of a functional Hyp–AG Ca2+-binding glycomotif of such elegant complexity (or simplicity?) harks back to the past origin of glycosylated Hyp in photosynthetic protists (Gotelli and Cleland, 1968; Lamport and Miller, 1971; Miller et al., 1972; Bollig et al., 2007). Tantalisingly, however, protists lack classical AGPs critically identified by chemical characterization. Indeed, we have been unable to isolate classical AGPs from Coleochaete despite the presence of Yariv-reactive material (Buglass et al., 2007)! The appearance of classical AGPs in bryophytes (Lamport, 1970) reflects the sea change that enabled the transition to terra firma and a new challenging environment (Popper and Fry, 2003).
The journey from HRGP structure to function begun half a billion years ago, in human terms only half a century ago, has finally arrived at molecular-level roles for both extensins (Lamport et al., 2011) and AGPs with the paradoxical conclusion that although often perceived, both figuratively and literally, as peripheral glycoproteins, in fact AGPs and extensins play a central role in plant growth and development as originally surmised (Lamport, 1963b).
ACKNOWLEDGEMENTS
The Royal Botanic Gardens, Kew, receives grant-in-aid funding from Defra. D.T.A.L. and P.V. are grateful to the University of Sussex for generous laboratory facilities. We are indebted to the very helpful and insightful advice from the handling editor, Dr Zoe Popper, and also to the referees for their perspicacious, detailed and extremely helpful comments. We dedicate this paper to the memory of D. H. Northcote, F.R.S., PhD supervisor of D.T.A.L. We also commemorate Dr Michael Jermyn who pioneered AGP research which he further stimulated by his generous gifts of Yariv reagent.
LITERATURE CITED
- Akiyama Y, Kato K. An extracellular arabinogalactan-protein from Nicotiana tabacum. Phytochemistry. 1981;20:2507–2510. [Google Scholar]
- Albersheim P, Darvill A, Roberts K, Sederoff R, Staehelin A. Plant cell walls. New York: Garland Science; 2011. [Google Scholar]
- Anderson DMW, Brown Douglas DM. The composition of the proteinaceous gums exuded by some Leucaena species, subspecies and hybrids. Food Hydrocolloids. 1988;2:247–253. [Google Scholar]
- Arber A. The natural philosophy of plant form. Cambridge: Cambridge University Press; 1950. [Google Scholar]
- Aspinall GO, Malloy JA, Craig JWT. Extracellular polysaccharides from suspension-cultured sycamore cells. Canadian Journal of Biochemistry. 1969;47:1063–1070. doi: 10.1139/o69-170. [DOI] [PubMed] [Google Scholar]
- Ayling SM, Brownlee C, Clarkson DT. The cytoplasmic streaming response of tomato root hairs to auxin; observations of cytosolic calcium levels. Journal of Plant Physiology. 1994;143:184–188. [Google Scholar]
- Bacic A, Churms SC, Stephen AM, Cohen PB, Fincher GB. Fine structure of the arabinogalactan-protein from Lolium multiflorum. Carbohydrate Research. 1987;162:85–93. [Google Scholar]
- Bacic A, Gell AC, Clarke AE. Arabinogalactan proteins from stigmas of Nicotiana alata. Phytochemistry. 1988;27:679–684. [Google Scholar]
- Bacic A, Du H, Stone BA, Clarke AE. Arabinogalactan proteins: a family of cell-surface and extracellular matrix plant proteoglycans. Essays in Biochemistry. 1996;31:91–101. [PubMed] [Google Scholar]
- Barkoulas M, Hay A, Kougioumoutzi E, Tsiantis M. A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nature Genetics. 2008;40:1136–1141. doi: 10.1038/ng.189. [DOI] [PubMed] [Google Scholar]
- Baster P, Robert S, Kleine-Vehn J, et al. SCF(TIR1/AFB)-auxin signalling regulates PIN vacuolar trafficking and auxin fluxes during root gravitropism. EMBO J. 2013;32:260–274. doi: 10.1038/emboj.2012.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu D, Liang Y, Liu X, et al. Functional identification of a hydroxyproline-O-galactosyltransferase specific for arabinogalactan protein biosynthesis in Arabidopsis. Journal of Bioogical Chemistry. 2013;288:10132–10143. doi: 10.1074/jbc.M112.432609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battey NH, James NC, Greenland AJ, Brownlee C. Exocytosis and endocytosis. Plant Cell. 1999;11:643–659. doi: 10.1105/tpc.11.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkova E, Michniewicz M, Sauer M, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Elementary and global aspects of calcium signalling. Journal of Physiology. 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollig K, Lamshoeft M, Schweimer K, Marner FJ, Budzikiewicz H, Waffenschmidt S. Structural analysis of linear hydroxyproline-bound O-glycans of Chlamydomonas reinhardtii-conservation of the inner core in Chlamydomonas and land plants. Carbohydrate Research. 2007;342:2557–2566. doi: 10.1016/j.carres.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Borner GHH, Sherrier DJ, Stevens TJ, Arkins IT, Dupree P. Prediction of glycosylphosphatidylinositol (GPI)-anchored proteins in Arabidopsis: a genomic analysis. Plant Physiology. 2002;129:486–499. doi: 10.1104/pp.010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner GHH, Lilley KS, Stevens TJ, Dupree P. Identification of glycosylphosphatidylinositol-anchored proteins in Arabidopsis. A proteomic and genomic analysis. Plant Physiology. 2003;132:568–577. doi: 10.1104/pp.103.021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose J, Pottosin II, Shabala SS, Palmgren MG, Shabala S. Calcium efflux systems in stress signaling and adaptation in plants. Frontiers in Plant Science. 2011;2:1–17. doi: 10.3389/fpls.2011.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche N, Yellin A, Snedden WA, Fromm H. Plant-specific calmodulin-binding proteins. Annual Review of Plant Biology. 2005;56:435–466. doi: 10.1146/annurev.arplant.56.032604.144224. [DOI] [PubMed] [Google Scholar]
- Boudjeko T, Rihouey C, Ndoumou DO, El Hadrami I. Characterisation of cell wall polysaccharides, arabinogalactans-proteins (AGPs) and phenolics of Cola nitida, Cola acuminata and Garcinia kola seeds. Carbohydrate Polymers. 2009;78:820–827. [Google Scholar]
- Buglass S, Lamport DTA, Xu J, Tan Li, Kieliszewksi MJ. Origin of the land plants: is coleochaete their closest living relative? The writing is on the wall. 2007. 11th Cell Wall Meeting, Copenhagen: Abstract 17.
- Cannon MC, Terneus K, Hall Q, et al. Self-assembly of the plant cell wall requires an extensin scaffold. Proceedings of the National Academy of Sciences U S A; 2008. pp. 2226–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casero PJ, Casimiro I, Knox JP. Occurrence of cell surface arabinogalactan-protein and extensin epitopes in relation to pericycle and vascular tissue development in the root apex of four species. Planta. 1998;204:252–259. [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell. 2001;13:843–852. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T, Pickard BG. Gravitropism in a starchless mutant of Arabidopsis. Planta. 1989;177:185–197. [PubMed] [Google Scholar]
- Castro AJ, Suarez C, Zienkiewicz K, Alche JdD, Zienkiewicz A, Rodriguez-Garcia MI. Electrophoretic profiling and immunocytochemical detection of pectins and arabinogalactan proteins in olive pollen during germination and pollen tube growth. Annals of Botany. 2013;112:503–513. doi: 10.1093/aob/mct118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A, Blervacq AS, Vasseur J, Hilbert JL. Arabinogalactan-proteins in Cichorium somatic embryogenesis: effect of β-glucosyl Yariv reagent and epitope localisation during embryo development. Planta. 2000;211:305–314. doi: 10.1007/s004250000299. [DOI] [PubMed] [Google Scholar]
- Chaves I, Regalado AP, Chen M, Ricardo CP, Showalter AM. Programmed cell death induced by β-d-galactosyl)3 Yariv reagent in Nicotiana tabacum BY-2 suspension-cultured cells. Physiologia Plantarum. 2002;116:548–553. [Google Scholar]
- Chebli Y, Geitmann A. Mechanical principles governing pollen tube growth. Functional Plant Science and Biotechnology. 2012;1:232–245. [Google Scholar]
- Chen K-M, Wu G-L, Wang YH, et al. The block of intracellular calcium release affects the pollen tube development of Picea wilsonii by changing the deposition of cell wall components. Protoplasma. 2008;233:39–49. doi: 10.1007/s00709-008-0310-2. [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wang H, Wu H. A floral transmitting tissue-specific (TTS) glycoprotein attracts pollen tubes and stimulates their growth. Cell. 1995;82:383–393. doi: 10.1016/0092-8674(95)90427-1. [DOI] [PubMed] [Google Scholar]
- Chrispeels MJ, Varner JE. Gibberellic acid-enhanced synthesis and release of α-amylase and ribonuclease by isolated barley aleurone layers. Plant Physiology. 1967;42:398–406. doi: 10.1104/pp.42.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Yang H, Richter GL, et al. phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS Biology. 2011;9:e1001076. doi: 10.1371/journal.pbio.1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churms SC, Merrifield EH, Stephen AM. Some new aspects of the molecular structure of Acacia senegal gum (gum arabic) Carbohydrate Research. 1983;123:267–279. [Google Scholar]
- Churms SC, Stephen AM, Steyn CB. Analytical comparison of gums from Acacia hebeclada and other gummiferae species. Phytochemistry. 1986;25:2807–2809. [Google Scholar]
- Clarke AE, Currie G, Gilson P, et al. University of Melbourne; 2000. Arabinogalactan-proteins in reproductive tissues of flowering plants: a historical perspective of work from the Plant Cell Biology Centre; pp. 121–131. Victoria, Australia. In: Nothnagel EA, Bacic A, Clarke, AE, eds. Proceedings of the 20th Symposium in Plant Physiology. University of California at Riverside, 1999. New York: Springer. [Google Scholar]
- Classen B, Witthohn K, Blaschek W. Characterization of an arabinogalactan-protein isolated from pressed juice of Echinacea purpurea by precipitation with the β-glucosyl Yariv reagent. Carbohydrate Research. 2000;327:497–504. doi: 10.1016/s0008-6215(00)00074-4. [DOI] [PubMed] [Google Scholar]
- Coimbra S, Duarte C. Arabinogalactan proteins may facilitate the movement of pollen tubes from the stigma to the ovules in Actinidia deliciosa and Amaranthus hypochondriacus. Euphytica. 2003;133:171–178. [Google Scholar]
- Coimbra S, Salema R. Immunolocalization of arabinogalactan proteins in Amaranthus hypochondriacus L. ovules. Protoplasma. 1997;199:75–82. [Google Scholar]
- Coimbra S, Oliveira H, Monteiro L, Sottomayor M, Pereira LG. Arabinogalactan proteins are present in Arabidopsis thaliana pollen tubes. 2004. Xth International Cell Wall Meeting Abstract 24. [DOI] [PubMed]
- Coimbra S, Costa M, Jones BJ, Mendes MA, Pereira LG. Pollen grain development is compromised in Arabidopsis agp6 agp11 null mutants. Journal of Experimental Botany. 2009;60:3133–3142. doi: 10.1093/jxb/erp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs A. Transforming a stem into a bush. PLoS Biology. 2013;11:e1001476. doi: 10.1371/journal.pbio.1001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ, Hedrich R. Stretch-activated chloride, potassium, and calcium channels coexisting in plasma membranes of guard cells of Vicia faba L. Planta. 1991;186:143–153. doi: 10.1007/BF00201510. [DOI] [PubMed] [Google Scholar]
- Costa M, Nobre MS, Becker JD, et al. Expression-based and co-localization detection of arabinogalactan protein 6 and arabinogalactan protein 11 interactors in Arabidopsis pollen and pollen tubes. BMC Plant Biology. 2013a;13:1–19. doi: 10.1186/1471-2229-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Pereira AMS, Rudall PJ, Coimbra S. Immunolocalization of arabinogalactan proteins (AGPs) in reproductive structures of an early-divergent angiosperm, Trithuria (Hydatellaceae) Annals of Botany. 2013b;111:183–190. doi: 10.1093/aob/mcs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I, Vanneste S, Inze D, Beeckman T. Lateral root initiation or the birth of a new meristem. Plant Molecular Biology. 2006;60:871–887. doi: 10.1007/s11103-005-4547-2. [DOI] [PubMed] [Google Scholar]
- Defaye J, Wong E. Structural studies of gum arabic, the exudate polysaccharide from Acacia senegal. Carbohydrate Research. 1986;150:221–231. [Google Scholar]
- Di Giacomo ED, Iannelli MA, Frugis G. TALE and shape: how to make a leaf different. Plants. 2013;2:317–342. doi: 10.3390/plants2020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JP, Pickard BG. Mechanosensory calcium-selective cation channels in epidermal cells. Plant Journal. 1993;3:83–110. doi: 10.1111/j.1365-313x.1993.tb00013.x. [DOI] [PubMed] [Google Scholar]
- Ding JP, Badot P-M, Pickard BG. Aluminium and hydrogen ions inhibit a mechanosensory calcium-selective cation channel. Australian Journal of Plant Physiology. 1993;20:771–778. doi: 10.1071/pp9930771. [DOI] [PubMed] [Google Scholar]
- Ding L, Zhu J-K. A role for arabinogalactan-proteins in root epidermal cell expansion. Planta. 1997;203:289–294. doi: 10.1007/s004250050194. [DOI] [PubMed] [Google Scholar]
- Ding Z, Galvan-Ampudia CS, Demarsy E, et al. Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nature Cell Biology. 2013;13:447–452. doi: 10.1038/ncb2208. [DOI] [PubMed] [Google Scholar]
- Dodd AN, Kudla J, Sanders D. The language of calcium signaling. Annual Review of Plant Biology. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- Dolan L, Linstead P, Roberts K. An AGP epitope distinguishes a central metaxylem initial from other vascular initials in the Arabidopsis root. Protoplasma. 1995;189:149–155. [Google Scholar]
- Dougall DK, Shimbayashi K. Factors affecting growth of tobacco callus tissue and its incorporation of tyrosine. Plant Physiology. 1960;35:396–404. doi: 10.1104/pp.35.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driouich A, Baskin TI. Intercourse between cell wall and cytoplasm exemplified by arabinogalactan proteins and cortical microtubules. American Journal of Botany. 2008;95:1491–1497. doi: 10.3732/ajb.0800277. [DOI] [PubMed] [Google Scholar]
- Du H, Clarke AE, Bacic A. Arabinogalactan-proteins: a class of extracellular matrix proteoglycans involved in plant growth and development. Trends in Cell Biology. 1996a;6:411–414. doi: 10.1016/s0962-8924(96)20036-4. [DOI] [PubMed] [Google Scholar]
- Du H, Simpson RJ, Clarke AE, Bacic A. Molecular characterization of a stigma-specific gene encoding an arabinogalactan-protein (AGP) from Nicotiana alata. Plant Journal. 1996b;9:313–323. doi: 10.1046/j.1365-313x.1996.09030313.x. [DOI] [PubMed] [Google Scholar]
- Ebashi S, Endo M. Calcium ion and muscle contraction. Progress in Biophysics and Molecular Biology. 1968;18:123–166. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Ellis M, Egelund J, Schultz CJ, Bacic A. Arabinogalactan-proteins: key regulators at the cell surface? Plant Physiology. 2010;153:403–419. doi: 10.1104/pp.110.156000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle HH. Auxin causes oscillations of cytosolic free calcium and pH in Zea mays coleoptiles. Planta. 1988;174:495–499. doi: 10.1007/BF00634478. [DOI] [PubMed] [Google Scholar]
- Fincher GB, Stone BA. A water-soluble arabinogalactan-peptide from wheat endosperm. Australian Journal of Biological Science. 1974;27:117–132. doi: 10.1042/bj1390535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincher GB, Stone BA, Clarke AE. Arabinogalactan-proteins: structure, biosynthesis, and function. Annual Review of Plant Physiology. 1983;34:47–70. [Google Scholar]
- Folta KM, Lieg EG, Durham T, Spalding EP. Primary inhibition of hypocotyl growth and phototropism depend differently on phototropin mediated increases in cytoplasmic calcium induced by blue light. Plant Physiology. 2003;133:1464–1470. doi: 10.1104/pp.103.024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin-Tong VE. Signaling and the modulation of pollen tube growth. Plant Cell. 1999;11:727–738. doi: 10.1105/tpc.11.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Wisniewski JP, Benkova E, Mengen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- Fu H, Yadav MP, Nothnagel EA. Physcomitrella patens arabinogalactan proteins contain abundant terminal 3-O-methyl-L-rhamnosyl residues not found in angiosperms. Planta. 2007;226:1511–1524. doi: 10.1007/s00425-007-0587-y. [DOI] [PubMed] [Google Scholar]
- Fukuda H. Signals that control plant vascular cell differentiation. Nature Reviews in Molecular Cell Biology. 2004;5:379–391. doi: 10.1038/nrm1364. [DOI] [PubMed] [Google Scholar]
- Gane AM, Weinhandl JA, Bacic A, Harris PJ. Histochemistry and composition of the cell walls of styles of Nicotiana alata Link et Otto. Planta. 1994;195:217–225. [Google Scholar]
- Gane AM, Clarke AE, Bacic A. Localization and expression of arabinogalactan-proteins in the ovaries of Nicotiana alata Link and Otto. Sexual Plant Reproduction. 1995a;8:278–282. [Google Scholar]
- Gane AM, Craik D, Munro SLA, Howlett GJ, Clarke AE, Bacic A. Structural analysis of the carbohydrate moiety of arabinogalactan-proteins from stigmas and styles of Nicotiana alata. Carbohydrate Research. 1995b;277:67–85. doi: 10.1016/0008-6215(95)00197-2. [DOI] [PubMed] [Google Scholar]
- Gao M, Showalter AM. Yariv reagent treatment induces programmed cell death in Arabidopsis cell cultures and implicates arabinogalactan protein involvement. Plant Journal. 1999;19:321–331. doi: 10.1046/j.1365-313x.1999.00544.x. [DOI] [PubMed] [Google Scholar]
- Gao M, Kieliszewski MJ, Lamport DTA, Showalter AM. Isolation, characterization and immunolocalization of a novel, modular tomato arabinogalactan-protein corresponding to the LeAGP-1 gene. Plant Journal. 1999;18:43–55. doi: 10.1046/j.1365-313x.1999.00428.x. [DOI] [PubMed] [Google Scholar]
- Gaspar Y, Johnson KL, McKenna JA, Bacic A, Schultz CJ. The complex structures of arabinogalactan-proteins and the journey towards understanding function. Plant Molecular Biology. 2001;47:161–176. [PubMed] [Google Scholar]
- Gaspar YM, Nam J, Schultz CJ, et al. Characterization of the Arabidopsis lysine-rich arabinogalactan-protein AtAGP17 mutant (rat1) that results in a decreased efficiency of Agrobacterium transformation. Plant Physiology. 2004;135:2162–2171. doi: 10.1104/pp.104.045542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring CA, Irving HR, Parish RW. Effects of auxin and abscisic acid on cytosolic calcium and pH in plant cells. Proceedings of the National Academy of Sciences USA. 1990a;87:9645–9649. doi: 10.1073/pnas.87.24.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring CA, Williams DA, Cody SH, Parish RW. Phototropism and geotropism in maize coleoptiles are spatially correlated with increases in cytosolic free calcium. Nature. 1990b;345:528–530. doi: 10.1038/345528a0. [DOI] [PubMed] [Google Scholar]
- Gens JS, Fujiki M, Pickard BG. Arabinogalactan protein and wall-associated kinase in a plasmalemmal reticulum with specialized vertices. Protoplasma. 2000;212:115–134. doi: 10.1007/BF01279353. [DOI] [PubMed] [Google Scholar]
- Geshi N, Johansen JN, Dilokpimol A, et al. A galactosyltransferase acting on arabinogalactan protein glycans is essential for embryo development in Arabidopsis. Plant Journal. 2013;76:128–137. doi: 10.1111/tpj.12281. [DOI] [PubMed] [Google Scholar]
- Goodrum LJ, Patel A, Leykam JF, Kieliszewski MJ. Gum arabic glycoprotein contains glycomodules of both extensin and arabinogalactan-glycoproteins. Phytochemistry. 2000;54:99–106. doi: 10.1016/s0031-9422(00)00043-1. [DOI] [PubMed] [Google Scholar]
- Gotelli IB, Cleland RE. Differences in the occurrence and distribution of hydroxyproline-proteins among the algae. American Journal of Botany. 1968;55:907–914. [PubMed] [Google Scholar]
- Groover A, Jones AM. Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiology. 1999;119:375–384. doi: 10.1104/pp.119.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald W, Cannoot B, Friml J, Gheysen G. Parasitic nematodes modulate PIN-mediated auxin transport to faciltate infection. PLoS Pathogens. 2009;5:7. doi: 10.1371/journal.ppat.1000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Nothnagel EA. Binding of arabinogalactan proteins by Yariv phenylglycoside triggers wound-like responses in arabidopsis cell cultures. Plant Physiology. 2004;135:1346–1366. doi: 10.1104/pp.104.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. Journal of Plant Research. 2003;116:483–505. doi: 10.1007/s10265-003-0110-x. [DOI] [PubMed] [Google Scholar]
- Held MA, Tan L, Kamyab A, Hare M, Shpak E, Kieliszewksi MJ. Di-isodityrosine is the intermolecular cross-link of isodityrosine-rich extensin analogs cross-linked in vitro. Journal of Biological Chemistry. 2004;279:55474–55482. doi: 10.1074/jbc.M408396200. [DOI] [PubMed] [Google Scholar]
- Hepler PK. Calcium: a central regulator of plant growth and development. Plant Cell. 2005;17:2142–2155. doi: 10.1105/tpc.105.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn ANJ. The physiology of cell elongation. Botanical Review. 1940;6:515–574. [Google Scholar]
- Himanen K, Boucheron E, Vanneste S, Engler JdA, Inze D, Beeckman T. Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell. 2002;14:2339–2351. doi: 10.1105/tpc.004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Weddle NM, Kim S, et al. Effect of extracellular calcium, pH and borate on growth oscillations in Lilium formosanum pollen tubes. Journal of Experimental Botany. 2003;54:65–72. doi: 10.1093/jxb/erg004. [DOI] [PubMed] [Google Scholar]
- Hunt GE. A comparative chromatographic survey of the amino acids in five species of legume roots and nodules. American Journal of Botany. 1951;38:452–457. [Google Scholar]
- Ikeda Y, Men S, Fischer U, et al. Local auxin biosynthesis modulates gradient-directed planar polarity in Arabidopsis. Nature Cell Biology. 2009;11:731–739. doi: 10.1038/ncb1879. [DOI] [PubMed] [Google Scholar]
- Irving HR, Gehring CA, Parish RW. Changes in cytosolic pH and calcium of guard cells precede stomatal movements. Proceedings of the National Academy of Sciences USA. 1992;89:1790–1794. doi: 10.1073/pnas.89.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani M, Liu J, Halfter U, Kim C-S, Shi W, Zhu J-K. SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding. Plant Cell. 2000;12:1667–1677. doi: 10.1105/tpc.12.9.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauh GY, Lord EM. Localization of pectins and arabinogalactan-proteins in lily (Lilium longiflorum L.) pollen tube and style, and their possible roles in pollination. Planta. 1996;199:251–261. [Google Scholar]
- Jermyn MA, Yeow YM. A class of lectins present in the tissues of seed plants. Australian Journal of Plant Physiology. 1975;2:501–531. [Google Scholar]
- Jiang L, Liu X, Xiong G. DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature. 2013;504:401–405. doi: 10.1038/nature12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM. Programmed cell death in development and defense. Plant Physiology. 2001;125:94–97. doi: 10.1104/pp.125.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AR, Kramer ER, Knox K, et al. Auxin transport through non-hair cells sustains root-hair development. Nature Cell Biology. 2009;11:78–84. doi: 10.1038/ncb1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose-Estanyol M, Puigdomenech P. Plant cell wall glycoproteins and their genes. Plant Physiology and Biochemistry (Paris; 2000) 2000;38:97–108. [Google Scholar]
- Joslyn MA, Stepka W. The free amino acids of fruits. Food Research. 1949;14:459–467. doi: 10.1111/j.1365-2621.1949.tb16256.x. [DOI] [PubMed] [Google Scholar]
- Kim T-H, Boehmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annual Review of Plant Biology. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa K, Tryfona T, Yoshimi Y, et al. β-Galactosyl Yariv reagent binds to the β-1,3-galactan of arabinogalactan proteins. Plant Physiology. 2013;161:1117–1126. doi: 10.1104/pp.112.211722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant Journal. 1997;12:1067–1078. doi: 10.1046/j.1365-313x.1997.12051067.x. [DOI] [PubMed] [Google Scholar]
- Knoch E, Dilokpimol A, Tryfona T, et al. A β-glucuronosyltransferase from Arabidopsis thaliana involved in biosynthesis of type-II arabinogalactan has a role in cell elongation during seedling growth. Plant Journal. 76:1016–1029. doi: 10.1111/tpj.12353. [DOI] [PubMed] [Google Scholar]
- Knox JP. Arabinogalactan-proteins (AGPs) and plant cell development. Foods & Food Ingredients Journal of Japan. 2006;211:26–31. [Google Scholar]
- Knox JP, Day S, Roberts K. A set of cell surface glycoproteins forms an early marker of cell position, but not cell type, in the root apical meristem of Daucus carota L. Development. 1989;106:56. [Google Scholar]
- Komalavilas P, Zhu J, Nothnagel EA. Arabinogalactan-proteins from the suspension culture medium and plasma membrane of rose cells. Journal of Biological Chemistry. 1991;266:15956–15965. [PubMed] [Google Scholar]
- Kreuger M, van Holst G-J. Arabinogalactan proteins are essential in somatic embryogenesis of Daucus carota L. Planta. 1993;189:243–248. [Google Scholar]
- Kreuger M, van Holst G-J. Arabinogalactan proteins and plant differentiation. Plant Molecular Biology. 1996;30:1077–1086. doi: 10.1007/BF00019543. [DOI] [PubMed] [Google Scholar]
- Kunkel ME, Seo A, Minten TA. Magnesium binding by gum arabic, locust bean gum, and arabinogalactan. Food Chemistry. 1997;59:87–93. [Google Scholar]
- Lamport DTA. Oxygen fixation into hydroxyproline of plant cell wall protein. Journal of Biological Chemistry. 1963a;238:1438–1440. [PubMed] [Google Scholar]
- Lamport DTA. The primary cell wall. 1963b. PhD thesis, University of Cambridge http://gradworks.umi.com/3504823.pdf .
- Lamport DTA. Cell wall metabolism. Annual Review of Plant Physiology. 1970;21:235–270. [Google Scholar]
- Lamport DTA. Structure, biosynthesis and significance of cell wall glycoproteins. In: Loewus FA, Runeckles VC, editors. Recent advances in phytochemistry. New York: Plenum Publishing Corp; 1977. pp. 79–115. [Google Scholar]
- Lamport DTA. Life behind cell walls: paradigm lost, paradigm regained. Cellular and Molecular Life Sciences. 2001;58:1363–1385. doi: 10.1007/PL00000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport DTA. Hydroxyproline assay using NaBr/NaOCl. 2013a. Bio-Protocol 3: http://www.bio-protocol.org/wenzhang.aspx?id=917 .
- Lamport DTA. Preparation of arabinogalactan glycoproteins from plant tissue? Bio-Protocol. 2013b;3 http://www.bio-protocol.org/wenzhang.aspx?id=918 . [Google Scholar]
- Lamport DTA, Miller DH. Hydroxyproline arabinosides in the plant kingdom. Plant Physiology. 1971;48:454–456. doi: 10.1104/pp.48.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport DTA, Northcote DH. Hydroxyproline in primary cell walls of higher plants. Nature. 1960;188:665–666. [Google Scholar]
- Lamport DTA, Varnai P. Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytologist. 2013;197:58–64. doi: 10.1111/nph.12005. [DOI] [PubMed] [Google Scholar]
- Lamport DTA, Kieliszewski MJ, Showalter AM. Salt stress upregulates periplasmic arabinogalactan proteins: using salt stress to analyze AGP function. New Phytologist. 2006;169:479–492. doi: 10.1111/j.1469-8137.2005.01591.x. [DOI] [PubMed] [Google Scholar]
- Lamport DTA, Kieliszewksi MJ, Chen Y, Cannon MC. Role of the extensin superfamily in primary cell wall architecture. Plant Physiology. 2011;156:11–19. doi: 10.1104/pp.110.169011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin PJ. Plant protoplast agglutination by artificial carbohydrate antigens. Journal of Cell Science. 1978;30:283–292. doi: 10.1242/jcs.30.1.283. [DOI] [PubMed] [Google Scholar]
- Lavenus J, Goh T, Roberts I, et al. Lateral root development in Arabidopsis: fifty shades of auxin. Trends in Plant Science. 2013;18:450–458. doi: 10.1016/j.tplants.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Lecourieux D, Ranjeva R, Pugin A. Calcium in plant defence-signalling pathways. New Phytologist. 2006;171:249–269. doi: 10.1111/j.1469-8137.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- Lee KJD, Sakata Y, Mao S-L, et al. Arabinogalactan proteins are required for apical cell extension in the moss Physcomitrella patens. Plant Cell. 2005;17:3051–3065. doi: 10.1105/tpc.105.034413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Pennell R, Alvarez ML, Palmer R, Lamb CJ. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Current Biology. 1996;6:427–437. doi: 10.1016/s0960-9822(02)00510-9. [DOI] [PubMed] [Google Scholar]
- Li S, Showalter AM. Cloning and developmental/stress regulated expression of a gene encoding a tomato arabinogalactan protein. Plant Molecular Biology. 1996;32:641–652. doi: 10.1007/BF00020205. [DOI] [PubMed] [Google Scholar]
- Liang Y, Basu D, Pattathil S, et al. Biochemical and physiological characterization of fut4 and fut6 mutants defective in arabinogalactan-protein fucosylation in Arabidopsis. Journal of Experimental Botany. 2013;64:5537–5551. doi: 10.1093/jxb/ert321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Mehdy MC. A nonclassical arabinogalactan protein gene highly expressed in vascular tissues, AGP31, is transcriptionally repressed by methyl jasmonic acid in Arabidopsis. Plant Physiology. 2007;145:863–874. doi: 10.1104/pp.107.102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosveld A-MA, Grobet PJ, Delcour JA. Contents and structural features of water-extractable arabinogalactan in wheat flour fractions. Journal of Agricultural and Food Chemistry. 1997;45:1998–2002. [Google Scholar]
- Lu H, Chen M, Showalter AM. Developmental expression and perturbation of arabinogalactan-proteins during seed germination and seedling growth in tomato. Physiologia Plantarum. 2001;112:442–450. doi: 10.1034/j.1399-3054.2001.1120319.x. [DOI] [PubMed] [Google Scholar]
- Ma H, Zhao J. Genome-wide identification, classification, and expression analysis of the arabinogalactan protein gene family in rice (Oryza sativa L.) Journal of Experimental Botany. 2010;61:2647–2668. doi: 10.1093/jxb/erq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Zhao Y, Walker RK, Berkowitz GA. Molecular steps in the immune signaling pathway evoked by plant elicitor peptides: Ca2+-dependent protein kinases, nitric oxide, and reactive oxygen species are downstream from the early Ca2+ signal. Plant Physiology. 2013;163:1459–1471. doi: 10.1104/pp.113.226068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehly AC, Paleus S. Paper chromatography of the amino-acids in horseradish peroxidase. Acta Chemica Scandinavica. 1950;4:508–511. [Google Scholar]
- Majewska-Sawka A, Nothnagel EA. The multiple roles of arabinogalactan proteins in plant development. Plant Physiology. 2000;122:3–9. doi: 10.1104/pp.122.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska-Sawka A, Munster A, Rodriguez-Garcia MI. Guard cell wall: immunocytochemical detection of polysaccharide components. Journal of Experimental Botany. 2002;53:1067–1079. doi: 10.1093/jexbot/53.371.1067. [DOI] [PubMed] [Google Scholar]
- Mascarenhas JP. Molecular mechanisms of pollen tube growth and differentiation. Plant Cell. 1993;5:1303–1314. doi: 10.1105/tpc.5.10.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas JP, Machlis L. Chemotropic response of Antirrhinum majus pollen to calcium. Nature. 1962;196:292–293. doi: 10.1104/pp.39.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiguchi K, Urakami E, Hasegama M, et al. Defense-related signaling by interaction of arabinogalactan proteins and β-glucosyl Yariv reagent inhibits gibberellin signaling in barley aleurone cells. Plant and Cell Physiology. 2008;49:178–190. doi: 10.1093/pcp/pcm175. [DOI] [PubMed] [Google Scholar]
- Miller DH, Lamport DTA, Miller M. Hydroxyproline heterooligosaccharides in Chlamydomonas. Science. 1972;176:918–920. doi: 10.1126/science.176.4037.918. [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Miller ND, Murphy AS, Gilroy JS. Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis. Plant Journal. 2011;65:309–318. doi: 10.1111/j.1365-313X.2010.04423.x. [DOI] [PubMed] [Google Scholar]
- Moore JP, Nguema-Ona E, Chevalier L, et al. Response of the leaf cell wall to desiccation in the resurrection plant Myrothamnus flabellifolius. Plant Physiology. 2006;141:651–662. doi: 10.1104/pp.106.077701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Nguema-Ona E, Vicre-Gibouin M, et al. Arabinose-rich polymers as an evolutionary strategy to plasticize resurrection plant cell walls against desiccation. Planta. 2013;237:739–754. doi: 10.1007/s00425-012-1785-9. [DOI] [PubMed] [Google Scholar]
- Motose H, Sugiyama M, Fukuda H. A proteoglycan mediates inductive interaction during plant vascular development. Nature. 2004;429:873–878. doi: 10.1038/nature02613. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Katagiri T, Shinozaki K, et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proceedings of the National Academy of Sciences USA; 2007. pp. 3639–3644. 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L, Dunoyer P, Jay F, et al. A plant miRNA contributes to antibacterial resistance by repressing auxin signalling. Science. 2006;312:436–439. doi: 10.1126/science.1126088. [DOI] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT. Hydrogen peroxide and nitric oxide as signalling molecules in plants. Journal of Experimental Botany. 2002;53:1237–1247. [PubMed] [Google Scholar]
- Nguema-Ona E, Andeme-Onzighi C, Aboughe-Angone S, et al. The reb1-1 mutation of arabidopsis. Effect on the structure and localization of galactose-containing cell wall polysaccharides. Plant Physiology. 2006;206:1406–1417. doi: 10.1104/pp.105.074997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguema-Ona E, Coimbra S, Vicre-Gibouin M, Mollet JC, Driouich A. Arabinogalactan proteins in root and pollen-tube cells: distribution and functional aspects. Annals of Botany. 2012;110:383–404. doi: 10.1093/aob/mcs143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguema-Ona E, Vicre-Gibouin M, Cannesan M-A, Driouich A. Arabinogalactan proteins in root–microbe interactions. Trends in Plant Science. 2013;18:440–449. doi: 10.1016/j.tplants.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Norman PM, Kjellbom P, Bradley DJ, Hahn MG, Lamb CJ. Immunoaffinity purification and biochemical characterization of plasma membrane arabino-galactan-rich glycoproteins of Nicotiana glutinosa. Planta. 1990;181:365–373. doi: 10.1007/BF00195889. [DOI] [PubMed] [Google Scholar]
- Nothnagel EA. Proteoglycans and related components in plant cells. International Review of Cytology. 1997;174:195–291. doi: 10.1016/s0074-7696(08)62118-x. [DOI] [PubMed] [Google Scholar]
- O'Neil MA, Ishii T, Albersheim P, Darvill AG. Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annual Review of Plant Biology. 2004;55:109–139. doi: 10.1146/annurev.arplant.55.031903.141750. [DOI] [PubMed] [Google Scholar]
- Oxley D, Bacic A. Structure of the glycosylphosphatidylinositol anchor of an arabinogalactan protein from Pyrus communis suspension-cultured cells. Proceedings of the National Academy of Sciences USA. 1999;96:14246–14251. doi: 10.1073/pnas.96.25.14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T, Zazimalova E, Ruthardt N, et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- Pagnussat GC, Alandete-Saez M, Bowman JL, Sundaresan V. Auxin-dependent patterning and gamete specification in the Arabidopsis female gametophyte. Science. 2009;324:1684–1689. doi: 10.1126/science.1167324. [DOI] [PubMed] [Google Scholar]
- Pal A, Das S. Arabinogalactan-proteins: role in plant tissue differentiation and commercial importance. Proceedings of the National Academy of Sciences; India, Sect B. 2006. pp. 312–320. [Google Scholar]
- Park MH, Suzuki Y, Chono M, Knox JP, Yamaguchi I. CsAGP1, a gibberellin-responsive gene from cucumber hypocotyls, encodes a classical arabinogalactan protein and is involved in stem elongation. Plant Physiology. 2003;131:1450–1459. doi: 10.1104/pp.015628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard BG. Action potentials in higher plants. Botanical Review. 1973;39:172–201. [Google Scholar]
- Pickard BG. Voltage transients elicited by sudden step-up of auxin. Plant Cell and Environment. 1984;7:171–178. [PubMed] [Google Scholar]
- Pickard BG. Delivering force and amplifying signals in plant mechanosensing. Current Topics in Plant Membranes. 2007;58:361–392. [Google Scholar]
- Pickard BG. Arabinogalactan proteins – becoming less mysterious. New Phytologist. 2013;197:3–5. doi: 10.1111/nph.12061. [DOI] [PubMed] [Google Scholar]
- Plieth CP, Trewavas AJ. Reorientation of seedlings in the earth's gravitational field induces cytosolic calcium transients. Plant Physiology. 2002;129:786–796. doi: 10.1104/pp.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson BJ, Davies C. Characterization of a cDNA encoding the protein moiety of a putative arabinogalactan protein from Lycopersicon esculentum. Plant Molecular Biology. 1995;28:347–352. doi: 10.1007/BF00020254. [DOI] [PubMed] [Google Scholar]
- Pope DG. Relationships between hydroxyproline-containing proteins secreted into the cell wall and medium by suspension-cultured Acer pseudoplatanus cells. Plant Physiology. 1977;59:894–900. doi: 10.1104/pp.59.5.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA, Fry SC. Primary cell wall composition of bryophytes and charophytes. Annals of Botany. 2003;91:1–12. doi: 10.1093/aob/mcg013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA, Sadler IH, Fry SC. 3-O-Methylrhamnose in lower land plant primary cell walls. Biochemical Systematics and Ecology. 2004;32:279–289. [Google Scholar]
- Popper ZA, Sadler IH, Fry SC. 3-O-Methyl-d-galactose residues in lycophyte primary cell walls. Phytochemistry. 2001;57:711–719. doi: 10.1016/s0031-9422(01)00140-6. [DOI] [PubMed] [Google Scholar]
- Qi W, Fong C, Lamport DTA. Gum arabic glycoprotein is a twisted hairy rope: a new model based on O-galactosylhydroxyproline as the polysaccharide attachment site. Plant Physiology. 1991;96:848–855. doi: 10.1104/pp.96.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Zhao J. Roles of arabinogalactan-proteins in sexual reproduction of angiosperms. Zhiwu Shengli Yu Fenzi Shengwuxue Xuebao. 2004;30:371–378. [PubMed] [Google Scholar]
- Qin Y, Zhao J. Localization of arabinogalactan proteins in egg cells, zygotes, and two-celled proembryos and effects of α-d-glucosyl Yariv reagent on egg cell fertilization and zygote division in Nicotiana tabacum L. Journal of Experimental Botany. 2006;57:2061–2074. doi: 10.1093/jxb/erj159. [DOI] [PubMed] [Google Scholar]
- Qin Y, Chen D, Zhao J. Localization of arabinogalactan proteins in anther, pollen, and pollen tube of Nicotiana tabacum L. Protoplasma. 2007;231:43–53. doi: 10.1007/s00709-007-0245-z. [DOI] [PubMed] [Google Scholar]
- Rauh RA, Basile DV. Phenovariation induced in Streptocarpus prolixus (Gesneriaceae) by β-glucosyl Yariv reagent. Canadian Journal of Botany. 2003;81:338–344. [Google Scholar]
- Rayle DL, Cleland RE. Evidence that auxin-induced growth of soybean hypocotyls involves proton excretion. Plant Physiology. 1980;66:433–437. doi: 10.1104/pp.66.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle DL, Cleland RE. The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiology. 1992;99:1271–1274. doi: 10.1104/pp.99.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgwell RJ, Curti D, Fischer M, Nicolas P, Fay LB. Coffee bean arabinogalactans: acidic polymers covalently linked to protein. Carbohydrate Research. 2002;337:239–253. doi: 10.1016/s0008-6215(01)00316-0. [DOI] [PubMed] [Google Scholar]
- Redgwell RJ, Fischer M, Curti D, Sutherland P, Hallett I, MacRae E. Arabinogalactan-proteins in coffee bean cell walls. Foods & Food Ingredients Journal of Japan. 2006;211:38–47. [Google Scholar]
- Rees DA. Polysaccharide shapes. London: Chapman and Hall; 1977. [Google Scholar]
- Reger BJ, Chaubal R, Pressey R. Chemotropic responses by pearl millet pollen tubes. Sexual Plant Reproduction. 1992;5:47–56. [Google Scholar]
- Robert S, Kleine-Vehn J, Barbez E, et al. ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell. 2010;143:111–121. doi: 10.1016/j.cell.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Jauh GY, Hepler PK, Lord EM. Effects of Yariv phenylglycoside on cell wall assembly in the lily pollen tube. Planta. 1998;204:450–458. doi: 10.1007/s004250050279. [DOI] [PubMed] [Google Scholar]
- Roy S, Holdaway-Clarke TL, Hackett GR, Kunkel JG, Lord EM, Hepler PK. Uncoupling secretion and tip growth in lily pollen tubes: evidence for the role of calcium in exocytosis. Plant Journal. 1999;19:379–386. doi: 10.1046/j.1365-313x.1999.00515.x. [DOI] [PubMed] [Google Scholar]
- Samaj J, Baluska F, Bobak M, Volkmann D. Extracellular matrix surface network of embryogenic units of friable maize callus contains arabinogalactan-proteins recognized by monoclonal antibody JIM4. Plant Cell Reports. 1999a;18:369–374. [Google Scholar]
- Samaj J, Braun M, Baluska F, Ensikat HJ, Tsumuraya Y, Volkmann D. Specific localization of arabinogalactan-protein epitopes at the surface of maize root hairs. Plant and Cell Physiology. 1999b;40:874–883. [Google Scholar]
- Samuelsen AB, Paulsen BS, Wold JK, Knutsen SH, Yamada H. Characterization of a biologically active arabinogalactan from the leaves of Plantago major L. Carbohydrate Polymers. 1998;35:145–153. [Google Scholar]
- Saulnier L, Brillouet J-M, Moutounet M, Herve du Penhoat C, Michon V. New investigations of the structure of grape arabinogalactan-protein. Carbohydrate Research. 1992;224:219–235. doi: 10.1016/0008-6215(92)84108-5. [DOI] [PubMed] [Google Scholar]
- Scarpella E, Marcos D, Friml J, Berleth T. Control of leaf vascular patterning by polar auxin transport. Genes Development. 2006;20:1027. doi: 10.1101/gad.1402406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer P. Cytochemical identification of arabinogalactan protein in the outer epidermal wall of maize coleoptiles. Planta. 1990;183:139–142. doi: 10.1007/BF00197578. [DOI] [PubMed] [Google Scholar]
- Schopfer P. Determination of auxin-dependent pH changes in coleoptile cell walls by a null-point method. Plant Physiology. 1993;103:351–357. doi: 10.1104/pp.103.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz CJ, Johnson KL, Currie G, Bacic A. The classical arabinogalactan protein gene family of Arabidopsis. Plant Cell. 2000;12:1751–1767. doi: 10.1105/tpc.12.9.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz CJ, Rumsewicz MP, Johnson KL, Jones BJ, Gaspar YM, Bacic A. Using genomic resources to guide research directions. The Arabinogalactan protein gene family as a test case. Plant Physiology. 2002;129:1448–1463. doi: 10.1104/pp.003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlbach M, Koenig S, Mormann M, Sendker J, Hensel A. Arabinogalactan protein cluster from Jatropha curcas seed embryo contains fasciclin, xylogen and LysM proteins. Carbohydrate Polymers. 2013;98:522–531. doi: 10.1016/j.carbpol.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Seifert GJ, Roberts K. The biology of arabinogalactan proteins. Annual Review of Plant Biology. 2007;58:137–161. doi: 10.1146/annurev.arplant.58.032806.103801. [DOI] [PubMed] [Google Scholar]
- Seifert GJ, Barber C, Wells B, Dolan L, Roberts K. Galactose biosynthesis in Arabidopsis. Genetic evidence for substrate channeling from UDP-D-galactose into cell wall polymers. Current Biology. 2002;12:1840–1845. doi: 10.1016/s0960-9822(02)01260-5. [DOI] [PubMed] [Google Scholar]
- Serpe MD, Nothnagel EA. Effects of Yariv phenylglycosides on Rosa cell suspensions: evidence for the involvement of arabinogalactan-proteins in cell proliferation. Planta. 1994;193:542–550. [Google Scholar]
- Serpe MD, Nothnagel EA. Heterogeneity of arabinogalactan-proteins on the plasma membrane of rose cells. Plant Physiology. 1996;112:1261–1271. doi: 10.1104/pp.112.3.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpe MD, Nothnagel EA. Arabinogalactan-proteins in the multiple domains of the plant cell surface. Advances in Botanical Research. 1999;30:207–289. [Google Scholar]
- Shinohara N, Taylor C, Leyser O. Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biology. 2013;11:1–14. doi: 10.1371/journal.pbio.1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishova M, Lindberg S. Auxin induces an increase of Ca2+ concentration in the cytosol of wheat leaf protoplasts. Journal of Plant Physiology. 2004;161:937–945. doi: 10.1016/j.jplph.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Shishova M, Lindberg S. A new perspective on auxin perception. Journal of Plant Physiology. 2010;167:417–422. doi: 10.1016/j.jplph.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Showalter AM. Arabinogalactan-proteins: structure, expression and function. Cellular Molecular and Life Sciences. 2001;58:1399–1417. doi: 10.1007/PL00000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter AM, Keppler B, Lichtenberg J, Gu D, Welch LR. A bioinformatics approach to the identification, classification, and analysis of hydroxyproline-rich glycoproteins. Plant Physiology. 2010;153:485–513. doi: 10.1104/pp.110.156554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak E, Leykam JF, Kieliszewski MJ. Synthetic genes for glycoprotein design and the elucidation of hydroxyproline-O-glycosylation codes. Proceedings of the National Academy of Sciences USA. 1999;96:14736–14741. doi: 10.1073/pnas.96.26.14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddig NE, Osman ME, Al Assaf S, Phillips GO, Williams P. Studies on acacia exudate gums, part IV. Distribution of molecular components in Acacia seyal in relation to Acacia senegal. Food Hydrocolloids. 2005;19:679–686. [Google Scholar]
- Sims IM, Middleton K, Lane AG, Cairns AJ, Bacic A. Characterization of extracellular polysaccharides from suspension cultures of members of the Poaceae. Planta (2000) 2000;210:261–268. doi: 10.1007/PL00008133. [DOI] [PubMed] [Google Scholar]
- Slocum RD, Roux SJ. An improved method for the subcellular localization of calcium using a modification of the antimonate precipitation technique. Journal of Histochemistry and Cytochemistry. 1982;30:617–629. doi: 10.1177/30.7.6179981. [DOI] [PubMed] [Google Scholar]
- Smith SM. Witchcraft and destruction. Nature. 2013;504:384–385. doi: 10.1038/nature12843. [DOI] [PubMed] [Google Scholar]
- Smolarkiewicz M, Dhonukshe P. Formative cell divisions: principal determinants of plant morphogenesis. Plant and Cell Physiology. 2013;54:333–342. doi: 10.1093/pcp/pcs175. [DOI] [PubMed] [Google Scholar]
- Sommer-Knudsen J, Clarke AE, Bacic A. A galactose-rich, cell-wall glycoprotein from styles of Nicotiana alata. Plant Journal. 1996;9:71–83. doi: 10.1046/j.1365-313x.1996.09010071.x. [DOI] [PubMed] [Google Scholar]
- Sommer-Knudsen J, Lush WM, Bacic A, Clarke AE. Re-evaluation of the role of a transmitting tract-specific glycoprotein on pollen tube growth. Plant Journal. 1998;13:529–535. [Google Scholar]
- Souter M, Lindsey K. Polarity and signalling in plant embryogenesis. Journal of Expermental Botany. 2000;51:971–983. doi: 10.1093/jexbot/51.347.971. [DOI] [PubMed] [Google Scholar]
- Steward FC, Thompson JF, Millar FK, Thomas MD, Hendricks RH. The amino acids of alfalfa as revealed by paper chromatography with special reference to compounds labelled with S35. Plant Physiology. 1951;26:123–135. doi: 10.1104/pp.26.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone BA, Valenta K. A brief history of arabinogalactan-proteins. Cell and Developmental Biology; Arabinogalactan-Proteins [Proceedings of the Symposium on Plant Physiology] 20th. 1999;1:1–10. [Google Scholar]
- Sun W, Kieliszewski MJ, Showalter AM. Overexpression of tomato LeAGP-1 arabinogalactan-protein promotes lateral branching and hampers reproductive development. Plant Journal. 2004;40:870–881. doi: 10.1111/j.1365-313X.2004.02274.x. [DOI] [PubMed] [Google Scholar]
- Sutherland PW, Hallett IC, MacRae E, Fischer M, Redgwell RJ. Cytochemistry and immunolocalization of polysaccharides and proteoglycans in the endosperm of green Arabica coffee beans. Protoplasma. 2004;223:203–211. doi: 10.1007/s00709-004-0036-8. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Kitagawa M, Knox JP, Yamaguchi I. A role for arabinogalactan proteins in gibberellin-induced α-amylase production in barley aleurone cells. Plant Journal. 2002;29:733–741. doi: 10.1046/j.1365-313x.2002.01259.x. [DOI] [PubMed] [Google Scholar]
- Svetek J, Yadav MP, Nothnagel EA. Presence of a glycosylphosphatidylinositol lipid anchor on rose arabinogalactan proteins. Journal of Biological Chemistry. 1999;274:14724–14733. doi: 10.1074/jbc.274.21.14724. [DOI] [PubMed] [Google Scholar]
- Swarbreck SM, Colaco R, Davies JM. Plant calcium-permeable channels. Plant Physiology. 2013;163:514–522. doi: 10.1104/pp.113.220855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Leykam JF, Kieliszewski MJ. Glycosylation motifs that direct arabinogalactan addition to arabinogalactan-proteins. Plant Physiology. 2003;132:1362–1369. doi: 10.1104/pp.103.021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Qiu F, Lamport DTA, Kieliszewski MJ. Structure of a hydroxyproline (Hyp)-arabinogalactan polysaccharide from repetitive Ala-Hyp expressed in transgenic Nicotiana tabacum. Journal of Biological Chemistry. 2004;279:13156–13165. doi: 10.1074/jbc.M311864200. [DOI] [PubMed] [Google Scholar]
- Tan L, Varnai P, Lamport DTA, et al. Plant O-hydroxyproline arabinogalactans are composed of repeating trigalactosyl subunits with short bifurcated side chains. Journal of Biological Chemistry. 2010;285:24575–24583. doi: 10.1074/jbc.M109.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Li, Eberhard S, Pattathil S, et al. An arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell. 2013;25:270–287. doi: 10.1105/tpc.112.107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XC, He YQ, Wang Y, Sun MX. The role of arabinogalactan proteins binding to Yariv reagents in the initiation, cell developmental fate, and maintenance of microspore embryogenesis in Brassica napus L. cv. Topas. Journal of Experimental Botany. 2006;57:2639–2650. doi: 10.1093/jxb/erl027. [DOI] [PubMed] [Google Scholar]
- Taoka K, Ohki I, Tsuji H, Kojima C, Shimamoto K. Structure and function of florigen and the receptor complex. Trends in Plant Science. 2013;18:287–294. doi: 10.1016/j.tplants.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Telewski FW. A unified hypothesis of mechanoperception in plants. American Journal of Botany. 2006;93:1466–1476. doi: 10.3732/ajb.93.10.1466. [DOI] [PubMed] [Google Scholar]
- Thompson HJM, Knox JP. Stage-specific responses of embryogenic carrot cell suspension cultures to arabinogalactan protein-binding β-glucosyl Yariv reagent. Planta. 1998;205:32–38. [Google Scholar]
- Toyota M, Gilroy S. Gravitropism and mechanical signaling in plants. American Journal of Botany. 2013;100:111–125. doi: 10.3732/ajb.1200408. [DOI] [PubMed] [Google Scholar]
- Toyota M, Furoichi T, Tatsumi H, Sokabe M. Cytoplasmic calcium increases in response to changes in the gravity vector in hypocotyls and petioles of Arabidopsis seedlings. Plant Physiology. 2008;146:505–514. doi: 10.1104/pp.107.106450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretyn A, Wagner G, Felle HH. Signal transduction in Sinapis alba root hairs: auxins as external messengers. Journal of Plant Physiology. 1991;139:187–193. [Google Scholar]
- Trewavas AJ. Signal perception and transduction. American Society of Plant Physiologists. In: Buchanan RB, Gruissem W, Jones R, editors. Biochemistry and molecular biology of plants. 2000. pp. 930–987. [Google Scholar]
- Tryfona T, Liang H-C, Kotake T, et al. Carbohydrate structural analysis of wheat flour arabinogalactan protein. Carbohydrate Research. 2010;345:2656. doi: 10.1016/j.carres.2010.09.018. [DOI] [PubMed] [Google Scholar]
- Tryfona T, Liang H-C, Kotake T, Tsumuraya Y, Stephens E, Dupree P. Structural characterization of arabidopsis leaf arabinogalactan polysaccharides. Plant Physiology. 2012;160:653–666. doi: 10.1104/pp.112.202309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai F-C, Seki A, Yang HW, et al. A polarized Ca2C, diacylglycerol and STIM1 signalling system regulates directed cell migration. Nature Cell Biology. 2014;16:133–144. doi: 10.1038/ncb2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumuraya Y, Ogura K, Hashimoto Y, Mukoyama H, Yamamoto S. Arabinogalactan-proteins from primary and mature roots of radish (Raphanus sativus L.) Plant Physiology. 1988;86:155–160. doi: 10.1104/pp.86.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja N, Sopory SK. Chemical signaling under abiotic stress environment in plants. Plant Signaling and Behavior. 2007;3:525–536. doi: 10.4161/psb.3.8.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe M, Raphael M, Lehen'kyi V, et al. ORAI1 calcium channel orchestrates skin homeostasis. Proceedings of the National Academy of Sciences USA. 2014;110:E4839–E4848. doi: 10.1073/pnas.1310394110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Friml J. Calcium: the missing link in auxin action. Plants. 2013;2:650–675. doi: 10.3390/plants2040650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, De Rybel S, Beemster GTS, et al. Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell. 2005;17:3035–3050. doi: 10.1105/tpc.105.035493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret F, Wheeler GL, Taylor AR, Farnham G, Brownlee C. Calcium channels in photosynthetic eukaryotes: implications for evolution of calcium-based signalling. New Phytologist. 2010;187:23–43. doi: 10.1111/j.1469-8137.2010.03271.x. [DOI] [PubMed] [Google Scholar]
- Weerasinghe RR, Swanson SJ, Okada SF, et al. Touch induces ATP release in Arabidopsis roots that is modulated by the heterotrimeric G-protein complex. FEBS Letters. 2009;583:2521–2526. doi: 10.1016/j.febslet.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler GL, Brownlee C. Ca2+ signalling in plants and green algae – changing channels. Trends in Plant Science. 2008;13:506–514. doi: 10.1016/j.tplants.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Willats WGT, Knox JP. A role for arabinogalactan-proteins in plant cell expansion: evidence from studies on the interaction of β-glucosyl Yariv reagent with seedlings of Arabidopsis thaliana. Plant Journal. 1996;9:919–925. doi: 10.1046/j.1365-313x.1996.9060919.x. [DOI] [PubMed] [Google Scholar]
- Wu H, Wang H, Cheung AY. A pollen tube growth stimulatory glycoprotein is deglycosylated by pollen tubes and displays a glycosylation gradient in the flower. Cell. 1995;82:395–403. doi: 10.1016/0092-8674(95)90428-x. [DOI] [PubMed] [Google Scholar]
- Wu HM, Wong E, Ogdahl J, Cheung AY. A pollen tube growth-promoting arabinogalactan protein from Nicotiana alata is similar to the tobacco TTS protein. Plant Journal. 2000;22:165–176. doi: 10.1046/j.1365-313x.2000.00731.x. [DOI] [PubMed] [Google Scholar]
- Wu Y, Williams M, Bernard S, Driouich A, Showalter AM, Faik A. Functional identification of two nonredundant Arabidopsis fucosyltransferases specific to arabinogalactan proteins. Journal of Biological Chemistry. 2010;285:13638–13645. doi: 10.1074/jbc.M110.102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Tan L, Goodrum KJ, Kieliszewski MJ. High-yields and extended serum half-life of human interferon alpha 2b expressed in tobacco cells as arabinogalactan-protein fusions. Biotechnolog and Bioengineering. 2007;97:997–1008. doi: 10.1002/bit.21407. [DOI] [PubMed] [Google Scholar]
- Xu J, Tan L, Lamport DTA, Showalter AM, Kieliszewksi MJ. The O-Hyp glycosylation code in tobacco and Arabidopsis and a proposed role of Hyp-glycans in secretion. Phytochemistry. 2008;69:1631–1640. doi: 10.1016/j.phytochem.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Yang G, Showalter AM. Expression and localization of AtAGP18, a lysine-rich arabinogalactan-protein in Arabidopsis. Planta. 2007;226:169–179. doi: 10.1007/s00425-007-0478-2. [DOI] [PubMed] [Google Scholar]
- Yang J, Sardar HS, McGovern KR, Zhang Y, Showalter AM. A lysine-rich arabinogalactan protein in Arabidopsis is essential for plant growth and development, including cell division and expansion. Plant Journal. 2007;49:629–640. doi: 10.1111/j.1365-313X.2006.02985.x. [DOI] [PubMed] [Google Scholar]
- Yang S-H, Wang H, Sathyan P, Stasolla C, Loopstra CA. Real-time RT-PCR analysis of loblolly pine (Pinus taeda) arabinogalactan-protein and arabinogalactan-protein-like genes. Physiologia Plantarum. 2005;124:94–106. [Google Scholar]
- Yariv J, Rapport MM, Graf L. The interaction of glycosides and saccharides with antibody to the corresponding phenylazo glycosides. Biochemical Journal. 1962;85:383–388. doi: 10.1042/bj0850383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youl JJ, Bacic A, Oxley D. Arabinogalactan-proteins from Nicotiana alata and Pyrus communis contain glycosylphosphatidylinositol membrane anchors. Proceedings of the National Academy of Science USA. 1998;95:7921–7926. doi: 10.1073/pnas.95.14.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemeckis R. Back to the Future. 1985. Amblin Entertainment.
- Zhao ZD, Tan L, Showalter AM, Lamport DTA, Kieliszewski MJ. Tomato LeAGP-1 arabinogalactan-protein purified from transgenic tobacco corroborates the Hyp contiguity hypothesis. Plant Journal. 2002;31:431–444. doi: 10.1046/j.1365-313x.2002.01365.x. [DOI] [PubMed] [Google Scholar]
- Zhou F, Lin Q, Zhu L, Ren Y, Zhou K, Shabek N. D14-SCFD3-dependent degradation of D53 regulates strigolactone signalling. Nature. 2013;504:406–412. doi: 10.1038/nature12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J-K. Plant salt tolerance. Trends in Plant Science. 2001;6:66–71. doi: 10.1016/s1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- Zonia L, Cordeiro S, Tupy J, Feijo A. Oscillatory chloride efflux at the pollen tube apex has a role in growth and cell volume regulation and is targeted by inositol 3,4,5,6-tetra kis phosphate. Plant Cell. 2002;14:2233–2249. [PubMed] [Google Scholar]