Abstract
Background
Although plants and many algae (e.g. the Phaeophyceae, brown, and Rhodophyceae, red) are only very distantly related they are united in their possession of carbohydrate-rich cell walls, which are of integral importance being involved in many physiological processes. Furthermore, wall components have applications within food, fuel, pharmaceuticals, fibres (e.g. for textiles and paper) and building materials and have long been an active topic of research. As shown in the 27 papers in this Special Issue, as the major deposit of photosynthetically fixed carbon, and therefore energy investment, cell walls are of undisputed importance to the organisms that possess them, the photosynthetic eukaryotes (plants and algae). The complexities of cell wall components along with their interactions with the biotic and abiotic environment are becoming increasingly revealed.
Scope
The importance of plant and algal cell walls and their individual components to the function and survival of the organism, and for a number of industrial applications, are illustrated by the breadth of topics covered in this issue, which includes papers concentrating on various plants and algae, developmental stages, organs, cell wall components, and techniques. Although we acknowledge that there are many alternative ways in which the papers could be categorized (and many would fit within several topics), we have organized them as follows: (1) cell wall biosynthesis and remodelling, (2) cell wall diversity, and (3) application of new technologies to cell walls. Finally, we will consider future directions within plant cell wall research. Expansion of the industrial uses of cell walls and potentially novel uses of cell wall components are both avenues likely to direct future research activities. Fundamentally, it is the continued progression from characterization (structure, metabolism, properties and localization) of individual cell wall components through to defining their roles in almost every aspect of plant and algal physiology that will present many of the major challenges in future cell wall research.
Keywords: Arabidopsis thaliana, arabinogalactan protein, callose, cellulose synthase, cell wall, Ceratopteris richardii, C-Fern, extracellular matrix, Fucales, glucuronoarabinoxylan, glycoprotein, haustoria, hyaline bodies, Miscanthus, mixed-linkage glucan, Orobanchaceae, pectin, pectin methylesterase, Penium margaritaceum, pollen, ripening, root, rhamnogalacturonan I, rhamnogalacturonan II, seed coat, xyloglucan endotransglucosylase/hydrolase, Zea mays
INTRODUCTION
Amongst the first microscope images are those of cork, which – reportedly due to their visual appearance – led Robert Hooke to coin the term ‘cell’ (Hooke, 1665). In fact what are most clearly seen in these images are the (extensively thickened cork) cell walls. Recognition that cell walls are not rigid but that they are instead metabolically active and responsible for expansive cell growth and differentiation occurred some 200 years later (Sachs, 1887). The development of, and advances in, many technologies (including microscopy; McCann and Knox, 2011; Domozych, 2012) have alternately challenged and progressed our understanding of the carbohydrate-rich cell walls possessed by plants and algae (Fry, 2000; Durachko and Cosgrove, 2009; Foster et al., 2010a, b; Albersheim et al., 2011; Popper, 2011; Avci et al., 2012; Moller et al., 2012; Pattathil et al., 2012; Agger et al., 2014; Mitra and Loqué, 2014). As the major deposit of photosynthetically fixed carbon (Krässig, 1993; Heinze, 2005), and therefore energy investment, cell walls are of undisputed importance to the organisms that possess them, photosynthetic eukaryotes (plants and algae). The complexity of cell wall components along with their interactions with the biotic and abiotic environment are becoming increasingly revealed (Sørensen et al., 2010; Fry, 2011; Popper et al., 2011; Fangel et al., 2012) and discussion of the role of cell walls in plant and algal evolution was recently included in the second edition of The Evolution of Plants (Willis and McElwain, 2014), highlighting the extent of reach into other areas within the plant sciences. Cell wall diversity is the result of differential expression of the vast array of genes, present in plants and algae that code for carbohydrate-active enzymes (Michel et al., 2010; Yin et al., 2010; Coutinho and Henrissat, 2011) according to cell, organ, tissue and plant/algal source, as well as their developmental stage, and impacts on their industrial applications as well as their biological functions (de Siqueira et al. 2010; Stengel et al., 2011; Burton and Fincher, 2014). As a consequence of the importance of cell walls and the numerous techniques required to characterize them, and their components, the community of researchers studying cell walls is both multi-disciplinary and highly dynamic. This is perhaps reflected by the number of cell wall-focussed activities. For example, in the last 5 years, there have been ‘cell wall’-themed focus editions in Plant Physiology (2010) and Frontiers in Plant Science (2012), and several more are scheduled, including within the Journal of Integrative Plant Biology and Plants. In the same time period, there have been a number of cell wall-specific conferences and sessions. Although not an exhaustive list these include: the XIIth International Plant Cell Wall Meeting (Porto, 2010), the 4th conference on Biosynthesis of Plant Cell Wall (Japan, 2011), The Gordon Research Seminar and Conference on Plant Cell Walls (USA, 2012), Society for Experimental Biology Cell Wall Session (Plant and Algal Cell Walls: Origin and Diversity; Valencia, 2013), XIIIth International Plant Cell Wall Meeting (Nantes, 2013), and the 5th International Conference on Plant Cell Wall Biology (Australia, 2014). ‘The increasing pace of remarkable discoveries in the field’ was remarked upon by Albersheim et al. (2011) in the preface to their textbook Plant Cell Walls, itself an indication of the demand for, and necessity and extent of current knowledge in this field.
This Issue
The collection of papers published in this Special Issue represents only a small selection of the expanse of contemporary plant and algal cell wall-focussed research, highlighting areas of recent progress; the number of papers submitted to this Special Issue illustrates the high level of activity in this area of biological research. The 27 featured papers include those focussed on: specific algae (the Fucales, charophycean green algae, Penium margaritaceum) and plants (Arabidopsis thaliana, Ceratopteris richardii, grapes, maize, Miscanthus and members of the Orobanchaceae), different plant organs (roots, pollen tubes, seed coats, pollen, and fruit), as well as individual cell wall components (arabinogalactan proteins, callose, galactans, rhamnogalacturonan II, pectins, and wall-localized or -acting proteins). The methodologies employed in the research described in these papers are also derived from rapidly evolving, state-of-the-art biochemical, molecular, cell biology, immunological and even ecological technologies, further supporting the modern synthesis of disciplines used to study cell walls. Even a precursory glance at this list suggests that many of the papers could easily fit within one or more of the categories that we have divided them into, highlighting the interconnected nature that is an inherent feature of much cell wall research.
CONTENT SUMMARIES
Cell wall biosynthesis and remodelling
Wall biosynthesis has been a predominant interest in cell wall research (Ulvskov, 2011). The papers in the first category of this Special Issue focus on how the complex components that make up the wall are made, and how they are modified during expansive cell growth and development. Krishnamoorthy et al. (2014) review how the construction and maintenance of cell wall components are dependent on endomembrane trafficking, and discuss the role of the signalling molecules known as phosphoinositides in vesicle trafficking and their potential impact on cell wall architecture. This is followed by a review by Bashline et al. (2014) that discusses new insights into trafficking of the cellulose synthase complex (CSC). CSCs are responsible for synthesis of cellulose, the most abundantly occurring and renewable wall component in land plants, and the wall component currently most exploited, for example as fuel (both traditional fuels such as wood and in the emerging biofuel industries) and for textile fibres, building materials (such as wood) and paper. Lamport et al. (2014) speculate on the role of arabinogalactan proteins (AGPs) in signalling and morphogenesis, whereas the next paper in this section (Hijazi et al., 2014) also focuses on AGPs but clearly shows the interaction and binding of AGP31 to galactan side-chains of rhamnogalacturonan I. The authors propose that AGP31 may form complex supra-molecular scaffolds with other wall components, thereby supporting rapid plant growth.
Following cessation of expansion, some land plant cell walls synthesize secondary walls, which often perform specialized functions; for example, the secondary cell wall of xylem cells facilitates water transport. In the next review paper of this issue, Ko et al. (2014) consider the how biosynthesis of the secondary wall, including cellulose, hemicellulose and lignin, is co-ordinated and regulated through the network regulated by the transcription factor MYB46.
Root biology has seen a recent renewal in research activity (Lux and Rost, 2012; Szymanowska-Pułka, 2013; White et al., 2013). The next five manuscripts in this issue examine the various aspects of the involvement of cell walls in root and root hair growth. Rajasundaram et al. (2014) carry out a detailed bioinformatics-based analysis of the arabidopsis root transcriptome and translatome to compare expression patterns of wall synthesis genes. Seifert et al. (2014) use mutant analysis in combination with growth regulators and inhibitors to study the role of fasciclin-like arabinogalactan proteins (FLAs), thought to modulate signalling upstream of wall component synthesis. Seifert et al. (2014) strengthen this hypothesis through their finding that FASCICLIN LIKE ARABINOGALACTAN PROTEIN 4 and abscisic acid act synergistically to regulate cell wall biosynthesis and normal root growth. Kozlova et al. (2014) investigate the arrangement of mixed-linkage glucan, glucoarabinoxylan and cellulose microfibrils in maize roots, and develop a model describing how the fine structure is modified during expansive cell growth. The theme of expansive growth is also the subject of the next paper in this section. In this case Larson et al. (2014) characterize VTI1 3 (a member of the SNARE gene family) showing that it has an essential role in root hair growth. In the remaining paper within this sub-section, regulation of a pectin methylesterase, PECTIN METHYLESTERASE17, is shown to be controlled by a subtilisin-type serine protease (Sénéchal et al., 2014). The authors find that the genes AtPME17 and AtSBT3.5 are co-expressed during root development and knock-outs exhibit impaired root growth.
The next two papers discuss the development of plant reproductive features. In the first paper Dumont et al. (2014) reveal that the stability of pollen tube walls, necessary for their elongation and successful fertilization, is dependent on rhamnogalacturonan II (RG-II). Their research also provides new insight into the biosynthesis of RG-II. In the final manuscript of this section, Quilichini et al. (2014) apply cryo-fixation and transmission electron microscopy to the study of anther development. The highly detailed images and information they are able to capture using this technique offer a new perspective on pollen development and are likely to be the springboard for many new advances in this area.
Cell wall diversity
Cell wall composition has been shown to differ between different plant groups (Popper et al., 2011; Sørensen et al., 2010; Fangel et al., 2012) and impacts on their interactions with the environment. The last decade has seen an enormous increase the number of plants and algae whose cell walls are being studied, as well as the specific conditions under which they are being investigated. The papers in this section focus on characterization of cell wall components from less-well-studied organisms and the natural and induced (e.g. through extraction) variability in cell wall components.
Brown algae are evolutionarily only distantly related to land plants but are photosynthetic, multicellular and possess cell walls, thereby allowing interesting comparisons. Deniaud-Bouët et al. (2014) use chemical and enzymatic fragmentation methods to aid characterization of the extracellular matrix in members of the Fucales and, based on their results, generate a cell wall model for this order that is analogous to those that have been developed for flowering plants. They consider this structure in relation to the evolution of brown algae and the rapidly changing coastal environment that they in habit.
The following two papers could equally be placed within cell wall biosynthesis and modelling but are considered in this section as they focus on members of the charophycean green algae (CGA), the closest living ancestors of land plants, which provide glimpses into how the land-plant cell wall evolved. Mikkelsen et al. (2014) analyse the currently available transcriptomes to gain insight into the evolution of the biosynthetic machinery of the CGA. Penium margaritaceum provides many advantages as a model for cell wall studies, particularly now methods for its transformation are available (Sørensen et al., 2014), and Ochs et al. (2014) investigate the developmental co-ordination of the cytoskeleton with cell wall development in this unicellular member of the CGA. They describe the unique cytoskeletal components, satellite microtubule bands, which are involved in cell division.
The next manuscript in this section exhibits how cell wall variation can occur within species. North et al. (2014) review mucilage production in seed coats of natural variants of arabidopsis and are able to link this to potential adaptive functions, such as dispersal mechanisms, as well as the potential of this variation to assist in the identification of genes involved in polysaccharide synthesis. Another paper highlighting within-species variation is presented by da Costa et al. (2014), who describe genotypically derived differences, including digestibility, in the cell wall composition of the biomass crop, Miscanthus. In addition, they point out that this source of variation is frequently masked by developmental- and tissue-derived variation, indicating the necessity to study cell walls at multiple levels. Developmental variation is also considered by Moore et al. (2014), who study grape berry (Vitis vinifera) fruit. They are able to identify specific changes in cell wall components, including decreases in pectic-β-(1,4)-galactan epitopes and increases in extensin and arabinogalactan protein epitopes as the cell walls are remodelled during ripening; specific wall components being linked with distinct ripening stages. Variation in cell wall composition can also be tissue- and cell-derived, and Eeckhout et al. (2014) describe one such case. Ceratopteris richardii (C-Fern), a model fern amenable to stable transformation (Plackett et al., 2014), is of particular interest for cell wall studies as features of its lifecycle allow relative ease of comparison between the (haploid) gametophyte and (diploid) sporophyte generations (Leroux et al., 2013). Detailed analysis of cell wall components from the two generations indicates a functional, rather than genetic, source for the variation found between them (Eeckhout et al., 2014).
Xyloglucans are found in most land plants that have been investigated; however, diversity has been found to exist in their composition (Hoffman et al., 2005; Peña et al. 2008) and in their abundance. For example, the cell walls of grasses (Poales) contain less xyloglucan than those of the eudicotyledons. This is seemingly mismatched by the similarity in gene family size in rice and arabidopsis of the xyloglucan endotransglucosylase/hydrolases (XTHs) responsible for xyloglucan remodelling. In the next manuscript of this issue, Hara et al. (2014) investigated the specificity of XTHs in rice and discover that redundancy may, at least partly, explain the disproportionate size of the XTH gene family in Poales compared with the low abundance of xyloglucan.
Cell wall components are often characterized following extraction. Many of the extraction conditions can alter the structure of the component and can, in some cases, have an impact on their properties. This induced variation is of particular importance in the context of commercially valuable components. A good example of this is the differences in composition of pectin (a wall component used for its viscosity in the food and pharmaceutical industries) extracted using different chemical conditions, as highlighted by Kaya et al. (2014). Buffetto et al. (2014) also describe chemically induced variation in wall composition; however, they additionally observe natural variation in rhamnogalacturonan II from a single wine cultivar.
Interactions with the environment
Plants and algae necessarily interact with their biotic and abiotic environment and the papers in this category look at how plant cell walls are impacted by a number of specific influences. In the first paper in this section, Engelsdorf and Hamann (2014) look at how plants sense cell wall functional integrity following damage caused by abiotic and biotic stresses, including pathogen attack. Ellinger and Voigt (2014) follow this with a viewpoint paper that considers what has been learned about callose biosynthesis in the last decade. Callose, (1→3)-β-glucan, is a polymer with specific importance in maintaining wall integrity that is frequently, although not exclusively, produced in response to stress. Ellinger and Voigt (2014) summarize what is known about the synthesis, deposition and effectiveness of callose as an induced barrier against pathogen (predominantly bacterial and microbial) attack. Some plants have evolved the ability to parasitize and obtain nutrients from other plants (Kuijt, 1969). Although they have received considerably less attention in this regard, in common with bacterial or fungal attack, parasitism involves breaching of host cell walls, and changes in host cell wall composition are likely. What is less intuitive, and is highlighted by Pielach et al. (2014), is the distinctive cell wall composition and architecture of the hyaline body, part of the specialized attachment and feeding structure known as the haustorium, and the compositional changes, including AGP-enrichment and pectin de-esterification, that occur within it during haustorial maturation.
Application of new technologies to cell walls
The papers in this category report advances gained through the application of specific techniques to cell walls; more specifically, atomic force microscopy (AFM), which is emerging as an important tool within cell wall biology. Paniagua et al. (2014) review the use of AFM toward characterizing changes in pectins, including their solubilization, depolymerization and demethylesterfication, that occur during fruit ripening and lead to the associated textural changes. AFM is also used by Zhou et al. (2014), this time to investigate the properties of AGPs ex situ. Although they have been attributed with many roles, the function of AGPs remains somewhat elusive. Zhou et al. (2014) find that an AGP isolated from arabidopsis could organize into specific structures on surfaces. This insight may lead to novel understanding of how AGPs are presented on cell surfaces and eventually to an improved understanding of their role(s).
CONCLUSIONS AND FUTURE RESEARCH DIRECTIONS
The fact that ‘plants [and many algae] invest much more effort in cell walls than in nuclei’ (Fry, 2006) demands that in order to know how plants and algae function, be it motivated purely by curiosity or for application, we must gain as much knowledge as possible about cell walls. It also suggests that there is rather a lot to find out about cell walls! Obtaining evidence to answer most questions leads, inevitably, to more questions being raised. This is of course true for all areas of knowledge, but within cell wall research the current rate of discoveries is extremely rapid – driven partly by technologies developed by researchers focussed on cell walls and related (and even sometimes quite disparately allied) research areas, but also by their application to novel questions. A clear example of this is the application to cell wall biology of the advances made in microscopy (Domozych, 2012; Quilichini et al., 2014; Zhou et al., 2014). Widespread recognition of the differences in cell wall composition between different plant and algal groups has lead to diversification of the plants and algae whose walls are being characterized by researchers (Fig. 1) and may lead to improved understanding of their evolution and survival strategies (e.g. as exemplified by the scenarios discussed by Pielach et al. 2014, Fig. 2, and North et al., 2014, Fig. 3), and to potentially novel uses of their cell wall components. Expansion of the industrial uses of cell walls is an avenue likely to direct future research activities. However, fundamentally, it is the continued progression from characterization (structure, metabolism, properties and localization) of individual cell wall components (although this is far from complete, and remains necessary) through to seeking evidence for their roles in almost every aspect of plant and algal physiology that presents many of the major challenges in cell wall research. We hope that this Special Issue will provide inspiration for future cell wall-focussed research.
Fig. 1.
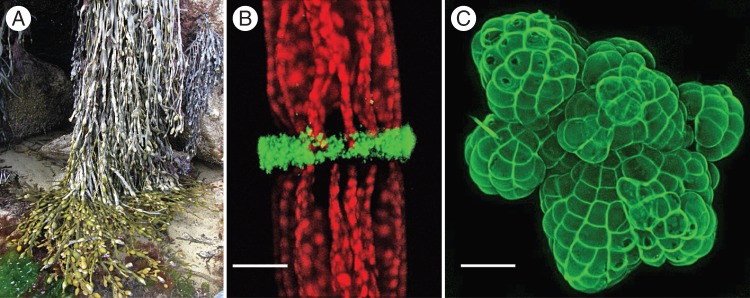
Algal cell walls are increasingly being more fully characterized at different levels including structure and genome (Michel et al., 2010; Deniaud-Bouët et al., 2014). (A) The seaweed Ascophyllum nodosum. Seaweeds are photosynthetic, multicellular, eukaryotic organisms and possess complex carbohydrate-rich cell walls, therefore sharing many characters with land plants whilst having a different evolutionary history. Many seaweed-derived polysaccharides have economic value. (B, C) Members of the charophycean green algae (CGA). (B) Penium margaritaceum immunolabelled with JIM7, which recognizes epitopes present in esterified homogalacturonan. The band shows the zone where high esterified homogalacturonan is being secreted. Scale bar = 8 μm. (C) Coleochaete orbicularis immunolabelled with the monoclonal antibody LM18 (which recognizes epitopes present in homogalacturonan). Scale bar = 100 μm. Freshwater green algae, specifically members of the CGA, are the closest living ancestors of land plants and their study gives new insight into many aspects of cell wall function (Domozych et al., 2014a, b; Ochs et al., 2014). Images: (A) Sandra Raimundo; (B, C) David S. Domozych.
Fig. 2.

Rhinanthus, a dicotyledonous plant that is parasitic on other plants. More precise tools allowing in situ localization of cell wall components have facilitated the investigation of complex interchanges between plants and their biotic environment, for example during pathogenic, parasitic and symbiotic interactions (Jackson et al., 2012; Pielach et al., 2014). Image: Anna Pielach.
Fig. 3.
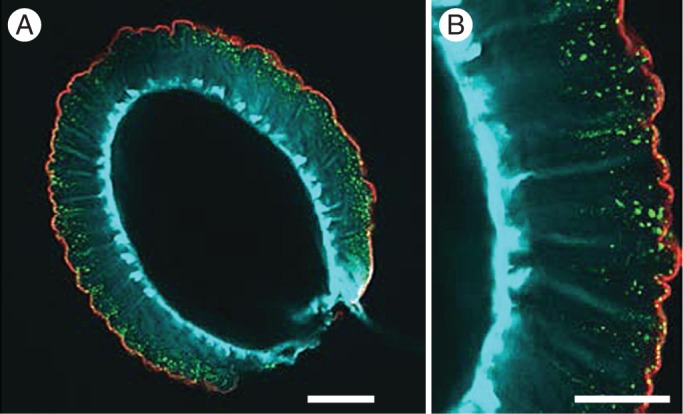
Labelling of polysaccharide components of seed coat epidermal cells and mucilage in arabidopsis. (A) Composite image of optical section through whole seed and mucilage. Scale bar = 100 μm. (B) Magnification of region in (A). Scale bar = 50 μm. Blue, calcofluor-labelled cellulose; red, INRA-RU1-immunolabelled rhamnogalacturonan; green, JIM7-immunolabelled highly methylesterified homogalacturonan. Image: Helen North.
ACKNOWLEDGEMENTS
We thank Dr David Frost and Prof. Pat Heslop-Harrison, Managing Editor and Chief Editor of Annals of Botany, respectively, for their enormous help at all stages of the organization and preparation of this Special Issue. We also thank all the authors who submitted their manuscripts for inclusion in this Special Issue, and we are extremely grateful to those members of the cell wall community, and related research areas, who generously gave their time and expertise to rigorously review the manuscripts. Sandra Raimundo, Anna Pielach and Helen North are thanked for providing beautiful images of their work for inclusion in this preface (Figs 1–3, respectively). This work was supported by an Irish Research Council New Foundations award, The Annals of Botany Company and The Society for Experimental Biology.
Dedication. Researchers focussed on plant and algal cell walls constitute a vibrant and dynamic community investigating many aspects of cell walls, from the fundamental to the applied. We would like to dedicate this Special Issue to all scientists who study cell walls and in particular our mentors, collaborators and colleagues, lab members and teachers.
LITERATURE CITED
- Agger JW, Isaksen T, Várnai A, et al. Discovery of the LPMO activity on hemicelluloses shows the importance of oxidative processes in plant cell wall degradation. Proceedings of the National Academy of Sciences USA; 2014. pp. 6287–6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albersheim P, Darvill A, Roberts K, Sederoff R, Staehelin A. Plant cell walls: from chemistry to biology. New York: Garland Science, Taylor Francis Group; 2011. [Google Scholar]
- Avci U, Pattathil S, Hahn MG. Immunological approaches to plant cell wall biomass characterization: immunolocalization of glycan epitopes. In: Himmel ME, editor. Biomass conversion: methods and protocols. Methods in Molecular Biology. Springer: 2012. pp. 73–82. [DOI] [PubMed] [Google Scholar]
- Bashline L, Li S, Gu Y. The trafficking of the cellulose synthase complex in higher plants. Annals of Botany. 2014;114:1059–1067. doi: 10.1093/aob/mcu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffetto F, Ropartz D, Zhang XJ, Gilbert HJ, Guillon F, Ralet M-C. Recovery and fine structure variability of RGII sub-domains in wine (Vitis vinifera Merlot) Annals of Botany. 2014;114:1327–1337. doi: 10.1093/aob/mcu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Fincher GB. Plant cell wall engineering: applications in biofuel production and improved human health. Current Opinion in Plant Biology. 2014;26:79–84. doi: 10.1016/j.copbio.2013.10.007. [DOI] [PubMed] [Google Scholar]
- da Costa RMF, Lee SJ, Allison GG, Hazen SP, Winters A, Bosch M. Genotype, development and tissue-derived variation in cell-wall properties in the lignocellulosic energy crop Miscanthus. Annals of Botany. 2014;114:1265–1277. doi: 10.1093/aob/mcu054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho PM, Henrissat B. Annotating carbohydrate-active enzymes in plant genomes: present challenges. In: Ulvskov P, editor. Plant polysaccharides, biosynthesis and bioengineering. Annual Plant Reviews. Oxford: Blackwell Publishing Ltd; 2011. [Google Scholar]
- Deniaud-Bouët E, Kervarec N, Michel G, Tonon T, Kloareg B, Hervé C. Chemical and enzymatic fractionation of cell walls from Fucales: insights into the structure of the extracellular matrix of brown algae. Annals of Botany. 2014;114:1203–1216. doi: 10.1093/aob/mcu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domozych DS. The quest for four-dimensional imaging in plant cell biology: it's just a matter of time. Annals of Botany. 2012;110:461–474. doi: 10.1093/aob/mcs107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domozych DS, Sørensen I, Popper ZA, et al. Pectin metabolism and assembly in the cell wall of the charophyte green alga Penium margaritaceum. Plant Physiology. 2014a;165:105–118. doi: 10.1104/pp.114.236257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domozych DS, Sørensen I, Sacks C, et al. Disruption of the microtubule network alters cellulose deposition and causes major changes in pectin distribution in the cell wall of the green alga, Penium margaritaceum. Journal of Experimental Botany. 2014b;65:465–479. doi: 10.1093/jxb/ert390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont M, Lehner A, Bouton S, Kiefer-Meyer MC, Voxeur A, Pelloux J, Lerouge P, Mollet J-C. The cell wall pectic polymer rhamnogalacturonan-II is required for proper pollen tube elongation: implications of a putative sialyltransferase-like protein. Annals of Botany. 2014;114:1177–1188. doi: 10.1093/aob/mcu093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durachko DM, Cosgrove DJ. Measuring plant cell wall extension (creep) induced by acidic pH and alpha-expansin. Journal of Visual Experimentation. 2009;25:1263. doi: 10.3791/1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhout S, Leroux O, Willats WGT, Popper ZA, Viane RLL. Comparative glycan profiling of Ceratopteris richardii ‘C-Fern’ gametophytes and sporophytes links cell-wall composition to functional specialization. Annals of Botany. 2014;114:1295–1307. doi: 10.1093/aob/mcu039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger D, Voigt CA. Callose biosynthesis in arabidopsis with a focus on pathogen response: what have we learned within the last decade. Annals of Botany. 2014;114:1349–1358. doi: 10.1093/aob/mcu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelsdorf T, Hamann T. An update on receptor-like kinase involvement in the maintenance of plant cell wall integrity. Annals of Botany. 2014;114:1339–1347. doi: 10.1093/aob/mcu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangel JU, Ulvskov P, Knox JP, et al. Cell wall evolution and diversity. Frontiers in Plant Science. 2012;3:152. doi: 10.3389/fpls.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CE, Martin TM, Pauly M. Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) Part I: lignin. Journal of Visual Experimentation. 2010a;37:1745. doi: 10.3791/1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CE, Martin TM, Pauly M. Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) Part II: carbohydrates. Journal of Visual Experimentation. 2010b;37:1837. doi: 10.3791/1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC. The growing plant cell wall: chemical and metabolic analysis (reprint edition) Caldwell, NJ: Blackburn Press; 2000. [Google Scholar]
- Fry SC. Wall-to-wall biochemistry: a personal perspective. In: Hayashi T, editor. The science and lore of the plant cell wall. Biosynthesis, structure and function. Boca Raton, FL: BrownWalker Press; 2006. [Google Scholar]
- Fry SC. Cell wall polysaccharide composition and covalent crosslinking. In: Ulvskov P, editor. Plant polysaccharides, biosynthesis and bioengineering. Annual Plant Reviews. Oxford: Blackwell Publishing Ltd; 2011. [Google Scholar]
- Hara Y, Yokoyama R, Osakabe K, Toki S, Nishitani K. Functional xyloglucan endotransglucosylase/hydrolases in rice. Annals of Botany. 2014;114:1309–1318. doi: 10.1093/aob/mct292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze T. Chemical functionalization of cellulose. In: Dumitriu S, editor. Polysaccharides: structural diversity and functional versatility. 2nd edn. New York: Marcel Dekker Inc; 2005. pp. 551–590. [Google Scholar]
- Hijazi M, Roujol D, Nguyen-Kim H, del Rocio Cisneros Castillo L, Saland E, Jamet E, Albenne C. Arabinogalactan protein 31 (AGP31), a putative network-forming protein in Arabidopsis thaliana cell walls? Annals of Botany. 2014;114:1087–1097. doi: 10.1093/aob/mcu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman M, Jia Z, Peña MJ, et al. Structural analysis of xyloglucans in the primary cell walls of plants in the subclass Asteridae. Carbohydrate Research. 2005;340:1826–40. doi: 10.1016/j.carres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Hooke R. Micrographia or some physiological descriptions of minute bodies made by magnifying glasses with observations and inquiries thereupon. London: The Royal Society; 1665. [Google Scholar]
- Jackson O, Taylor O, Adams DG, Knox JP. Arabinogalactan proteins occur in the free-living cyanobacterium genus Nostoc and in plant–Nostoc symbioses. Molecular Plant–Microbe interactions. 2012;25:1338–1349. doi: 10.1094/MPMI-04-12-0095-R. [DOI] [PubMed] [Google Scholar]
- Kaya M, Sousa AG, Crépeau M-J, Sørensen SO, Ralet M-C. Characterization of citrus pectin samples extracted under different conditions: influence of acid type and pH of extraction. Annals of Botany. 2014;114:1319–1326. doi: 10.1093/aob/mcu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J-H, Jeon H-W, Kim W-C, Kim J-Y, Han K-H. The MYB46/MYB83-mediated transcriptional regulatory programme is a gatekeeper of secondary wall biosynthesis. Annals of Botany. 2014;114:1099–1107. doi: 10.1093/aob/mcu126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlova LV, Ageeva MV, Ibragimova NN, Gorshkova TA. Arrangement of mixed-linkage glucan and glucuronoarabinoxylan in the cell walls of growing maize roots. Annals of Botany. 2014;114:1135–1145. doi: 10.1093/aob/mcu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krässig HA. Cellulose — structure, accessibility and reactivity. Amsterdam: Gordon and Breach; 1993. [Google Scholar]
- Krishnamoorthy P, Sanchez-Rodriguez C, Heilmann I, Persson S. Regulatory roles of phosphoinositides in membrane trafficking and their potential impact on cell-wall synthesis and re-modelling. Annals of Botany. 2014;114:1049–1057. doi: 10.1093/aob/mcu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijt J. The biology of parasitic flowering plants. Berkeley, CA: University of California Press; 1969. [Google Scholar]
- Lamport DTA, Varnai P, Seal CE. Back to the future with the AGP–Ca2+ flux capacitor. Annals of Botany. 2014;114:1069–1085. doi: 10.1093/aob/mcu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson ER, Domozych DS, Tierney ML. SNARE VTI13 plays a unique role in endosomal trafficking pathways associated with the vacuole and is essential for cell wall organization and root hair growth in arabidopsis. Annals of Botany. 2014;114:1147–1159. doi: 10.1093/aob/mcu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux O, Eeckhout S, Viane RLL, Popper ZA. Frontiers in Plant Science. Vol. 4. 367; 2013. Ceratopteris richardii (C-Fern): a model for investigating adaptive modification of vascular plant cell walls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux A, Rost TL. Plant root research: the past, the present and the future. Annals of Botany. 2012;110:201–204. doi: 10.1093/aob/mcs156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MC, Knox JP. Plant cell wall biology: polysaccharides in architectural and developmental contexts. In: Ulvskov P, editor. Plant polysaccharides, biosynthesis and bioengineering. Annual Plant Reviews. Oxford: Blackwell Publishing Ltd; 2011. [Google Scholar]
- Michel G, Tonon T, Scornet D, Cock JM, Kloareg B. Central and storage carbon metabolism of the brown alga Ectocarpus siliculosus: insights into the origin and evolution of storage carbohydrates in Eukaryotes. New Phytologist. 2010;188:67–81. doi: 10.1111/j.1469-8137.2010.03345.x. [DOI] [PubMed] [Google Scholar]
- Mikkelsen MD, Harholt J, Ulvskov P, Johansen IE, Fangel JU, Doblin MS, Bacic A, Willats WGT. Evidence for land plant cell wall biosynthetic mechanisms in charophyte green algae. Annals of Botany. 2014;114:1217–1236. doi: 10.1093/aob/mcu171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra PP, Loqué D. Histological staining of Arabidopsis thaliana secondary cell wall elements. Journal Visual Experimentation. 2014;87:e51381. doi: 10.3791/51381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller IE, Pettolino FA, Hart C, Lampugnani ER, Willats WGT, Bacic A. Glycan profiling of plant cell wall polymers using microarrays. Journal of Visual Experimentation. 2012;70:e4238. doi: 10.3791/4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Fangel JU, Willats WGT, Vivier MA. Pectic-β(1,4)-galactan, extensin and arabinogalactan–protein epitopes differentiate ripening stages in wine and table grape cell walls. Annals of Botany. 2014;114:1279–1294. doi: 10.1093/aob/mcu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North HM, Berger A, Saez-Aguayo S, Ralet M-C. Understanding polysaccharide production and properties using seed coat mutants: future perspectives for the exploitation of natural variants. Annals of Botany. 2014;114:1251–1263. doi: 10.1093/aob/mcu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs J, LaRue T, Tinaz B, Yongue C, Domozych DS. The cortical cytoskeletal network and cell-wall dynamics in the unicellular charophycean green alga Penium margaritaceum. Annals of Botany. 2014;114:1237–1249. doi: 10.1093/aob/mcu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniagua C, Posé S, Morris VJ, Kirby AR, Quesada MA, Mercardo JA. Fruit softening and pectin disassembly: an overview of nanostructural pectin modifications assessed by atomic force microscopy. Annals of Botany. 2014;114:1375–1383. doi: 10.1093/aob/mcu149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattathil S, Avci U, Miller JS, Hahn MG. Immunological approaches to plant cell wall and biomass characterization: glycome profiling. In: Himmel ME, editor. Biomass conversion: methods and protocols. Methods in Molecular Biology. Springer: 2012. [DOI] [PubMed] [Google Scholar]
- Peña MJ, Darvill AG, Eberhard S, Work WS, O'Neill MA. Moss and liverwort xyloglucans contain galacturonic acid and are structurally distinct from the xyloglucans synthesised by hornworts and vascular plants. Glycobiology. 2008;18:891–940. doi: 10.1093/glycob/cwn078. [DOI] [PubMed] [Google Scholar]
- Pielach A, Leroux O, Domozych DS, Knox JP, Popper ZA. Arabinogalactan protein-rich cell walls, paramural deposits and ergastic globules define the hyaline bodies of rhinanthoid Orobanchaceae haustoria. Annals of Botany. 2014;114:1359–1373. doi: 10.1093/aob/mcu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plackett AR, Huang L, Sanders HL, Langdale JA. High efficiency stable transformation of the model fern species Ceratopteris richardii via microparticle bombardment. Plant Physiology. 2014;165:3–14. doi: 10.1104/pp.113.231357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA, editor. The plant cell wall. Methods and protocols. Methods in Molecular Biology. Springer: Humana Press; 2011. [Google Scholar]
- Popper ZA, Michel G, Hervé C, et al. Evolution and diversity of plant cell walls: from algae to flowering plants. Annual Review Plant Biology. 2011;62:567–590. doi: 10.1146/annurev-arplant-042110-103809. [DOI] [PubMed] [Google Scholar]
- Quilichini TD, Douglas CJ, Samuels AL. New views of tapetum ultrastructure and pollen exine development in Arabidopsis thaliana. Annals of Botany. 2014;114:1189–1201. doi: 10.1093/aob/mcu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasundaram D, Selbig J, Persson S, Klie S. Co-ordination and divergence of cell-specific transcription and translation of genes in arabidopsis root cells. Annals of Botany. 2014;114:1109–1123. doi: 10.1093/aob/mcu151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Sachs J. Lectures on the physiology of plants. Oxford: Clarendon Press; 1887. Translated by Ward H. Marshall. [Google Scholar]
- Seifert GJ, Xue H, Acet T. The Arabidopsis thaliana FASCICLIN LIKE ARABINOGALACTAN PROTEIN 4 gene acts synergistically with abscisic acid signalling to control root growth. Annals of Botany. 2014;114:1125–1133. doi: 10.1093/aob/mcu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sénéchal F, Graff L, Surcouf O, Marcelo P, Rayon C, Bouton S, Mareck A, Mouille G, Stintzi A, Höfte H, Lerouge P, Schaller A, Pelloux J. Arabidopsis PECTIN METHYLESTERASE17 is co-expressed with and processed by SBT3.5, a subtilisin-like serine protease. Annals of Botany. 2014;114:1161–1175. doi: 10.1093/aob/mcu035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Siqueira FG, Filho F, Ximenes E. Plant cell wall as a substrate for the production of enzymes with industrial applications. Mini-Reviews in Organic Chemistry. 2010;7:54. [Google Scholar]
- Sørensen I, Domozych D, Willats WGT. How have plant cell walls evolved? Plant Physiology. 2010;153:366–372. doi: 10.1104/pp.110.154427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen I, Fei Z, Andreas A, Willats WGT, Domozych DS, Rose JKC. Stable transformation and reverse genetic analysis of Penium margaritaceum: a platform for studies of charophyte green algae, the immediate ancestors of land plants. Plant Journal. 2014;77:339–351. doi: 10.1111/tpj.12375. [DOI] [PubMed] [Google Scholar]
- Stengel DB, Connan S, Popper ZA. Algal chemodiversity and bioactivity: sources of natural variability and implications for commercial applications. Biotechnology Advances. 2011;29:483–501. doi: 10.1016/j.biotechadv.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Szymanowska-Pułka J. Form matters: morphological aspects of lateral root development. Annals of Botany. 2013;112:1643–1654. doi: 10.1093/aob/mct231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulvskov P. Plant polysaccharides: biosynthesis and bioengineering. Annual Plant Reviews. Oxford: Wiley-Blackwell; 2011. [Google Scholar]
- White PJ, George TS, Gregory PJ, Bengough AG, Hallett PD, McKenzie BM. Matching roots to their environment. Annals of Botany. 2013;112:207–222. doi: 10.1093/aob/mct123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis KJ, McElwain JC. The evolution of plants. 2nd edn. Oxford: Oxford University Press; 2014. [Google Scholar]
- Yin Y, Chen H, Hahn MG, Mohnen D, Xu Y. Evolution and function of the plant cell wall synthesis-related glycosyltransferase family 8. Plant Physiology. 2010;153:1729–1746. doi: 10.1104/pp.110.154229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou LH, Weizbauer RA, Singamaneni S, Xu F, Genin GM, Pickard BG. Structures formed by a cell membrane-associated arabinogalactan-protein on graphite or mica alone and with Yariv phenylglycosides. Annals of Botany. 2014;114:1385–1397. doi: 10.1093/aob/mcu172. [DOI] [PMC free article] [PubMed] [Google Scholar]


