Abstract
For tissue engineering applications, the preparation of biodegradable and biocompatible scaffolds is the most desirable but challenging task. Among the various fabrication methods, electrospinning is the most attractive one due to its simplicity and versatility. Additionally, electrospun nanofibers mimic the size of natural extracellular matrix ensuring additional support for cell survival and growth. This study showed the viability of the fabrication of long fibers spanning a larger deposit area for a novel biodegradable and biocompatible polymer named poly(glycerol-dodecanoate) (PGD)1 by using a newly designed collector for electrospinning. PGD exhibits unique elastic properties with similar mechanical properties to nerve tissues, thus it is suitable for neural tissue engineering applications. The synthesis and fabrication set-up for making fibrous scaffolding materials was simple, highly reproducible, and inexpensive. In biocompatibility testing, cells derived from mouse embryonic stem cells could adhere to and grow on the electrospun PGD fibers. In summary, this protocol provided a versatile fabrication method for making PGD electrospun fibers to support the growth of mouse embryonic stem cell derived neural lineage cells.
Keywords: Bioengineering, Issue 88, tissue engineering, electrospinning, fiber scaffolds, Poly(glycerol-dodecanedioate) (PGD), gelatin, Mouse embryonic stem cells
Introduction
Electrospinning is one of the effective processing methods to produce micro-to-nanometer size fiber scaffolds. The basic principle of electrospinning involves a Taylor cone of solution that is held at the orifice of a needle by applying high voltage between the tip of the needle and a grounded collector. When the electrostatic repulsion in the solution overcomes the surface tension, a charged fluid jet is ejected out of the needle tip, travels through the air with solvent evaporation, and is finally deposited on the grounded collector. The syringe pump provides a continuous flow of solution emerging from the spinneret and thus multiple copies of the electrospun fibers can be fabricated within a short period of time. During the course of leaving the spinneret to arrive at the collector, the charged jet will undergo stretching and whipping according to a number of parameters that include the viscosity and surface tension of the polymeric solution, the electrostatic force in the solution, and the interaction of the external electric field, etc2.
In the electrospinning process, a collector serves as a conductive substrate where the micro-to-nanometer fibers could be deposited. In this study, a new type of fiber collector was designed to obtain fiber mats with the desired size (length x width). Traditionally, aluminum foil is used as a collector but it is difficult to transfer the fibers from the flat surface to another substrate. The difficulty of harvesting an intact fiber mat from a traditional collector was mainly due to the fact that the electrospun fibers attach strongly to the collector's surface. Therefore, we modified the collector by folding a piece of aluminum foil into a rectangular strip and attaching it perpendicular to a flat metal plate. The electrospun fibers are stretched across the area between the tip of the strip and the metal plate, which can be easily transferred to another substrate.
Interest in thermally crosslinked elastomeric polymers is rapidly growing because of the pioneering work of Robert Langer's group, who introduced poly(glycerol sebacate) (PGS), a polyester which is analogous to vulcanized rubber in 2002 3. Similar to PGS, we have successfully developed poly(glycerol-dodecanoate) (PGD) by thermal condensation of glycerol and dodecanedioic acid and demonstrated its unique shape memory property1. Unlike stiffer synthetic materials poly(hydroxyl butyrate) or poly(L-lactide) (Young's moduli of 250 MPa and 660 MPa, respectively), PGD exhibits elastomeric property like rubber, with a Young's modulus of 1.08 MPa when the temperature is above 37 °C, which is a close match to the in-situ peripheral nerve (0.45 MPa). In addition, PGD is biodegradable and the degradation time can be fine-tuned by varying the ratio of glycerol and dodecanedioic acid. Dodecanedioic acid is a twelve-carbon substance with two terminal carboxylic groups, HOOC(CH2)10COOH. Even numbered dicarboxylic acids like sebacic acid and dodecanedioic acid can be metabolized to acetyl-CoA and enter the tricarboxylic acid (TCA)/(citric acid) cycle. The metabolic product of dicarboxylic acids, succinyl-CoA, is a gluconeogenetic precursor and intermediate of TCA cycle4. Thus, some studies suggested that they could be utilized as an alternative fuel substrate for enteral and parenteral nutrition, especially in the pathological conditions. In addition, PGD exhibits unique shape memory because its glass transition temperature is 31 °C, thus it shows distinct mechanical properties at room temperature and at body temperature. In sum, PGD is biodegradable, biocompatible, exhibiting unique elastic properties with mechanical properties similar to nerve tissues; therefore, it is a suitable material for nerve tissue engineering applications. In this protocol, the electrospun long fibers spanning a large deposit area were fabricated via the newly-designed collector from PGD. The fiber scaffolds can support the mouse pluripotent stem cells growth and differentiation.
Protocol
1. Electrospinning Collector Setup
Cut the aluminum foil into a rectangular piece.
Fold the rectangular piece into a rectangular strip, and attach it perpendicular to a flat metal plate with tape (Figure 1). Note: The size of the fiber mat depends on the length and width of the strip. Thus, the strip dimensions can be adjusted as needed.
2. Polymeric Solution Preparation
Mix glycerol and dodecanedioic acid (DDA) in 1:1 molar ratio in a beaker at 120 °C for 100 hr to obtain PGD polymer.
Dissolve poly(ethylene oxide) (PEO) and gelatin in 65% ethanol with a weight ratio of 1.5:3:95.5 in a 15 ml tube, tighten the cap and heat the mixture in an oven at 60 °C for 1 hr with stirring until it becomes a homogeneous solution (Basal solution).
For electrospinning, mix the PGD polymer and the basal solution in 4:6 weight ratio. Note: PGD concentration has a big effect on fiber diameter. 30%-50% percent PGD is acceptable to produce fibers longer than 5 cm with increased fiber diameters.
Add 0.1% riboflavin to the polymeric solution and mix well.
3. Electrospinning
Feed the polymeric solution into a 5 ml standard syringe with an 18 G blunted stainless steel needle.
Insert the syringe into a syringe pump.
Attach the grounded lead of a high voltage power source to the metal plate and the positively charged lead to the needle.
Adjust the distance between the needle and the aluminum foil strip to 15 cm.
Place the syringe pump in an angle of about 15° with the horizontal to prevent aggregation of the fibers at the front of the strip.
Turn on the syringe pump and adjust the flow rate of the pump to 0.6 ml/hr.
Turn on the high voltage power source and set the operating voltage to 14.6 kV.
4. Fiber Processing
After the collection is complete, expose the fiber mat to UV light for 60 min for cross-linking.
Transfer the fiber mat from the aluminum foil strip to a 100 mm Petri dish, and expose it to UV light for another 20 min for sterilization.
In a biosafety cabinet, cut the fiber mat into round pieces of the same size with a surgical blade and place the pieces into a 24-well plate.
For cell pre-seeding treatment, immerse fiber samples in 1 ml of phosphate buffered saline (PBS) and incubate at 37 °C overnight.
On the following day, aspirate PBS carefully, add 1 ml of differentiation medium (DMEM/F12, N2, and FGF2) (see recipe in Materials table) to each well and incubate at 37 °C for 3 hr.
Aspirate differentiation medium carefully, add 0.2 ml of matrigel to each fiber sample and incubate at 37 °C for 30 min. Note: Laminin can also be used for fiber coating. Add 0.2 ml of 20 µg/ml laminin to the fiber and incubate at room temperature for 3 hr.
Carefully remove excess matrigel from each well. Rinse with 2 ml of differentiation medium once. Note: The fiber samples are ready for cell culture.
5. Cell Seeding on Fibers
Add 1 ml of Accutase to the mES cell culture dish and incubate for 10 min at 37 °C.
After 10 min, the mES cells are detached from the culture dish. Add 4 ml of differentiation medium to the plate. Collect the floating mES cells into a 15 ml tube and pipette cells up and down to break colonies (about 15x).
Centrifuge at 400 x g for 5 min and re-suspend cells in 4 ml of differentiation medium.
Count the cells using a hemocytometer. Transfer 200 µl of the cell suspension into a 15 ml tube and dilute it 10x with differentiation medium. Transfer 15 µl of the diluted cell suspension to a chamber on the hemocytometer with a cover-slip in place. Count the cells in the 1 mm center square and the four corner squares of the hemocytometer under the microscope. Note: Cell per ml = the average count per square x the dilution factor x 104
Place approximately 5 x 104 mES cells onto each well. Slowly drop the cell suspension onto the middle of the fiber samples, instead of sliding off to the side of the well, to prevent the solution from draining off the fiber mat.
Add 1 ml of differentiation medium to each well, and keep the plate in an incubator at 37 °C and 5% CO2 to allow cells attachment, growth and differentiation.
Aspirate the old medium from each well and replace with 1 ml of fresh differentiation medium every other day.
6. Cell Viability
Aspirate the old medium from each well.
Mix 1/10th volume of Resazurin fluorescence reagent with culture medium and add 1 ml to each well.
Incubate at 37 °C and 5% CO2 for 4 hr, protected from direct light.
After 4 hr, transfer 100 µl 3x of the reagent from each well to a 96-well plate, and then the samples are ready to be measured on a fluorescence spectrophotometer using 560EX nm/590EM filter settings.
7. Real Time PCR
After 14 days of culture, add lysis buffer to the samples and isolate total RNA from the cells on the fiber samples.
Use 4 µl of total RNA to synthesize cDNA in 20 µl reaction scales by reverse transcription.
Prepare the PCR reaction mixture in each optical tube: 10 µl Master Mix (2x), 0.2 µl dye, 8 µl Nuclease-Free water, 1 µl cDNA, and 1 µl primer (Primers used in this study are listed in Table 1).
Set up the PCR program: a. 95 °C 2:20 min, 1 cycle b. 95 °C 3 sec→60° C 1 min, 40 cycle c. 95° C 15 sec, 1 cycle d. 60° C 1 min, 1 cycle e. 95° C 15 sec, 1 cycle. Choose comparative CT method to determine relative gene expression levels.
After PCR is finished, analyze the real-time PCR results with StepOne software and export the results to Excel.
Representative Results
The major components of the electrospinning are shown in Figure 1. A large size fiber mat was typically obtained through the perpendicularly attached aluminum foil strip and a flat metal plate. Figure 2 shows the collector design and the electrospinning fiber mat. The width and length can be adjusted for different applications. The length of the fiber made with PGD polymer and basal solution mixture is up to 10 cm. The morphology of electrospun fibers is shown in Figure 3. The diameters of fibers made from 40% PGD concentration are in the micrometer range. Confocal microscopy images of differentiated cells derived from mES cells cultured for 3 and 6 days on fibers are depicted in Figure 4. The green fluorescent signals came from the over-expression of green fluorescent protein (GFP) in the cells. The result of Resazurin fluorescence reagent in Figure 5 showed that mES cells grown on the PGD fibers coating with matrigel and laminin had equivalent cell viability and had relatively higher proliferation compared to the uncoated group. The gene expression of pluripotency and neural cells markers was quantified by real time PCR (Figure 6). The majority of mES cells cultured on fibers expressed the pluripotency markers OCT4, Nanog and Sox2 while the minority of cells expressed the neural stem cell marks PAX6 and Nestin. After 2 weeks culture, mES grown on fibers showed increased expression levels of neural cell marks such as MAP2 and DCX, as well as oligodendrocyte marker Oligo1 and astrocyte marker GFAP.
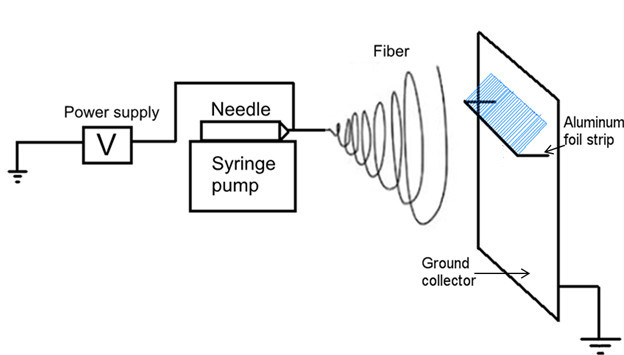 Figure 1. Electrospinning set up. The polymeric solution is ejected from a blunted needle. A high voltage power source grounds a flat metal plate and an aluminum foil strip between which micro-to-nano meter fibers are deposited (blue).
Figure 1. Electrospinning set up. The polymeric solution is ejected from a blunted needle. A high voltage power source grounds a flat metal plate and an aluminum foil strip between which micro-to-nano meter fibers are deposited (blue).
 Figure 2. Collector design and electrospun fiber mat. The width and length of the fiber mat can be easily changed by adjusting the size of the aluminum foil strip. There is no limit for mat width, and the longest fibers can be up to 10 cm long.
Figure 2. Collector design and electrospun fiber mat. The width and length of the fiber mat can be easily changed by adjusting the size of the aluminum foil strip. There is no limit for mat width, and the longest fibers can be up to 10 cm long.
 Figure 3. SEM images of electrospun of PGD and basal solution 4:6 (w/w). The average diameter of the fibers is around 2 µm. When PGD concentration decreases to 30%, the average diameter of the fibers falls into nanometer range. The white scale bar represents 10 µm.
Figure 3. SEM images of electrospun of PGD and basal solution 4:6 (w/w). The average diameter of the fibers is around 2 µm. When PGD concentration decreases to 30%, the average diameter of the fibers falls into nanometer range. The white scale bar represents 10 µm.
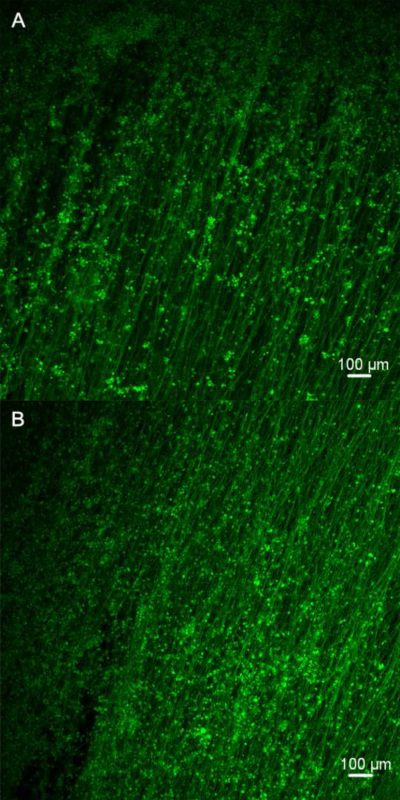 Figure 4. Confocal microscopy images of differentiated mES cells on fibers. The cells carrying the GFP exhibit bright green fluorescence when exposed to light in blue to ultraviolet range. The increased number of green fluorescent cells on Day 6 indicates that the fiber scaffolds can support the cell adhesion and proliferation. The white scale bar represents 100 µm. (Day 3 and Day 6).
Figure 4. Confocal microscopy images of differentiated mES cells on fibers. The cells carrying the GFP exhibit bright green fluorescence when exposed to light in blue to ultraviolet range. The increased number of green fluorescent cells on Day 6 indicates that the fiber scaffolds can support the cell adhesion and proliferation. The white scale bar represents 100 µm. (Day 3 and Day 6).
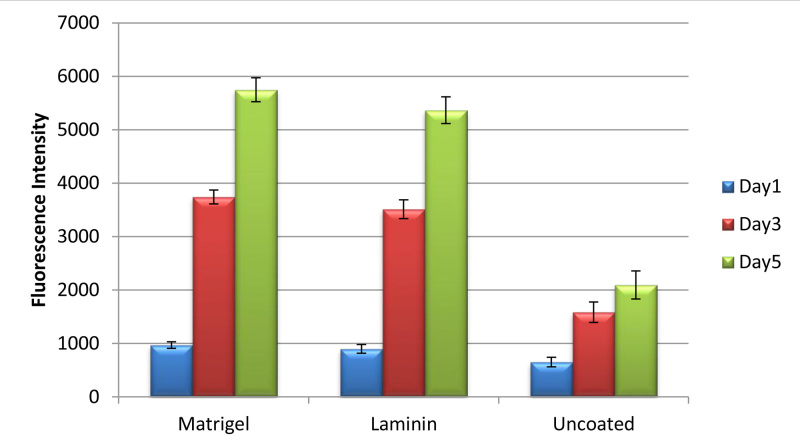 Figure 5. The cell viability of mES cells on PGD fibers coating with Matrigel and laminin at 1, 3, and 5 days as determined by Resazurin fluorescence reagent. Cells cultured on the uncoated fibers used as control (p<0.05).
Figure 5. The cell viability of mES cells on PGD fibers coating with Matrigel and laminin at 1, 3, and 5 days as determined by Resazurin fluorescence reagent. Cells cultured on the uncoated fibers used as control (p<0.05).
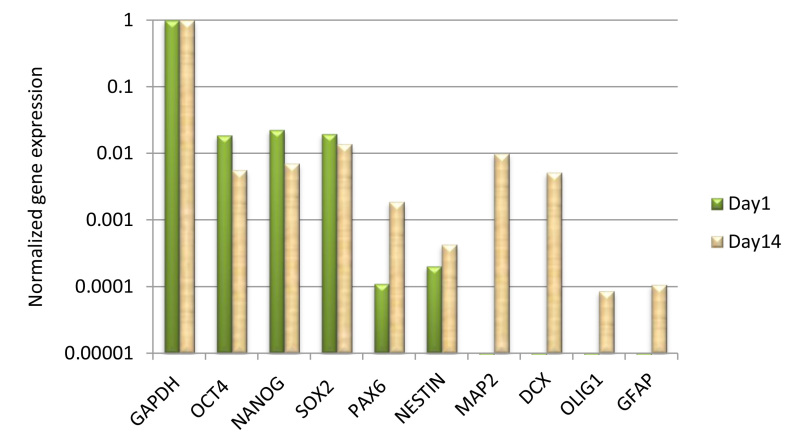 Figure 6. qRT-PCR analysis of gene expression in differentiated mES cells on PGD fibers. The neural cell markers evident after 2 weeks demonstrated that the mES cells on the scaffolds have differentiated into neural cells.
Figure 6. qRT-PCR analysis of gene expression in differentiated mES cells on PGD fibers. The neural cell markers evident after 2 weeks demonstrated that the mES cells on the scaffolds have differentiated into neural cells.
| mGAPDH-L | AACTTTGGCATTGTGGAAGG |
| mGAPDH-R | ACACATTGGGGGTAGGAACA |
| mOct4-L | CACGAGTGGAAAGCAACTCA |
| mOct4-R | AGATGGTGGTCTGGCTGAAC |
| mNanog-L | AAGTACCTCAGCCTCCAGCA |
| mNanog-R | GTGCTGAGCCCTTCTGAATC |
| mSox2-L | CACAGTTCAGCCCTGAGTGA |
| mSox2-R | AGGCCACAACAACAACAACA |
| mPax6-L | AACAACCTGCCTATGCAACC |
| mPax6-R | ACTTGGACGGGAACTGACAC |
| mNestin-L | CCAGAGCTGGACTGGAACTC |
| mNestin-R | ACCTGCCTCTTTTGGTTCCT |
| mMAP2-L | CTTATGGGAATGTGGGATGG |
| mMAP2-R | AAAAAGTGGGCCTTGGAACT |
| mDCX-L | ATGCAGTTGTCCCTCCATTC |
| mDCX-R | ATGCCACCAAGTTGTCATCA |
| mOligo1-L | CTTGCTCTCTCCAGCCAAAC |
| mOligo1-R | GCGAGCCTGAAAAACAGAAC |
| mGFAP-L | CACGAACGAGTCCCTAGAGC |
| mGFAP-R | ATGGTGATGCGGTTTTCTTC |
Table 1. List of PCR primers.
Discussion
The limitations of simple collectors or the complexities of rotating collectors that are currently used for electrospinning increase the restriction of obtaining the desired length and size of fiber mat for some applications. Additionally, transferring fibers from the ground collector to the culture dish or other substrates is a challenge5. In this report, a newly designed collector, made simply by attaching an aluminum foil strip to the grounded collector, was able to obtain large size fiber mats up to 10 cm by 20 cm at a time. Figures 1 and 2 illustrate the schematic setup designed for fabricating up to 10 cm long electrospun fibers with controlled mat width. Influenced by the electrostatic field between the needle tip and collector, the electrospun fibers were stretched across the area between the aluminum foil strip and the grounded collector to form a smooth fiber mat, which can be easily transferred to another substrate. Electrospun fibers provide porous fibrous structures that allow cells to bridge and attach to multiple fibers in a truly three-dimensional environment. The fiber mats generated in this study are large enough to make them ideal candidates for a wide range of applications such as wound healing and neural regeneration.
Fiber diameters can be adjusted by controlling several variables in the electrospinning procedure. These variables include the polymer concentration, magnitude of applied voltage, polymer delivery rate, distance from the needle to the collector, etc6-8. In this study, the testing solutions, which were composed with 50% PGD and 50% BS, 40% PGD and 60% BS, 30% PGD and 70% BS, and 20% PGD and 80% BS, respectively, were prepared for making electrospun fibers. The fibrous scaffolds were examined using SEM to determine the diameters and morphologies of the fibers (Figure 3). As expected, higher PGD concentrations produced larger diameters of electrospun fibers. Also, it is critical to adjust the strip length according to the different PGD concentrations. A lower PGD concentration requires a shorter strip length to ensure fibers formation.
Numerous efforts have been made to explore the biocompatibility of the electrospun fibrous scaffolds through the study of cell culture9-13. The confocal microscopy images in Figure 4 show that the attached cells with the strong green fluorescent signals indicated the cell survival on PGD fibers. In addition, the cell density increasing from day 3 to day 6 also suggested the cell proliferation on PGD fibers. The cell viability test in figure 5 also confirmed this result. The gene expression of neural cell marks MAP2 and DCX demonstrated that the mES cells were able to differentiate into neural cells on the scaffolds (Figure 6). In conclusion, PGD fibrous scaffolds could support cell adhesion and proliferation, and thus exhibit the potential for neural tissue engineering applications.
Here are some general troubleshooting guidelines: if the fiber coming out of the needle is discontinuous, warm up the polymeric solution again and mix it well or if the fiber is sticking to the aluminum foil strip with no attraction to the metal plate, reduce the strip length or increase the PGD concentration. Sometimes large polymer globs form at the needle tip, turn off the high voltage power source, wipe it with a paper towel, and reduce the delivery rate of the pump. In addition, sometimes fibers aggregate on the upper edge of the aluminum foil strip try adjusting the angle of the syringe pump.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was conducted using the facilities of the Biomedical Engineering Department at Florida International University.
References
- Migneco F, Huang Y-C, Birla RK, Hollister SJ. Poly (glycerol-dodecanoate), a biodegradable polyester for medical devices and tissue engineering scaffolds. Biomaterials. 2009;30:6479–6484. doi: 10.1016/j.biomaterials.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneker DH, Yarin AL. Electrospinning jets and polymer nanofibers. Polymer. 2008;49:2387–2425. [Google Scholar]
- Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nature biotechnology. 2002;20:602–606. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- Panunzi S, De Gaetano A, Mingrone G. Approximate linear confidence and curvature of a kinetic model of dodecanedioic acid in humans. American Journal of Physiology-Endocrinology And Metabolism. 2005;289 doi: 10.1152/ajpendo.00503.2003. [DOI] [PubMed] [Google Scholar]
- Park S, et al. Apparatus for preparing electrospun nanofibers: designing an electrospinning process for nanofiber fabrication. Polymer Internationa l. 2007;56:1361–1366. [Google Scholar]
- Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Advanced drug delivery reviews. 2007;59:1413–1433. doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Li W-J, Mauck RL, Tuan RS. Electrospun nanofibrous scaffolds: production, characterization, and applications for tissue engineering and drug delivery. Journal of Biomedical Nanotechnology. 2005;1:259–275. [Google Scholar]
- Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue engineering. 2006;12:1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- Lim SH, Mao H-Q. Electrospun scaffolds for stem cell engineering. Advanced drug delivery reviews. 2009;61:1084–1096. doi: 10.1016/j.addr.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Lowery JL, Datta N, Rutledge GC. Effect of fiber diameter, pore size and seeding method on growth of human dermal fibroblasts in electrospun poly (epsilon-caprolactone) fibrous mats. Biomaterials. 2010;31:491–504. doi: 10.1016/j.biomaterials.2009.09.072. [DOI] [PubMed] [Google Scholar]
- Tillman BW, et al. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials. 2009;30:583–588. doi: 10.1016/j.biomaterials.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Ju YM, Choi JS, Atala A, Yoo JJ, Lee SJ. Bilayered scaffold for engineering cellularized blood vessels. Biomaterials. 2010;31:4313–4321. doi: 10.1016/j.biomaterials.2010.02.002. [DOI] [PubMed] [Google Scholar]
- McCullen SD, et al. In situ collagen polymerization of layered cell-seeded electrospun scaffolds for bone tissue engineering applications. Tissue Engineering Part C: Methods. 2010;16:1095–1105. doi: 10.1089/ten.tec.2009.0753. [DOI] [PubMed] [Google Scholar]


