Supplemental Digital Content is Available in the Text.
Key Words: corneal endothelial dystrophy, Harboyan syndrome, sensorineural hearing loss, SLC4A11
Abstract
Purpose:
Homozygous mutations in SLC4A11 cause 2 rare recessive conditions: congenital hereditary endothelial dystrophy (CHED), affecting the cornea alone, and Harboyan syndrome consisting of corneal dystrophy and sensorineural hearing loss. In addition, adult-onset Fuchs endothelial corneal dystrophy (FECD) is associated with dominant mutations in SLC4A11. In this report, we investigate whether patients with CHED go on to develop hearing loss and whether their parents, who are carriers of an SLC4A11 mutation, show signs of having FECD.
Methods:
Patients with CHED were screened for mutations in the SLC4A11 gene and underwent audiometric testing. The patients and their parents underwent a clinical examination and specular microscopy.
Results:
Molecular analyses confirmed SLC4A11 mutations in 4 affected individuals from 3 families. All the patients were found to have varying degrees of sensorineural hearing loss at a higher frequency range. Guttate lesions were seen in 2 of the 4 parents who were available for examination.
Conclusions:
Our observations suggest that CHED caused by homozygous SLC4A11 mutations progresses to Harboyan syndrome, but the severity of this may vary considerably. Patients with CHED should therefore be monitored for progressive hearing loss. We could not determine conclusively whether the parents of the patients with CHED were at increased risk of developing late-onset FECD.
Loss of corneal clarity accounts for 2% of blind registrations in children below the age of 16 years in the United Kingdom.1 This is frequently because of inherited corneal endothelial dystrophies,2 which include posterior polymorphous corneal dystrophy and congenital hereditary endothelial dystrophy (CHED). Fuchs endothelial corneal dystrophy (FECD), the most common endothelial dystrophy, tends to occur as a late-onset disease. Although clinically distinct, these disorders share many signs and pathological features including altered morphology and loss of endothelial cells, secretion of an abnormal collagenous layer posterior to the basement membrane, and corneal edema.
CHED may be inherited in a dominant (CHED1, MIM %121700) or a recessive manner (CHED2, MIM #217700).3 CHED2 is more common and is characterized by a more severe phenotype than CHED1. Clinical signs include bilateral cloudy corneas at birth or in the early neonatal period, and sometimes, the condition is associated with congenital glaucoma.4,5 Because the severity of corneal opacity in CHED varies considerably, observation is an appropriate management option for patients with mild to moderate corneal clouding. Penetrating keratoplasty is the mainstay of surgical management, although successful Descemet stripping endothelial keratoplasty has recently been reported.6 Mutations in SLC4A11 (MIM *610206) on chromosome 20p13 cause CHED2.7 Harboyan syndrome (MIM #217400) is characterized by CHED in association with sensorineural hearing loss. The hearing deficit in Harboyan syndrome is not present at birth but is typically progressive with onset around the age of 10 to 15 years. Recessive mutations in SLC4A11 also account for this phenotype.8 More than 60 different homozygous or compound heterozygous mutations in SLC4A11 have been reported to cause either CHED or Harboyan syndrome with little evidence to support a genetic difference between these phenotypes.9,10
FECD generally presents in the fifth or sixth decade. Corneal examination reveals endothelial guttae before the patient becomes symptomatic, but as the disease progresses, corneal edema develops. The patient may complain of glare, diminished vision, and discomfort, which is typically worse on awakening because of overnight hypoxia resulting in increased epithelial and stromal edema. FECD accounts for 22% of corneal transplants in the United Kingdom.11 It is usually sporadic, but familial forms with dominant inheritance have been documented. To date, dominant mutations in 4 genes, SLC4A11,12 COL8A2 (MIM *120252),13 ZEB1/TCF8 (MIM *189909),14 and LOXHD1 (MIM *613072)15 and 3 loci on chromosomes 5 (FECD5, MIM %613269),16 9 (FECD7, MIM %613271),14 and 13 (FECD2, MIM %610158)17 have been reported. In addition, an X-linked locus has been described.18
To determine whether recessively inherited CHED, Harboyan syndrome, and late-onset FECD may all coexist as allelic disorders within 1 family, we revisited 2 previously reported CHED families7 and also analyzed an unrelated CHED patient and 1 of his parents who was available for the examination.
MATERIALS AND METHODS
Recruitment and Clinical Assessment
Patients and their families were recruited to the study after their informed consent was obtained using a process that was approved by the local Research Ethics committee. The study adhered to the tenets of the Declaration of Helsinki. Three families (Fig. 1A), 2 of Pakistani origin living in the United Kingdom and the other of Mexican origin, had in total 4 affected members that had been diagnosed with CHED, by an experienced ophthalmologist. A full medical history was taken from all the family members, who were also subject to a detailed ophthalmic examination. In addition, patients with CHED underwent audiometry screening using either the Madsen Aurical audiometer (GN Otometrics A/S, Taastrup, Denmark) or an AD229 diagnostic audiometer (Interacoustics A/S, Assens, Denmark) according to the manufacturers' instructions. The affected cases and their parents were also examined using specular microscopy (Tomey EM-3000 specular microscope, Tomey GmbH, Erlangen-Tennenlohe, Germany).
FIGURE 1.
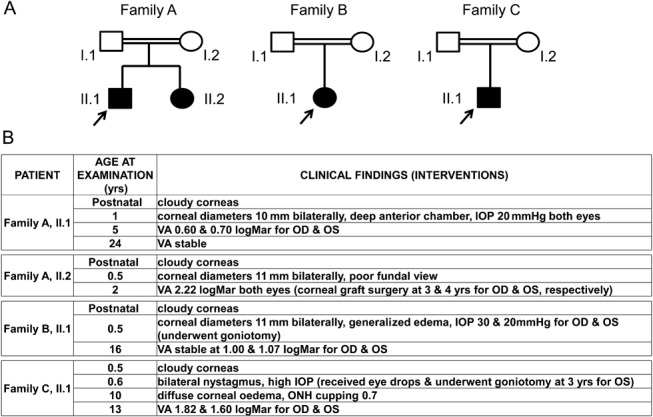
Family structure and ophthalmic history of patients with CHED. A, Families A, B, and C are shown. The proband is indicated by an arrow. B, Age at the examination and clinical findings are shown for each patient. Any ophthalmic interventions are also highlighted. IOP, intraocular pressure; OD, right eye; ONH, optic nerve head; OS, left eye; VA, visual acuity.
Molecular Analysis
Peripheral blood samples were collected from the affected patients and unaffected relatives. Genomic DNA was extracted from blood according to standard procedures and used as a template in the polymerase chain reaction to screen for mutations in the 19 exons of SLC4A11 as described previously.7
RESULTS
Ophthalmic History and Examination
Clinical examination of the patients with endothelial dystrophy suggested a diagnosis of CHED, whereas their family history suggested recessive inheritance (Fig. 1 and Supplementary Figure S1; http://links.lww.com/ICO/A190).
Mutation Screening
Sequencing of SLC4A11 confirmed the presence of homozygous mutations in all the families and the diagnosis of CHED. The patients from families A and B were all homozygous for the previously reported mutation c.1391G->A, p.G464D in exon 11,7 whereas the patient from family C was homozygous for a novel mutation c.397T->C, p.F133L in exon 4 (Supplementary Figure S2; http://links.lww.com/ICO/A190).
Audiometric Testing
Audiometric examination of patient II.1 from family A at 12 years of age revealed bilateral mid- to high-frequency sensorineural hearing loss consistent with a diagnosis of Harboyan syndrome (Fig. 2A). Audiometric examination was repeated at age 21, when some deterioration of hearing was measured. His younger affected sibling, whose corneal opacity was more marked at presentation, had subjective hearing problems at the age of 21, expressing a difficulty in hearing others' conversations when studying at college. The audiometry results showed a midfrequency bilateral sensorineural hearing loss consistent with these symptoms (Fig. 2A). The findings of the audiometry test in the patient from family B at age 11 were unremarkable. This was repeated at age 15 when a bilateral high-frequency hearing loss was found (Fig. 2B). Audiometric testing in the patient from family C at the age of 12 disclosed a bilateral sensorineural hearing loss in the range of 30 to 60 dB, mainly affecting the higher frequencies (Fig. 2C).
FIGURE 2.
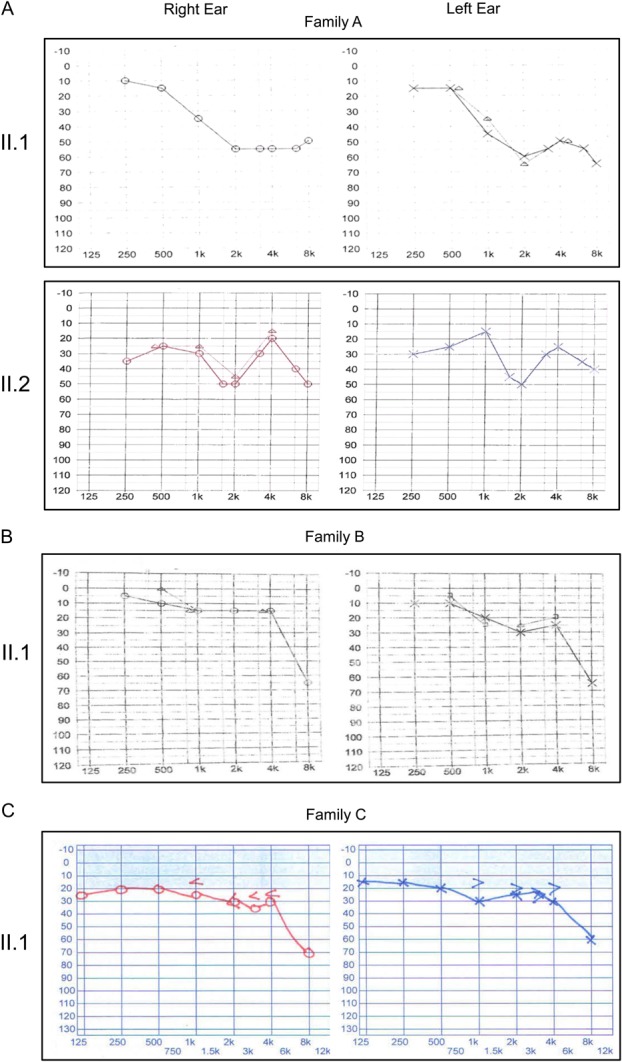
Hearing tests. Audiometry of the CHED2-affected patients from families A, B, and C. The graphs show the frequency in hertz (x axis) and the hearing level in decibels (y axis). The test was performed on the patient at 12 years (family A, II.1), 21 years (family A, II.2), 15 years (family B, II.1), and 12 years (family C, II.1), respectively. Note the reduced sensorineural hearing loss in the range 30 to 50 dB at the higher frequencies.
Specular Microscopy Examination
For each family, the parents, who were related by consanguinity, did not manifest obvious visual problems. However, a corneal examination of the parents in family A using a specular microscope showed guttae in the 44-year-old father, whereas the 46-year-old mother's scan showed moderate pleomorphism with a normal cell count (Fig. 3). A specular microscopic examination of the mother at age 38 in family B showed unremarkable images with a normal endothelial cell count (data not shown). The father in family B aged 40 was unavailable for examination. The specular microscopic examination of the mother in family C, aged 37 years, revealed guttae but a normal cell count (Fig. 3). The father, aged 42 years, was unavailable for clinical examination.
FIGURE 3.
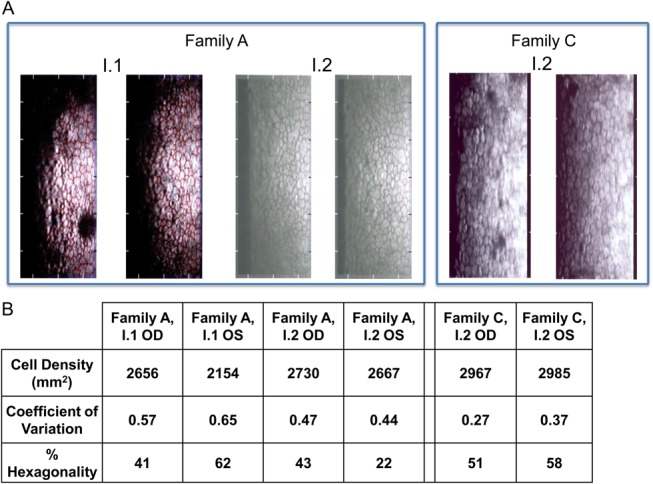
Corneal endothelium analysis. A, Specular microscopy images of the father (I.1) and mother (I.2) of family A and also the mother (I.2) of family C are shown. B, The table highlights the values for cell density (per square millimeter), coefficient of variation, and percentage hexagonality for the specular microscopic images. As a guideline, cell density of 2500 cells per square millimeter at middle age is within the normal range, and this value decreases with age, a coefficient of variation >0.4 and <50% hexagonality are indicative of an abnormal endothelium.
DISCUSSION
Given that CHED,7 Harboyan syndrome,8 and some FECD12 are caused by mutations in SLC4A11, it is perhaps surprising that all these conditions have never been described within the same family. In this article, we report that all 4 patients originally diagnosed with CHED2 were subsequently found to have varying degrees of sensorineural hearing loss at the higher frequency range after audiometric examination, suggesting that CHED2 invariably progresses to Harboyan syndrome. We also investigated whether parents of these patients, who are themselves heterozygous SLC4A11 mutation carriers, have early signs of FECD and found that 2 out of the 4 parents who were available to be examined had guttae in their endothelium. However, the relatively young age of the parents makes it difficult to reach a conclusion regarding their risk of developing FECD. Although based on a small number and therefore speculative, these observations are nevertheless valuable given that CHED is a rare condition. Our findings, together with data from other similar studies, will lead to a better understanding of this rare condition. Interestingly, this pattern of inheritance for a mutation in SLC4A11 is somewhat similar to that recently reported for LOXHD1, in which dominant mutations cause FECD,15 whereas recessive mutations cause deafness.19
The basis for the phenotypic heterogeneity seen in homozygous SLC4A11 mutation carriers with nonsyndromic CHED2 or Harboyan syndrome has been the subject of much speculation. The onset of progressive hearing loss in Harboyan syndrome has been described previously in children as young as 2 years and in adults as old as 33 years.6,8 However, auditory abnormalities have not been directly tested for in most cases of isolated CHED2,7,20–26 suggesting that Harboyan syndrome may have gone undetected. Because the proportion of null and missense SLC4A11 mutations identified in both conditions is similar, with no obvious clustering9 and the coexistence of the conditions within a family has been reported,10 there is little evidence to support a genetic basis for the difference between them. Instead, our longitudinal study suggests that CHED2 cases eventually experience some degree of sensorineural hearing loss and that variable age of onset of these symptoms may be related to some unknown differences in the expression of genetic modifiers or exposure to environmental triggers.
The auditory phenotype seen in Harboyan syndrome is consistent with the observation that SLC4A11 is not only expressed in corneal endothelial cells but it is also expressed in fibrocytes of the stria vascularis in the inner ear27,28 cells with a common embryonic origin in the neural crest. SLC4A11 exists as a transmembrane homodimer and functions as a sodium ion transporter,29 so its absence in knockout mice causes accumulation of sodium chloride in the corneal stroma, collection of water in the normally hydrated cornea, and morphological changes in fibrocytes resulting in deafness.27,28 Examination of cells transfected with mutant SLC4A11 constructs shows that the mutant protein fails to glycosylate and is retained intracellularly, never reaching the cell surface.7,12
Dominant mutations in SLC4A11 cause FECD.12 Most of these cases are caused by missense changes. Cell-based biochemical assays using SLC4A11 constructs seem to distinguish between the mutations that cause FECD and those that cause CHED2.30 Coexpression of mutant SLC4A11 with the wild-type construct causes a partial rescue of most CHED-causing mutants but not those implicated in FECD. This is thought to occur because, although most SLC4A11 mutations do not affect cell surface processing of the wild-type SLC4A11, the presence of the FECD mutant protein reduces wild-type processing at the cell surface, suggesting a possible explanation for the dominant inheritance pattern for this disorder. However, our study reveals that the parents of patients with CHED2, carrying heterozygous missense mutations in SLC4A11, develop guttae, which may be early signs of the onset of FECD. The cell-based biochemical approach highlighted would provide confirmatory evidence that these signs indeed predict FECD and are not age-related changes.
To conclude, we report here that CHED2 progresses to Harboyan syndrome and that both conditions are the same disease at different stages of development. We advise that patients with CHED2 should be monitored for progressive hearing loss and that their parents might be at an increased risk of developing late-onset FECD.
ACKNOWLEDGMENTS
The authors thank the families who participated in this study, as well as Dr. Nick Thyer, University of Leeds, who helped with this study.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.corneajrnl.com).
Supported by MRC grant number 408757 (S.S. is an MRC Clinical Research Training Fellow) and by Yorkshire Eye Research grant number 022.
The authors have no other funding or conflicts of interest to disclose.
S. Siddiqui, J.C. Zenteno, and A. Rice contributed equally to this work and should be considered equivalent first authors.
REFERENCES
- 1.Rahi JS, Cable N. Severe visual impairment and blindness in children in the UK. Lancet. 2003;362:1359–1365 [DOI] [PubMed] [Google Scholar]
- 2.Klintworth GK. Corneal dystrophies. Orphanet J Rare Dis. 2009;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callaghan M, Hand CK, Kennedy SM, et al. Homozygosity mapping and linkage analysis demonstrate that autosomal recessive congenital hereditary endothelial dystrophy (CHED) and autosomal dominant CHED are genetically distinct. Br J Ophthalmol. 1999;83:115–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullaney PB, Risco JM, Teichmann K, et al. Congenital hereditary endothelial dystrophy associated with glaucoma. Ophthalmology. 1995;102:186–192 [DOI] [PubMed] [Google Scholar]
- 5.Ramamurthy B, Sachdeva V, Mandal AK, et al. Coexistent congenital hereditary endothelial dystrophy and congenital glaucoma. Cornea. 2007;26:647–649 [DOI] [PubMed] [Google Scholar]
- 6.Mittal V, Mittal R, Sangwan VS. Successful Descemet stripping endothelial keratoplasty in congenital hereditary endothelial dystrophy. Cornea. 2011;30:354–356 [DOI] [PubMed] [Google Scholar]
- 7.Vithana EN, Morgan P, Sundaresan P, et al. Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystrophy (CHED2). Nat Genet. 2006;38:755–757 [DOI] [PubMed] [Google Scholar]
- 8.Desir J, Moya G, Reish O, et al. Borate transporter SLC4A11 mutations cause both Harboyan syndrome and non-syndromic corneal endothelial dystrophy. J Med Genet. 2007;44:322–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desir J, Abramowicz M. Congenital hereditary endothelial dystrophy with progressive sensorineural deafness (Harboyan syndrome). Orphanet J Rare Dis. 2008;3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta JS, Hemadevi B, Vithana EN, et al. Absence of phenotype–genotype correlation of patients expressing mutations in the SLC4A11 gene. Cornea. 2010;29:302–306 [DOI] [PubMed] [Google Scholar]
- 11.Keenan TD, Jones MN, Rushton S, et al. Trends in the indications for corneal graft surgery in the United Kingdom: 1999 through 2009. Arch Ophthalmol. 2012;130:621–628 [DOI] [PubMed] [Google Scholar]
- 12.Vithana EN, Morgan PE, Ramprasad V, et al. SLC4A11 mutations in Fuchs endothelial corneal dystrophy. Hum Mol Genet. 2008;17:656–666 [DOI] [PubMed] [Google Scholar]
- 13.Biswas S, Munier FL, Yardley J, et al. Missense mutations in COL8A2, the gene encoding the 2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum Mol Genet. 2001;10:2415–2423 [DOI] [PubMed] [Google Scholar]
- 14.Riazuddin SA, Zaghloul NA, Al-Saif A, et al. Missense mutations in TCF8 cause late-onset Fuchs corneal dystrophy and interact with FCD4 on chromosome 9p. Am J Hum Genet. 2010;86:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riazuddin SA, Parker DS, McGlumphy EJ, et al. Mutations in LOXHD1, a recessive-deafness locus, cause dominant late-onset Fuchs corneal dystrophy. Am J Hum Genet. 2012;90:533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riazuddin SA, Eghrari AO, Al-Saif A, et al. Linkage of a mild late-onset phenotype of Fuchs corneal dystrophy to a novel locus at 5q33.1-q35.2. Invest Ophthalmol Vis Sci. 2009;50:5667–5671 [DOI] [PubMed] [Google Scholar]
- 17.Sundin OH, Jun AS, Broman KW, et al. Linkage of late-onset Fuchs corneal dystrophy to a novel locus at 13pTel-13q12. 13. Invest Ophthalmol Vis Sci. 2006;47:140–145 [DOI] [PubMed] [Google Scholar]
- 18.Schmid E, Lisch W, Philipp W, et al. A new, X-linked endothelial corneal dystrophy. Am J Ophthalmol. 2006;141:478–487 [DOI] [PubMed] [Google Scholar]
- 19.Grillet N, Schwander M, Hildebrand MS, et al. Mutations in LOXHD1, an evolutionarily conserved stereociliary protein, disrupt hair cell function in mice and cause progressive hearing loss in humans. Am J Hum Genet. 2009;85:328–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiao X, Sultana A, Garg P, et al. Autosomal recessive corneal endothelial dystrophy (CHED2) is associated with mutations in SLC4A11. J Med Genet. 2007;44:64–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Bhattacharjee S, Prakash DR, et al. Genetic analysis of two Indian families affected with congenital hereditary endothelial dystrophy: two novel mutations in SLC4A11. Mol Vis. 2007;13:39–46 [PMC free article] [PubMed] [Google Scholar]
- 22.Ramprasad VL, Ebenezer ND, Aung T, et al. Novel SLC4A11 mutations in patients with recessive congenital hereditary endothelial dystrophy (CHED2). Hum Mutat. 2007;28:522–523 [DOI] [PubMed] [Google Scholar]
- 23.Aldave AJ, Yellore VS, Bourla N, et al. Autosomal recessive CHED associated with novel compound heterozygous mutations in SLC4A11. Cornea. 2007;26:896–900 [DOI] [PubMed] [Google Scholar]
- 24.Sultana A, Garg P, Ramamurthy B, et al. Mutational spectrum of the SLC4A11 gene in autosomal recessive congenital hereditary endothelial dystrophy. Mol Vis. 2007;13:1327–1332 [PubMed] [Google Scholar]
- 25.Hemadevi B, Veitia RA, Srinivasan M, et al. Identification of mutations in the SLC4A11 gene in patients with recessive congenital hereditary endothelial dystrophy. Arch Ophthalmol. 2008;126:700–708 [DOI] [PubMed] [Google Scholar]
- 26.Paliwal P, Sharma A, Tandon R, et al. Congenital hereditary endothelial dystrophy-mutation analysis of SLC4A11 and genotype–phenotype correlation in a North Indian patient cohort. Mol Vis. 2010;16:2955–2963 [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez IA, Rosenblatt MI, Kim C, et al. Slc4a11 gene disruption in mice: cellular targets of sensorineural abnormalities. J Biol Chem. 2009;284:26882–26896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gröger N, Fröhlich H, Maier H, et al. SLC4A11 prevents osmotic imbalance leading to corneal endothelial dystrophy, deafness, and polyuria. J Biol Chem. 2010;285:14467–14474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park M, Li Q, Shcheynikov N, et al. NaBC1 is a ubiquitous electrogenic Na+-coupled borate transporter essential for cellular boron homeostasis and cell growth and proliferation. Mol Cell. 2004;16:331–341 [DOI] [PubMed] [Google Scholar]
- 30.Vilas GL, Loganathan SK, Quon A, et al. Oligomerization of SLC4A11 protein and the severity of FECD and CHED2 corneal dystrophies caused by SLC4A11 mutations. Hum Mutat. 2012;33:419–428 [DOI] [PubMed] [Google Scholar]


