Abstract
Among chronic smokers, individual differences in subjective reactions to smoking may characterize important facets of nicotine dependence that relate to abstinence-induced craving, withdrawal symptom profiles, and risk for relapse. Although the negative reinforcing properties of smoking have achieved prominent positions in models of relapse (Baker, Brandon, & Chassin, 2004), vulnerability to relapse risk may also arise from seeking positive reinforcement from smoking (Shiffman & Kirchner, 2009). In this study, 183 cessation-motivated smokers provided subjective craving, positive and negative reactions to standardized cigarettes following overnight abstinence. Level of craving, negative mood, and positive mood after overnight abstinence were significantly predictive of withdrawal on quit-day. Increased positive reactions to smoking were uniquely predictive of relapse after quitting (Hazard Ratio = 1.22, p < .001). Individual differences in positive reactions to smoking may be important markers of neurobiological systems that promote dependence and interfere with cessation efforts.
Keywords: positive affect, smoking relapse, smoking reinforcement
Acute tobacco administration produces characteristic subjective effects, which may play important roles in the dependence process. For example, tobacco is believed to have hedonic properties in that it induces positive affect and attenuates negative affect (Baker et al., 2004). Such hedonic properties are attributed to nicotine activating reward neurocircuitry (Kenny & Markou, 2001; Pontieri, Tanda, Orzi, & Di Chiara, 1996; Volkow & Fowler, 2000). The actions of nicotine on reward systems suggest both a functional role of smoking in regulating positive mood, anhedonia, and negative mood (Baker et al., 2004). Although the relationship between urge relief and increased positive effects may become attenuated, on average, significant positive associations between urges and positive effects from smoking persist among chronic smokers (Shiffman & Kirchner, 2009). Evidence suggests that subjective effects of acute tobacco administration are stable during regular smoking patterns (Lerman, Perkins, & Gould, 2009), may increase significantly following brief periods of abstinence (Kalman, 2002; Perkins et al., 1994), and therefore may be a powerful driving force that maintains the daily reinstatement of smoking.
In support of these claims, laboratory investigations indicate that, among overnight abstinent smokers, tobacco or nicotine administration acutely (a) enhances positive affect, (b) alleviates negative affect, and (c) suppresses urges (Cinciripini et al., 2006; Eissenberg, Adams, Riggins, & Likness, 1999; Gilbert, Dibb, Plath, & Hiyane, 2000; Heishman, 1999; Perkins et al., 1994). There is also evidence of substantial between-person variation in subjective reactivity (Kalman, 2002). A recent study showed that genetic variance accounted for a significant portion of variability in acute nicotine response among twins (Swan et al., 2007). Thus, acute tobacco reactivity may be an important phenotypic marker of heterogeneity within the tobacco dependence syndrome (Lerman, Perkins, & Gould, 2009; Pomerleau, 1995).
The phenotypic significance of acute tobacco reactivity would be supported if it were associated with key indices of tobacco dependence such as (1) withdrawal symptoms upon tobacco deprivation and (2) relapse after attempts at cessation (Perkins, 2002). Although there is some evidence that individual differences in subjective reactivity predict aspects of withdrawal and relapse, the findings are generally mixed (Kaufmann et al., 2004; Niaura, Shadel, Goldstein, Hutchinson, & Abrams, 2001; Perkins et al., 2002; Shiffman, Ferguson, & Gwaltney, 2006).
We previously explored the predictive influence of reactivity to a controlled dose of tobacco in a sample of 183 chronic smokers prior to a self-guided quit-attempt (Niaura et al., 2001). The strength of the physiological reactivity to tobacco and degree of tolerance to tobacco effects (i.e., reductions in responses after multiple cigarettes) were not strongly related to either cessation-related withdrawal symptoms or cessation outcomes. However, the results suggested patterns of rapid tolerance to tobacco effects did characterize a sizable subgroup of chronic smokers who reported more negative affect after quitting.
The current study is an analysis of additional measures collected by Niaura and colleagues (2001) that extends examination beyond physiological effects to abstinence-related changes among distinct facets of subjective withdrawal symptoms (i.e., urges, reduced positive affect, and negative affect) that have demonstrated influences on smoking relapse vulnerability (McCarthy, Piasecki, Fiore, & Baker, 2006; Perkins, Jetton, & Keenan, 2003; Strasser et al., 2005; Strong et al., 2009). The purpose of the present report was to (1) evaluate the nature of tobacco reactivity phenotypes by exploring the effects of tobacco administration on urge to smoke, positive affect, and negative affect; (2) examine if decreased urges, increased positive and decreased negative affect after tobacco use predicted cessation-related reactions in corresponding aspects of withdrawal (i.e., urges, positive affect, and negative affect); and (3) investigate the predictive influence of tobacco reactivity phenotypes on smoking cessation outcomes. To elucidate the potential significance of these results, we examine whether these effects are incremental predictors of relapse that are independent of tobacco dependence and the severity of withdrawal symptoms during cessation.
Methods
Participants
The study sample consisted of 183 smokers who were interested in quitting smoking and participated in a smoking-dependence study (Niaura et al., 2001). The mean age of the sample was 42.8 years (SD = 11.7). Fifty-five percent of the participants were female and the majority (95%) was Caucasian. On average, participants smoked 25.8 cigarettes per day (SD = 10.5) and reported being regular smokers for 24.6 years (SD = 11.4). Participants had an average of 3.3 (SD = 2.4) prior quit attempts and reported being able to abstain from smoking for a median of six days (interquartile range = 2–50) during their most recent quit attempt, and 96% reported current intentions to “quit and stay off cigarettes forever”.
Procedures
Detailed study procedures were reported in a prior investigation (Niaura et al., 2001). One week after pretreatment assessments, participants attended a laboratory visit where they smoked three experimentally controlled cigarettes. At the end of the laboratory session, participants were given smoking cessation materials and instructed to prepare to quit smoking one week following the laboratory visit. Participants visited the laboratory for self-report and biochemical verification on target quit day (TQD) and 2 (2-TQD), 7 (7-TQD), 14 (14-TQD), and 30 (30-TQD) days following quit day.
Smoking procedure
Participants were instructed to refrain from smoking, drinking alcohol, or ingesting caffeine for 12 hours prior to the laboratory session. On the morning of the challenge procedure (between 8 a.m. and 11 a.m.), smoking abstinence was biochemically verified with an expired alveolar carbon monoxide (CO) reading of ≤15 ppm. During the session, participants smoked three 1.0-mg cigarettes (i.e., Marlboro King Size Hard Pack) spaced 30 minutes apart (B2, B3, and B4). Cigarettes were smoked in a standardized fashion, 10 puffs at a rate of one puff every 30 seconds, using the Quantified Smoke Delivery System (QSDS: Gilbert & Meliska, 1991). A self-report assessment of the subjective effects from the cigarette was completed prior to smoking (B1) and after each smoking period (B2–B4). We focus on the ratings obtained immediately before (B1) and after smoking the first cigarette (B2). We believe that capturing this first experience best isolated the acute effects of an entire cigarette on changes in specific aspects of the withdrawal syndrome that are known to predict relapse after cessation. Changes in subjective effects after subsequent doses of tobacco were not evaluated.
Smoking cessation outcomes
Seven-day point prevalence abstinence (PPA) was biochemically verified by expired CO readings of <8 ppm. PPA rates were 26% at 7 days, 27% at 14 days, and 22% at 30 days (Shadel et al., 1998). Using a calendar-based interview during each assessment, we calculated the number of days from TQD that a participant smoked for seven consecutive days. Unverified and missing reports of abstinence were designated as relapsed seven days from the previous verified abstinent assessment.
Measures
Fagerström Test for Nicotine Dependence (FTND)
The FTND (Heatherton, Kozlowski, Frecker, & Fagerström, 1991) was collected at baseline to measure severity of nicotine dependence.
Nicotine Effects Questionnaire (NEQ)
The NEQ is a 16-item measure with one urge to smoke item, a set of four items measuring positive affect effects (happy, friendly, calm, and content; α = .85), three items measuring negative affect effects (worried, tense, and sad; α = .69), six items measuring unpleasant physical effects (buzzing, nausea, unpleasant, dizzy, palm sweating, and heart pounding; α = .69), and two items assessing cognitive effects (trouble concentrating, drowsy; r = .49). Items were scaled from 0 (Not at all) to 10 (Very much) with instructions to report how the respondent felt in that moment. The NEQ was administered immediately prior to (B1) and following the first cigarette after overnight abstinence (B2). Analyses focused on urges, positive affect, and negative affect effects given the demonstrated relationships of corresponding withdrawal components with smoking outcomes (McCarthy, Piasecki, Fiore, & Baker, 2006; Perkins, Jetton, & Keenan, 2003; Strasser et al., 2005; Strong et al., 2009).
Positive and Negative Affect Schedule (PANAS)
The PANAS (Watson, Clark, & Tellegen, 1988) is a reliable measure of global positive and negative affect. Participants completed PANAS assessments at baseline, B1, quit day (TQD), and all postquit follow-up sessions (2, 7, 14, and 30 days after TQD).
Analysis Plan
Cigarette challenge
We estimated mean differences (Cohen’s d) across gender and intercorrelations among baseline levels of nicotine dependence, baseline levels of PANAS-positive and PANAS-negative scales, and each of the examined NEQ ratings prior to smoking (B1). NEQ ratings prior to smoking (B1) and changes in ratings after smoking (B2–B1) also were examined.
Predicting withdrawal and relapse to smoking
Our primary outcomes included (a) repeated assessments of urges to smoke, PANAS-positive affect, and PANAS-negative affect ratings after TQD and at 2, 7, 14, 30 days after quitting regardless of their smoking statuses; and (b) the number days until smokers relapsed. We used a latent variable framework that included growth curve models to examine individual variation in urge and affect trajectories. Continuous latent variables represented TQD ratings (postquit intercept: s0) and the change rates of the postquit ratings over time (postquit slopes: 2-TQD, 7-TQD, 14-TQD, 30-TQD). Models assessed the association of these growth parameters with survival to smoking relapse using a Cox proportional hazards regression model within Mplus version 5.0 (Muthen & Muthen, 2007). Urge, positive- and negative-affect trajectories were estimated without regard to lapse status. The joint analysis of repeated assessments and times to events is described in Xu and Zeger (2001), and a generalization for applications within Mplus software is described in Asparouhov, Masyn, and Muthen (2006).
Latent growth models were used to test (a) whether overnight deprivation prior to the challenge procedure generated ratings of urges, positive affect, and negative affect that were associated prospectively with urges to smoke, positive affect, and negative affect, respectively, after TQD (postquit intercept) and throughout follow-ups (postquit slopes: 2-TQD to 30-TQD); and (b) whether decreases in urges, increases in positive and decreases in negative cigarette effects after the cigarette challenge predicted survival to smoking relapse. Gender, level of nicotine dependence, and corresponding baseline values for urges, positive and negative affect were included as covariates and postquit changes (postquit intercepts and postquit slopes) were estimated in all survival analyses of relapse.
Results
Ratings After Overnight Abstinence
Table 1 presents means and correlations describing the relationships of gender, level of nicotine dependence, and baseline levels of PANAS-positive and PANAS-negative affect with laboratory session measures of urge and affective ratings after overnight abstinence (B1), and changes in ratings following the first cigarette of the day (B2). On average, smokers reported moderate levels of urges to smoke, moderate levels of positive affect, and low levels of negative affect.
Table 1.
Evaluation of Urges, Positive Affect, and Negative Affect Ratings During Acute Withdrawal After Overnight Abstinence and in Reaction to Smoking One Standard Cigarette (n = 174)
| Instrument | After overnight abstinence (B1)
|
Changes after the first cigarette (B2–B1)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Urge | Positive | Negative | Mean | SD | Urge | Positive | Negative | |
| Covariate | ||||||||||
| Female | (55%) | 0.11a | 0.03a | 0.31a,* | 0.10a | 0.26a | 0.00a | |||
| FTND | 6.80 | 2.69 | 0.39** | −0.28** | 0.01 | −0.30** | 0.08 | 0.02 | ||
| Baseline Affect (B0) | ||||||||||
| PANAS Positive | 32.50 | 7.68 | 0.15* | 0.18* | −0.15* | 0.05 | 0.14 | 0.04 | ||
| PANAS Negative | 14.65 | 5.29 | 0.10 | −0.13* | 0.48** | −0.01 | 0.04 | −0.12 | ||
| Cigarette Challenge Rating | ||||||||||
| Urge to Smoke | 5.89 | 2.93 | −3.74** | 3.15 | −0.47** | |||||
| Positive Affect | 5.47 | 1.70 | −0.23** | 0.20 | 1.62 | −0.06 | −0.27** | |||
| Negative Affect | 1.38 | 1.59 | 0.22** | −0.29** | 0.06 | 1.32 | 0.13 | −0.22** | −0.39** | |
Note. Means, standard deviations, standardized mean differences, and Pearson correlations are presented.
Effect size estimate (Cohen’s d).
p < .05.
p < .01.
Although women and men reported similar levels of urges to smoke and positive affect ratings, women reported significantly higher levels of negative affect ratings after overnight deprivation, t(172) = 2.1, p = .04. Higher levels of nicotine dependence were associated with significantly higher urges to smoke and lower levels of positive affect after overnight abstinence.
Pretreatment PANAS-positive and PANAS-negative affect scales were related significantly to levels of positive and negative affect after overnight abstinence. Higher pretreatment PANAS-positive affect was also associated with higher urges after overnight abstinence. Among the concurrent ratings during abstinence, higher urges were related to lower positive and higher negative affect ratings.
Ratings After the First Cigarette
There was a significant decrease in urges to smoke after smoking a cigarette, t(171) = −15.58, p < .001. Average changes (B2–B1) in positive, t(171) = −1.60, p < .11, and negative, t(171) = 0.59, p < .55 affect were not significantly different than zero. Although changes in positive affect were associated inversely with changes in negative affect, changes in urges to smoke were independent of changes in positive or negative affect (see Table 1).
Women and men reported similar changes in cigarette effects ratings. Smokers with higher levels of nicotine dependence reported significantly greater reductions in the urge to smoke after one cigarette. Reports of pretreatment PANAS-positive and PANAS-negative affect were not related to changes in urges or changes in positive or negative cigarette effects.
Overnight Abstinence Ratings and Withdrawal After Cessation
Table 2 presents results from the latent growth models of cessation-related changes in urges to smoke, PANAS-positive and PANAS-negative affect, respectively. Model fit indices of unconditional growth models supported linear and quadratic components for urge (χ2(6) = 7.54, p = .27; RMSEA = 0.04, CI = 0.00 – 0.11) and PANAS-negative (χ2(6) = 12.61, p = .05; RMSEA = 0.07, CI = 0.03–0.14) to describe a decline over the first week and relative stability through TQD-30. A linear component was sufficient for PANAS-positive trajectories (χ2(10) = 4.95, p = .89; RMSEA = 0.00, CI = 0.00–0.04). Each model included gender, FTND, the corresponding baseline measure, and individual latent growth factors for levels after TQD (postquit intercepts) and changes over time (postquit quadratic and/or linear slopes) coded to reflect unequal intervals between assessments and centered after TQD-7 (i.e., −0.7, −0.5, 0, 0.7, and 2.3). Smokers with higher levels of tobacco dependence reported significantly higher levels of PANAS-negative affect and steeper decreases in PANAS-negative affect after TQD. Gender and nicotine dependence were not related to changes in urges to smoke or levels of PANAS-positive affect after cessation.
Table 2.
Relationship Between Nicotine Effects Questionnaire Ratings and Cessation-Related Changes in Urges to Smoke, Positive and Negative Mood After Quitting (Postquit Intercept), and Changes Over Time (Postquit Linear and Quadratic Slopes)
| Fixed Effect | Urge to smoke
|
PANAS Positive
|
PANAS Negative
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Postquit intercept | p | Postquit slope | p | Postquit intercept | p | Postquit slope | p | Postquit intercept | p | Postquit slope | p | |
| Covariate | ||||||||||||
| Female | −0.17 (0.18) | 0.35 | −0.12 (0.19)l | 0.53 | −0.94 (0.93) | 0.31 | −0.01 (0.32) | 0.97 | 1.05 (0.86) | 0.22 | −1.93 (1.04)l | 0.06 |
| 0.07 (0.18)q | 0.70 | 0.85 (0.46)q | 0.07 | |||||||||
| FTND | −0.08 (0.11) | 0.45 | −0.05 (0.10)l | 0.62 | −0.78 (0.50) | 0.12 | −0.01 (0.19) | 0.98 | 1.28 (0.42) | 0.00 | −1.26 (0.48)l | 0.01 |
| 0.14 (0.09)q | 0.13 | 0.51 (0.23)q | 0.03 | |||||||||
| Urge to Smoke | ||||||||||||
| Overnight Abstinence | 0.13 (0.04) | 0.00 | −0.14 (0.04)l | 0.00 | ||||||||
| 0.09 (0.04)q | 0.03 | |||||||||||
| Change After 1 Cigarette | 0.01 (0.04) | 0.73 | −0.05 (0.04)l | 0.23 | ||||||||
| 0.03 (0.05)q | 0.50 | |||||||||||
| Positive Affect | ||||||||||||
| Overnight Abstinence | 1.09 (0.34) | 0.00 | −0.08 (0.12)l | 0.49 | ||||||||
| Change After 1 Cigarette | 0.19 (0.39) | 0.63 | 0.15 (0.11) | 0.15 | ||||||||
| Negative Affect | ||||||||||||
| Overnight Abstinence | 1.07 (0.37) | 0.00 | −0.28 (0.43)l | 0.51 | ||||||||
| 0.25 (0.21)q | 0.23 | |||||||||||
| Change After 1 Cigarette | 0.18 (0.35) | 0.60 | 0.09 (0.42)l | 0.83 | ||||||||
| 0.02 (0.23)q | 0.91 | |||||||||||
| Growth Factor Variancea | 1.05 (0.14) | 0.00 | 0.72 (0.18)l | 0.00 | 50.60 (5.81) | 0.00 | 4.26 (1.19) | 0.00 | 34.05 (4.27) | 0.00 | 15.97 (4.03)l | 0.00 |
| 0.20 (0.05)q | 0.00 | 4.63 (1.15)q | 0.00 | |||||||||
Note. Individual model parameters are presented with standard errors in parentheses. p = 2-tailed.
Unconditional model estimates.
Linear slope estimate.
Quadratic slope estimate.
Ratings of urges, positive affect, and negative affect after overnight abstinence (B1) were predictive of corresponding measure of urges, PANAS-positive and PANAS-negative affect scales (postquit intercepts) after TQD (ps < 0.01). Further, changes in urges (i.e., postquit linear and quadratic slopes) also were predicted by ratings of urges collected after overnight abstinence. These results support the predictive validity of urge and positive and negative affect ratings obtained after overnight abstinence. However, tobacco reactivity measures (B2–B1) were not predictive of corresponding changes in these withdrawal symptoms over time.
Tobacco Reactivity Ratings Predict Smoking Relapse
Of the 183 smokers, 81.4% (n = 149) had relapsed to seven consecutive days of smoking prior to the final assessment. Evaluation of Schoenfeld residuals (Grambsch & Therneau, 1994) suggested that the proportional hazards assumption was tenable for the covariate-adjusted Cox model (χ2 = 5.01; p = .08).
Table 3 presents survival analysis effect estimates (bs) of urge to smoke, positive affect, and negative affect ratings after overnight abstinence and changes after smoking a cigarette along with corresponding baseline values, values after TQD (postquit intercept), and changes in corresponding values after cessation (postquit slopes). Ratings of the urge to smoke after overnight abstinence (Risk Ratio (RR) = 1.03; 95% CI = 0.95–1.11, p = .57) and changes in ratings after smoking a cigarette were not predictive of relapse (RR = 1.01; 95% CI = 0.95–1.07, p = .72). Higher levels of urges after TQD and increases during cessation were strong predictors of relapse.
Table 3.
Results of Proportional Hazards Regression Models Relating NEQ Ratings and Withdrawal Symptoms to Risk for Smoking Relapse
| Fixed Effect | Relapse
|
|||
|---|---|---|---|---|
| Effect | SE | Effect/SE | p | |
| Covariatesa | ||||
| Female | 0.10 | 0.15 | 0.67 | .52 |
| FTND | 0.09 | 0.08 | 1.12 | .28 |
| Urge to Smokeb | ||||
| Overnight Abstinence (B1) | 0.03 | 0.04 | 0.57 | .57 |
| Change After 1 Cigarette (B2–B1) | 0.01 | 0.03 | 0.36 | .72 |
| Baseline Level | 0.01 | 0.06 | 0.17 | .86 |
| Postquit Intercept | 0.39 | 0.09 | 4.08 | .00 |
| Postquit Slope (Linear) | 0.30 | 0.12 | 2.56 | .01 |
| Postquit Slope (Quadratic) | 0.64 | 0.16 | 4.03 | .00 |
| Positive Affect Ratingb | ||||
| Overnight Abstinence (B1) | 0.09 | 0.05 | 1.63 | .10 |
| Change After 1 Cigarette (B2–B1) | 0.20 | 0.05 | 4.00 | .00 |
| PANAS Positive | ||||
| Baseline Level | 0.04 | 0.01 | 3.61 | .00 |
| Postquit Intercept | −0.08 | 0.01 | −5.56 | .00 |
| Postquit Slope (Linear) | 0.01 | 0.05 | 0.14 | .89 |
| Negative Affect Ratingb | ||||
| Overnight Abstinence (B1) | −0.13 | 0.08 | −1.54 | .12 |
| Change After 1 Cigarette (B2–B1) | 30.05 | 0.08 | 30.66 | .51 |
| PANAS Negative | ||||
| Baseline Level | −0.01 | 0.02 | −0.47 | .64 |
| Postquit Intercept | 0.07 | 0.02 | 4.50 | .00 |
| Postquit Slope (Linear) | 0.09 | 0.04 | 2.25 | .03 |
| Postquit Slope (Quadratic) | 0.01 | 0.11 | 0.12 | .91 |
Model estimates are not adjusted for the effects of the examined predictors.
Model estimates adjusted for the effects of covariates and predictor effects.
Although ratings of the positive affect after overnight abstinence (RR = 1.09; 95% CI = 0.99–1.21, p = .10) were not significantly related to relapse, changes in positive affect (B2–B1) after smoking a cigarette were highly predictive of increased risk of relapse (RR = 1.22; 95% CI = 1.11–1.35, p = .00). Figure 1 displays the probability of relapse for smokers across increasing levels (25th, 50th, and 75th percentiles) of changes in positive affect ratings after smoking a cigarette. Higher ratings of negative affect after overnight abstinence (RR = 0.88; 95% CI = 0.75–1.03, p = .12) were associated with reduced risk of relapse. However, changes in ratings after smoking a cigarette were not predictive of relapse (RR = 0.95; 95% CI = 0.81–1.11, p = .51). Higher levels of negative affect after TQD and increases during cessation were strong predictors of relapse. Survival analyses did not support relationships between NEQ cognitive (RR = 1.14; 95% CI = 0.89–1.45, p = .31) or unpleasant physical effects measures (RR = 1.22; 95% CI = 0.88–1.69, p = .22) and smoking relapse.
Figure 1.
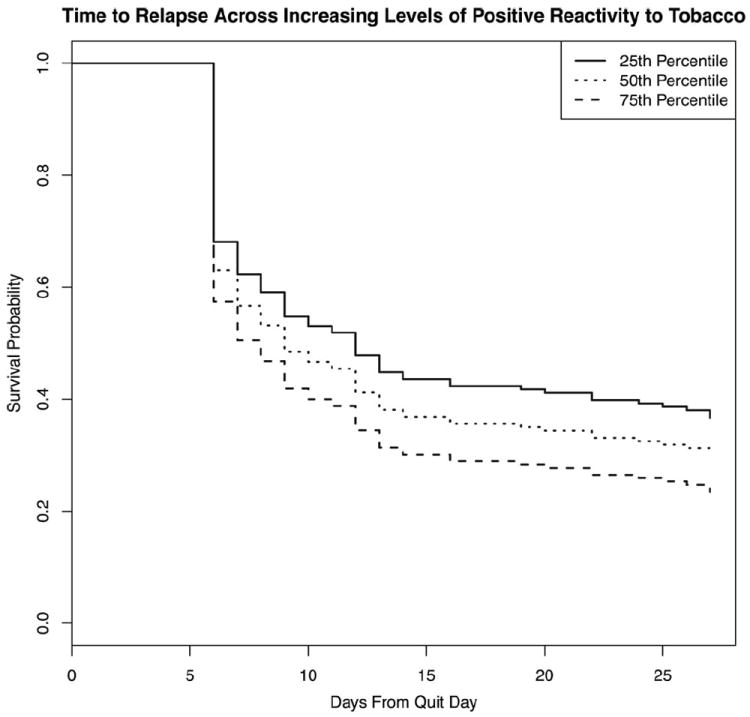
Survival curves for smokers across increasing levels (25th, 50th, and 75th percentiles) of change in positive affect ratings after smoking a cigarette.
Discussion
This study documents the predictive utility of precessation craving and withdrawal after overnight abstinence and positive affect reactions after cigarette smoking. Each of the laboratory-based ratings of urges and positive and negative affect after overnight abstinence were reliable predictors of corresponding levels assessed on quit day. Higher urges, lower positive affect, and higher negative affect after quit day were associated with significant risk for early relapse. Of the tobacco reactivity measures, only changes in positive affect were significantly predictive of risk for smoking relapse. For every unit increase in ratings of positive affect reactions after smoking, the risk for relapse was 22% higher. This index of hedonic reactivity to smoking suggests a putative phenotypic marker of tobacco dependence.
After overnight abstinence, more dependent smokers had stronger urges to smoke, lower levels of positive affect, and experienced greater relief from their urge to smoke after a single cigarette. Despite significant concurrent relationships between strength of urges and levels of positive and negative affect after overnight abstinence, ratings obtained after smoking suggested that relief from urges after smoking was independent from changes in positive and negative affect ratings. Further, neither dependence nor prechallenge affective states were related to affective changes in reaction to smoking. These results suggest the importance of dependence in shaping experienced relief of urges and raise the possibility that affective reactions to smoking may not be tightly linked to core dependence construct (Piper et al., 2008).
Increases in positive affect alone was prospectively related to the increased risk for relapse. Research evaluating day-to-day smoking reactions before a quit attempt suggests that smokers with the highest precessation satisfaction ratings were most likely to relapse (Shiffman & Kirchner, 2009). Higher satisfaction ratings after an initial lapse also has been shown to predict subsequent relapse (Shiffman et al., 2006). In Positron Emission Tomography studies, greater improvements in mood after smoking have been associated with lower levels of resting dopamine concentrations and heightened dopamine release in the striatum, and may indicate dysfunction in the regulation of reward systems (Barrett, Boileau, Okker, Pihl, & Dagher, 2004; Brody et al., 2009; Montgomery, Lingford-Hughes, Egerton, Nutt, & Grasby, 2007). Greater increases in positive mood after smoking and potentially low positive mood after overnight deprivation may identify smokers with expected difficulty regulating moods during cessation. Although the mechanism by which tobacco reactivity may result in relapse remains uncertain, these data do suggest that greater reactivity to smoking with increased positive mood may be an important aspect of tobacco dependence.
Limitations
Given the evidence for the subjective positive effects from sensory aspects of smoking de-nicotinized cigarettes (Brauer et al., 2001), we cannot attribute changes in positive mood to the effects of nicotine alone. A primary limitation in relating mood effects to smoking relapse was the use of intermittent rather than ecological momentary assessments (Shiffman et al., 2006). The lack of detailed assessment limits our ability to link phasic shifts in mood during early periods of abstinence to relapse risk. Again, a more detailed assessment of multiple-item urge measures may have increased our ability to detect more graded urge responses during smoking and after cessation. Finally, this study is a secondary analysis of auxiliary data from a larger study of tolerance reported previously (Niaura et al., 2001), and the significance of the current findings has yet to be replicated.
Summary
This study highlighted the utility of an overnight abstinence challenge when predicting withdrawal intensity after attempts at cessation. Reactions to smoking that were accompanied by increased positive affect were uniquely predictive of relapse to smoking and may be important markers of the tobacco dependence phenotype.
Contributor Information
David R. Strong, Alpert Medical School of Brown University and Butler Hospital
Daniel P. Evatt, Johns Hopkins University School of Medicine
Benjamin D. Greenberg, Alpert Medical School of Brown University and Butler Hospital
Adam M. Leventhal, Department of Preventive Medicine, University of Southern California
Suzanne Haber, Department of Pharmacology and Physiology, University of Rochester School of Medicine and Dentistry.
David Abrams, The Schroeder Institute for Tobacco Research and Policy Studies.
Raymond Niaura, The Schroeder Institute for Tobacco Research and Policy Studies.
References
- Asparouhov T, Masyn K, Muthen B. Continuous time survival in latent variable models. Proceedings of the Joint Statistical Meeting. American Statistical Association section on Biometrics; Seattle, WA: 2006. Aug, pp. 180–187. [Google Scholar]
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annual Review of Psychology. 2004;55:463–491. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Boileau I, Okker J, Pihl RO, Dagher A. The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C]raclopride. Synapse. 2004;54(2):65–71. doi: 10.1002/syn.20066. [DOI] [PubMed] [Google Scholar]
- Behm FM, Lane JD, Westman EC, Perkins C, Rose JE. Individual differences in smoking reward from de-nicotinized cigarettes. Nicotine & Tobacco Research. 2001;3(2):101–109. doi: 10.1080/14622200123249. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Allen-Martinez Z, Scheibal D, Abrams AL, et al. Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology. 2009;34(2):282–289. doi: 10.1038/npp.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Carter BL, Lam C, Wu X, de Moor CA, et al. The effects of smoking deprivation and nicotine administration on emotional reactivity. Nicotine & Tobacco Research. 2006;8(3):379–392. doi: 10.1080/14622200600670272. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Adams C, Riggins EC, 3, Likness M. Smokers’ sex and the effects of tobacco cigarettes: Subject-rated and physiological measures. Nicotine & Tobacco Research. 1999;1(4):317–324. doi: 10.1080/14622299050011441. [DOI] [PubMed] [Google Scholar]
- Gilbert D, Meliska C. Individual differences in and reliability of electroencephalogram, cortisol, beta-endorphin, heart rate, and subjective responses to smoking multiple cigarettes via a quantified smoke delivery system. In: Lippiello PM, Collins A, Grey J, Robinson J, editors. The Biology of Nicotine. New York: Raven Press; 1991. pp. 141–155. [Google Scholar]
- Gilbert DG, Dibb WD, Plath LC, Hiyane SG. Effects of nicotine and caffeine, separately and in combination, on EEG topography, mood, heart rate, cortisol, and vigilance. Psychophysiology. 2000;37(5):583–595. [PubMed] [Google Scholar]
- Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ. Behavioral and cognitive effects of smoking: Relationship to nicotine addiction. Nicotine and Tobacco Research. 1999;1(Supplement 2):S143–147. doi: 10.1080/14622299050011971. discussion S165–146. [DOI] [PubMed] [Google Scholar]
- Kalman D. The subjective effects of nicotine: Methodological issues, a review of experimental studies, and recommendations for future research. Nicotine & Tobacco Research. 2002;4(1):25–70. doi: 10.1080/14622200110098437. [DOI] [PubMed] [Google Scholar]
- Kaufmann V, Jepson C, Rukstalis M, Perkins K, Audrain-McGovern J, Lerman C. Subjective effects of an initial dose of nicotine nasal spray predict treatment outcome. Psychopharmacology (Berl) 2004;172(3):271–276. doi: 10.1007/s00213-003-1659-8. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacology, Biochemistry and Behavior. 2001;70(4):531–549. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Lerman C, Perkins K, Gould TJ. Phenotypes and Endophenotypes: Foundations for Genetic Studies of Nicotine Use and Dependence, Tobacco Control Monograph No. 20. In: Swan Gary E, Ph D, Baker Timothy B, Chassin Laurie, Conti David V, Lerman Caryn, Perkins Kenneth A., editors. Nicotine Dependence Endophenotypes in Chronic Smokers. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. NIH Publication No. 09–6366; 2009. Aug, [Google Scholar]
- McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: An electronic diary study. Journal of Abnormal Psychology. 2006;115(3):454–466. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, Lingford-Hughes AR, Egerton A, Nutt DJ, Grasby PM. The effect of nicotine on striatal dopamine release in man: A [11C]raclopride PET study. Synapse. 2007;61(8):637–645. doi: 10.1002/syn.20419. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus user’s guide. 5. Los Angeles, CA: Muthen & Muthen; 2007. [Google Scholar]
- Niaura R, Shadel WG, Goldstein MG, Hutchinson KE, Abrams DB. Individual differences in responses to the first cigarette following overnight abstinence in regular smokers. Nicotine & Tobacco Research. 2001;3(1):37–44. doi: 10.1080/14622200020032088. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Chronic tolerance to nicotine in humans and its relationship to tobacco dependence. Nicotine Tobacco Research. 2002;4(4):405–422. doi: 10.1080/1462220021000018425. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Broge M, Gerlach D, Sanders M, Grobe JE, Cherry C, Wilson AS. Acute nicotine reinforcement, but not chronic tolerance, predicts withdrawal and relapse after quitting smoking. Health Psychology. 2002;21(4):332–339. doi: 10.1037//0278-6133.21.4.332. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Fonte C, Goettler J, Cagguila AR, Reynolds WA, Jacob RG. Chronic and acute tolerance to subjective, behavioral and cardiovascular effects of nicotine in humans. Journal of Pharmacology and Experimental Therapeutics. 1994;272:628–638. [PubMed] [Google Scholar]
- Perkins KA, Jetton C, Keenan J. Common factors across acute subjective effects of nicotine. Nicotine & Tobacco Research. 2003;5(6):869–875. doi: 10.1080/14622200310001614629. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF. Individual differences in sensitivity to nicotine: Implications for genetic research on nicotine dependence. Behavior Genetics. 1995;25(2):161–177. doi: 10.1007/BF02196925. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382(6588):255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Shadel WG, Niaura R, Abrams DB, Goldstein MG, Rohsenow DJ, Sirota AD, Monti PM. Scripted imagery manipulaitons and smoking cue reactivity in a clinical sample of self-quitters. Experimental and Clinical Psychopharmacology. 1998;6:179–186. doi: 10.1037//1064-1297.6.2.179. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ. Immediate hedonic response to smoking lapses: Relationship to smoking relapse, and effects of nicotine replacement therapy. Psychopharmacology (Berl) 2006;184(3–4):608–618. doi: 10.1007/s00213-005-0175-4. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Kirchner TR. Cigarette-by-cigarette satisfaction during ad libitum smoking. Journal of Abnormal Psychology. 2009;118(2):348–359. doi: 10.1037/a0015620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser AA, Kaufmann V, Jepson C, Perkins KA, Pickworth WB, Wileyto EP, Lerman C. Effects of different nicotine replacement therapies on postcessation psychological responses. Addictive Behaviors. 2005;30(1):9–17. doi: 10.1016/j.addbeh.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Strong DR, Kahler CW, Leventhal AM, Abrantes AM, Lloyd-Richardson E, Niaura R, Brown RA. Impact of bupropion and cognitive-behavioral treatment for depression on positive affect, negative affect, and urges to smoke during cessation treatment. Nicotine & Tobacco Research. 2009;11:1142–1153. doi: 10.1093/ntr/ntp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN, Krasnow RE, Wilhelmsen KC, Jacob P, 3, Benowitz NL. Genetic and environmental sources of variation in heart rate response to infused nicotine in twins. Cancer Epidemiology, Biomarkers and Prevention. 2007;16(6):1057–1064. doi: 10.1158/1055-9965.EPI-06-1093. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cerebral Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Xu J, Zeger SL. Joint analysis of longitudinal data comprising repeated measures and time to events. Applied Statistics. 2001;50:375–387. [Google Scholar]


