Abstract
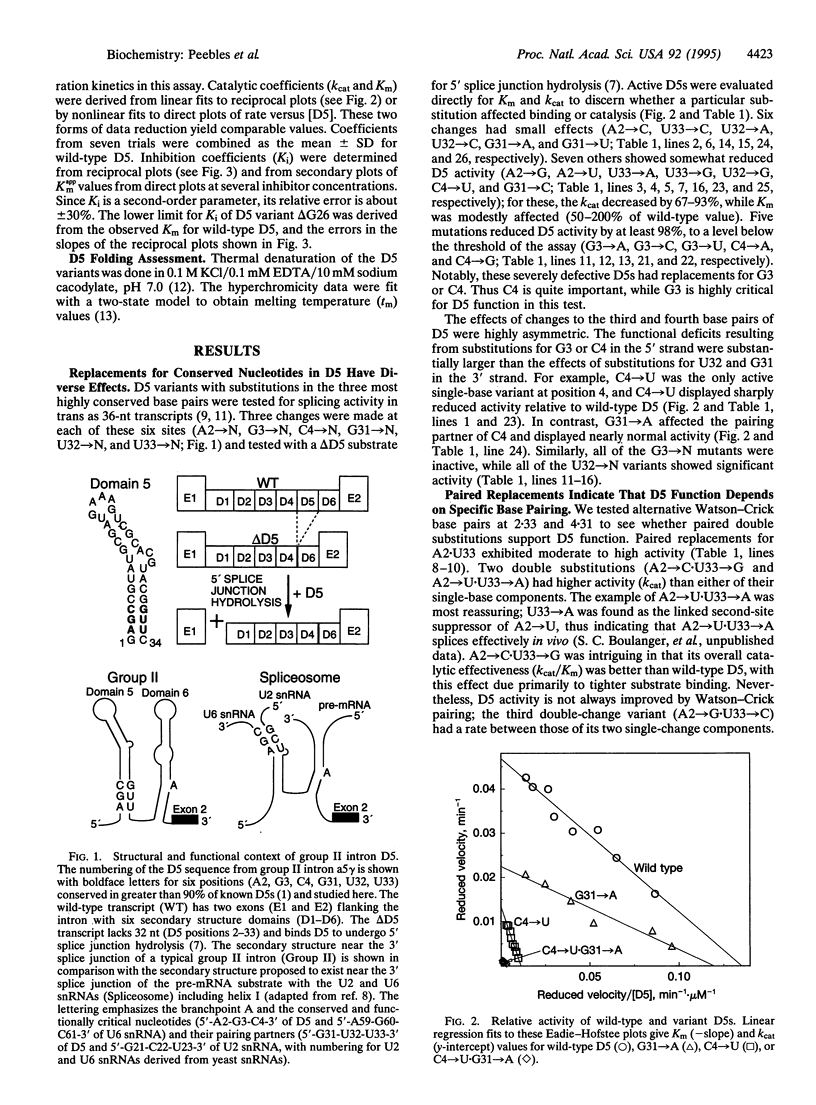
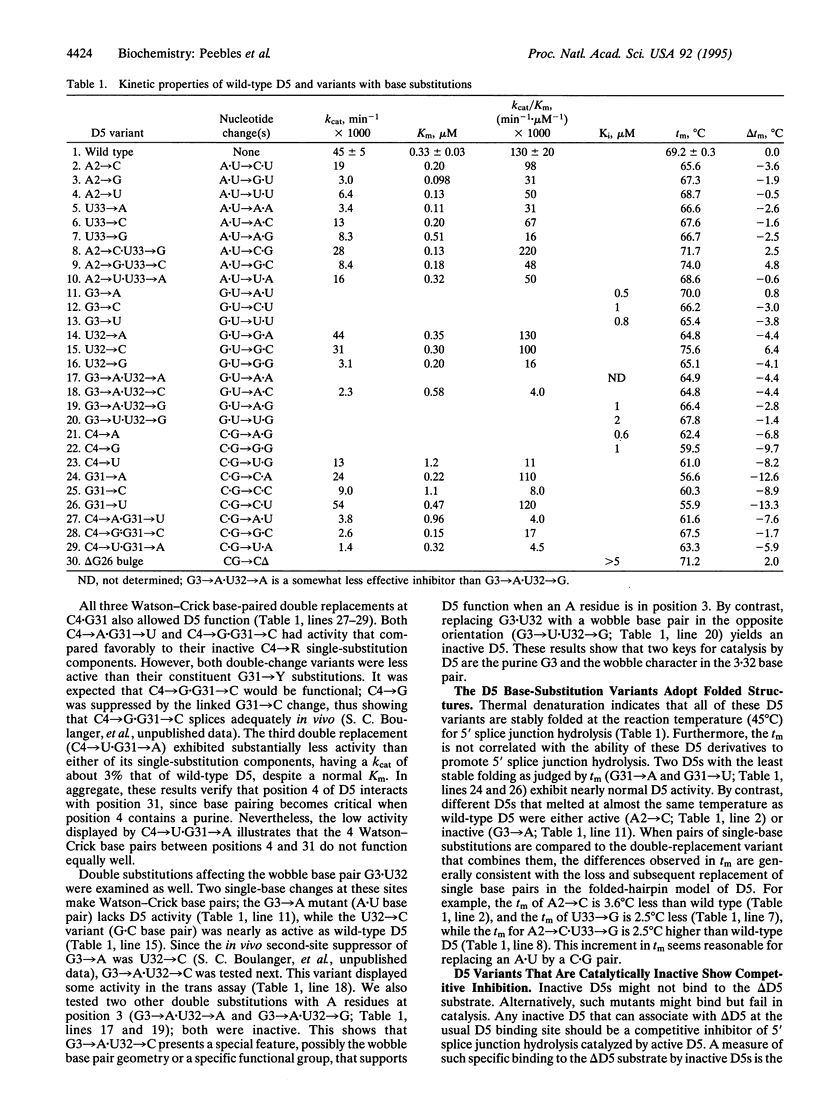
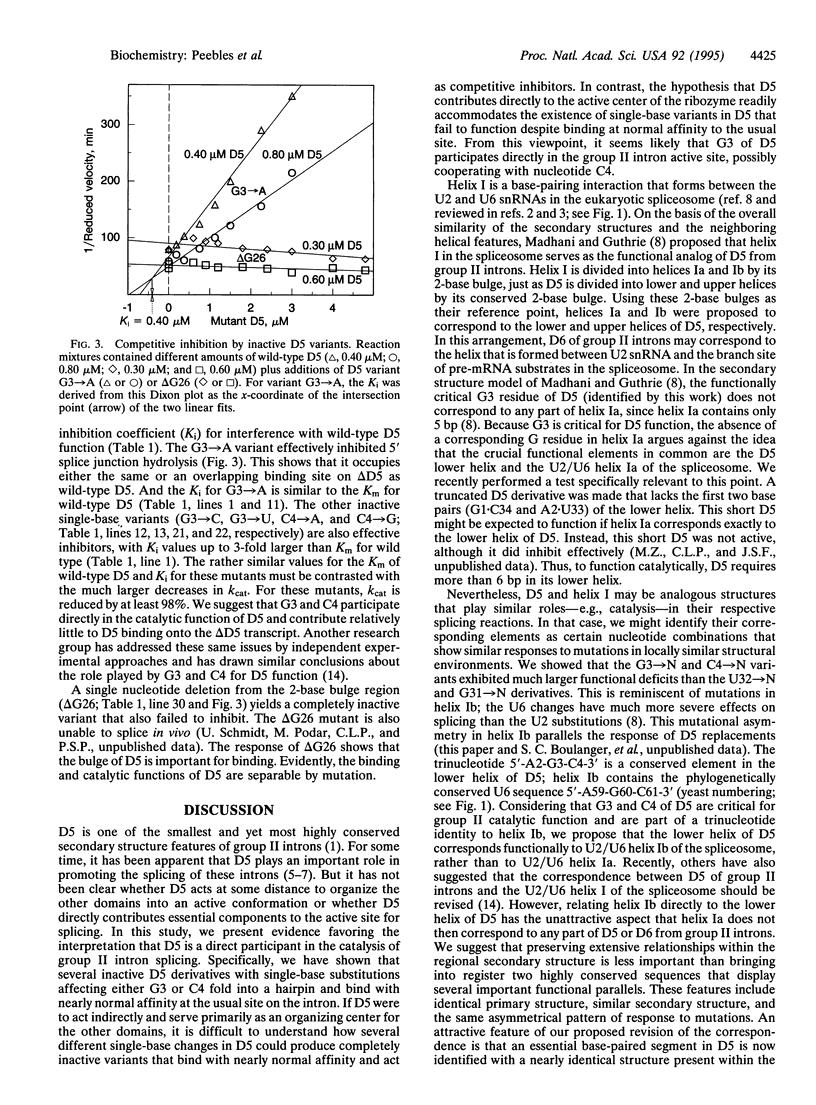
Domain 5 (D5) is a small hairpin structure within group II introns. A bimolecular assay system depends on binding by D5 to an intron substrate for self-splicing activity. In this study, mutations in D5 identify two among six nearly invariant nucleotides as being critical for 5' splice junction hydrolysis but unimportant for binding. A mutation at another site in D5 blocks binding. Thus, mutations can distinguish two D5 functions: substrate binding and catalysis. The secondary structure of D5 may resemble helix I formed by the U2 and U6 small nuclear RNAs in the eukaryotic spliceosome. Our results support a revision of the previously proposed correspondence between D5 and helix I on the basis of the critical trinucleotide 5'-AGC-3' present in both. We suggest that this trinucleotide plays a similar role in promoting the chemical reactions for both splicing systems.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chanfreau G., Jacquier A. Catalytic site components common to both splicing steps of a group II intron. Science. 1994 Nov 25;266(5189):1383–1387. doi: 10.1126/science.7973729. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj S. D., Boulanger S. C., Hebbar S. K., Peebles C. L., Franzen J. S., Perlman P. S. Domain 5 interacts with domain 6 and influences the second transesterification reaction of group II intron self-splicing. Nucleic Acids Res. 1993 Apr 25;21(8):1797–1804. doi: 10.1093/nar/21.8.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen J. S., Zhang M., Chay T. R., Peebles C. L. Thermal activation of a group II intron ribozyme reveals multiple conformational states. Biochemistry. 1994 Sep 20;33(37):11315–11326. doi: 10.1021/bi00203a029. [DOI] [PubMed] [Google Scholar]
- Franzen J. S., Zhang M., Peebles C. L. Kinetic analysis of the 5' splice junction hydrolysis of a group II intron promoted by domain 5. Nucleic Acids Res. 1993 Feb 11;21(3):627–634. doi: 10.1093/nar/21.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K. A., Dietrich R. C., Perlman P. S. Group II intron domain 5 facilitates a trans-splicing reaction. Mol Cell Biol. 1988 Jun;8(6):2361–2366. doi: 10.1128/mcb.8.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch J. L., Boulanger S. C., Dib-Hajj S. D., Hebbar S. K., Perlman P. S. Group II introns deleted for multiple substructures retain self-splicing activity. Mol Cell Biol. 1992 May;12(5):1950–1958. doi: 10.1128/mcb.12.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhani H. D., Guthrie C. A novel base-pairing interaction between U2 and U6 snRNAs suggests a mechanism for the catalytic activation of the spliceosome. Cell. 1992 Nov 27;71(5):803–817. doi: 10.1016/0092-8674(92)90556-r. [DOI] [PubMed] [Google Scholar]
- Madhani H. D., Guthrie C. Dynamic RNA-RNA interactions in the spliceosome. Annu Rev Genet. 1994;28:1–26. doi: 10.1146/annurev.ge.28.120194.000245. [DOI] [PubMed] [Google Scholar]
- Michel F., Umesono K., Ozeki H. Comparative and functional anatomy of group II catalytic introns--a review. Gene. 1989 Oct 15;82(1):5–30. doi: 10.1016/0378-1119(89)90026-7. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Uhlenbeck O. C. Synthesis of small RNAs using T7 RNA polymerase. Methods Enzymol. 1989;180:51–62. doi: 10.1016/0076-6879(89)80091-6. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Podar M., Boulanger S. C., Perlman P. S. The stereochemical course of group II intron self-splicing. Science. 1994 Dec 9;266(5191):1685–1688. doi: 10.1126/science.7527587. [DOI] [PubMed] [Google Scholar]
- Petersheim M., Turner D. H. Base-stacking and base-pairing contributions to helix stability: thermodynamics of double-helix formation with CCGG, CCGGp, CCGGAp, ACCGGp, CCGGUp, and ACCGGUp. Biochemistry. 1983 Jan 18;22(2):256–263. doi: 10.1021/bi00271a004. [DOI] [PubMed] [Google Scholar]
- Pyle A. M., Green J. B. Building a kinetic framework for group II intron ribozyme activity: quantitation of interdomain binding and reaction rate. Biochemistry. 1994 Mar 8;33(9):2716–2725. doi: 10.1021/bi00175a047. [DOI] [PubMed] [Google Scholar]



