Abstract
Aging is characterized by progressive, degenerative changes in many tissues. To elucidate the relationships among degenerative changes in Caenorhabditis elegans, we developed methods to measure age-related changes quantitatively and analyzed correlations among these changes by using a longitudinal study. The age-related declines of pharyngeal pumping and body movement were positively correlated with each other and lifespan. These findings suggest that the declines of pharyngeal pumping and body movement cause a decline in survival probability or that a shared regulatory system mediates the declines in pharyngeal pumping, body movement, and survival probability. Furthermore, measurements of these processes can be used to predict lifespan and detect premature aging. The declines of physiological processes were measured in daf-2, age-1, daf-16, eat-2, and clk-1 mutants that have altered lifespans. Each mutant strain displayed changes in one or more age-related declines, but the correlations among age-related changes were similar to WT. These measurements were used to generate a system of four stages that describes the aging process and is useful for the analysis of genetic and environmental effects on aging.
The identification and measurement of age-related changes, or markers of aging, is a major element of aging research. It is necessary because an understanding of the aging process requires a description of the specific changes that occur. Furthermore, many of the hypotheses about the cause of aging are based on the identification of an age-related change. Because age-related changes are widespread, it is particularly important to determine the relationships among these changes and identify age-related changes that affect lifespan. Three main experimental approaches can be used to investigate these relationships. Longitudinal studies that include serial measurements of the same individual can identify correlations among age-related changes that are measured at different times in the lifespan (1, 2). Cross-sectional studies that include one evaluation of each individual can identify correlations among age-related changes that are measured at the same time in the lifespan. Both longitudinal and cross-sectional data can be used to analyze correlations by comparing two or more populations of animals that have different aging properties as a result of genetic or environmental factors (3, 4). Longitudinal studies provide the most information about the relationships among changes that occur at different times in the lifespan, but longitudinal studies have not been performed frequently because they require substantial time and effort. Here we used longitudinal studies to analyze the relationships among age-related declines of physiological processes and lifespan of the nematode Caenorhabditis elegans.
C. elegans is an important model organism for aging research, because it has a short lifespan, and many mutations have been identified that extend the adult lifespan (reviewed by Kenyon in ref. 5). The most extensively characterized pathway that regulates C. elegans lifespan is an insulin/insulin-like growth factor 1 (IGF-1) signal transduction pathway (6). Mutations that reduce the activity of the daf-2 gene, which encodes the IGF receptor, or the age-1 gene, which encodes a phosphatidylinositol-3-OH (PI3) kinase, cause significant extensions of adult lifespan (7–12). These genes act upstream of and negatively regulate the daf-16 gene, which encodes a forkhead transcription factor; a daf-16 loss-of-function (lf) mutation suppresses the lifespan extensions caused by a daf-2 or age-1 mutation (9, 13–15). A second set of genes that affect C. elegans aging are the clock (clk) genes; mutations in clk genes cause extensions of adult lifespan and reduce the rate of many other physiological processes (16). The clk-1 gene encodes a homolog of the yeast CAT5/COQ7 and appears to be important for mitochondrial function (17). A third set of genes that affect C. elegans aging are the eat genes (18, 19). A mutation of eat-2 reduces pharyngeal pumping and food intake and causes a lifespan extension. eat mutations have been interpreted to act by causing caloric restriction, which extends the adult lifespan of many animals (20).
Several age-related changes have been identified and measured in C. elegans, including declines in physiological processes such as body movement (3, 21, 22), pharyngeal pumping (21), egg-laying (3, 21), and defecation frequency (21, 22); changes in appearance such as loss of tissue integrity (23, 24), altered yolk protein distribution (24), reduced sperm number (25), increased body size (21), and accumulation of fluorescent material (26); and changes in biochemical properties such as DNA integrity (27). Very little is known about how these changes are related to each other and to lifespan. Here, we describe methods for making quantitative measurements of age-related declines of self-fertile reproduction, body movement, and pharyngeal pumping. Longitudinal studies were used to determine the relationships among these age-related changes and between age-related changes and adult lifespan. The age-related declines in body movement and pharyngeal pumping were positively correlated with each other and with adult lifespan. These measurements were used to define stages of senescence.
Materials and Methods
General Methods and Strains. C. elegans strains were cultured at 20°C unless stated otherwise on 6-cm Petri dishes containing nematode growth media agar and a lawn of Escherichia coli strain OP50 (28). The WT N2 strain was obtained from the Caenorhabditis Genetics Center (St. Paul). The following well characterized mutations that affect lifespan were used: daf-2 (e1370 P1465S) is a partial loss-of-function mutation that affects the kinase domain of the DAF-2 receptor tyrosine kinase (12); age-1 (hx546) is a partial loss-of-function mutation that affects the AGE-1 PI3 kinase and has not been molecularly defined (10); clk-1 (e2519 E148K) is a partial loss-of-function mutation that affects the CLK-1 CAT5/COQ7 (17); eat-2 (ad465) is a strong loss-of-function or null mutation that would result in the production of only the first 45 amino acids of the EAT-2 non-alpha nicotinic acetylcholine receptor subunit (29, 30); and daf-16 (m26) is a partial loss-of-function mutation that disrupts mRNA splicing of the DAF-16 Forkhead transcription factor (14, 15).
Analyses of Aging Phenotypes. Animals were synchronized by using a dissecting microscope to identify fourth larval stage (L4) hermaphrodites based on the size of the animal and the appearance of the vulva as a dark half circle. Animals have this appearance for ≈6 h (data not shown). Studies of aging were begun on day zero by placing one L4 hermaphrodite on a Petri dish. Hermaphrodites were transferred to a fresh Petri dish about every 2 days during the reproductive period (approximately the first 7 days) to eliminate self-progeny and transferred as necessary thereafter. Thus, hermaphrodites were analyzed in isolation rather than groups and provided abundant food in the form of live E. coli. Each hermaphrodite was examined every 1 or 2 days by using a dissecting microscope for the following phenotypes. (i) Self-fertile reproduction was assessed by the presence of larvae on the Petri dish. If the last progeny were produced during a 2-day scoring interval, then we assigned the last day of self-fertile reproduction as the first day of that scoring interval. (ii) Body movement was assessed by observation for 10–30 sec. Petri dishes were tapped against the microscope stage to stimulate movement before scoring. Animals were classified as fast moving if they exhibited continuous and well coordinated sinusoidal movements and not fast moving if they displayed discontinuous or sluggish movements. Fast body movement corresponds to a locomotion rate of >1 mm per 10 sec when a ruler is used to measure the linear distance that the head of the animal moves. Our classification of fast body movement corresponds to type I of Hosono et al. (31) and class A of Herndon et al. (24). (iii) Pharyngeal pumping was assessed by observing the number of pharyngeal contractions during a 10-sec interval for longitudinal studies or a 60-sec interval for the data shown in Fig. 1. If pharyngeal pumping displayed an irregular rhythm, then we made multiple measurements and calculated an average value. Animals that displayed 0, 1–24, and ≥25 pharyngeal contractions per 10 sec were classified as not pumping, slow pumping, or fast pumping, respectively. (iv) An animal was classified as dead if it displayed no spontaneous movement and no responsive movement after prodding with a platinum wire. Lifespan (LS) was defined as the time from day zero to the last day of survival. Animals that displayed traumatic or accidental death resulting from internally hatched progeny, an extruded gonad, or desiccation on the edge of the Petri dish were excluded from the data.
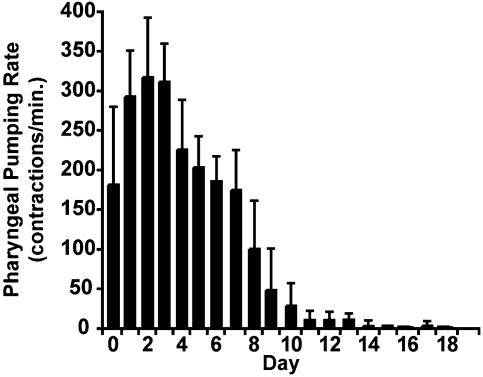
Fig. 1.
Age-related change of pharyngeal pumping rate. Day zero animals were WT L4 hermaphrodites. Vertical bars represent the average number of pharyngeal contractions per minute and the standard deviation for live animals on each day (n = 11 animals on day zero and fewer animals on later days). The standard deviation of replicate measurements of day 2 individuals is ≈9% of the average value, indicating that the most of the variability is due to differences between individuals.
Statistical Analyses. The statistical comparison of two different strain distributions was done by the nonparametric log rank test (32). The correlation analysis was based on the Spearman rank correlation (33). The test of significance for these correlations was based on the corresponding asymptotic standard normal tests. The analysis of the prediction of age at death by variables self-fertile reproductive span, fast body movement span, fast pharyngeal pumping span, and pharyngeal pumping span was done by the logistic regression on ordinal response variable lifespan (34). All these estimates and tests were carried out by using proc lifetest/sas, proc corr/sas, and proc logistic/sas (sas/stat user's guide, Version 6, SAS Institute, Cary, NC).
Results
Quantitative Measurements of Age-Related Changes of Physiological Functions. To analyze relationships among age-related changes, we chose to measure three physiological processes that can be observed with a dissecting microscope with minimal disturbance of the animal: pharyngeal pumping, body movement, and self-progeny production. The pharynx is a neuromuscular organ that undergoes rhythmic contractions (5). The contractions can be counted, and the rate displays an age-related decline (21, 31). WT L4 hermaphrodites, defined as day 0 for our aging studies, displayed 180 ± 100 pharyngeal pumps per minute, and the rate increased to 320 ± 80 by day 2 (Fig. 1). The pharyngeal pumping rate declined gradually from day 2 until day 7 to 180 ± 50 per minute and rapidly from day 7 until day 10 to 30 ± 30 per minute; pharyngeal pumping ceased entirely over the next several days.
To develop quantitative measurements that are well suited for correlation studies, we used this analysis to define three states of pharyngeal pumping: fast pharyngeal pumping is >149 per minute, slow pharyngeal pumping is 6–149 per minute, and no significant pharyngeal pumping is <6 per minute. These rates were calculated from observations of individual hermaphrodites for 10-sec periods (see Materials and Methods). We refer to the time from day zero to the last day of fast pharyngeal pumping as the fast pharyngeal pumping span and the time from day zero to the last day of slow pharyngeal pumping as the pharyngeal pumping span. For WT animals, the mean fast pharyngeal pumping span was 8.1 (±2.1) days and the mean pharyngeal pumping span was 11.8 (±3.0) days (Table 1). This approach yields two values that measure the middle and the end of the decline of pharyngeal pumping.
Table 1. Age-related declines of physiological processes in WT and mutant worms.
| Genotype | Self-fertile reproductive span† | Fast body movement span† | Fast pharyngeal pumping span† | Pharyngeal pumping span† | Lifespan† | N‡ |
|---|---|---|---|---|---|---|
| WT 20°C | 5.8 ± 2.0 | 8.2 ± 1.7 | 8.1 ± 2.1 | 11.8 ± 3.0 | 15.2 ± 3.6 | 180 |
| WT 15°C | 8.9 ± 2.2** | 14.0 ± 4.5** | 12.4 ± 3.6** | 19.3 ± 5.9** | 25.0 ± 6.6** | 87 |
| daf-2 (e1370) | 6.3 ± 1.5 | 15.6 ± 3.7** | 10.1 ± 6.9** | 21.0 ± 7.8** | 34.6 ± 10.2** | 114 |
| age-1 (h×546) | 5.8 ± 1.5 | 10.8 ± 2.1** | 8.5 ± 1.9 | 15.2 ± 4.7** | 24.8 ± 7.1** | 134 |
| daf-16 (m26) | 6.0 ± 1.6 | 7.5 ± 1.4** | 7.1 ± 1.3** | 9.1 ± 2.2** | 12.8 ± 2.5** | 84 |
| eat-2 (ad465) | 10.0 ± 3.3** | 10.5 ± 3.1** | N.A.§ | 13.1 ± 3.9** | 18.0 ± 4.5** | 74 |
| clk-1 (e2519) | 6.9 ± 2.1** | 8.1 ± 2.5 | 7.4 ± 3.7 | 12.6 ± 5.6** | 16.7 ± 7.7** | 74 |
Values in days are the mean of the population and the standard deviation. Each mutant was compared to WT 20°C by the nonparametric log rank test that compares strain distributions. *, P < 0.05; **, P < 0.005.
The number of hermaphrodites analyzed.
This value cannot be determined for eat-2 mutants because they typically do not display fast pharyngeal pumping.
The body movement of a young adult hermaphrodite is relatively continuous and exhibits a well coordinated, sinusoidal pattern. As an animal ages, its body movement becomes progressively less continuous and coordinated and ultimately stops altogether. This process has been measured quantitatively by counting backward waves per minute on each day of adulthood (21). A single value for each individual or population that can be used for correlation studies has been derived from such data by calculating the rate of decline of body movement and/or the x-intercept of this line (3, 22). The decline of body movement has also been measured by classifying worms into three groups based on qualitative descriptions of body movement (24, 31). We used a qualitative description to classify worms as fast moving if they displayed continuous, well coordinated sinusoidal movement and not fast moving if they displayed discontinuous or sluggish movement (see Materials and Methods). To derive a single value that is well suited for correlation studies, we measured the time of the transition from fast body movement to non-fast body movement. We refer to the time from day zero to the last day of fast body movement as the fast body movement span. WT hermaphrodites had a mean fast body movement span of 8.2 (±1.7) days (Table 1).
Self-fertile hermaphrodites display an age-related decline in progeny production that has been measured by determining the number of progeny produced on each day of adulthood (3, 21, 26). We classified animals as producing live progeny or failing to do so and measured the time of this transition. We refer to the time from day zero to the last day of self-progeny production as the self-fertile reproductive span. WT hermaphrodites had a mean self-fertile reproductive span of 5.8 (±2.0) days (Table 1).
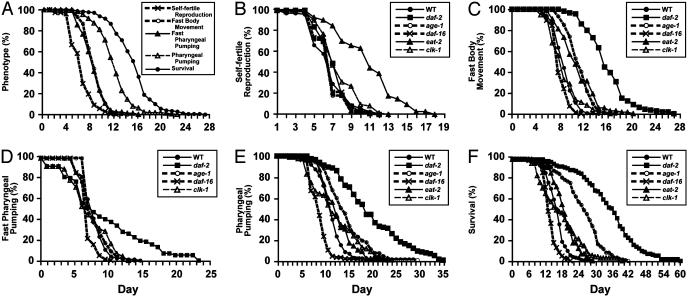
Fig. 2A shows the age-related declines in self-fertile reproduction, fast body movement, fast pharyngeal pumping, pharyngeal pumping, and survival as a function of time. These five measures represent the progressive, age-related deterioration that occurs in adult animals.
Fig. 2.
Mutations of daf-2, age-1, daf-16, eat-2, and clk-1 affect specific aging phenotypes. (A) WT L4 hermaphrodites were placed on individual Petri dishes and monitored for self-progeny production, fast body movement, fast pharyngeal pumping, any pharyngeal pumping, and survival. The percent of animals in the population (n = 180) that displayed the phenotype is graphed versus time in days. (B–E) Mutant animals were analyzed similarly to WT animals. Table 1 shows the alleles, number of animals analyzed, and calculated mean values. The plots show self-fertile reproduction (B), fast body movement (C), fast pharyngeal pumping (D), pharyngeal pumping (E), and survival (F) versus age in days.
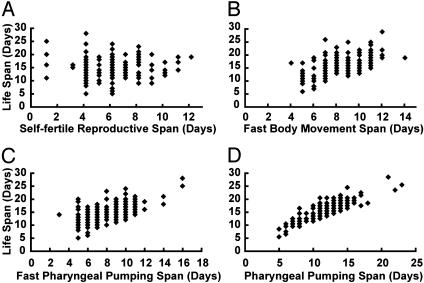
Correlations Among Age-Related Changes in WT Animals. To determine the relationships among age-related changes in physiological processes and lifespan, we performed a longitudinal study by separately maintaining 180 WT hermaphrodites and serially measuring self-progeny production, body movement, pharyngeal pumping, and survival for each worm. One method to analyze these data is to plot these traits versus lifespan for each individual. Self-fertile reproductive span was not correlated with lifespan (Fig. 3A), whereas fast body movement span, fast pharyngeal pumping span, and pharyngeal pumping span were positively correlated with lifespan (Fig. 3 B–D). A second method of data analysis was the Spearman rank correlation (33). This method generates a list of the same individuals ranked from highest to lowest for each trait. A comparison of two lists determines an r value between +1 and –1. An r value of +1 indicates a perfect positive correlation and means that the same individual scored first for traits A and B, the same individual scored second for traits A and B, etc. An r value of –1 indicates a perfect negative correlation and means that the individual that scored first for trait A scored last for trait B, etc. An r value of 0 indicates no correlation and means that the individual that scored first for trait A scored approximately in the middle for trait B, etc. Self-fertile reproductive span was not correlated with any other phenotypes (Table 2, first through fourth rows). By contrast, lifespan displayed a positive correlation with fast body movement span (r = 0.45, P < 0.0001), fast pharyngeal pumping span (r = 0.49, P < 0.0001), and pharyngeal pumping span (r = 0.83, P < 0.0001). Fast body movement span was also positively correlated with fast pharyngeal pumping span (r = 0.69, P < 0.0001) and pharyngeal pumping span (r = 0.48, P < 0.0001). Fast pharyngeal pumping span was positively correlated with pharyngeal pumping span (r = 0.61, P < 0.0001) (Table 2).
Fig. 3.
Longitudinal analysis of correlations between the declines of physiological processes and lifespan. Each point represents data for one WT hermaphrodite or more than one hermaphrodite in cases where multiple animals had identical values (n = 180). The lifespan and self-fertile reproductive (A), fast body movement (B), fast pharyngeal pumping (C), and pharyngeal pumping (D) spans are shown.
Table 2. Spearman rank correlation values.
| Spans† | WT‡ | daf-2‡ | age-1‡ | daf-16‡ | eat-2‡ | clk-1‡ | Interstrain‡ |
|---|---|---|---|---|---|---|---|
| RS/MS | 0.06 | -0.01 | -0.10 | -0.002 | 0.15 | -0.11 | -0.03 |
| RS/FPS | -0.05 | 0.01 | 0.04 | -0.15 | N.A.§ | -0.08 | -0.15 |
| RS/PS | -0.05 | 0.12 | -0.18* | -0.08 | 0.06 | -0.15 | 0.17 |
| RS/LS | 0.03 | 0.03 | -0.17 | -0.07 | 0.11 | -0.15 | 0.17 |
| MS/FPS | 0.69** | 0.21* | 0.49** | 0.50** | N.A. | 0.64** | 1.00** |
| MS/PS | 0.48** | 0.23* | 0.58** | 0.43** | 0.68** | 0.70** | 0.94* |
| MS/LS | 0.45** | 0.35** | 0.48** | 0.34** | 0.58** | 0.67** | 0.94* |
| FPS/PS | 0.61** | 0.33** | 0.40** | 0.46** | N.A. | 0.72** | 0.90* |
| FPS/LS | 0.49** | 0.14 | 0.33** | 0.31** | N.A. | 0.74** | 0.90* |
| PS/LS | 0.83** | 0.50** | 0.58** | 0.68** | 0.76** | 0.95** | 1.00** |
Each line shows the correlation between two spans. RS, self-fertile reproductive span; MS, fast body movement span; FPS, fast pharyngeal pumping span; PS, pharyngeal pumping span; LS, lifespan.
Mutant alleles and the number of animals analyzed are shown in Table 1. Interstrain values were derived by using the mean of the six populations. Values were determined by using Spearman rank correlation. *, P < 0.05; **, P < 0.005.
FPS was not determined for eat-2 mutants because they typically do not display fast pharyngeal pumping.
The positive correlations between lifespan and fast body movement span, fast pharyngeal pumping span, and pharyngeal pumping span indicate that these measurements can be used to predict lifespan. For example, if worm A displays pharyngeal pumping for 1 day longer than worm B, then worm A will probably live longer than worm B. The prediction of lifespan is expressed as the probability or odds that death will occur at or after a specified future time, and a higher value predicts a longer lifespan. Two individuals are compared by dividing the odds that death will occur at or after a specified time to yield an estimated odd ratio. Our data show that when the fast body movement span is increased by 1 day, the estimated odd ratio of death at or after a specified time is 1.75 (95% confidence interval from 1.48 to 2.05, P < 0.0001). When fast pharyngeal pumping span is increased by 1 day, the estimated odd ratio of death at or after a specified time is 1.71 (95% confidence interval from 1.49 to 1.96, P < 0.0001). When pharyngeal pumping span is increased by 1 day, the estimated odd ratio of death at or after a specified time is 2.80 (95% confidence interval from 2.39 to 3.28, P < 0.0001).
Analysis of Declining Physiological Functions in daf-2, age-1, daf-16, eat-2, and clk-1 Mutants. To determine how mutations that affect lifespan affect these age-related changes, we performed a longitudinal analysis of daf-2 (e1370), age-1 (hx546), daf-16 (m26), eat-2 (ad465), and clk-1 (e2519) (see Materials and Methods for a description of these alleles). daf-2 (e1370) and age-1 (hx546) caused a similar pattern of changes. Both mutations significantly extended the fast body movement span, pharyngeal pumping span, and lifespan and did not significantly affect the self-fertile reproductive span (Table 1, third and fourth rows, and Fig. 2). daf-2 (e1370) significantly extended the fast pharyngeal pumping span, whereas age-1(hx546) caused a small extension that was not statistically significant. daf-16 (m26) significantly shortened the fast body movement span, fast pharyngeal pumping span, pharyngeal pumping span, and lifespan and did not significantly affect the self-fertile reproductive span (Table 1 and Fig. 2). eat-2 (ad465) significantly extended the self-fertile reproductive span, fast body movement span, pharyngeal pumping span, and lifespan (Table 1 and Fig. 2). The fast pharyngeal pumping span cannot be scored in eat-2 mutants because they usually do not display fast pharyngeal pumping even as young adults. clk-1 (e2519) caused a small but significant extension of the self-fertile reproductive span, pharyngeal pumping span, and lifespan (Table 1 and Fig. 2).
To determine the relationships among these age-related changes in the mutant strains, we analyzed the longitudinal data by using the Spearman rank correlation method (Table 2). The correlations among aging phenotypes in the mutant strains were very similar to those of WT animals. For WT hermaphrodites, the four correlation values involving self-fertile reproductive span were not significantly different from zero. For the mutants, 18 of 19 correlation values involving self-fertile reproductive span were not significantly different from zero (Table 2, first through fourth rows). For WT hermaphrodites, the six correlations among fast body movement span, fast pharyngeal pumping span, pharyngeal pumping span, and lifespan were positive. For the mutants, 26 of 27 of these correlations were positive (Table 2, fifth through tenth rows).
The mean values for WT and the five mutant strains (total n = 6) were analyzed by using the Spearman rank correlation method. This analysis identified the same pattern of correlations as the longitudinal analysis of WT (Table 2, column 8). Thus, the same pattern of correlations was observed within six genetically homogeneous populations analyzed by longitudinal studies and in a comparison among these six different strains.
Stages of C. elegans Aging. The measurements of age-related declines of physiological processes were used to define stages of C. elegans aging. Stage I is the period of progeny production and is defined as the time from L4 to the end of the self-fertile reproductive span. Stage II is a post reproductive period characterized by vigorous motor activity and is defined as the time from the end of stage I to the end of the fast body movement span. Stage III is a period of reduced motor activity and is defined as the time from the end of stage II to the end of the pharyngeal pumping span. Stage IV is a period of minimal motor activity and is defined as the time from the end of stage III to the end of the lifespan.
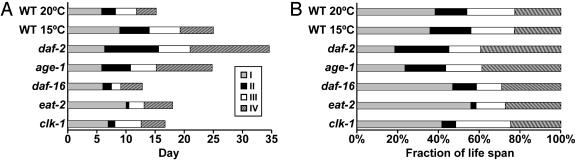
Fig. 4A displays the duration by using an absolute time scale; Fig. 4B uses a relative time scale to display the proportion of each stage in the adult lifespan. To investigate the ability of this staging system to distinguish lifespan extensions caused by different mechanisms, we analyzed the effects of temperature and five mutations. Lower temperatures extend C. elegans lifespan, whereas higher temperatures shorten lifespan (26). Temperature has similar effects on many poikilotherm animals, and one model is that these effects are caused by a global change in the rates of chemical reactions (26). Fig. 4A shows that WT animals raised at 15°C displayed a significant lifespan extension compared to animals raised at the normal culture temperature of 20°C. Fig. 4B shows that the proportion of the lifespan represented by each stage is almost the same for WT animals raised at 15°C and 20°C. By contrast, the five mutant strains did not display proportional changes of each stage. daf-2 and age-1 mutants displayed strikingly similar patterns; compared to WT animals, stage I was reduced and stage IV was expanded (Fig. 4B). daf-16 mutants displayed a reduction of stages II and III and an increase of stages I and IV compared to WT. eat-2 mutants displayed a very long stage I and a significantly reduced stage II compared to WT. clk-1 mutants displayed a reduction of stage II and small increases of stages I and III compared to WT.
Fig. 4.
Stages of C. elegans aging. (A) Horizontal bars represent the average time in days spent in stages I–IV (described in text). The mutant alleles, number of animals analyzed, and mean values for age-related traits are shown in Table 1. Animals were analyzed at 20°C except for WT 15°C. (B) The fraction of the mean lifespan occupied by each stage was calculated by setting the mean lifespan equal to 100%.
Discussion
Relationships Among Age-Related Changes. Aging is characterized by concurrent declines in the function of most or all tissues. It is important to determine the extent to which these declines are coordinated and the mechanisms that regulate this coordination. Studies of humans indicate that an individual can display some traits that are more aged and some traits that are less aged than the norm for individuals of that chronological age, raising the possibility that the rate of aging of different tissues is not highly coordinated (35). We used longitudinal studies that involved serial measurements of five traits in each individual to investigate the relationships among age-related changes that occur at different times. In WT animals, the declines of body movement, pharyngeal pumping, and lifespan were all positively correlated, whereas the self-fertile reproductive span did not correlate with these traits. Longitudinal analyses with daf-2, age-1, daf-16, eat-2, and clk-1 mutant strains revealed essentially the same correlations. The correlations among the five mutant strains and WT also yielded the same pattern, although this analysis involved the smallest data set (n = 6) and was complicated by the fact that each strain was genetically different. Thus, these correlations are robust and reproducible. To analyze physiological processes that decline continually, such as pharyngeal pumping, we chose to measure specific transitions. The result that fast pharyngeal pumping span and pharyngeal pumping span were positively correlated and had similar correlations with lifespan validates this approach and indicates that the choice of these specific transitions does not significantly affect the outcome of the study.
Several previous studies have analyzed relationships among age-related changes of C. elegans. Bolanowski et al. (22) observed no correlation between the lifespan and the rate of decline in movement wave frequency or the decline of defecation frequency when using a longitudinal analysis of WT hermaphrodites. Hosono et al. (31) showed that WT hermaphrodites that display rhythmic sinusoidal body movement have a longer lifespan and a higher pharyngeal pumping rate than similarly aged worms that lack this body movement. Johnson (3) analyzed correlations among long-lived recombinant inbred C. elegans lines. There was no correlation between the length of the reproductive period and lifespan and a positive correlation between the calculated time of movement cessation and lifespan. The studies described here differ from previous analyses in the following important respects. (i) Different measurements of age-related changes of pharyngeal pumping and body movement were used. (ii) The Spearman rank correlation method was used for data analysis. (iii) The five mutant strains were not included in previous studies. (iv) Six of the 10 correlations analyzed in Table 2 have not been previously characterized. Whereas previous studies have yielded various conclusions about the relationship between body movement and lifespan, our findings strongly support the positive correlation between the decline of body movement and lifespan.
A correlation between two age-related changes indicates that the declines are regulated by a common mechanism and/or the two processes are causally connected. The declines of pharyngeal pumping and body movement are positively correlated. Thus, a common regulatory system may mediate the decline of body movement and pharyngeal pumping; alternatively, the decline in pharyngeal pumping may cause a decline in body movement and/or the decline in body movement may cause a decline in pharyngeal pumping. Furthermore, the decline of body movement and pharyngeal pumping were positively correlated with lifespan. Thus, a common regulatory system may mediate all three declining processes and/or the declines of pharyngeal pumping and body movement may cause a reduced life expectancy. If a common mechanism coordinates the aging rate of different tissues, what is the nature of this mechanism? One possibility is an endocrine mechanism: a hormone that diffuses throughout the body and controls the aging rate of cells. The extended longevity caused by reduced function of the DAF-2 insulin-like growth factor receptor (9, 12) suggests that an insulin-like protein functions as a hormone that accelerates aging of C. elegans. A second possibility is that aging is coordinated by a developmental program that brings each tissue to peak function at approximately the same time. Tissues then degenerate because they lack a program to maintain function, and coordination results from a simultaneous onset of the degenerative processes.
The self-fertile reproductive span was not correlated with the declines of body movement, pharyngeal pumping, and survival probability, suggesting that the self-fertile reproductive span is regulated independently of these traits and the cessation of self-fertile reproduction does not significantly affect the subsequent declines in body movement, pharyngeal pumping, and survival probability. However, these findings do not exclude the possibility that there are important relationships between reproductive and somatic aging. Self-fertile hermaphrodites produce ≈250 sperm (25), and the self-fertile reproductive span is likely to be largely determined by the rate of sperm utilization. It is possible that a different measurement of reproductive aging, such as the mated reproductive span, would display a different relationship with lifespan. Our analysis of the eat-2 and clk-1 mutations is intriguing in this regard. Although these mutations do not increase the number of self-fertile progeny, both mutations extended the self-fertile reproductive span and the lifespan, suggesting that there is a connection between the regulation of these processes.
A Staging System for C. elegans Aging. The staging system was used to evaluate temperature and mutations that affect lifespan. Worms raised at 15°C had a significantly longer lifespan than worms raised at 20°C, but the proportion of the lifespan represented by each stage was similar. By contrast, each of the five mutations changed the proportions of the four stages compared to WT (Fig. 4B). Thus, none of these mutations is likely to affect lifespan by the same mechanism as temperature. The daf-2 and age-1 genes function together in a well defined signaling pathway, and daf-2 and age-1 mutations delay a variety of age-related changes (23, 24, 36). The daf-2 (e1370) and age-1 (hx546) mutations extended the lifespan to different extents but caused similar changes to the proportion of the stages. These findings suggest that the staging system can be used to group mutations that act by a similar mechanism.
One important use of aging markers is to distinguish lifespan shortening treatments that accelerate aging from those that cause novel pathologies. The daf-16 gene was implicated in lifespan control because it suppresses the Dauer constitutive and lifespan extension phenotypes caused by daf-2 and age-1 mutations (9, 13). daf-16 (lf) mutations cause a shorter lifespan, and Garigan et al. (23) reported that daf-16 (lf) mutations accelerate the appearance of tissue degeneration. We found that the daf-16 (m26) allele caused a small but significant reduction of fast body movement span, fast pharyngeal pumping span, pharyngeal pumping span, and lifespan. These findings are consistent with the interpretation that daf-16 mutants undergo accelerated aging and suggest that the measurements of age-related changes described here will be useful for evaluating other lifespan-shortening treatments or mutations.
Acknowledgments
We thank Diane Redmond for assistance with graphics. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by the National Center for Research Resources of the National Institutes of Health. K.K. and C.H. were supported by a Scholar Award from the Leukemia and Lymphoma Society and a Predoctoral Fellowship from the Howard Hughes Medical Institute, respectively.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: L4, fourth larval stage.
References
- 1.Shock, N. W. (1985) in Handbook of the Biology of Aging, eds. Finch, C. E. & Schneider, E. L. (Van Nostrand Reinhold, New York), pp. 721–743.
- 2.Miller, R. A. (2001) J. Gerontol. A Biol. Sci. Med. Sci. 56, B180–B186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson, T. E. (1987) Proc. Natl. Acad. Sci. USA 84, 3777–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arking, R. & Wells, R. A. (1990) Dev. Genet. 11, 141–148. [DOI] [PubMed] [Google Scholar]
- 5.Riddle, D. L. (1997) C. elegans II (Cold Spring Harbor Laboratory Press, Plainview, NY). [PubMed]
- 6.Guarente, L. & Kenyon, C. (2000) Nature 408, 255–262. [DOI] [PubMed] [Google Scholar]
- 7.Klass, M. R. (1983) Mech. Ageing Dev. 22, 279–286. [DOI] [PubMed] [Google Scholar]
- 8.Friedman, D. B. & Johnson, T. E. (1988) Genetics 118, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenyon, C., Chang, J., Gensch, E., Rudner, A. & Tabtiang, R. (1993) Nature 366, 461–464. [DOI] [PubMed] [Google Scholar]
- 10.Morris, J. Z., Tissenbaum, H. A. & Ruvkun, G. (1996) Nature 382, 536–539. [DOI] [PubMed] [Google Scholar]
- 11.Malone, E. A., Inoue, T. & Thomas, J. H. (1996) Genetics 143, 1193–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura, K. D., Tissenbaum, H. A., Liu, Y. & Ruvkun, G. (1997) Science 277, 942–946. [DOI] [PubMed] [Google Scholar]
- 13.Dorman, J. B., Albinder, B., Shroyer, T. & Kenyon, C. (1995) Genetics 141, 1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogg, S., Paradis, S., Gottlieb, S., Patterson, G. I., Lee, L., Tissenbaum, H. A. & Ruvkun, G. (1997) Nature 389, 994–999. [DOI] [PubMed] [Google Scholar]
- 15.Lin, K., Dorman, J. B., Rodan, A. & Kenyon, C. (1997) Science 278, 1319–1322. [DOI] [PubMed] [Google Scholar]
- 16.Hekimi, S., Lakowski, B., Barnes, T. M. & Ewbank, J. J. (1998) Trends Genet. 14, 14–20. [DOI] [PubMed] [Google Scholar]
- 17.Ewbank, J. J., Barnes, T. M., Lakowski, B., Lussier, M., Bussey, H. & Hekimi, S. (1997) Science 275, 980–983. [DOI] [PubMed] [Google Scholar]
- 18.Avery, L. (1993) Genetics 133, 897–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakowski, B. & Hekimi, S. (1998) Proc. Natl. Acad. Sci. USA 95, 13091–13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koubova, J. & Guarente, L. (2003) Genes Dev. 17, 313–321. [DOI] [PubMed] [Google Scholar]
- 21.Croll, N. A., Smith, J. M. & Zuckerman, B. M. (1977) Exp. Aging Res. 3, 175–189. [DOI] [PubMed] [Google Scholar]
- 22.Bolanowski, M. A., Russell, R. L. & Jacobson, L. A. (1981) Mech. Ageing Dev. 15, 279–295. [DOI] [PubMed] [Google Scholar]
- 23.Garigan, D., Hsu, A. L., Fraser, A. G., Kamath, R. S., Ahringer, J. & Kenyon, C. (2002) Genetics 161, 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herndon, L. A., Schmeissner, P. J., Dudaronek, J. M., Brown, P. A., Listner, K. M., Sakano, Y., Paupard, M. C., Hall, D. H. & Driscoll, M. (2002) Nature 419, 808–814. [DOI] [PubMed] [Google Scholar]
- 25.Ward, S. & Carrel, J. S. (1979) Dev. Biol. 73, 304–321. [DOI] [PubMed] [Google Scholar]
- 26.Klass, M. R. (1977) Mech. Ageing Dev. 6, 413–429. [DOI] [PubMed] [Google Scholar]
- 27.Klass, M., Nguyen, P. N. & Dechavigny, A. (1983) Mech. Ageing Dev. 22, 253–263. [DOI] [PubMed] [Google Scholar]
- 28.Brenner, S. (1974) Genetics 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raizen, D. M., Lee, R. Y. & Avery, L. (1995) Genetics 141, 1365–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKay, J. P., Raizen, D. M., Gottschalk, A., Schafer, W. R. & Avery, L. (2004) Genetics, in press. [DOI] [PMC free article] [PubMed]
- 31.Hosono, R., Sato, Y., Aizawa, S. I. & Mitsui, Y. (1980) Exp. Gerontol. 15, 285–289. [DOI] [PubMed] [Google Scholar]
- 32.Lawless, J. F. (1982) Statistical Models and Methods for Lifetime Data (Wiley, New York).
- 33.Kendall, M. & Gibbons, J. D. (1990) Rank Correlation Methods (Edward Arnold, London).
- 34.Agresti, A. (1984) Analysis of Ordinal Categorical Data (Wiley, New York).
- 35.Borkan, G. A. & Norris, A. H. (1980) J. Gerontol. 35, 177–184. [DOI] [PubMed] [Google Scholar]
- 36.Duhon, S. A. & Johnson, T. E. (1995) J. Gerontol. A Biol. Sci. Med. Sci. 50, B254–B261. [DOI] [PubMed] [Google Scholar]