Abstract
This study was conducted to evaluate the effects of Platycarya strobilacea S. et Z. (PSE) extract on mouse hair growth and to determine the mechanism of action of PSE. PSE was purchased and its antioxidant activities, such as electron donating ability, total polyphenol content, and flavonoid content were tested. Toxicity during topical treatment was determined by the CCK-8 assay, a cell viability test. Fifteen 4-week-old male C57BL/6 mice were assigned to receive one of three treatments: dimethyl sulfoxide (negative control), minoxidil (positive control) or PSE. Test materials were topically applied to the shaved dorsal skin of each mouse daily for 3 weeks. After 21 days, we observed skin tissue hair follicle morphology and length, mast cell number, and stem cell factor (SCF) expression using hematoxylin and eosin (H&E), toluidine blue, and immunohistochemical staining, respectively. Furthermore, the expression of cytokines involved in hair growth [i.e., insulin-like growth factor (IGF)-1, keratinocyte growth factor (KGF), and transforming growth factor (TGF)-β1] was determined by PCR. PSE was found to have very high antioxidant activity. The cell viability rate of PSE-treated mice was markedly higher than that of mice in the control group. We also observed an increase in hair follicle length, strong SCF staining, and a decrease in mast cell number in the PSE group. In addition, PSE-treated mice had higher IGF-1 and KGF expression and lower TGF-β1 expression than mice in the minoxidil-treated group. These results suggest that topical application of PSE promotes hair growth by intensifying SCF, suppressing mast cell production, and increasing hair growth-promoting cytokine expression.
Keywords: Platycarya strobilacea S. et Z., hair growth, hair growth factors, antioxidant activity, C57BL/6 mouse
INTRODUCTION
Although global economic growth has enriched the overall quality of life since the 1980s, individuals are still exposed to external stimulation and numerous diseases. Alopecia is defined as a state in which hair does not exist in typical areas of the body, with a general loss of thick and dark hair. The primary cause of alopecia is genetic, but many other factors may participate in hair loss, including social, psychological, and mental stress, local infection, and endocrine disorders (1–4). When a human hair follicle is exposed to external stimulation, immunocytes and neutrophils are activated to cause an inflammatory response. This accelerates the follicle’s transition to the catagen and telogen phases, causing destruction of the hair follicle system and inducing hair follicle cell apoptosis and hair loss (5).
Alopecia areata occurs in 2% of the population. Hair loss may affect up to 70% of men and 50% of women at some point in their lifetime. The frequency and severity of hair loss increases with age (6). Currently, two hair-growth stimulating agents are approved by the United States Food and Drug Administration: minoxidil, an ointment; and finasteride, an oral medication (7). In its early stages, minoxidil was used as an antihypertensive, but because patients who took this drug developed hirsutism, it began to be used as treatment for alopecia instead (8). Minoxidil is known to induce dilation of scalp blood vessels, increase blood flow, and activate hair mother cells, thus decelerating the progress of alopecia and accelerating hair growth (9). Finasteride was the first oral medicine used for androchronogenetic alopecia, and is known to prevent alopecia and promote hair growth by suppressing the formation of type II 5α-reductase (10).
The effects of minoxidil on hair growth are observed in >50% of patients; however, there is a very high rate of alopecia recurrence when treatment is discontinued (10,11). Reported side effects of minoxidil and finasteride use include weight gain, edema, cardiac impulse increase, angina, dermatitis, pruritus, and male sexual dysfunction (12,13). Both minoxidil and finasteride cause more serious side effects (e.g., hormone imbalance and fetal deformities) in fertile women (7,14). For this reason, many people are interested in natural materials that can accelerate hair growth and minimize adverse effects.
Platycarya strobilacea S. et Z. (PSE) is a small deciduous broadleaf tree belonging to the wild walnut family. PSE trees are distributed throughout Korea. Traditionally, PSE fruits and roots have been used medicinally (15). According to previous studies on PSE, 5-hydroxy-2-methoxy-1,4-naphthoquinone contained in the aerial root of PSE is an antifungal agent against tomato late blight (16), and a compound isolated from PSE leaves has shown strong anticancer activity against solid cancer cells (17). Thus far, studies on PSE have been limited to fractionation studies on the compounds contained in PSE. Research on the physiological activation, anti-inflammatory effects, and immune effects of PSE is very limited, and there have been no studies on the effects of PSE on alopecia or hair growth.
Therefore, this study was undertaken to investigate the effect and mechanism of action of PSE on hair growth. Hair growth and cytokine expression were observed and hair follicles were examined histologically after the topical application of PSE extract to mouse dorsal skin.
MATERIALS AND METHODS
Sample pretreatment and dilution
PSE was purchased from the Korea Plant Extract Bank, Korea Research Institute of Bioscience and Biotechnology (Deajeon, Korea). For the analysis of total polyphenols, total flavonoids, and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging capacity, 20 mg of PSE was added per 1 mL of 50% methanol and extracted for 24 h in a water bath set to 37ºC. The extract was then filtered through a 0.2 μm syringe filter (PALL Life Sciences, Port Washington, NY, USA) and diluted to a concentration of 1 mg/mL. The diluted sample was protected from light and stored in a refrigerator for future analysis.
Total polyphenol content
The total polyphenol content of the PSE extract was measured using the modified Folin-Denis method (18). After dilution with 50% methanol, a 0.5 mL sample was mixed with 0.5 mL of Folin-Denis reagent (Fluka, Buchs, Switzerland) and incubated for 3 min at room temperature. After 3 min, 0.5 mL of 10% sodium carbonate solution (Samchun Pure Chemical, Pyeongtaek, Korea) was added to the sample, the mixture was incubated for 1 h at room temperature, and the absorbance was measured at 760 nm using a 7315 UV-VIS spectrophotometer (Jenway, Bibby Scientific Ltd., Staffordshire, UK). The measured absorbance was used to determine the poly-phenol concentration of PSE against a tannic acid standard curve (Yakuri Pure Chemicals Co., Ltd., Kyoto, Japan).
Total flavonoid content
To determine the total flavonoid content, 0.5 mL of the pretreated sample was mixed with 5 mL of diethylene glycol (Junsei Chemicals, Tokyo, Japan) and 0.5 mL of 1 N NaOH (Duksan Pure Chemicals Inc., Ansan, Korea). The resulting mixture was mixed well and then incubated for 1 h in a 37ºC water bath. The absorbance of the mixture was measured 3 times at 420 nm by a 7315 UV-VIS spectrophotometer. To determine the total flavonoid content, the measured absorbance was converted to % naringin equivalents using a naringin (Tokyo Kasei Kogyo Co., Ltd., Tokyo, Japan) standard curve.
DPPH radical scavenging capacity
To determine the DPPH radical scavenging capacity, a 0.5 mL PSE sample was diluted to 25 mg/100 mL and added to 3 mL of DPPH reagent. The mixture was allowed to react for 20 min in the dark room. The absorbance of the resulting mixture was measured at 517 nm by a 7315 UV-VIS spectrophotometer. The formula below was used to calculate the electron donating ability (EDA) from the measured absorbance.
CCK-8 assay of cytotoxicity
The CCK-8 assay (Cell Counting Kit, Sigma, St. Louis, MO, USA) was used to determine cell viability. The cells used in the cytotoxicity test were originally derived from the human follicle dermal papilla cells (HFDPC) of a 33-year-old Caucasian woman and were purchased from Promocell (Heidelberg, Germany). The HFDPC were incubated to eight succeeding generations and then transferred into 96-well plates and pre-incubated for 24 h at 5% CO2 and 37ºC. The PSE was diluted to 8 different concentrations (4.9 ppm, 9.8 ppm, 19.5 ppm, 39.1 ppm, 156.3 ppm, 625.0 ppm, 2,500.0 ppm, and 10,000.0 ppm), and 10 μL of each concentration was added to each well; DMEM media was used as a negative control, minoxidil was used as a positive control, and triton X-100 (Sigma) was used as a blank. The plates were incubated for 24 h at 5% CO2 and 37ºC. Then, 10 μL of CCK-8 reagent was added to each well and the cells were incubated for approximately 2 h. When the color differences between the comparison groups were maximal, the absorbance of each well was measured at 450 nm (test wavelength) and 650 nm (reference wavelength) by an ELISA reader (IDEXX Laboratories, Inc., Westbrook, ME, USA). The measured absorbances were used to determine cell viability according to the formula given below.
Experimental design of in vivo study
Fifteen 4-week-old male C57BL/6 mice were purchased from the Central Lab, Animal Inc. (Seoul, Korea). The mice were allowed to acclimate to their surroundings for 2 weeks, to lead to the early onset of the telogen phase in mice hair cycles. At week 6, the mice were divided into 3 experimental groups: the dimethyl sulfoxide (DMSO)-treated group (i.e., negative control group; NC group), the minoxidil-treated group (i.e., positive control group; PC group), and the PSE extract-treated group. Mice were randomly assigned to the experimental groups (5 mice per group) and were singly housed in cages to minimize the loss of the topically applied extracts by contact with other mice.
To prepare the PSE extract for topical application, 20 mg of PSE extract was added to 1 mL of DMSO (Samchun Pure Chemical) and warmed for 24 h in a 37ºC water bath. Then the mixture was filtered through a 0.2 μm syringe filter (PALL Life Sciences) and diluted with DMSO to a concentration of 1 mg/1 mL. A 5% minoxidil solution (Hyundai Pharm. Co., Ltd., Seoul, Korea) was purchased from a drugstore for use as a positive control, and DMSO was used as a negative control. The dorsal skin of mice was shaved 1 day prior to the start of the experiment. The samples (100 μL) were applied to the dorsal skin at the same time daily for 3 weeks. The mice were maintained in a temperature-controlled (22±1ºC) room with 50~60% relative humidity and a 12-h light/dark cycle; food and water were provided ad libitum. For visual observation of hair growth, the dorsal skin to which the each experimental treatment was applied was photographed on days 0, 7, 14, and 21. All animal work was approved by the Animal Ethic Committee of Chung-Ang University (approval no. 13-0058).
Euthanasia of experimental animals and skin tissue preparation
On day 22 of the experiment, CO2 was used to euthanize the experimental animals and the skin tissue was removed from the treated area of each mouse. Exactly 0.05 g of the removed tissue was put in a standard bag (BA6040; Seward Ltd., Worthing, UK) and 500 μL of diethyl pyrocarbonate-distilled water (DEPC-DW; Bioneer, Daejeon, Korea) was added to destroy the tissue structure. A 250 μL aliquot of the treated sample was placed in an Eppendorf tube for RNA extraction. Other tissues were fixed in formalin (Sigma) and stored until future analyses.
Hematoxylin and eosin (H&E) staining for hair follicle morphology
H&E staining was performed by the routine Mayer method (19). After formalin fixation, each tissue piece was sliced with a knife and put in a cassette to be infiltrated with paraffin for 24 h in an Tissue-Tek® Auto TEC® Automated Embedder (Sakura, San Francisco, CA, USA), and then mounted to a paraffin block (Tissue-Tek® TEC™ 5; Sakura). Using a microtome (RM 2145, Leica, Wetzlar, Germany), 3 μm sections were cut from each paraffin-embedded tissue and affixed to a glass slide. Each slide was stained with H&E (Sigma) with a Slide Stainer (Shandon Linistain GLX, Shandon Inc., Pittsburgh, PA, USA). The shape of the follicles from each stained section were visualized with a Leica DM 500 optical microscope (Leica) at 100× magnification, and the skin thickness and follicle depth were measured with the scale bar of the microscope program.
Toluidine blue staining for mast cell count
Toluidine blue staining was performed by modifying the method of Schroeder (20). Just as in the H&E staining method, 3 μm sections were cut from each paraffin block and affixed to a glass slide. After the paraffin was removed, the slide was dehydrated in xylene and ethanol and then stained for 2 min in a working solution that was prepared by mixing 5 mL toluidine blue (Fluka) and 45 mL 1% sodium chloride. A Leica DM 500 optical microscope with a 200× objective was used to count the number of mast cells in the skin and hypoderm.
Immunohistochemical staining for stem cell factor (SCF)
The immunohistochemical staining was conducted by modifying the method of Kong (21). Each tissue piece was sliced to a 3 μm thickness, affixed to a glass slide, and placed in xylene and ethanol to be dehydrated. Dehydrated sections were treated with 300~400 μL of a 200:1 solution of phosphate buffered saline (PBS; Invitrogen Co., Carlsbad, CA, USA): proteinase K (Promega Co., Madison, WI, USA) for 25 min at 37ºC and then 200 μL of 5% normal goat serum (G9023) diluted in PBS with 0.1% Tween 20 (MP Biomedicals, Solon, OH, USA; PBST) for approximately 20 min at room temperature. Tissue sections were then incubated in 40 μL of the primary antibody solution, a 1:50 mixture of anti-SCF antibody (ab64677, Abcam, Cambridge, MA, USA) and 0.1 M PBST, at 36ºC for 1 h. After incubation, the primary antibody was removed by washing with PBST, and the tissue sections were incubated in the secondary antibody solution, a 1:50 mixture of anti-rabbit IgG HRP-conjugated antibody (Dako, Glostrup, Denmark) diluted in PBST, at 37ºC for 1 h. The secondary antibody was removed by washing in PBST, and then the tissue section was treated with 300 μL of the staining agent, a 1:9 dilution of 3,3′ diaminobenzidine (DAB; Roche Diagnostics GmbH, Mannheim, Germany) in 0.1 M PBS. Hematoxylin (Sigma) was used as a control stain. The morphology of follicles, mast cells, and skin was observed through Leica DM 500 optical microscope at 200×.
Cytokine analysis
To determine the levels of cytokines involved in hair growth, the mRNA expression of 5 cytokines, insulin-like growth factor (IGF)-1, keratinocyte growth factor (KGF), and transforming growth factor (TGF)-β1 were relatively quantified. cDNA was synthesized by reverse transcription after total mRNA extracted from the dorsal skin tissue of each C57BL/6 mouse. Polymerase chain reaction (PCR) was used to amplify each gene of interest after mixing the resulting cDNA with each gene primer set.
IGF-1 and KGF were quantified by gene amplification (denaturation, 30 s at 95ºC; annealing, 60 s at 60ºC; and extension, 30 s at 72ºC) by PTC-150 MiniCycler (MJ Research, Inc., Waltham, MA, USA), dielectrolysis (Bio-Rad, Hercules, CA, USA), image capturing (Gel Doc XR UV transilluminator; Bio-Rad), and digitizing band length (MBF ImageJ, Version 6.0, MBF Bioscience Inc., Williston, VT, USA). TGF-β1 was quantified using real-time PCR (TCR0096 Thermo Fisher Scientific Inc.), Waltham, MA, USA) for gene amplification (denaturation, 15 s at 95ºC; annealing, 15 s at 60ºC; and extension, 40 s at 72ºC) and VeriQuest SYBR Green qPCR Master Mix (2X) (Affymetrix Inc., Santa Clara, CA, USA). The primer sets were purchased from Bioneer and used at a 100 pmol concentration in DEPC-DW; the primer sequence of each gene is shown in Table 1.
Table 1.
Cytokine primer sequences
| Cytokine | Sequence | Size (bp) | |
|---|---|---|---|
| IGF-1 | Forward | 5′-AGAGACCCTTTGCGGGGTGA-3′ | 238 |
| Reverse | 5′-CTTCTGAGTCTTGGGCATGT-3′ | ||
| KGF | Forward | 5′-AGGGTGAGAAGACTGTTCTG-3′ | 151 |
| Reverse | 5′-CTTTCCACCCCTTTGATTGC-3′ | ||
| VEGF | Forward | 5′-TGCTTATAGGCTGTTGTGGGCTCT-3′ | 187 |
| Reverse | 5′-ACACACCTTGACTCTTCACCTGCT-3′ | ||
| HGF | Forward | 5′-TTGAGACCAGCCAGAGGTGAACAA-3′ | 170 |
| Reverse | 5′-ATGCTGAGTAGGTGGGCAAGCTAA-3′ | ||
| TGF-β1 | Forward | 5′-ATGGCAGCGACCATACTCCTCTTT-3′ | 121 |
| Reverse | 5′-AAAGACAGCCACTCAGGCGTATCA-3′ | ||
Statistical analysis
Collected data were analyzed by one-way ANOVA using the SPSS Statistics vers 17.0 (SPSS Inc., Chicago, IL, USA). Duncan’s multiple range test was used for post-hoc analysis. Differences between groups were considered significant when P<0.05. All experiments were repeated at least 3 times to assess reproducibility, and data are provided as mean±standard error (SE).
RESULTS AND DISCUSSION
Antioxidant activity of PSE
Because the current alopecia treatments, minoxidil and finasteride, have temporary effects and cause numerous side effects, there has been a growing interest in natural materials that encourage hair growth and have no side effects. Previous research indicates that high-antioxidant materials such as fermented Liriope platyphylla, green tea, onion extract, and wild rose root extract, have high anti-oxidant activities and high hair growth-promoting effects (22–25), with suppression of alopecia-promoting cytokine activities. Researchers are actively seeking natural materials that promote hair growth (26). Kim et al. (27) compared the antioxidant activity of 40 different native plants and herb-medicine resource extracts. They found that Lespedeza cuneata G. Don and Artemisia scoparia Waldst. et Kitamura had the highest polyphenol (223.90 mg/g and 228.45 mg/g, respectively) and flavonoid (90.15 mg/g and 77.65 mg/g, respectively) contents of the samples tested. The results of the present study indicate that the polyphenol and flavonoid contents of PSE are 366.44±3.81 mg/g extract weight and 152.65±0.21 mg/g extract weight, respectively (Table 2), which is 1.6 times higher than the polyphenol and flavonoid contents of Lespedeza cuneata G. Don and Artemisia scoparia Waldst. et Kitamura.
Table 2.
Total polyphenol content, total flavonoid content, and electron donating ability of Platycarya strobilacea S. et Z. extract
| Total polyphenols (mg/g tannic acid equivalent) | Total flavonoids (mg/g naringin equivalent) | EDA (%)1) | |
|---|---|---|---|
| Platycarya strobilacea S. et Z. | 366.44±3.81 | 152.65±0.21 | 73.73±0.06 |
Data shown are the means±SE from triplicate measurements.
EDA: Electron donating ability.
EDA (electron donating ability) is a representative index of antioxidant activity and is defined as the ability to maintain stable condition of substance by donating electrons to active oxygen molecules. Without stabilization, active oxygen can cause infection, damage blood vessels, and cause cardiovascular and cerebrovascular diseases by creating oxide stress in the human body (28). The EDA of PSE is 73.73±0.06% (Table 2), which is similar to that of Lespedeza cuneata G. Don (75.69%) but is 1.7 times higher than the free-radical scavenging capacity of Artemisia scoparia Waldst. et Kitamura (44.69%). The EDA of PSE extract is similar to the previously reported EDA value (74%) for butylated hydroxytoluene, a synthetic antioxidant (29).
Cell viability
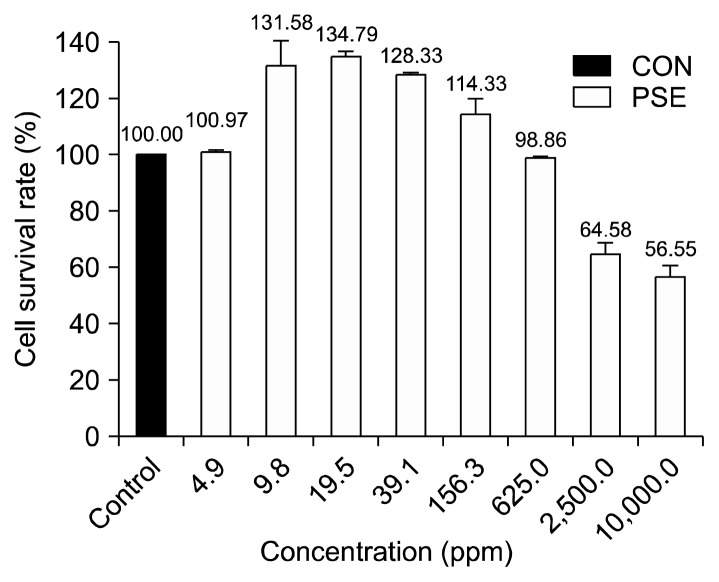
The viability of HFDPC treated with varying concentrations of PSE is shown in Fig. 1. When the viability of the NC group was adjusted to 100%, the viability of cells in the 4.9 ppm PSE group was 101.0%, which is similar to the viability rate of the NC group. The cell viability rate increased to 131.6% with the 9.8 ppm PSE treatment and peaked at 134.8% (i.e., approximately 1.34 times higher than that of the NC group) with the 19.5 ppm PSE treatment. However, the cell viability rate decreased steadily from 128.3% to 114.3% to 98.9%, at the 39.1 ppm, 156.3 ppm, and 625.0 ppm treatment levels, respectively. At the 1,250 ppm and 2,500 ppm PSE treatment levels, the cell viability rate decreased considerably. The lowest cell viability rate (56.6%) was observed at the highest PSE concentration (10,000 ppm), indicating that high PSE concentrations are associated with cytotoxicity.
Fig. 1.
Cell survival rate (%) after 24 h was measured by CCK-8 assay in HFDPC treated with Platycarya strobilacea S. et Z. extract (4.9 ppm, 9.8 ppm, 19.5 ppm, 39.1 ppm, 156.3 ppm, 625.0 ppm, 2,500.0 ppm, and 10,000.0 ppm). Values are expressed as the mean±SE of triplicate measurements.
Hair growth
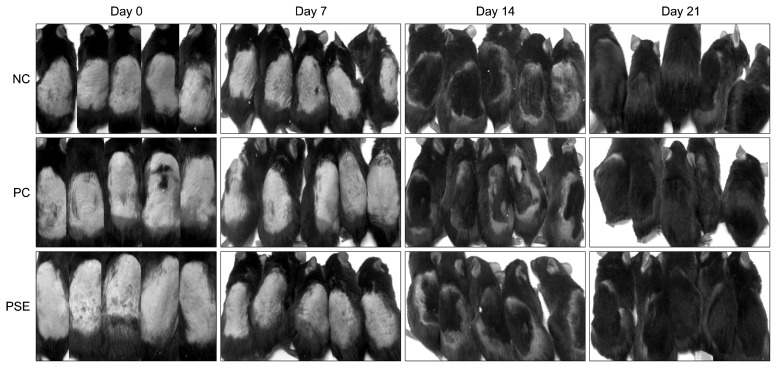
Results of the visual observation of hair growth after 3 weeks of topical PSE extract application are shown in Fig. 2. Seven days after PSE extract application, 2 mice in the NC group, 1 mouse in the PC group, and 3 mice in the PSE-treated group exhibited melanin pigmentation that caused the skin to darken, indicating that melanin had migrated to the skin surface during the transition from telogen to anagen (30,31). In addition, 2 mice in the PSE group exhibited hair growth seven days after PSE extract application. Fourteen days after PSE extract application, 4 mice in the NC group, 3 mice in the PC group, and all 5 mice in the PSE group exhibited hair growth at the sample-treated spot. Mice in the PSE-treated group experienced a shorter time before hair re-growth and had a higher number of re-grown hairs than mice in the NC and PC groups. After 21 days, all groups exhibited hair growth.
Fig. 2.
The dorsal skin of the mice was shaved and DMSO, minoxidil, or Platycarya strobilacea S. et Z. extract was applied topically for 3 weeks. NC, dimethyl sulfoxide (DMSO); PC, minoxidil; PSE, Platycarya strobilacea S. et Z. extract with DMSO.
Histological observation
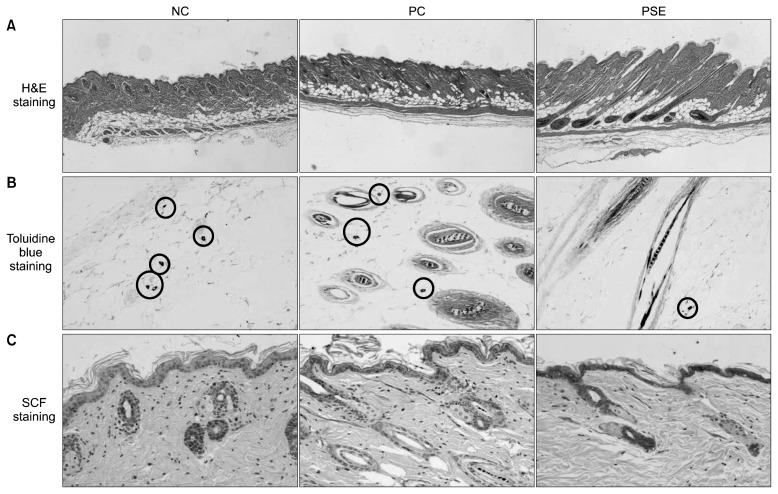
Results of the H&E, toluidine blue, and immunohistochemical staining of the dorsal skin tissue after 3 weeks of PSE extract application are shown in Fig. 3. Observation of the shape and depth of H&E stained follicles revealed that the hair follicles of the NC group and PC group were irregularly shaped and located closer to the skin surface, whereas those of the PSE group had a regular shape and were stably located–a better result than that of the other groups. Measurement of hair follicle depth using the scale bar program of an optical microscope (Table 3) revealed that the NC group had a hair follicle depth of 758.33±150.05 μm, the PC group had a hair follicle depth of 655.00±145.45 μm, and the PSE-treated group had a hair follicle depth of 843.33± 84.33 μm. Although no significant differences were found, the hair follicle depth of the PSE-treated group was the deepest of all the groups, confirming that the hair roots were formed very firmly.
Fig. 3.
Representative histological observation, comparison of number of mast cells, and immunohistochemistry of SCF antigens in an alopecia model of C57BL/6 mice treated with topical test compounds for 3 weeks. Shape and depth of H&E stained follicles (A), toluidine blue-stained sections showing mast cell count (B), and immunohistochemical staining of SCF in the follicles, mast cells, and skin (C). NC, dimethyl sulfoxide (DMSO); PC, minoxidil; PSE, Platycarya strobilacea S. et Z. extract with DMSO.
Table 3.
Hair follicle length and number of mast cells in an alopecia model of C57BL/6 mice treated with topical test compounds for 3 weeks
| Group1) | Length (μm) | Mast cell (no.) |
|---|---|---|
| NC | 758.33±150.05NS | 6.33±0.88b |
| PC | 655.00±145.45 | 7.00±0.71b |
| PSE | 843.33±84.33 | 1.50±0.29a |
Data shown are the means±SE from triplicate measurements.
NC, dimethyl sulfoxide (DMSO); PC, minoxidil; PSE, Platycarya strobilacea S. et Z. extract with DMSO.
Not significant.
Within each column, means with different superscripts are significantly different by Duncan’s multiple range test (P<0.05).
It has been widely accepted that minoxidil encourages hair growth by inducing dilation of scalp blood vessels, increasing blood flow, and activating hair mother cells (9). In our study, the PC group did show delayed hair growth compared to the NC and PSE groups. This may be because minoxidil normally works on follicles in the telogen phase first to shed, which are then replaced by thicker hairs in a new anagen phase. That is why the PC group showed delayed hair growth. It seemed that there was time difference in hair growth depending on the treatments. From the findings of the present study, we believe that hair growth was fastest in the PSE-treated group due to the accelerated transition from telogen to anagen.
Mast cells control hair growth by creating and releasing growth control factors such as cytokines, eicosanoids, proteases, glycosaminolycans, and inflammatory mediators (32,33). Paus et al. (34) reported that the histamine released by mast cells undergoing degranulation delays rodent hair growth and causes alopecia. In addition, mast cells may participate in regulation of hair growth (35). In the present study, the number of mast cells stained by toluidine blue is as follows: PC (7.00±0.71)> NC (6.33±0.88)> PSE (1.50±0.29). Notably, there are markedly fewer mast cells in the PSE-treated group than in the other groups (P<0.001). These findings suggest that PSE may improve hair growth by suppressing excessive mast cell proliferation better than minoxidil, which had the highest number of mast cells.
Immunohistochemical staining revealed that SCF expression in the hair follicles, mast cells, and skin of the NC group and the PC group is lower than SCF expression in the hair follicles, mast cells, and skin of the PSE-treated group. This finding indicates that SCF stimulation may be responsible for the enhanced hair growth observed in the PSE-treated group.
Quantification of cytokine gene expression
Various cytokines play essential roles in the growth and differentiation of cells. Cytokines that are important to hair growth include IGF-1, KGF, and TGF-β1. IGF-1 is synthesized in dermal papilla cells and is a controlling factor that suppresses the transformation of the hair growth cycle to catagen. KGF stimulates hair follicle proliferation. In contrast, TGF-β1 inhibits hair follicle growth. TGF-β1 also accelerates transformation of the hair growth cycle from anagen to catagen and suppresses the proliferation and spread of hair follicles (36,37).
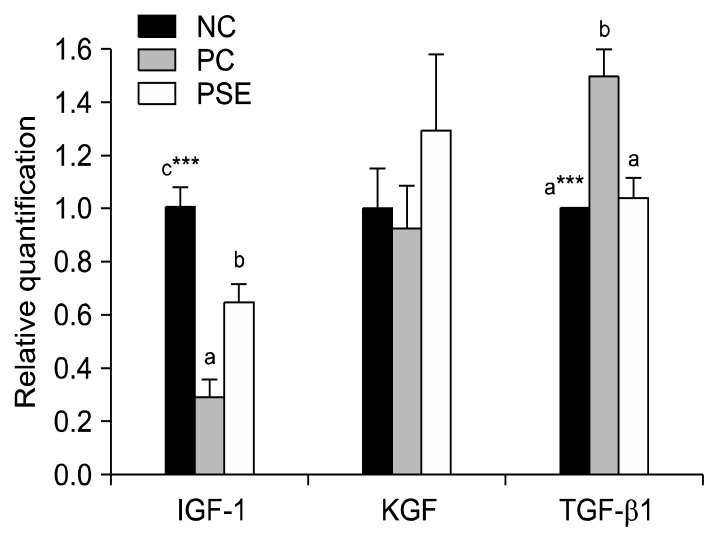
IGF-1 and KGF are cytokines that improve hair growth, while TGF-β1 is an element that suppresses hair growth. After the gene expression level of the NC group was set to 1.0, statistical comparison revealed that IGF-1 expression was highest (P<0.001) in the NC group, followed by the PSE group (0.65±0.07), and the PC group (0.29±0.07) (Fig. 4). These results are similar to those of previous research on the hair growth effect of Sophora flavescens extract (38), which revealed that the IGF-1 expression level of the extract-treated group was higher than that of the minoxidil-treated group. In the present study, KGF expression was highest in the PSE group (1.30±0.28) and lowest in the PC group (0.93±0.16). This may indicate that PSE treatment improves hair growth by increasing the expression of hair-growth promoting cytokines such as IGF-1 and KGF, which may act synergistically to further enhance hair growth.
Fig. 4.
Relative quantification of the mRNA expression levels of growth factors (IGF-1, KGF, and TGF-β1). Values are expressed as the mean±SE of triplicate measurements. NC, dimethyl sulfoxide (DMSO); PC, minoxidil; PSE, Platycarya strobilacea S. et Z. extract with DMSO. Means with different letters (a–c) are significantly different at P<0.05 by Duncan’s multiple range test. ***P<0.001.
TGF-β1 induces alopecia through its involvement in the induction of the catagen phase of the hair growth cycle; the mechanism of action for this effect is increased hair follicle matrix cell apoptosis (39). With increased TGF-β1 expression, hair follicle proliferation is not completed and reproduced follicles are reduced, resulting in thin and short hairs (40). In this study, TGF-β1 expression was 1.49±0.10 in the PC group and 1.04±0.08 in the PSE-treated group, indicating that the expression of hair growth-suppressing elements was higher after minoxidil application than after DMSO or PSE application (P<0.001). For this reason, it is believed that PSE would be a better hair growth treatment than minoxidil.
In conclusion, the topical application of PSE induced better hair follicle morphology, decreased mast cell number, and increased SCF expression. In addition, PSE intensified the expression of several hair growth-promoting cytokines and thus modulated the transformation of the hair growth cycle. Therefore, we conclude that PSE may be a more potent agent for the prevention and treatment of alopecia than minoxidil, the existing chemical ointment for alopecia and hair loss. However, further research is necessary to determine the bioactive component and mechanism of action responsible for PSE-related hair loss prevention.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2011-0013949).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Brauner GJ, Goodheart HP. Dermatologic care behind bars. J Am Acad Dermatol. 1988;18:1066–1073. doi: 10.1016/s0190-9622(88)70107-3. [DOI] [PubMed] [Google Scholar]
- 2.Muller HK, Rook AJ, Kubba R. Immunohistology and autoantibody studies in alopecia areata. Br J Dermatol. 1980;102:609–610. doi: 10.1111/j.1365-2133.1980.tb07664.x. [DOI] [PubMed] [Google Scholar]
- 3.Selmanowitz VJ, Victor S, Warburton D, Orentreich N. Fingerprints arches in alopecia areata. Arch Dermatol. 1974;110:570–571. [PubMed] [Google Scholar]
- 4.Bergfeld WF. Diffuse hair loss in women. Cutis. 1978;22:190–195. [PubMed] [Google Scholar]
- 5.Jiang H, Yamamoto S, Kato R. Induction of anagen in telogen mouse skin by topical application of FK506, a potent immunosuppressant. J Invest Dermatol. 1995;104:523–525. doi: 10.1111/1523-1747.ep12606018. [DOI] [PubMed] [Google Scholar]
- 6.Aljuffali IA, Sung CT, Shen FM, Huang CT, Fang JY. Squarticles as a lipid nanocarrier for delivering diphencyprone and minoxidil to hair follicles and human dermal papilla cells. AAPS J. 2014;16:140–150. doi: 10.1208/s12248-013-9550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClellan KJ, Markham A. Finasteride: a review of its use in male pattern hair loss. Drugs. 1999;57:111–126. doi: 10.2165/00003495-199957010-00014. [DOI] [PubMed] [Google Scholar]
- 8.Limas CJ, Fresis ED. Minoxidil in severe hypertension with renal failure. Effect of its addition to conventional antihypertensive drugs. Am J Cardiol. 1973;31:353–361. doi: 10.1016/0002-9149(73)90268-3. [DOI] [PubMed] [Google Scholar]
- 9.Messenger AG, Rundegren J. Minoxidil: Mechanisms of action on hair growth. Br J Dermatol. 2004;150:186–194. doi: 10.1111/j.1365-2133.2004.05785.x. [DOI] [PubMed] [Google Scholar]
- 10.Trüeb RM, Itin P. Photographic documentation of the effectiveness of 1 mg. oral finasteride in treatment of androgenic alopecia in the man in routine general practice in Switzerland. Praxis. 2001;90:2087–2093. [PubMed] [Google Scholar]
- 11.Arca E, Açikgöz G, Ta tan HB, Köse O, Kurumlu Z. An open, randomized, comparative study of oral finasteride and 5% topical minoxidil in male androgenetic alopecia. Dermatology. 2004;209:117–125. doi: 10.1159/000079595. [DOI] [PubMed] [Google Scholar]
- 12.Arcella D, Le Donne C, Piccinelli R, Leclercq C. Dietary estimated intake of intense sweeteners by Italian teenagers. Present levels and projections derived from the INRAN-RM-2001 food survey. Food Chem Toxicol. 2004;42:677–685. doi: 10.1016/j.fct.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y. Daily intake of food additives in Japan determination of food additives residues in food–from 1976 to 2000 year by market basket method. Foods Food Ingredients J Jpn. 2007;212:815–839. [Google Scholar]
- 14.Rogers NE, Avram MR. Medical treatments for male and female pattern hair loss. J Am Acad Dermatol. 2008;59:547–566. doi: 10.1016/j.jaad.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Lee CB. Coloured flora of Korea. Hyangmoon Publishing Co. Ltd; Seoul, Korea: 2003. p. 70. [Google Scholar]
- 16.Choi YH, Chae SG, Kim JH, Kang SJ, Baek NI, Han JT. Isolation of an antifungal compound from aerial parts of Platycarya strobilacea. J Korea Soc Agric Chem Biotechnol. 2003;46:268–270. [Google Scholar]
- 17.Kim YI, Lee SH, Cho TS. Isolation of anticancer agents from the leaves of Platycarya strobilacea S. et Z. Kor J Pharmacogn. 1996;27:238–245. [Google Scholar]
- 18.Duval B, Shetty K. The stimulation of phenolics and antioxidant activity in pea (Pisum sativum) elicited by genetically transformed anise root extract. J Food Biochem. 2001;25:361–377. [Google Scholar]
- 19.Garvey W. Modification of the Mayer hematoxylin stain. J Histotechnol. 1991;14:163–165. [Google Scholar]
- 20.Schroeder HE. Ultrastructure of the junctional epithelium of the human gingiva. Helv Odontol Acta. 1969;13:65–83. [PubMed] [Google Scholar]
- 21.Kong MK, Kim YC. Promotion effect of black sesame oil on hair growth in an alopecia model of C57BL/6 mice. J Biomed Res. 2010;11:103–116. [Google Scholar]
- 22.Lee HA, Han JS. Hair growth promoting effect of fermented Liriope platyphylla on hair loss-induced C57BL/6 mice. Abstract No P8-168 presented at 2010 KFN International Symposium and Annual Meeting; Daegu, Korea. 2010. pp. 347–348. [Google Scholar]
- 23.Esfandiari A, Kelly AP. The effects of tea polyphenolic compounds on hair loss among rodents. J Natl Med Assoc. 2005;97:1165–1169. [PMC free article] [PubMed] [Google Scholar]
- 24.Joo HH, Kwon SH, Shin HJ. The influence of Allium cepa extracts on promotion of hair growth. Presented at 2009 Korean Society for Biotechnology and Bioengineering Meeting & International Symposium; Pohang, Gyeongbuk, Korea. 2009. p. 197. [Google Scholar]
- 25.Kim JH, Hong SK, Hwang SJ, Son SW, Choi YS. The preclinical and clinical effects of herbal product containing Rosa mutiflora roots extracts as a main component on the hair growth promotion. Korean J Medicinal Crop Sci. 2012;20:108–116. [Google Scholar]
- 26.Kwon OS, Han JH, Yoo HG, Chung JH, Cho KH, Eun HC, Kim KH. Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG) Phytomedicine. 2007;14:551–555. doi: 10.1016/j.phymed.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Kim EJ, Choi JY, Yu MR, Kim MY, Lee SH, Lee BH. Total polyphenols, total flavonoid contents, and antioxidant activity of Korean natural and medicinal plants. Korean J Food Sci Technol. 2012;44:337–342. [Google Scholar]
- 28.Kim MJ, Cho SY. Effects of dandelion on oxygen free radical generating and scavenging system of brain in streptozotocin-induced diabetic rats. J Korean Soc Food Sci Nutr. 2002;31:500–505. [Google Scholar]
- 29.Sa YJ, Kim JS, Kim MO, Jeong HJ, Yu CY, Park DS, Kim MJ. Comparative study of electron donating ability, reducing power, antimicrobial activity and inhibition of α-glucosidase by Sorghum bicolor extracts. Korean J Food Sci Technol. 2010;42:598–604. [Google Scholar]
- 30.Kim YI, Lee MW, Kwon J, Song CH, Lee CH. Experimental studies on the factors related to hair loss in spontaneous hair loss C57BL/6N mice models. Korean J Anat. 2005;38:113–143. [Google Scholar]
- 31.Hong JT, Lee SR, Kim HH, Jo YK, Baek IJ, Yon JM, Nahm SS, Kwack DH, Lee JE, Lee BJ, Yun YW, Kim CJ, Nam SY. Hair growth promotion effect of a bio-active shampoo, Bonogen in C57BL/6 mice. J Toxicol Pub Health. 2006;22:221–228. [Google Scholar]
- 32.Paul WE, Seder RA, Plaut M. Lymphokine and cytokine production by FcɛRI+ cells. Adv Immunol. 1993;53:1–29. [PubMed] [Google Scholar]
- 33.Kumamoto T, Shalhevet D, Matsue H, Mummert ME, Ward BR, Jester JV, Takashima A. Hair follicles serve as local reservoirs of skin mast cell precursors. Blood. 2003;102:1654–1660. doi: 10.1182/blood-2003-02-0449. [DOI] [PubMed] [Google Scholar]
- 34.Paus R, Stenn KS, Elgjo K. The epidermal pentapeptide pyroGlu-Glu-Asp-Ser-GlyOH inhibits murine hair growth in vivo and in vitro. Dermatologica. 1991;183:173–178. doi: 10.1159/000247664. [DOI] [PubMed] [Google Scholar]
- 35.Lee MW, Oh CH, Kwon J, Song CH, Lee CH. The studies on the factors related to normal hair growth during postnatal growth periods in C57BL/6N, hairless and alopecia areata mice model. Korea J Phys Anthropol. 2005;18:13–27. [Google Scholar]
- 36.Danilenko DM, Ring BD, Pierce GF. Growth factors and cytokines in hair follicle development and cycling: recent insights from animal models and the potentials for clinical therapy. Mol Med Today. 1996;2:460–467. doi: 10.1016/1357-4310(96)10045-9. [DOI] [PubMed] [Google Scholar]
- 37.Tsuboi R, Imai R, Takamori K, Rubin JS, Ogawa H. Hepatocyte growth factor/scatter factor stimulates hair growth of mouse vibrissae in organ culture. J Invest Dermatol. 1994;103:306–309. doi: 10.1111/1523-1747.ep12394731. [DOI] [PubMed] [Google Scholar]
- 38.Roh SS, Kim CD, Lee MH, Hwang SL, Rang MJ, Yoon YK. The hair growth promoting effect of Sophora flavescens extract and its molecular regulation. J Dermatol Sci. 2002;30:43–49. doi: 10.1016/s0923-1811(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 39.Tsuji Y, Denda S, Soma T, Raftery L, Momoi T, Hibino T. A potential suppressor of TGF-β delays catagen progression in hair follicles. J Investig Dermatol Symp Proc. 2003;8:65–68. doi: 10.1046/j.1523-1747.2003.12173.x. [DOI] [PubMed] [Google Scholar]
- 40.Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Androgen-inducible TGF-β1 from balding dermal papilla cells inhibits epithelial cell growth: a clue to understanding paradoxical effects of androgen on human hair growth. FASEB J. 2002;16:1967–1969. doi: 10.1096/fj.02-0043fje. [DOI] [PubMed] [Google Scholar]