Abstract
Vascular dementia is characterized by white matter lesions involving the demyelination and activation of astrocytes and microglia. In a previous study, we showed that the supernatant of a laboratory-scale, hot water extract of ground whole wheat (TALE) attenuated white matter injury and astrocytic activation in a rat model of bilateral common carotid artery occlusion (BCCAO). In the present study, we made several modifications to the hot water extraction process to remove starch and enable large-scale production. We used wheat bran (WB), which contains less starch, instead of ground whole wheat. In addition, we removed starch granules with a decanter before hot water extraction. The final product, wheat bran extract (WBE), contained 2.42% arabinose, a surrogate marker of arabinoxylan, which is an active constituent of WBE. Supplementation of the rat model of BCCAO with WBE (400 mg/kg/day) for 33 days attenuated white matter injury, which was assessed by Luxol Fast Blue staining, in the corpus callosum (cc) and optic tract (opt) regions. Attenuation of white matter injury in the opt region was accompanied by improvement of the pupillary light reflex. Immunochemical staining revealed that supplementation with WBE reduced astrocytic activation in the cc and opt regions and reduced microglial activation in the opt region. These findings indicate that supplementation with WBE is effective at attenuating white matter injury accompanied by the inhibition of astrocytic and microglial activation. Therefore, extracts from WB, a cheap by-product of wheat milling, can be developed as a nutraceutical to prevent vascular dementia, a disease for which there is no approved pharmaceutical treatment.
Keywords: wheat bran extract, vascular dementia, BCCAO, white matter injury, nutraceutical
INTRODUCTION
Vascular dementia is the second most common form of dementia, after Alzheimer’s disease. Vascular dementia accounts for approximately 30% of dementia cases in Asian countries and 10% of dementia cases in Western countries (1,2). Vascular dementia is caused by vascular lesions and can be classified into three major types: subcortical vascular dementia, multi-infarct dementia, and strategic dementia. Subcortical vascular dementia is the most prevalent type of dementia, accounting for approximately 50% of vascular dementia cases (3). The prominent pathological features of subcortical vascular dementia are confluent white matter lesions characterized by a loss of oligodendrocytes that leads to demyelination, vacuolization, astrocytic activation (also known as astroglial), and microglial activation (4–6). These pathological features are caused by incomplete white matter infarction due to reduced blood supply to the region, such as chronic hypoperfusion (4). While a variety of pharmaceutical clinical trials have been conducted, no drug to prevent or treat vascular dementia has been approved by any regulatory agency (7). Thus, there is an urgent need to develop nutraceuticals to prevent vascular dementia.
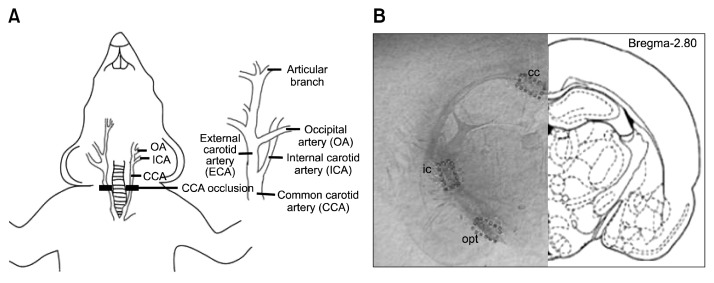
The rat bilateral common carotid artery occlusion (BCCAO) model has been widely used to study subcortical vascular dementia, as it mimics the pathological events that occur in this disease (Fig. 1A) (8,9). The BCCAO model reflects human subcortical vascular dementia well in that it induces white matter injury with demyelination, vacuolization, and astrocytic and microglial activation (10,11).
Fig. 1.
Schematic diagrams of BCCAO model and white matter locations. (A) Bilateral common carotid arteries (BCCA) were occluded by ligation with 4-0 silk sutures. CCA, ICA, and OA represent common carotid artery, internal carotid artery, and occipital artery, respectively. (B) The three white matter locations that were examined in this study: the corpus callosum (cc), internal capsule (ic), and optic tract (opt) regions. The section shown is located at −2.8 mm from the bregma (23).
A wheat grain is comprised of the endosperm, the bran layers, and the germ (12). Each part of the grain is composed of a varying distribution of starch, protein, and cell wall (13). The cell walls consist predominantly of nonstarch-polysaccharides (NSP), such as arabinoxylan (AX) and β-glucan, with minor amounts of arabinogalactan-peptide and glucomannans (13,14). AX is comprised of a linear D-xylose (xyl) chain backbone with L-arabinose (ara) side chains (13,14). The process of milling produces wheat flour from the wheat grain endosperm, leaving the rest of the grain [i.e., the wheat bran (WB) layers with variable amounts of remaining endosperm] as wheat bran (15). Whole wheat grains are approximately 65% starch, 15% protein, and 10% NSP, whereas wheat bran is approximately 25% starch, 20% protein, and 30% NSP (15). Although whole wheat grains and wheat bran contain very different levels of total AX (6% wt/wt vs. 17% wt/wt), they contain comparable levels of water-extractable AX (approximately 0.5% wt/wt) (16).
In a previous attempt to develop a nutraceutical that prevents vascular dementia, we found that the supernatant of a laboratory-scale, hot water extract of ground whole wheat (Triticum aestivum L.) (TALE) with batch type centrifuges attenuated white matter injury and astrocytic activation in a rat BCCAO model, leading to memory improvement (17). To determine the active constituents of TALE, we used the same animal model to test the effects of several TALE components on white matter lesions. The results of those experiments revealed that AX was an active constituent (17). The results of our previous experiment encouraged us to develop TALE as a nutraceutical that contains AX as an active component. However, when we attempted to produce TALE on an industrial scale with continuous-type centrifuges, we encountered difficulties with the removal of gelatinized starch, which is necessary to maintain a high level of AX in the TALE.
Once starch granules from wheat have reached the gelatinization temperature (about 60°C), they gelatinize to form a paste. The resultant paste cannot be restored to its starch-granule form and transforms to a gel when cooled below the gelatinization temperature (18). Both the paste and the gel were difficult to remove with a continuous-type centrifuge (data not shown). In the present study, we used wheat bran, which contains less starch than whole grains, as a starting material to circumvent the problem of removing the gelatinized starch. In addition, we used a decanter centrifuge to remove starch granules before they could be gelatinized by heating (19). Using this method, we were able to produce a wheat bran extract (WBE) with approximately 10% yield. In the present study, we examined whether WBE could protect white matter against chronic hypoperfusion injury in a rat BCCAO model. To ensure the quality of the WBE produced, it was necessary to measure the concentration of AX, an active constituent in WBE. However, it is difficult to measure the content of AX in WBE directly. Thus, we measured the ara content of WBE after its polysaccharides had been hydrolyzed by acid. The ara content of the acid hydrolyzed WBE was used as a surrogate marker of the AX content of WBE because ara is also an active component of WBE that has been shown to attenuate white matter injury (17).
MATERIALS AND METHODS
Preparation of WBE
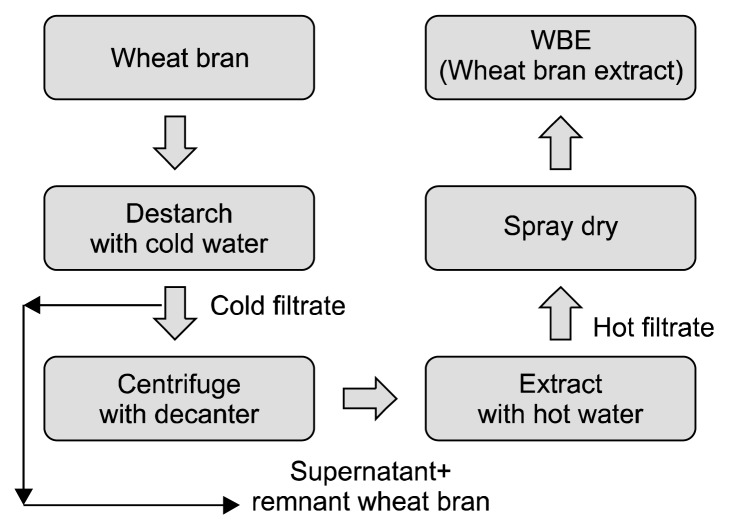
Wheat bran, a milling by-product of a domestic wheat variety, was purchased from a cultivator in Korea. The following procedure was used to produce WBE used in the current study (Fig. 2): (1) The wheat bran was sieved to remove small particles, which were mostly composed of starch; (2) The wheat bran was stirred with 5-parts (wt/wt) cold water in a 500 L extractor (BestKorea, Daejon, Korea) to remove starch granules from the wheat bran; (3) The cold extract was sieved and washed with 5-parts (wt/wt) cold water a second time to separate the filtrate from the remnant wheat bran, the filtrate was designated “cold filtrate”; (4) The cold filtrate was centrifuged with a decanter centrifuge (PTM 300, TOMOE Engineering Co., Ltd., Tokyo, Japan) to obtain a supernatant; (5) The supernatant was mixed with the remnant wheat bran and stirred in an extractor at approximately 95°C; (6) The hot extract obtained was sieved to yield “hot filtrate”; (7) The hot filtrate was concentrated with a vacuum dryer (HyoSung, Incheon, Korea) and dried with a spray dryer (YooJin Tech., Pyeongtaek, Korea) to yield the final product, which was designated “wheat bran extract” (WBE). The WBE was kindly provided by DongA One Corp. (Seoul, Korea).
Fig. 2.
A schematic diagram of wheat bran extract (WBE) preparation. Wheat bran was stirred in an extractor with cold water to separate the starch granules from the wheat bran, yielding cold filtrate and remnant wheat bran. The cold filtrate fraction was centrifuged with a decanter to separate the supernatant from the precipitated starch granules. Then, the supernatant and remnant wheat bran were combined and extracted with hot water to produce hot filtrate. The hot filtrate was concentrated and dried with a spray dryer to produce WBE.
WBE chemical composition analysis
The WBE nutrient analysis was performed according to the Korean Food Standard Codex (20). The moisture, fat, protein, and ash contents (wt%) were measured by the heating drying method, the ester extraction method, the Kjeldahl method, and the ash test method, respectively. The carbohydrate content (wt%) was calculated by difference. In addition, the total, insoluble, and soluble dietary fiber contents were measured by the enzymatic-gravimetric method.
Analysis of neutral monosaccharide composition of WBE
The neutral monosaccharide composition of WBE was determined as previously described (21). WBE (5~20 mg) was hydrolyzed with 0.5 mL of 2 M trifluoroacetic acid (TFA) at 121°C for 60 min. After cooling to room temperature, 25 μL of 20 mg/mL allose (internal standard) was added and the solution was mixed well. Each mixture was filtered through a 0.22 μm PTFE filter unit (13 mm) equipped with a 3 mL syringe. The filtrate was evaporated to dryness under a stream of nitrogen gas. One hundred microliters of water and 20 μL of 15 M ammonia were added to the dried hydrolysate, and the hydrolysate was reduced with 1 mL of 0.5 M sodium borohydride in dimethyl sulfoxide (DMSO) for 90 min 40°C. At the end of the reaction, the excess sodium borohydride was destroyed with 100 μL of 18 M acetic acid. Then, 200 μL of 1-methylimidazole and 2 mL of acetic anhydride were added and the mixture was incubated for 10 min at room temperature. To destroy the excess acetic anhydride, 5 mL of water was added and the mixture was allowed to cool. Alditol acetates were extracted from the mixture with 1 mL of dichloromethane (DCM) two times. The DCM extracts were combined and washed four times with 4 mL aliquots of water. Alditol acetates were obtained after evaporating the DCM under a stream of nitrogen gas. The resulting samples (i.e., alditol acetates) were dissolved in 2 mL of DCM, and 1 μL of each sample was injected for gas chromatography (GC) analysis. The alditol acetates were separated on a BPX70 column (SGE; 25 m×0.32 mm i.d.; 0.25 μm film thickness) in an Agilent 7890A gas chromatograph (Agilent Technologies, Loveland, CO, USA) equipped with an autosampler, splitter injection port (split ratio 10:1), and flame ionization detector (FID). The carrier gas was helium (1.2 mL/min). The initial oven temperature was set at 38°C and maintained for 30 s, increased to 170°C at 50°C/min, and then increased to 230°C at 2°C/min and held at 230°C for 5 min. The injection temperature was 230°C and the detector temperature was held at 250°C. The total run time was about 38 min.
Animals
Eight-week-old, male Sprague-Dawley (SD) rats with a body weight of approximately 300 g were purchased from Samtako Inc. (Osan, Korea). Experiments were carried out according to the Guide for the Care and Use of Laboratory Animals. Protocols were approved by the Institutional Animal Care and Research Advisory Committee of Catholic University, Daegu, Korea. Animals were housed with diet and water available ad libitum under diurnal lighting conditions, and in a temperature-controlled environment until the start of the experiment.
Diet administration
The rats were randomly assigned to one of the three groups: (1) sham (n=6), (2) control (n=6), and (3) WBE-treated (400 mg WBE/kg/day) (n=6). Rats in the WBE-treated group were fed 15 g of WBE diet per day for 5 days before and for 4 weeks after undergoing BCCAO injury. The WBE diet was prepared by mixing 8 g of WBE with 992 g of the diet. Once the rats consumed all the WBE diet, more diet was provided ad libitum. Rats in the sham and control groups, rats were fed the diet only. The body mass, diet consumption, and water consumption of each rat were measured daily throughout the experimental period.
Generation of BCCAO model
BCCAO was used as previously described (Fig. 1A) (17) to induce a moderate reduction of cerebral blood flow to the forebrain of rats in the control and WBE groups. Prior to BCCAO, the rats in all groups were anesthetized with isoflurane (Hana Pharmaceutical Inc., Seoul, Korea) in a mixture of oxygen/nitrous oxide (20%/80%) during surgical procedures. In the control and WBE-treated groups, the left and right common carotid arteries were exposed through a midline incision and ligated with 4-0 silk suture. In the sham group, the experimental procedures were the same, except that the common carotid arteries were not ligated. During surgery, rectal temperatures were maintained at 37±0.5°C with a thermostatically controlled warming plate (Harvard Apparatus, Holliston, MA, USA). After 4 weeks of BCCAO, the pupillary reflex of all rats was assessed. Subsequently, the rats were euthanized and the brains were harvested for the further study.
Assessment of pupillary light reflex (PLR)
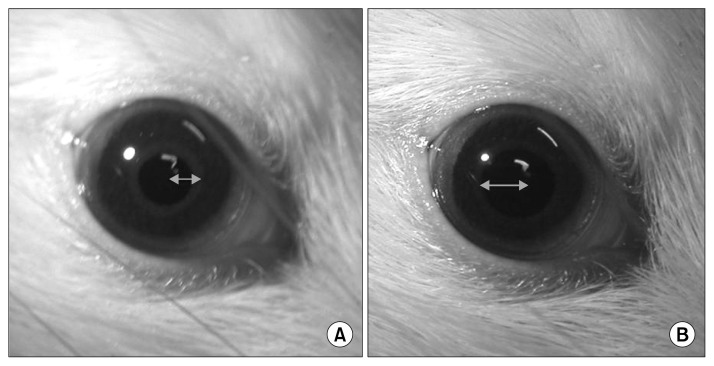
PLR was assessed as an indicator of optic nerve degeneration using a slightly modified adaptation of a previously described method (22). The pupillary reflexes of each rat were examined before surgery to confirm normal functioning. Briefly, each rat was adapted to darkness for at least 5 min. Then one eye was exposed to a beam of light from an otoscope and the reflex response was assessed. The rat was allowed to readapt to darkness for approximately 1 min and the reflex response of the other eye was assessed in the same way as the first. Loss of PLR was defined as failure of the pupil to constrict after a 10-sec exposure to light (Fig. 3).
Fig. 3.
Pupillary light reflex of a rat’s eye. When a rat has a normal pupillary light reflex, the pupil (A) constricts in the presence of light and (B) expands in the darkness.
Luxol Fast Blue staining
Injury to the white matter region of the brain was assessed by Luxol Fast Blue staining as previously described (17). Briefly, the brain was excised under anesthesia and cut into slices. The slices included the corpus callosum (cc), the internal capsule (ic), and the optic tract (opt) regions, which, according to the atlas of Paxinos and Watson (23), are located −2.64 to 3.14 mm from the bregma (Fig. 1B). The brain slices were fixed in formalin, embedded in paraffin, and cut into 5 μm sections. After deparaffinization, three sections from each rat brain were selected and stained with Luxol Fast Blue (24). The cc, ic, and opt regions of each section were identified (25) (Fig. 1B). The severity of injury in the three regions was assessed by an examiner blinded to the experimental conditions and graded as normal (Grade 0), presence of disarranged nerve fibers (Grade 1), formation of marked vacuoles (Grade 2), or absence of myelinated fibers (Grade 3) (26).
Immunohistochemical staining
Morphological changes to astrocytes and microglia in the white matter region were assessed by immunohistochemical staining as described previously (17). Five-micrometer thick sections were cut from paraffin-embedded brain slices. After deparaffinization, the sections were treated with 0.03% H2O2 and blocked in 1% bovine serum albumin and 5% normal serum. The sections were then incubated with primary antibodies against glial fibrillary acidic protein (GFAP, 1:100; BD Biosciences, San Diego, CA, USA) or ionized calcium binding adaptor molecule 1 (Iba1, 1:200; Wako, Osaka, Japan). After washing, the anti-GFAP treated sections were incubated in biotinylated horse anti-mouse IgG secondary antibody (1:200; Vector Laboratories, Inc., Burlingame, CA, USA) and the anti-Iba1 treated sections were incubated in HRP conjugated goat anti-rabbit IgG secondary antibody (1:200; Vector Laboratories, Inc.). Then all sections were incubated with an avidin-biotin peroxidase complex solution using the Vector ABC kit (Elite Vectastain ABC kit, Vector Laboratories, Inc.). The immunoreacted sections were visualized with a solution containing diaminobenzidine (DAB substrate; Roche Diagnostics Deutschland GmbH, Mannheim, Germany). Images of the immunostained sections were captured with an Olympus microscope (Olympus, Tokyo, Japan). The GFAP- and Iba1-positive cells were counted, and the relative values of GFAP- and Iba1-positive cells for the control and WBE-treated groups were determined by normalization to the mean number of GFAP- and IBA1-positive cells counted in the sham group sections.
Statistical analyses
Values are expressed as mean±SEM. A statistical analysis for multiple comparisons was performed with one-way ANOVA, followed by the Tukey post-hoc test. SPSS software (IBM SPSS Statistics version 19, IBM, Armonk, NY, USA) was used for all statistical analyses. P<0.05 was used as the criterion for statistical significance.
RESULTS
Effect of starch granule removal by decanter centrifugation on ara content
A decanter centrifuge was used to remove starch granules from wheat bran because this type of centrifuge can be used to separate a mixture containing a larger amount of solids and solids of a larger particle size than a disc stack centrifuge (Fig. 2) (27). Approximately half (46%) of the solids in the cold filtrate were removed by decanter centrifugation, which increased the ara content of the supernatant from 1.1% to 2.0%. Therefore, the use of decanter centrifugation to remove starch granules from wheat bran was a suitable method for increasing the AX concentration of WBE.
Characterization of WBE
The nutrient analysis revealed that WBE consists of 3.3 wt% moisture, 0.9 wt% fat, 15.2 wt% protein, 14.1 wt% ash, and 66.5 wt% carbohydrate. The dietary fiber analysis revealed that WBE also contains 11.8 wt% water-soluble dietary fiber, which represents 17.7% of the wt% value obtained for carbohydrate.
The following sequence of steps was used to assess the compositions of the neutral monosaccharides present in WBE: (1) TFA was used to hydrolyze WBE to its constituent monosaccharides; (2) The aldose monosaccharides generated were reduced to their corresponding alditols; (3) Alditols were acetylated to their corresponding alditol acetates; (4) The resultant alditol acetates were identified and quantified by gas chromatography, allose was used as an internal standard (21). The neutral monosaccharides composition of WBE is presented in Table 1. Of the monosaccharides detected, arabinose (2.42 wt%), xylose (3.78 wt%), and mannose (0.42 wt%) are derived only from the cell wall polysaccharides. Galactose and glucose are derived from nutrients and cell wall polysaccharides. Because TFA was used to hydrolyze WBE, cellulose was not hydrolyzed; thus, the glucose contained in cellulose was not included in the monosaccharide analysis. In total, 56% of the total dietary fiber detected in WBE was in the form of arabinose and xylose, which are components of AX, or mannose. The remaining 44% of the total dietary fiber present in WBE is likely composed of galactose, which is found in arabinogalactan, and glucose, which is found in β-glucan.
Table 1.
Neutral monosaccharide composition of WBE
| Mean (wt%) | SEM | |
|---|---|---|
| Rhamnose | 0.00 | 0.00 |
| Fucose | 0.00 | 0.00 |
| Ribose | 0.00 | 0.00 |
| Arabinose | 2.42 | 0.02 |
| Xylose | 3.78 | 0.05 |
| Mannose | 0.42 | 0.01 |
| Galactose | 2.27 | 0.03 |
| Glucose | 41.79 | 0.67 |
| Total | 50.68 | 0.77 |
Glucose is derived from starch and β-glucan.
Beneficial effect of WBE on white matter injury
To assess the beneficial effect of WBE on white matter injury, rats were fed a diet containing WBE (400 mg WBE/kg/day) for 5 days before and for 4 weeks after undergoing BCCAO injury. The dosage of WBE (400 mg/kg/day) was chosen based on a previous finding that showed a protective effect of 400 mg TALE/kg/day in the same BCCAO model (17). After 4 weeks of BCCAO, there was no significant difference in weight gain, daily diet consumption, or daily water consumption among groups. For example, the average body weight of rats in the sham, control, and WBE-treated groups was 424± 13.3 g, 422±2.0 g, and 416±4.0 g, respectively (P>0.05, n=6). Luxol Fast Blue staining was used to stain the myelin present in formalin-fixed brain sections. Among the white matter regions examined in this study, previous work indicates that the opt region is the most vulnerable to BCCAO injury, due to its dependence on the direct supply of blood from the internal carotid artery (Fig. 1A). The cc region is also susceptible to damage, whereas the ic region appears to be better protected (10). Thus, we focused on the damage that occurred in the opt and cc regions of the brain.
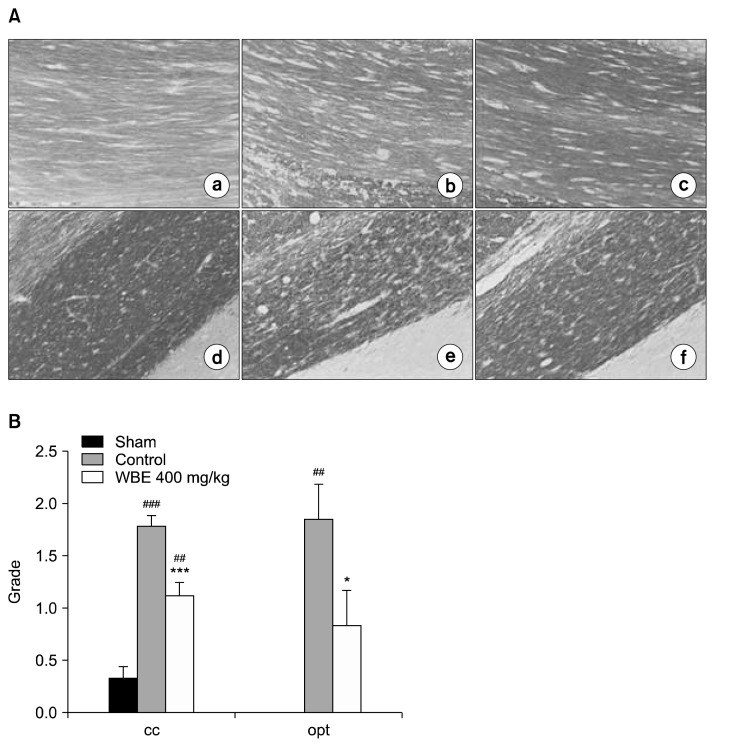
In the qualitative assessment, representative sections from the control group showed that BCCAO caused white matter injury in the cc and opt regions. The BCCAO-induced injury involved disarrangement and disappearance of the axons and myelin sheaths of nerve fibers, which led to white matter rarefaction, including vacuolization (Fig. 4A). Similar signs of injury were observed in the TALE-supplemented rats of our previous study (17). Representative sections from the WBE-treated group showed that supplementation with WBE attenuated BCCAO-induced injury (Fig. 4A). In the quantitative assessment, the severity of white matter injury was scored from Grade 0 to Grade 3. In the cc region, white matter injury was significantly reduced by 36.9% in the WBE-treated group compared with the control group (1.13±0.13 vs. 1.79±0.11, P<0.001) (Fig. 4B). In the opt region, white matter injury was significantly reduced by 56.1% in the WBE-treated group compared with the control group (0.83±0.34 vs. 1.89±0.34, P<0.05).
Fig. 4.
Luxol Fast Blue staining of white matter. (A) Photomicrographs of Luxol Fast Blue-stained white matter sections (200×): (a), (d) sham group; (b), (e) control group; (c), (f) WBE-treated group; (a)–(c) and (d)–(f) depict the corpus callosum (cc) and optic tract (opt) regions, respectively. (B) Quantitative analysis of the extent of white matter damage (n=6 rats per group). The white matter damage in the cc and opt regions were graded as normal (Grade 0), presence of disarranged nerve fibers (Grade 1), formation of marked vacuoles (Grade 2), and absence of myelinated fibers (Grade 3). ***P<0.001, *P<0.05 vs. control group. ###P<0.001, ##P<0.01 vs. sham group.
Effect on PLR
Because ischemic damage to the optic nerve causes loss of PLR in the BCCAO model (22) and WBE supplementation attenuated the damage observed in the opt region of the brains in this study, we assessed whether or not WBE supplementation could improve the PLR. In the sham group, 6 (100%) rats were positive for the PLR in both eyes (Table 2). However, in the control group, 3 (50%) rats lost the PLR in both eyes, 2 (33%) rats lost the PLR in one eye, and only 1 (17%) rat retained the PLR in both eyes. In contrast, in the WBE-treated group, 2 (33%) rats lost the PLR in both eyes, 1 (17%) rat lost the PLR in one eye, and 3 (50%) rats retained the PLR in both eyes. Thus, WBE intake-related preservation of white matter in the optic tract is associated with improvement of the PLR.
Table 2.
Effect of wheat bran extract (WBE) intake on pupillary light reflex (PLR)
| Experimental group | Loss of PLR in both eyes | Loss of PLR in one eye | PLR maintained in both eyes |
|---|---|---|---|
| Sham | 0 (0%) | 0 (0%) | 6 (100%) |
| Control | 3 (50%) | 2 (33%) | 1 (17%) |
| WBE (400 mg/kg/day) | 2 (33%) | 1 (17%) | 3 (50%) |
Effect of WBE on astrocytic activation
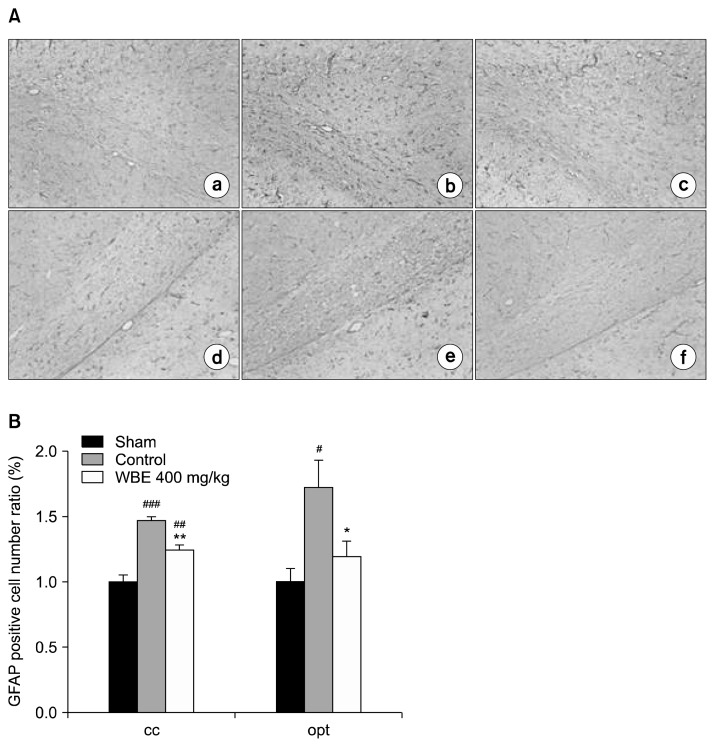
Because cerebral ischemia generated by chronic hypoperfusion triggers astrocytic activation (i.e., astroglial activation), which is manifested by an increase in intermediate filaments, cellular hypertrophy, and astrocyte proliferation (10,28,29), we assessed astrocytic activation using immunohistochemical staining against GFAP, an astrocyte-specific intermediate filament (30). In the qualitative assessment, representative sections from the control group showed that BCCAO increased the number of GFAP-positive astrocytes, and that each positive cell from the control group was more intensely immunostained than those from the opt and cc regions of the sham group (Fig. 5A). These observations are consistent with previous reports that astrocytic activation involves cellular hypertrophy and proliferation (17). On the other hand, representative sections from the WBE-treated group showed that supplementation with WBE reduced the number of GFAP-positive cells, and that each stained cell was less intensely immunostained than those from the opt and cc regions of the control group (Fig. 5A). In the quantitative assessment, the extent of astrocytic proliferation was evaluated by counting the number GFAP-positive cells (Fig. 5B) in each section. The number of GFAP-positive cells was significantly attenuated in the WBE-treated group compared with the cc (1.24±0.04 vs. 1.47±0.04, P<0.01) and opt (1.20±0.12 vs. 1.72±0.21 P<0.05) regions from the control group. These findings indicate that supplementation with WBE attenuates astrocytic activation in the cc and opt regions.
Fig. 5.
Immunochemical staining of astrocytes for GFAP. (A) Photomicrographs of white matter sections showing immunochemical staining of astrocytes for GFAP (100×): (a), (d) sham group; (b), (e) control group; (c), (f) WBE-treated group; (a)–(c) and (d)–(f) depict the corpus callosum (cc) and optic tract (opt) regions, respectively. (B) Quantitative analysis of GFAP-positive cells. The relative numbers of GFAP-positive cells in the cc and opt regions were determined by normalization to the number of GFAP-positive cells detected in the sham group (n=6 rats per group). **P<0.01, *P<0.05 vs. control group. ###P<0.001, ##P<0.01, #P<0.05 vs. sham group.
Effect of WBE on microglial activation
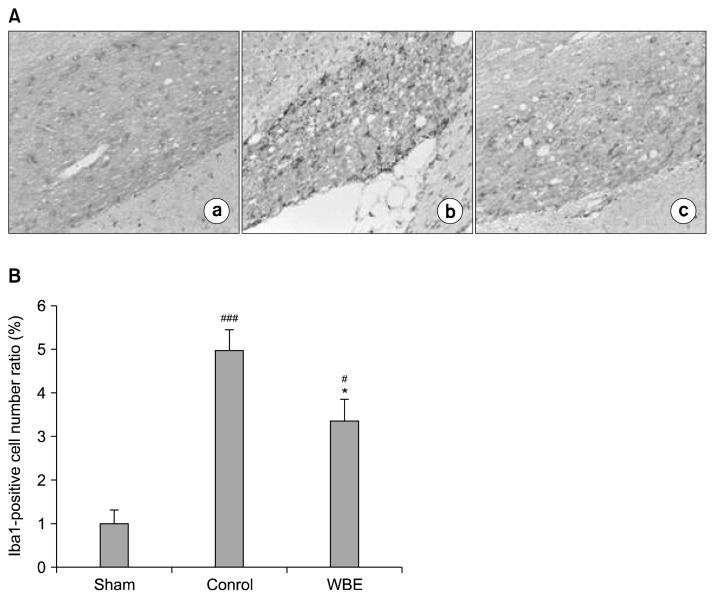
Because cerebral ischemia generated by chronic hypoperfusion induces rapid microglial activation that involves morphological changes and proliferation (10,31), we assessed microglial activation using immunohistochemical staining against Iba1, a microglia-specific calcium-binding protein (32). In the qualitative assessment, representative sections from the control group showed that BCCAO increased the number of Iba1-positive microglia in the opt region, and that each positive cell was more intensely immunostained than those from the sham group (Fig. 6A). These observations were consistent with previous reports that microglial activation involves microglial hypertrophy and proliferation (31,33). On the other hand, representative sections from the WBE-treated group showed that supplementation with WBE reduced the number of Iba1-positive cells and that each positive cell was less intensely immunostained than those from the opt region of the control group (Fig. 6A). In the quantitative assessment, the extent of microglial proliferation was evaluated by counting Iba1-positive cells (Fig. 6B). The number of Iba1-positive cells was significantly attenuated in the opt region of the WBE-treated group compared with the opt region of the control group (3.35±0.49 vs. 4.95±0.49, P<0.05). These findings indicate that supplementation with WBE attenuates microglial activation in the opt region.
Fig. 6.
Immunochemical staining of microglia for Iba1. (A) Photomicrographs of white matter sections showing immunochemical staining of microglia for Iba1 (200×): (a) sham group; (b) control group; (c) WBE-treated group; (a)–(c) depict the optic tract region (opt). (B) Quantitative analysis of Iba1-positive cells. The relative numbers of Iba1-positive cells in the opt region were determined by normalization to the number of Iba1-positive cells detected in the sham group (n=6 rats per group). *P<0.05 vs. control group. ###P<0.001, #P<0.05 vs. sham group.
DISCUSSION
In the present study, we showed that supplementation with WBE protected against white matter injury in the cc and opt regions of a rat BCCAO model. Attenuation of damage in the opt region was associated with improvement in PLR. This protection was accompanied by a reduction of astrocytic activation in the cc and opt regions, and by reduction of microglial activation in the opt region.
In our previous study, we prepared TALE by grinding whole wheat, extracting the ground wheat with hot water (approximately 90~95°C), centrifuging the extract in a batch-type centrifuge at 14,000 g for 30 min, and then drying the supernatant. We showed that supplementation with TALE (400 mg/kg/day), AX (20 mg/kg/day), and ara (50 mg/kg/day) attenuated white matter injury in a rat BCCAO model (17). However, when we tried to prepare TALE on a larger scale with a disc stack centrifuge, which is continuous-type centrifuge (27,34), we found that a lower residence time and clogging made it difficult to efficiently remove the gelatinized starch from the TALE. To solve the problem of removing starch, we first decided to substitute wheat bran for the whole wheat used in the previous study. Wheat bran was selected because it contains less starch than whole wheat, is a by-product of wheat milling and is therefore more affordable than whole wheat (35), and contains a comparable level of water-extractable AX (approximately 0.5 wt%) (16). Next, we decided to remove the starch from the wheat bran before the starch is gelatinized by heating (i.e., when they were still in granule form) because the starch granules can be easily removed from wheat bran by stirring in low temperature water (4°C) (36). A decanter centrifuge was used to remove the starch granules from the wheat bran because this type of centrifuge can be used to separate a mixture containing larger amounts of solids and larger-sized particles than a disc stack centrifuge (27). Decanters have been used to separate starch granules from wheat flour during the Alfa-Laval/Raisio process (19). Approximately half (46 wt%) of the solid in the filtrate was removed by applying decanter centrifugation. This increased the content of ara, a surrogate marker of AX, from 1.1 wt% to 2.0 wt%. Therefore, the use of decanter centrifugation to remove starch granules from wheat bran is a suitable procedure for increasing the concentration of AX, an active constituent, during WBE production. The final ara concentration of the WBE produced with this method was 2.42 wt%.
To investigate whether WBE might be effective at preventing vascular dementia, we chose a rat BCCAO model because this model mimics the pathological events occurring in subcortical vascular dementia (SVD) (8,9). Because one prominent feature of SVD is diffuse white matter damage, a reduction of white matter damage might be beneficial for preventing vascular dementia. Supplementation with WBE protected the cc and opt regions of white matter against the effects of BCCAO-induced chronic hypoperfusion and prevented loss of the PLR. Thus, protection of white matter in the opt region by supplementation with WBE might result in prevention of PLR loss because white matter injury in the opt region is associated with loss of the PLR (22,37). This beneficial effect is applicable to humans because the retinal ischemia that is observed in the BCCAO model can be caused by carotid artery disease (38).
During brain injury caused under ischemic conditions, microglia (i.e., the tissue macrophages of the brain) become activated first, which involves microglial hypertrophy and proliferation (29,31,39). In the present study, we showed that supplementation with WBE suppressed microglial activation in the opt region. This outcome can be explained by two different underlying mechanisms. Firstly, microglial activation may serve as an index of brain injury (29,40). Thus, attenuation of brain injury through WBE intake should result in the suppression of microglial activation. Secondly, microglial activation may act as a direct cause of brain injury. Overactivated microglia can induce highly neurotoxic effects by releasing excessive cytotoxic factors such as reactive oxygen species and proinflammatory cytokines (29,41). Furthermore, agents that suppress microglial activation can protect against brain ischemia. For example, minocycline, a tetracycline family antibiotic, and edaravone, a novel free radical scavenger, inhibit microglial activation, leading to a reduction of brain injury (10,42). Thus, suppression of microglial activation through WBE intake should attenuate brain injury. Cerebral ischemia also triggers astrocytic activation, which is manifested by an increase in the number of intermediate filaments, cellular hypertrophy, and astrocyte proliferation (10,28,29). In this study, we showed that supplementation with WBE suppressed astrocytic activation in the cc and opt regions. This finding may be associated with an attenuation of brain injury because astrocytic activation occurs in most pathologies of the brain and retina (28).
In our previous study, we showed that AX was an active constituent of WBE. However, wheat bran does contain phenolic compounds (43), and phenolic compounds prepared from green tea have exhibited beneficial effects in the same rat model used in this study (44). Thus, we explored the possibility that the phenolic compounds present in WBE may also contribute significantly to the observed beneficial effects. WB contains approximately 3~4 mg of gallic acid equivalents of phenolic compounds/g, of which ferulic acid is the major component (43). The 300 g rats used in this study consumed approximately 120 mg of WBE/day, which is equivalent to approximately 1.2 g of WB (10% WBE yield from WB). Therefore, the rats in this study consumed approximately 4~5 mg of phenolic compounds/day. However, each rat probably consumed less than 5 mg of phenolic compounds/day because the maximum calculated concentration of ferulic acid in WBE (3%; 4 mg ferulic acid/120 WBE) is much higher than the saturation concentration of ferulic compounds in 50°C water (0.2%) (45). A previously published manuscript reported that 4 to 8 weeks of supplementation with 120 mg of green tea polyphenols/300 g rat/day yielded beneficial effects in a rat model of BCCAO (44). As a result, it is most likely that the phenolic compounds in WBE did not contribute significantly to the observed beneficial effects because the amount of phenolic compounds (5 mg/day) consumed by the rats in this study was too small. Therefore, we conclude that the phenolic compounds present in WBE may not significantly contribute to the beneficial effects of WBE.
In summary, we developed a process for removing starch with a decanter centrifuge before the starch could be gelatinized by heating. Application of this process increased the concentration of arabinoxylan, an active constituent, in wheat bran extract (WBE). We also demonstrated that supplementation with WBE attenuates white matter injury in the cc and opt regions of the brain and suppresses astrocytic and microglial activation in a rat BCCAO model. These results indicate that WBE produced from wheat bran, a cheap by-product of wheat milling, can be developed as a nutraceutical to prevent vascular dementia.
ACKNOWLEDGEMENTS
This research was supported by the High Value-added Food Technology Development Program, Ministry of Agriculture, Food and Rural Affairs. We thank Yaesil Kim for the monosaccharide analysis of WBE. We also thank Yong Hyun Choi and Jina Park for their cooperation in producing the WBE.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflicts of interest.
REFERENCES
- 1.Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front Aging Neurosci. 2013;5:17. doi: 10.3389/fnagi.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, Luchsinger JA, Ogunniyi A, Perry EK, Potocnik F, Prince M, Stewart R, Wimo A, Zhang ZX, Antuono P. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomimoto H. Subcortical vascular dementia. Neurosci Res. 2011;71:193–199. doi: 10.1016/j.neures.2011.07.1820. [DOI] [PubMed] [Google Scholar]
- 4.Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Sucortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 5.Black S, Gao FQ, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40:S48–S52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- 6.Thal DR, Grinberg LT, Attems J. Vascular dementia: different forms of vessel disorders contribute to the development of dementia in the elderly brain. Exp Gerontol. 2012;47:816–824. doi: 10.1016/j.exger.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baskys A, Cheng JX. Pharmacological prevention and treatment of vascular dementia: approaches and perspectives. Exp Gerontol. 2012;47:887–891. doi: 10.1016/j.exger.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Jiwa NS, Garrard P, Hainsworth AH. Experimental models of vascular dementia and vascular cognitive impairment: a systematic review. J Neurochem. 2010;115:814–828. doi: 10.1111/j.1471-4159.2010.06958.x. [DOI] [PubMed] [Google Scholar]
- 9.Kitamura A, Fujita Y, Oishi N, Kalaria RN, Washida K, Maki T, Okamoto Y, Hase Y, Yamada M, Takahashi J, Ito H, Tomimoto H, Fukuyama H, Takahashi R, Ihara M. Selective white matter abnormalities in a novel rat model of vascular dementia. Neurobiol Aging. 2012;33:1012.e25–1012.e35. doi: 10.1016/j.neurobiolaging.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Farkas E, Luiten PGM, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: A model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev. 2007;54:162–180. doi: 10.1016/j.brainresrev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Hainsworth AH, Markus HS. Do in vivo experimental models reflect human cerebral small vessel disease? a systematic review. J Cereb Blood Flow Metab. 2008;28:1877–1891. doi: 10.1038/jcbfm.2008.91. [DOI] [PubMed] [Google Scholar]
- 12.Dexter JE, Wood PJ. Recent applications of debranning of wheat before milling. Trends Food Sci Technol. 1996;7:35–41. [Google Scholar]
- 13.Shewry PR, Hawkesford MJ, Piironen V, Lampi AM, Gebruers K, Boros D, Andersson AAM, Aman P, Rakszegi M, Bedo Z, Ward JL. Natural variation in grain composition of wheat and related cereals. J Agric Food Chem. 2013;61:8295–8303. doi: 10.1021/jf3054092. [DOI] [PubMed] [Google Scholar]
- 14.Guillon F, Tranquet O, Quillien L, Utille JP, Ortiz JJO, Saulnier L. Generation of polyclonal and monoclonal antibodies against arabinoxylans and their use for immunocytochemical location of arabinoxylans in cell walls of endosperm of wheat. J Cereal Sci. 2004;40:167–182. [Google Scholar]
- 15.Rosenfelder P, Eklund M, Mosenthin R. Nutritive value of wheat and wheat by-products in pig nutrition: A review. Animal Feed Sci Technol. 2013;185:107–125. [Google Scholar]
- 16.Gebruers K, Dornez E, Bedõ Z, Rakszegi M, Frás A, Boros D, Courtin CM, Delcour JA. Environment and genotype effects on the content of dietary fiber and its components in wheat in the HEALTHGRAIN diversity screen. J Agric Food Chem. 2010;58:9353–9361. doi: 10.1021/jf100447g. [DOI] [PubMed] [Google Scholar]
- 17.Han HS, Jang JH, Jang JH, Choi JS, Kim YJ, Lee C, Lim SH, Lee HK, Lee JW. Water extract of Triticum aestivum L. and its components demonstrate protective effect in a model of vascular dementia. J Med Food. 2010;13:572–578. doi: 10.1089/jmf.2009.1242. [DOI] [PubMed] [Google Scholar]
- 18.Goesaert H, Brijs K, Veraverbeke WS, Courtin CM, Gebruers K, Delcour JA. Wheat flour constituents: how they impact bread quality, and how to impact their functionality. Trends Food Sci Technol. 2005;16:12–30. [Google Scholar]
- 19.Sayaslan A. Wet-milling of wheat flour: industrial processes and small-scale test methods. LWT–Food Sci Technol. 2004;37:499–515. [Google Scholar]
- 20.KFDA. Korea Food Standards Codex. Korea Food and Drug Administration; Chungbuk, Korea: 2008. [Google Scholar]
- 21.Melton LD, Smith BG. Determination of neutral sugars by gas chromatography of their alditol acetates. Current Protocols in Food Analytical Chemistry. 2001:E3.2.1–E3.2.13. [Google Scholar]
- 22.Stevens WD, Fortin T, Pappas BA. Retinal and optic degeneration after chronic carotid ligation: Time course and role of light exposure. Stroke. 2002;33:1107–1112. doi: 10.1161/01.str.0000014204.05597.0c. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. Academic Press Inc; Orland, FL, USA: 1998. Figure 31. [Google Scholar]
- 24.Pistorio AL, Hendry SH, Wang X. A modified technique for high-resolution staining of myelin. J Neurosci Methods. 2006;153:135–146. doi: 10.1016/j.jneumeth.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Cho KO, La HO, Cho YJ, Sung KW, Kim SY. Minocycline attenuates white matter damage in a rat model of chronic cerebral hypoperfusion. J Neurosci Res. 2006;83:285–291. doi: 10.1002/jnr.20727. [DOI] [PubMed] [Google Scholar]
- 26.Wakita H, Tomimoto H, Akiguchi I, Kimura J. Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: an immunohistochemistry. Acta Neuropathol. 1994;87:484–492. doi: 10.1007/BF00294175. [DOI] [PubMed] [Google Scholar]
- 27.Milledge JJ, Heaven S. A review of the harvesting of micro-algae for biofuel production. Rev Environ Sci Biotechnol. 2013;12:165–178. [Google Scholar]
- 28.Pekny M, Wilhelmsson U, Pekna M. The dual role of astrocyte activation and reactive gliosis. Neurosci Lett. 2014;565:30–38. doi: 10.1016/j.neulet.2013.12.071. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D, Hu X, Qian L, O’Callaghan JP, Hong JS. Astrogliosis in CNS pathologies: is there a role for microglia? Mol Neurobiol. 2010;41:232–241. doi: 10.1007/s12035-010-8098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- 31.Zielasek J, Hartung HP. Molecular mechanisms of microglial activation. Adv Neuroimmunol. 1996;6:191–222. doi: 10.1016/0960-5428(96)00017-4. [DOI] [PubMed] [Google Scholar]
- 32.Imai Y, Kohsaka S. Intracellular signaling in M-CSF-induced microglia activation: role of Iba1. Glia. 2002;40:164–174. doi: 10.1002/glia.10149. [DOI] [PubMed] [Google Scholar]
- 33.Ziebell JM, Adelson PD, Lifshitz J. Microglia: dismantling and rebuilding circuits after acute neurological injury. Metab Brain Dis. 2014 doi: 10.1007/s11011-014-9539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutherland K. Filtration and separation technology: what’s new with centrifuges? Filtr Sep. 2009;46:30–32. [Google Scholar]
- 35.Swennen K, Courtin CM, Lindemans GCJE, Delcour JA. Large-scale production and characterization of wheat bran arabinoxylooligosaccharides. J Sci Food Agric. 2006;86:1722–1731. [Google Scholar]
- 36.Zhou S, Liu X, Guo Y, Wang Q, Peng D, Cao L. Comparison of the immunological activities of arabinoxylans from wheat bran with alkali and xylanase-aided extraction. Carbohydr Polym. 2010;81:784–789. [Google Scholar]
- 37.Davidson CM, Pappas BA, Stevens WD, Fortin T, Bennett SA. Chronic cerebral hypoperfusion: loss of pupillary reflex, visual impairment and retinal neurodegeneration. Brain Res. 2000;859:96–103. doi: 10.1016/s0006-8993(00)01937-5. [DOI] [PubMed] [Google Scholar]
- 38.Minhas G, Morishita R, Anand A. Preclinical models to investigate retinal ischemia: advances and drawbacks. Front Neurol. 2012;3:75. doi: 10.3389/fneur.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinstein JR, Koerner IP, Möller T. Microglia in ischemic brain injury. Future Neurol. 2010;5:227–246. doi: 10.2217/fnl.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perry VH, Nicoll JAR, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 41.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 42.Kawabori M, Yenari MA. The role of the microglia in acute CNS injury. Metab Brain Dis. 2014 doi: 10.1007/s11011-014-9531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim KK, Tsao R, Yang R, Cui SW. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006;95:466–473. [Google Scholar]
- 44.Xu Y, Zhang JJ, Xiong L, Zhang L, Sun D, Liu H. Green tea polyphenols inhibit impairment induced by chronic cerebral hypoperfusion via modulating oxidative stress. J Nutr Biochem. 2010;21:741–748. doi: 10.1016/j.jnutbio.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Mota FL, Queimada AJ, Pinho SP, Macedo EA. Aqueous solubility of some natural phenolic compounds. Ind Eng Chem Res. 2008;47:5182–5189. [Google Scholar]