Abstract
The bioactive compounds and antioxidant activity of aronia leaves at different stages of maturity were identified and evaluated. Young and old leaves were approximately 2 months of age and 4 months of age, respectively. The young leaves contained more polyphenols and flavonoids than the old leaves. Three phenolic compounds (i.e., chlorogenic acid, p-coumaric acid, and rutin) were detected by HPLC. Antioxidant activity was measured using 2,2-di-phenyl-1-picrylhydrazyl (DPPH) radical, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical, and superoxide anion radical scavenging assays. The reducing power of aronia leaf extracts increased in a concentration-dependent manner (0~100 μg/mL). The antioxidant activity of the 80% ethanol extract was greater than that of distilled water extract. The high phenolic compound content indicated that these compounds contribute to antioxidant activity. The overall results indicate that aronia leaves contain bioactive compounds, and that younger aronia leaves may be more favorable for extracting antioxidative ingredients because they contain more polyphenols.
Keywords: aronia leaves, phenolic, flavonoids, carotenoids, antioxidant activity
INTRODUCTION
Aronia (Aronia melanocarpa, commonly called the black chokeberry, wild gooseberry, or dogberry) belongs to the Rosacea family and is a shrub that is native to North America. Aronia was introduced and became popular in Europe about a century ago (1–3). The cultivation of aronia is becoming more popular because components of the plant contain several useful bioactive compounds. The aronia plant is known to be one of the richest natural sources of polyphenols such as hydroxycinnamic acid, flavanols, and anthocyanin (1,4,5).
During the past few years, many studies have been conducted on aronia because of its health-related properties. Aronia has been used as an antioxidant, an anti-atherosclerotic drug, an antidiabetic agent, an anti-inflammatory agent, an antiviral agent, and an antimutagenic agent (2,6). It also has antiproliferative effects on various solid tumor models (7) and anticancer, chemo-preventive effects on the appearance and growth of cancer stem cells (3,4). Many of the previous studies focused on the juice of the aronia fruit (2–4). However, the aronia wastes obtained after juice extraction contain many phenolic compounds, including anthocyanins (8). Although the bioactivity of the aronia fruit has been well characterized, there have been few studies investigating the bioactivity of aronia leaves.
Aronia leaves, which are affordable and an abundant raw material, are byproducts of aronia grove farming and accumulate during the pruning of aronia trees. They are expected to contain bioactive compounds that have various applications in the cosmetic, therapeutic, and food industries. Previous studies on aronia have reported that the leaves of several aronia species [e.g., Rubus ulmifolius (9) and Crataegus aronia (10)] are used in traditional medicine because of their anti-inflammatory, antiviral, antimicrobial, and antiproliferative activities against cancer cells. Therefore, aronia leaves might contain bio-active compounds and have biological effects resulting from the polyphenols, flavonoids, and chlorophylls that they contain. They are also expected to have a positive effect on human health as potential sources of natural antioxidants.
During leaf maturation, changes in the oxidative metabolism of plant tissues occur (11,12). The accumulation and export of products also changes throughout leaf development. Therefore, the bioactive compounds and the antioxidant activity of aronia leaves collected at different stages of maturity (i.e., young and old) were measured in this study. Furthermore, the effects of different solvents on the extraction of bioactive compounds from aronia leaves were determined. The bioactive compound contents of distilled water extracts and 80% ethanol extracts from aronia leaves collected at two times of harvest were measured. Several assays were used to evaluate the antioxidative properties of each extract.
MATERIALS AND METHODS
Chemicals
Folin-Ciocalteu’s phenol reagent, DPPH, ABTS, gallic acid, catechin, nitroblue tetrazolium chloride (NBT), nicotinamide adenine dinucleotide (NADH), Tris-HCl, potassium hexacyanoferrate, trichloroacetic acid, ferric chloride, and para-methyl styrene (PMS) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Polyphenol standards (i.e., chlorogenic acid, p-coumaric acid, rutin, quercetin, and catechin) for HPLC analysis were also purchased from Sigma-Aldrich Co. HPLC-grade water, methanol, acetonitrile, and trifluoroacetic acid (TFA) were purchased from Fisher Scientific Company Llc. (Fair Lawn, NJ, USA). All chemicals used in the described experiments were of an analytical grade.
Sample preparation
Leaves of the ‘Nero’ cultivar of aronia were manually picked from a local farm in Korea. Aronia leaves were picked at different stages of growth (i.e., young or old, depending on the sampling date). Young and old leaves were approximately 2 months of age and 4 months of age, respectively. The picked leaves were free from insect and mechanical damage. After washing, the leaves were frozen at −80°C overnight and freeze-dried for 2 days. The freeze-dried samples were finely ground in a food grinder (Hanil, Seoul, Korea) and stored at −80°C until extraction.
The powdered samples were extracted with distilled water at 100°C or 80% ethanol at 85°C for 2 h. Briefly, the powdered samples were mixed with distilled water or 80% ethanol at a ratio of 1:25 (g/mL), and the bio-active compounds were extracted. Then, the supernatant was saved and filtered through Whatman no. 2 filter paper (Whatman International Ltd., Maidstone, UK) with a vacuum filter; this process was repeated in triplicate. The extracted filtrate was then evaporated using a rotary evaporator (EYELA, Tokyo, Japan) under reduced pressure at 40°C. After evaporation, 50 mL of distilled water was added to the evaporated solution. The solution was then freeze-dried (Ilshin Biobase Co., Ltd., Yangju, Korea) and stored at −20°C until analysis.
Determination of total chlorophyll and carotenoid contents
The chlorophyll and carotenoid contents of aronia leaves were analyzed using the method of Lichtenthaler and Buschmann (13). Freeze-dried powder (20 mg) was mixed with 5 mL of dimethyl sulfoxide (DMSO) and incubated at 65°C for 6 h. After incubation, the mixture was centrifuged at 15,000 g for 5 min. The supernatant was collected, and the absorbance was read at 663 nm to measure chlorophyll a content, 647 nm to measure chlorophyll b content, and 470 nm to measure carotenoid content. The chlorophyll and carotenoid concentrations were calculated using the following equations:
Determination of total polyphenol and flavonoid contents
The total polyphenol content was analyzed using Folin-Ciocalteu’s phenol reagent following the method of Zhou et al. (14). The total flavonoid contents of aronia leaves were analyzed by the method of Woisky and Salatino (15).
Extraction and quantification of polyphenols by HPLC
Powdered samples (0.1 g) of the water extracts and the 80% ethanol extracts were mixed with 5 mL of methanol containing 0.1% formic acid and vortexed for 1 min. Each mixture was then centrifuged for 5 min, and the upper fraction was transferred to another glass tube. The aqueous layer (lower fraction) was re-extracted using another 5 mL of methanol containing 0.1% formic acid. This extraction was performed 3~4 times until the extracts were colorless. The methanolic fractions were combined and evaporated in a rotary evaporator. The residue was redissolved in the extraction solvent at an appropriate concentration for HPLC analysis with an injection volume of 10 μL. Polyphenol concentrations were analyzed using HPLC (Ultimate 3000, Dionex, Sunnyvale, CA, USA) on an Agilent XDB C18 column (4.6×150 mm, 5 μm). The solvent system used was (A) water with 0.3% TFA and (B) acetonitrile. The samples were separated with the following gradient: A/B=95/5 (0~39 min), 40/60 (40 min), 0/100 (45~50 min), and 95/5 (55~60 min) at a flow rate of 0.8 mL/min. The peaks were detected with a UV/Visible Detector (190–800 DAD scanning; Waters Co., Milford, MA, USA) at 280 nm.
DPPH radical scavenging activity
The DPPH radical scavenging activities of distilled water extracts and 80% ethanolic extracts of aronia leaves were determined by the method of Cheung et al. (16) with minor modifications. First, a 192-μL solution of 50 μM DPPH was mixed with 48 μL of a diluted sample. The mixture was then covered with aluminum foil and incubated in the dark at room temperature for 30 min. The extent to which the DPPH had decolored was read at 517 nm with a microplate reader (Spectra MAX M2, Molecular Device, Sunnyvale, CA, USA). Distilled water was used as a blank. DPPH radical scavenging activity was calculated with the following equation:
where Ablank is the absorbance of the control reaction (containing all reagents except the test compound), and Asample is the absorbance of the sample reaction (containing all reagents including the test compound).
ABTS radical scavenging activity
The ABTS radical scavenging activity of the aronia leaf extracts was determined using the method of Re et al. (17) with minor modifications. First, ABTS was dissolved in distilled water to obtain a 7 mM ABTS stock solution. The ABTS radical cation (i.e., ABTS reagent) was produced by reacting the ABTS stock solution with 2.45 mM K2S2O8 (at a ratio of 2:1) in the dark and covered with aluminum foil for 24 h before use. The ABTS reagent was diluted with 94% ethanol until the absorbance of the solution at 734 nm reached 0.7±0.03. Then, 950 μL of the diluted ABTS reagent was mixed with 50 μL of various concentrations of the experimental samples. The mixture was covered with aluminum foil and incubated in the dark at room temperature for 10 min. Then the absorbance of the solution at 734 nm was measured with a microplate reader. Distilled water was used as a blank. Each sample was measured in triplicate, and the percent inhibition (%) was calculated using the following equation:
where Ablank is the absorbance of the control reaction (containing all reagents except the test compound), and Asample is the absorbance of the sample reaction (containing all reagents including the test compound).
Superoxide anion scavenging activity
The superoxide radical generated in the xanthine-xanthine oxidase system was determined spectrophotometrically by measuring NBT as the end product (18). The reaction mixture was prepared with 50 μL of each sample, 0.5 mL of a 1:1 mixture of 0.4 mM xanthine and 0.24 mM NBT, 0.5 mL of 0.049 U/mL xanthine oxidase, and distilled water (final volume: 2.0 mL). After incubation at 37°C for 40 min, 2 mL of 69 mM SDS was added to stop the reaction. The absorbance of the resulting solution was measured at 560 nm and compared with the absorbance of control samples that were run without xanthine oxidase. Ascorbic acid was used as the positive control. Each sample was measured in triplicate, and the percent inhibition (%) was calculated using the following equation:
where Ablank is the absorbance of the control reaction (containing all reagents except the test compound), and Asample is the absorbance of the sample reaction (containing all reagents including the test compound).
Reducing power activity
The Fe3+ reducing power of the extracts was determined using the method of Oyaizu (19) with minor modifications. Various concentrations of each sample (0.25 mL) were mixed with 0.25 mL of phosphate buffer (0.2 M, pH 6.6) and 0.25 mL of potassium hexacyanoferrate [K3Fe(CN)6] (1% w/v). After incubating the mixture at 50°C in a water bath for 20 min, the reaction was stopped by adding 0.25 mL of trichloroacetic acid solution (10% w/v). Then, the mixture was centrifuged at 15,000 g for 10 min. Subsequently, 0.5 mL of the supernatant was mixed with 0.5 mL of distilled water and 0.1 mL of a ferric chloride (FeCl3) solution (0.1% w/v) for 10 min. The absorbance of the resulting mixture was immediately measured at 700 nm with a microplate reader to determine the reducing power. Ascorbic acid (0 μg/mL to 200 μg/mL) was used to generate the standard curve for this assay.
Statistical analysis
All results are presented as the means±standard deviation. A statistical analysis system (SPSS software package, version 17.0, SPSS Inc., Chicago, IL, USA) was used to perform all statistical analyses. Data were compared by one-way analysis of variance; P<0.05 was considered significantly different.
RESULTS AND DISCUSSION
Total chlorophyll and carotenoid contents
The total chlorophyll and carotenoid contents of aronia leaves at different stages of growth are presented in Table 1. The old leaves contained more chlorophylls than the young leaves, but this difference was not significant. Both young and old leaves contained more chlorophyll a than chlorophyll b. The chlorophyll contents were higher in the 80% ethanol extracts from aronia leaves than in the distilled water extracts from aronia leaves. The highest total chlorophyll content was obtained from the old leaves extracted with 80% ethanol (66.32 mg/g dry weight). In contrast, the lowest chlorophyll content (8.48 mg/g dry weight) was obtained from the young leaves extracted with distilled water.
Table 1.
Chlorophyll and carotenoid contents of aronia leaves collected at different stages of growth (unit: mg/g dry weight)
| Growth stage | Extraction solvent | Chlorophyll a | Chlorophyll b | Total chlorophylls | Carotenoids |
|---|---|---|---|---|---|
| Young | Water | 4.76±0.94a | 3.00±0.24a | 8.48±0.56a | 1.36±0.04a |
| 80% ethanol | 41.72±1.04b | 11.20±0.08b | 58.40±1.48b | 9.36±0.20b | |
| Old | Water | 5.59±0.40a | 3.88±0.20a | 10.36±0.64a | 2.04±0.08a |
| 80% ethanol | 48.40±0.68b | 11.68±0.72b | 66.32±0.28b | 9.88±0.24b |
Data represent the means±SD of three separate experiments.
Within each column, values with different superscript letters are significantly different at P<0.05.
The total carotenoid content of the old leaves was slightly higher than that of the young leaves, but the difference was minimal. The total carotenoid contents of the 80% ethanol extracts of young and old leaves were 9.36 mg/g dry weight and 9.88 mg/g dry weight, respectively. With respect to carotenoid recovery, 80% aqueous ethanol was a more efficient extraction solvent than distilled water. For example, 6.88-fold and 4.84-fold more carotenoids were detected in the 80% ethanol extracts of young and old leaves than in the distilled water extracts of young and old leaves, respectively.
The chlorophyll and carotenoid contents of plant leaves vary according to several biotic factors, including species, variety, cultivar, production practice, maturity, and abiotic factors, including light, temperature, and soil properties (20–22). Žnidarčič et al. measured chlorophyll concentrations in leafy vegetables that are commonly consumed in Mediterranean countries and found that the total chlorophyll content of the vegetables ranged from 2.00 mg/g to 3.59 mg/g (21). Žnidarčič et al. also found that the concentration of chlorophyll a (1.42 mg/g to 2.61 mg/g) was greater than that of chlorophyll b (0.58 mg/g to 0.98 mg/g) in the vegetables tested (21). The results of the present study indicate that aronia leaves contain more total chlorophyll and carotenoids than other plant species and products.
Total polyphenol and flavonoid contents
The concentrations of total polyphenols and flavonoids in aronia leaves at different stages of growth are presented in Table 2. The highest total polyphenol content was obtained using the 80% ethanol extract. The young leaves tested in this study contained more polyphenols than the old leaves. The highest phenolic content [250.8 mg gallic acid equivalent (GAE)/g dry weight] was obtained from the young leaves extracted with 80% ethanol. In contrast, the lowest phenolic content (69.5 mg GAE/g dry weight) was obtained from the old leaves extracted with distilled water.
Table 2.
Total polyphenol and flavonoid contents of aronia leaves collected at different stages of growth
| Growing stage | Extraction solvent | Total polyphenols (mg GAE/g dry weight) | Total flavonoids (mg CE/g dry weight) |
|---|---|---|---|
| Young | Water | 141.6±0.9b | 110.7±1.5b |
| 80% ethanol | 250.8±2.4c | 163.7±1.0c | |
| Old | Water | 69.5±2.7a | 56.4±0.9a |
| 80% ethanol | 139.3±2.1ab | 103.6±1.8b |
Data represent the means±SD of three separate experiments.
Within each column, values with different superscript letters are significantly different at P<0.05.
GAE, gallic acid equivalent; CE, catechin equivalent.
The total flavonoid contents of the young leaves were higher than that of the old leaves. The total flavonoid contents of the 80% ethanol extracts of young and old leaves were 163.7 mg catechin equivalent (CE)/g dry weight and 103.6 mg CE/g dry weight, respectively. With regard to the extraction of flavonoid compounds, 80% aqueous ethanol was a better extraction solvent than distilled water. When compared to the distilled water extracts, the 80% ethanol extracts yielded 1.47-fold and 1.84-fold more flavonoids from the young and old leaves, respectively.
The antioxidant profile of aronia leaves varied throughout maturation. Specifically, the total phenolic and flavonoid contents of the young leaves were approximately twice those of the old leaves. During growth period, the plants synthesize the secondary metabolites and accumulate different amounts of the bioactive compounds (23).
In our previous research, the polyphenol and flavonoid contents of aronia fruit were measured and compared to the polyphenol and flavonoid contents of aronia leaves. The aronia leaves contained a large proportion of the total phenolic and flavonoid contents of the aronia plant. The total phenolic content of the aronia leaves was lower than that of the aronia fruit, while the total flavonoid content of the aronia leaves was approximately twice that of the aronia fruit (data not shown). In addition, the total phenol content of the aronia leaves was higher than that of the leaves of several plants, including Anacardium occidentale (58.57 mg/g), Mangifera indica (65 mg/g), Azadiracta indica (14.43 mg/g), Cymbopogon citratus (28.30 mg/g), and Carica papaya L. (21.80 mg/g) (24). These findings reveal the potential of aronia to become a useful and natural source of biologically active compounds.
Arabshahi-Delouee et al. (25) tested three different solvent extracts (i.e., water, methanol, and acetone) of mulberry leaves. The antioxidant activities, as measured by different assay systems, were affected by the solvent extract used. Arabshahi-Delouee et al. found that, compared to water and acetone, methanol was the most effective solvent for the extraction of polyphenols from mulberry leaves. The methanolic extracts of mulberry leaves contained the highest amount of total phenolics and had the highest radical scavenging activity, followed by the acetone and water extracts. Similar to our results, Arabshahi-Delouee et al. found that the antioxidant activity of an extract correlated to the amount of total phenolics present in the sample (25). The age of the plant materials was an important factor, which determined photosynthesis and metabolism of the plants (26).
Extraction and quantification of polyphenols by HPLC
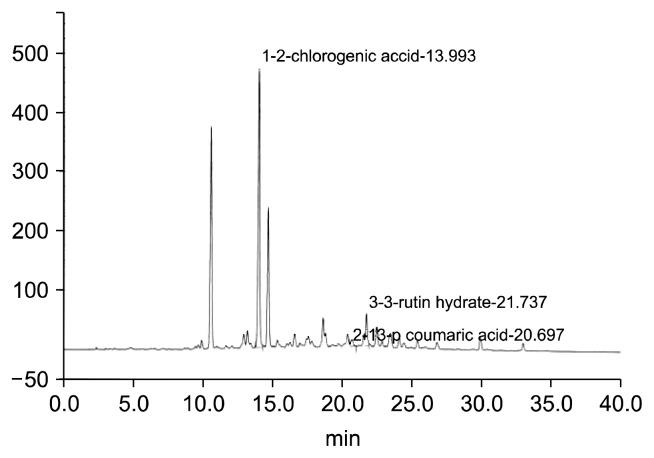
Fig. 1 shows a chromatogram of phenolic compounds in aronia leaf extracts. We detected three phenolic compounds, including chlorogenic acid, p-coumaric acid, and rutin, which had retention times of 14.0 min, 20.7 min, and 21.7 min, respectively. Chlorogenic acid was the major phenolic compound, followed by rutin and p-coumaric acid. Table 3 shows the concentrations of the detected polyphenols in the aronia leaves harvested at different stages of growth and extracted by distilled water or 80% ethanol. The different extraction solvents affected the polyphenol contents of the aronia leaf extracts. The chlorogenic acid concentrations of the distilled water and 80% ethanol extracts from aronia leaves were 17.2 mg/g and 22.8 mg/g, respectively. The rutin concentrations of the distilled water and 80% ethanol extracts from young leaves were 3.4 mg/g and 4.1 mg/g, respectively.
Fig. 1.
A typical HPLC chromatogram of the polyphenols in aronia leaf extracts.
Table 3.
Polyphenol contents of aronia leaves collected at different stages of growth (unit: mg/g dry weight)
| Extraction solvent | Growing stage | Chlorogenic acid | p-Coumaric acid | Rutin |
|---|---|---|---|---|
| Water | Young | 17.2±0.51b | ND1) | 3.4±0.05ab |
| Old | 9.9±0.23a | ND | 3.0±0.06a | |
| 80% ethanol | Young | 22.8±0.65c | 0.3±6.7a | 4.1±0.01b |
| Old | 10.8±0.26a | 0.1±5.8a | 2.9±0.01a |
Data represent the means±SD of three separate experiments.
Within each column, values with different superscript letters are significantly different at P<0.05.
ND, not determined.
The polyphenol contents of the extracts from old leaves were lower than those of the extracts from young leaves. Specifically, the distilled water and 80% ethanol extracts of old leaves contained 0.58-fold and 0.47-fold less chlorogenic acid, respectively, than the distilled water and 80% ethanol extracts of young leaves. The rutin concentrations of the distilled water and 80% ethanol extracts from old leaves were 3.0 mg/g dry weight and 2.9 mg/g dry weight, respectively. p-Coumaric acid was not detected in the distilled water extracts, but minimal amounts were detected in the 80% ethanol extracts.
Lee et al. (27) determined the polyphenol components of aronia leaves across three different stages of maturity (i.e., young, mature, and aged) using LC-tandem mass spectrometry. They quantified 12 polyphenols in fresh aronia leaves, namely caffeoylquinic acid isomer (356.4~6,659.4 mg/kg), apigenin 7,4′-di-O-rhamnoside (101.1~289.2 mg/kg), quercetin dirhamnosylhexoside (41.1~280.5 mg/kg), quercetin rhamnosylhexoside (19.1~77.1 mg/kg), dicaffeoylquinic acid (38.6~1,936.1 mg/kg), quercetin 3-O-vicianoside (90.0~363.2 mg/kg), quercetin 3-O-glucoside (59.2~130.3 mg/kg), quercetin 3-O-rutinoside (202.9~2,340.9 mg/kg), kaempferol coumaroyl glucoside (31.6~153.5 mg/kg), and isorhamnetin rhamnosylhexoside isomer (42.6~17,039 mg/kg) (28). They found that the type and content of polyphenols were influenced by maturity; the young leaves contained higher amounts of polyphenol compounds than the mature leaves and the aged leaves. This finding suggests that younger aronia leaves may be more favorable for processing into higher functioning antioxidative ingredients because they contain higher amounts of polyphenols.
Polyphenolic compounds can be found in all plants, as they are secondary metabolites. For example, HPLC analysis has revealed that olive plant (Olea europaea L.) leaves contain hydroxytyrosol, tyrosol, rutin, luteolin-7-glucoside, verbascoside, apigenin-7-glucoside, oleuropein, and luteolin (28). Eleven polyphenol compounds have been detected in Morus alba leaves (29). Nine poly-phenols have been identified in tobacco leaves, including 5-O-caffeoylquinic acid (3.3 mg), chlorogenic acid (21.5 mg), 4-O-caffeoyquinic acid (3.1 mg), caffeic acid (3.2 mg), esculetin (2.7 mg), chrysatropic acid (3.2 mg), rutin (27.8 mg), kaempferol (3.8 mg), and quercetin (3.7 mg) (30).
The 80% aqueous ethanol was a better solvent for safely extracting the antioxidant compounds from aronia leaves than distilled water. It is possible that aronia leaves contain diverse phenolic compounds with a range of polarities. Therefore, the solvents used for these experiments may have only extracted phytochemicals from the aronia leaves.
Antioxidant activity
The radical scavenging capacities of aronia leaves were determined by measuring the DPPH, ABTS, and super-oxide anion radical scavenging activities and the reducing power activities of each extract in vitro. Differences between the radical scavenging potentials of young and old leaves were found in the current study.
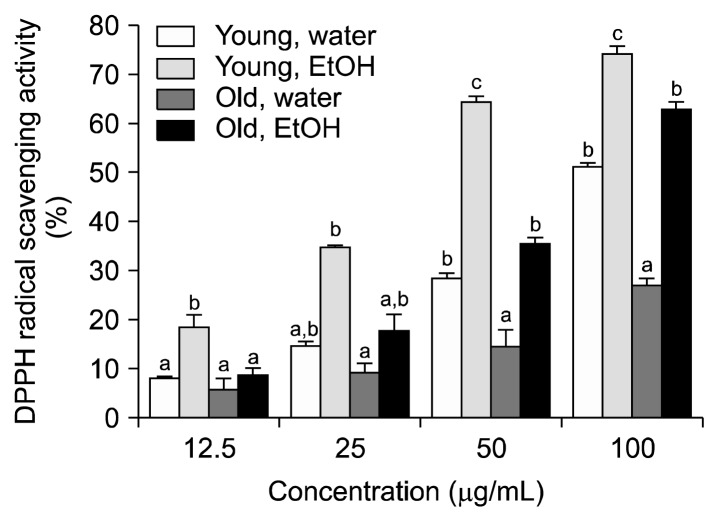
The DPPH radical scavenging activities of aronia leaves at different stages of growth are shown in Fig. 2. The DPPH radical scavenging activity of the distilled water and 80% ethanol extracts of all samples increased in a concentration-dependent manner (12.5~100 μg/mL). The extracts from young leaves and the 80% ethanol extracts had greater DPPH radical scavenging activities than the extracts from old leaves and the distilled water extracts. On average, 50 μg/mL concentrations of the distilled water and 80% ethanol extracts of young leaves were associated with a 28.5% and 64.4% inhibition of the DPPH radical, respectively, whereas the distilled water and 80% ethanol extracts of old leaves were associated with a 14.6% and 35.3% inhibition of the DPPH radical, respectively. At the 100 μg/mL concentration, the strongest DPPH radical scavenging activity (i.e., 74.2%) was observed in the 80% ethanol extract from young leaves. In contrast, the lowest DPPH radical scavenging activity (i.e., 26.9%) at the 100 μg/mL concentration was observed in the distilled water extract from old leaves.
Fig. 2.
The DPPH radical scavenging activity of aronia leaves collected at different stages of growth. Data are the means±SD of three separate experiments. For each concentration, values with the same letters are not significantly different at P<0.05.
DPPH radical scavenging activity has been investigated in other plant leaf extracts, including Psidium guajava L. (50% at 460.37 μg/mL) (31), Melia azedarach Linn (68.38% at 60 μg/mL) (32), Liriope spicata L. (50% at 24.55~378.97 μg/mL) (33), blackberry species (50% at 186.0~414.0 μg/mL) (34), and Bridelia ferruginea (50% at 201.10 μg/mL) (35). These results indicate that the DPPH radical scavenging activity of leaves varies by species. The results of the present study indicate that the DPPH radical scavenging activities of extracts from aronia leaves are higher than those of leaves from the aforementioned species, with the exception of leaves from Liriope spicata L. and Melia azedarach L. DPPH radical scavenging capacity is dependent on leaf species and extraction solvent (36). In the current study, 80% ethanol is preferred over distilled water as a solvent for the extraction of antioxidant compounds. This finding is similar to that reported by Ahmed et al. (32).
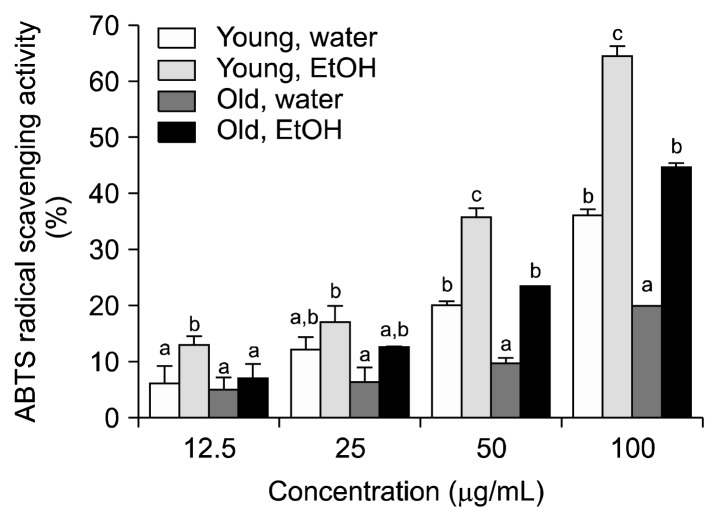
The ABTS radical scavenging activity of aronia leaves is shown in Fig. 3. ABTS radical scavenging activity increased in a concentration-dependent manner (12.5~100 μg/mL). At all concentrations tested, ABTS radical scavenging activities (i.e., the ability of a sample to inhibit the oxidant compound) were higher in the 80% ethanol extracts of aronia leaves than in the distilled water extracts of aronia leaves. At the 50 μg/mL concentration, the average ABTS radical scavenging activities of the distilled water and 80% ethanol extracts of young leaves were 20.1% and 35.9%, respectively, and the average ABTS radical scavenging activities of the distilled water and 80% ethanol extracts of old leaves were 9.5% and 23.4%, respectively. The highest inhibition of the ABTS radical was found in the 80% ethanol extracts of young leaves (64.2% at 100 μg/mL). The distilled water extracts of old leaves had the lowest inhibitory effect on the ABTS radical (20.1% at 100 μg/mL). ABTS radical scavenging activities were lower than DPPH radical scavenging activities for the extracts tested in this study. The ABTS radical scavenging assay is one of the most common assays used to measure the antioxidant activity of plants. Several published papers have reported ABTS radical scavenging activities from other plant leaves, including Camellia sinensis (50% at 0.17~0.19 mg/mL) (36), Celtis africana (>80% at 0.02 mg/mL) (37), Solanum surattense (50% at 89.28 μg/mL) (38), and Annona species (50% at 206~3,051 μg/mL) (39).
Fig. 3.
The ABTS radical scavenging activity in of aronia leaves collected at different stages of growth. Data are the means±SD of three separate experiments. For each concentration, values with the same letters are not significantly different at P<0.05.
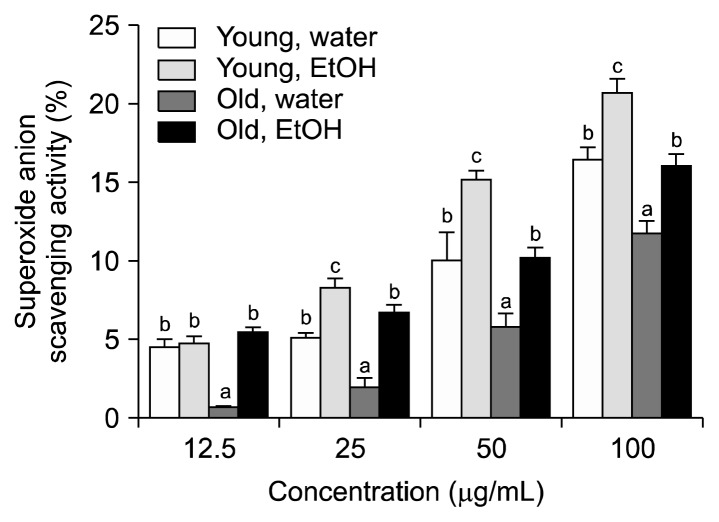
The superoxide anion scavenging activities of aronia leaves collected at different stages of growth are shown in Fig. 4. The inhibitory activities of the 80% ethanol extracts of aronia leaves were higher than those of the distilled water extracts of aronia leaves. The overall inhibitory effects of the aronia leaves against the super-oxide anion were weaker than the DPPH and ABTS radical scavenging activities of the aronia leaf extracts tested in the present study. At a 100 μg/mL concentration, the distilled water and 80% ethanol extracts of young aronia leaves inhibited superoxide anion formation by 16.3% and 20.5%, respectively. At the same concentration, the distilled water and 80% ethanol extracts of old leaves were 11.6% and 16.0%, respectively.
Fig. 4.
The superoxide anion radical scavenging activity of aronia leaves collected at different stages of growth. Data are the means±SD of three separate experiments. For each concentration, values with the same letters are not significantly different at P<0.05.
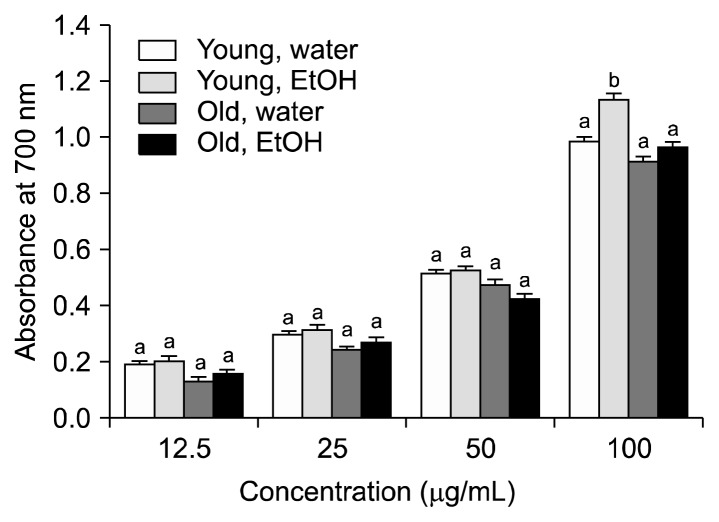
The reducing power activities of aronia leaves at different stages of maturity are presented in Fig. 5. In the reducing power assay, Fe3+ was reduced to Fe2+ in the presence of an antioxidant (i.e., aronia leaf extracts); high absorbance values indicated a strong reducing power (19). The absorbance of the young and old aronia leaves increased in a concentration-dependent manner, thus indicating that higher concentrations of the extracts were associated with higher reducing power activities. The 80% ethanol extracts of aronia leaves had greater reducing power activities than the distilled water extracts of aronia leaves. There were only minimal differences in the reducing power of aronia leaves collected at different stages of maturity (i.e., young vs. old leaves).
Fig. 5.
The reducing power activity of aronia leaves collected at different stages of growth. Data are the means±SD of three separate experiments. For each concentration, values with the same letters are not significantly different at P<0.05.
The data from most assays showed the concentration-dependent manner of aronia leave extracts, with the exception of the hydroxyl radical scavenging assay in the 80% ethanol extract samples. The 80% ethanol extracts of young aronia leaves had the highest effect on radical scavenging activity. This result indicates that there is a possible relationship between the antioxidant activity levels and the total phenolic and flavonoid contents of plant extracts (40,41).
ACKNOWLEDGEMENTS
This research was supported by the High Value-added Food Technology Development Program (112078-3), the Ministry of Agriculture, Food and Rural Affairs, Korea.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Kulling SE, Rawel HM. Chokeberry (Aronia melanocarpa)–A review on the characteristic components and potential health effects. Planta Med. 2008;74:1625–1634. doi: 10.1055/s-0028-1088306. [DOI] [PubMed] [Google Scholar]
- 2.Sainova I, Pavlova V, Alexieva B, Vavrek I, Nikolova E, Valcheva-Kuzmanova S, Markova T, Krachanova M, Denev P. Chemoprotective, antioxidant and immunomodulatory in vitro effects of Aronia melanocarpa total extract on laboratory-cultivated normal and malignant cells. J Biosci Biotech. 2012:35–43. SE/ONLINE. [Google Scholar]
- 3.Kim JH, Auger C, Kurita I, Anselm E, Rivoarilala LO, Lee HJ, Lee KW, Schini-Kerth VB. Aronia melanocarpa juice, a rich source of polyphenols, induces endothelium-dependent relaxations in porcine coronary arteries via the redox-sensitive activation of endothelial nitric oxide synthase. Nitric Oxide. 2013;35:54–64. doi: 10.1016/j.niox.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Sharif T, Stambouli M, Burrus B, Emhemmed F, Dandache I, Auger C, Etienne-Selloum N, Schini-Kerth VB, Fuhrmann G. The polyphenolic-rich Aronia melanocarpa juice kills teratocarcinomal cancer stem-like cells, but not their differentiated counterparts. J Funct Foods. 2013;5:1244–1252. [Google Scholar]
- 5.Malinowska J, Babicz K, Olas B, Stochmal A, Oleszek W. Aronia melanocarpa extract suppresses the biotoxicity of homocysteine and its metabolite on the hemostatic activity of fibrinogen and plasma. Nutrition. 2012;28:793–798. doi: 10.1016/j.nut.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Chrubasik C, Li G, Chrubasik S. The clinical effectiveness of chokeberry: a systematic review. Phytother Res. 2010;24:1107–1114. doi: 10.1002/ptr.3226. [DOI] [PubMed] [Google Scholar]
- 7.Bermúdez-Soto MJ, Tomás-Barberán FA, García-Conesa MT. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007;102:865–874. [Google Scholar]
- 8.D’Alessandro LG, Vauchel P, Przybylski R, Chataigné G, Nikov I, Dimitrov K. Integrated process extraction-adsorption for selective recovery of antioxidant phenolics from Aronia melanocarpa berries. Sep Purif Technol. 2013;120:92–101. [Google Scholar]
- 9.Martini S, D’Addario C, Colacevich A, Focardi S, Borghini F, Santucci A, Figura N, Rossi C. Antimicrobial activity against Helicobacter pylori strains and antioxidant properties of blackberry leaves (Rubus ulmifolius) and isolated compounds. Int J Antimicrob Agents. 2009;34:50–59. doi: 10.1016/j.ijantimicag.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Ljubuncic P, Portnaya I, Cogan U, Azaizeh H, Bomzon A. Antioxidant activity of Crataegus aronia aqueous extract used in traditional Arab medicine in Israel. J Ethnopharmacol. 2005;101:153–161. doi: 10.1016/j.jep.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Lepeduš H, Gaća V, Viljevac M, Kovač S, Fulgosi H, Simić D, Jurković V, Cesar V. Changes in photosynthetic performance and antioxidative strategies during maturation of Norway maple (Acer platanoides L.) leaves. Plant Physiol Biochem. 2011;49:368–376. doi: 10.1016/j.plaphy.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Bunchanan-Wolllaston V. The molecular biology of leaf senescence. J Exp Bot. 1997;48:181–199. [Google Scholar]
- 13.Lichtenthaler HK, Buschmann C. Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. In: Lichtenthaler HK, editor. Current Protocols in Food Analytical Chemistry (CPFA) spectroscopy. Supplement 1. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2001. pp. F4.3.1–F4.3.8. [Google Scholar]
- 14.Zhou K, Su L, Yu L. Phytochemicals and antioxidant properties in wheat bran. J Agric Food Chem. 2004;52:6108–6114. doi: 10.1021/jf049214g. [DOI] [PubMed] [Google Scholar]
- 15.Woisky RG, Salatino A. Analysis of propolis: some parameters and procedures for chemical quality control. J Apic Res. 1998;37:99–105. [Google Scholar]
- 16.Cheung LM, Cheung PCK, Ooi VEC. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003;81:249–255. [Google Scholar]
- 17.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Yuan X, Jin Z, Tian Y, Song H. Free radical and reactive oxygen species scavenging activities of peanut skins extract. Food Chem. 2007;104:242–250. [Google Scholar]
- 19.Oyaizu M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jap J Nutr Diet. 1986;44:307–315. [Google Scholar]
- 20.van den Berg H, Faulks R, Fernando Granado H, Hirschberg J, Olmedilla B, Sandmann G, Southon S, Stahl W. The potential for the improvement of carotenoid levels in foods and the likely systemic effects. J Sci Food Agric. 2000;80:880–912. [Google Scholar]
- 21.Žnidarčič D, Ban D, Šircelj H. Carotenoid and chlorophyll composition of commonly consumed leafy vegetables in Mediterranean countries. Food Chem. 2011;129:1164–1168. doi: 10.1016/j.foodchem.2011.05.097. [DOI] [PubMed] [Google Scholar]
- 22.Loranty A, Rembiałkowska E, Rosa EAS, Bennett RN. Identification, quantification and availability of carotenoids and chlorophylls in fruit, herb and medicinal teas. J Food Compos Anal. 2010;23:432–441. [Google Scholar]
- 23.Li Y, Kong D, Huang R, Liang H, Xu C, Wu H. Variations in essential oil yields and compositions of Cinnamomum cassia leaves at different developmental stages. Ind Crop Prod. 2013;47:92–101. [Google Scholar]
- 24.Iyawe HOT, Azih MC. Total phenolic contents and lipid peroxidation potentials of some tropical antimalarial plants. Eur J Med Plants. 2011;1:33–39. [Google Scholar]
- 25.Arashahi-Delouee S, Urooj A. Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem. 2007;102:1233–1240. [Google Scholar]
- 26.Klavsen SK, Madsen TV. Effect of leaf age on CAM activity in Littorella uniflora. Aquat Bot. 2008;89:50–56. [Google Scholar]
- 27.Lee JE, Kim GS, Park SM, Kim YH, Kim MB, Lee WS, Jeong SW, Lee SJ, Jin JS, Shin SC. Determination of chokeberry (Aronia melanocarpa) polyphenol components using liquid chromatography-tandem mass spectrometry: Overall contribution to antioxidant activity. Food Chem. 2014;146:1–5. doi: 10.1016/j.foodchem.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 28.Altıok E, Bayçın D, Bayraktar O, Ülkü S. Isolation of polyphenols from the extracts of olive leaves (Olea europaea L.) by adsorption on silk fibroin. Sep Purif Technol. 2008;62:342–348. [Google Scholar]
- 29.Dugo P, Donato P, Cacciola F, Germanò MP, Rapisarda A, Mondello L. Characterization of the polyphenolic fraction of Morus alba leaves extracts by HPLC coupled to a hybrid IT-TOF MS system. J Sep Sci. 2009;32:3627–3634. doi: 10.1002/jssc.200900348. [DOI] [PubMed] [Google Scholar]
- 30.Ji X, Wei Y, Liu G, Chen H. Quantitative determination of polyphenols in tobacco leaves by HPLC. J Food Agric Environ. 2013;11:868–870. [Google Scholar]
- 31.Lee WC, Mahmud R, Pillai S, Perumal S, Ismail S. Antioxidant activities of essential oil of Psidium guajava L. leaves. APCBEE Procedia. 2012;2:86–91. [Google Scholar]
- 32.Ahmed MF, Rao AS, Ahemad SR, Ibrahim M. Phytochemical studies and antioxidant activity of Melia azedarach Linn leaves by DPPH scavenging assay. Int J Pharm Appl. 2012;3:271–276. [Google Scholar]
- 33.Hou WC, Wu WC, Yang CY, Chen HJ, Liu SY, Lin YH. Antioxidant activities of methanolic and hot-water extracts from leaves of three cultivars of Mai-Men-Dong (Liriope spicata L.) Botanic Bull Acad Sinica. 2004;45:285–290. [Google Scholar]
- 34.Gawron-Gzella A, Dudek-Makuch M, Matlawska I. DPPH radical scavenging activity and phenolic compound content in different leaf extracts from selected blackberry species. Acta Biol Cracov Ser Bot. 2012;54:32–38. [Google Scholar]
- 35.Fabiyi OA, Atolani O, Adeyemi OS, Olatunji GA. Anti-oxidant and cytotoxicity of β-amyrin acetate fraction from Bridelia ferruginea leaves. Asian Pacific J Tropic Biomed. 2012;2:S981–S984. [Google Scholar]
- 36.Nor Qhairul Izzreen MN, Mohd Fadzelly AB. Phyto-chemicals and antioxidant properties of different parts of Camellia sinensis leaves from Sabah Tea Plantation in Sabah, Malaysia. Int Food Res J. 2013;20:307–312. [Google Scholar]
- 37.Adedapo AA, Jimoh FO, Afolayan AJ, Masika PJ. Antioxidant properties of the methanol extracts of the leaves and stems of Celtis africana. Rec Nat Prod. 2009;3:23–31. [Google Scholar]
- 38.Muruhan S, Selvaraj S, Viswanathan PK, Chandramohan G. In vitro antioxidant activities of Solanum surattense leaf extract. Asian Pac J Trop Biomed. 2013;3:28–34. doi: 10.1016/S2221-1691(13)60019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baskar R, Rajeswari V, Kumar TS. In vitro antioxidant studies in leaves of Annona spieces. Indian J Exp Biol. 2006;45:480–484. [PubMed] [Google Scholar]
- 40.Fidrianny I, Windyaswari AS, Wirasutisna KR. Anti-oxidant capacities of various leaves extract from five colors varieties of sweet potatoes tubers using ABTS, DPPH assays and correlation with total flavonoid, phenolic, carotenoid content. Res J Med Plant. 2013;7:130–140. [Google Scholar]
- 41.Chew KK, Ng SY, Thoo YY, Khoo MZ, Wan Aida WM, Ho CW. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Centella asiatica extracts. Int Food Res J. 2011;18:571–578. [Google Scholar]