Abstract
Bacillus pumilus 2.g isolated from gembus, an Indonesian fermented soybean cake, secretes several proteases that have strong fibrinolytic activities. A fibrinolytic enzyme with an apparent molecular weight of 20 kDa was purified from the culture supernatant of B. pumilus 2.g by sequential application of ammonium sulfate precipitation, ion-exchange chromatography, and hydrophobic chromatography. The partially purified enzyme was stable between pH 5 and pH 9 and temperature of less than 60°C. Fibrinolytic activity was increased by 5 mM MgCl2 and 5 mM CaCl2 but inhibited by 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM sodium dodecyl sulfate (SDS), and 1 mM ethylenediaminetetraacetic acid (EDTA). The partially purified enzyme quickly degraded the α and β chains of fibrinogen but was unable to degrade the γ chain.
Keywords: fibrinolytic enzyme, Bacillus pumilus, fermented food, gembus
INTRODUCTION
Cardiovascular diseases are the number one cause of death globally. According to a report published by the World Health Organization (WHO) in 2011, an estimated 17.3 million people died from cardiovascular diseases in 2008, representing 30% of all global deaths (1). The number of people who die from cardiovascular diseases is expected to increase to 23.3 million people by 2030. Heart disease and stroke are projected to remain the leading causes of death throughout this period (2).
Intravascular thrombosis (i.e., the clotting of blood in blood vessels), is one of the major causes of cardiovascular diseases. Clots formed from insoluble fibrin restrict the smooth flow of blood in blood vessels, leading to thrombosis and heart attacks. Insoluble fibrin is the major protein component of blood clots, which are formed from fibrinogen by thrombin (3). This insoluble fibrin could be hydrolyzed into fibrin degradation products by plasmin, which is generated from plasminogen by plasminogen activators (4). The basis of fibrinolytic therapy is intravenous administration of an exogenous plasminogen activator, that lyses the thrombus and restores blood flow to the area of ischemia. The three fibrinolytic agents that are currently being used for this purpose are urokinase, streptokinase, and genetically engineered tissue plasminogen activator (t-PA). However, these enzymes are expensive, thermolabile and can produce undesirable side effects such as gastrointestinal bleeding, allergic reactions, and resistance to repercussion (5,6).
Recently, potent fibrinolytic enzymes were discovered in various fermented food products, including Japanese natto (7–9), skipjack shiokara (10), Korean cheonggukjang (11–13), doenjang (14), a traditional Asian fermented shrimp paste seasoning (15), and a traditional Chinese soybean food, douchi (16). Nattokinase, an extracellular fibrinolysin produced by Bacillus subtilis natto, can directly hydrolyze fibrin in blood clots and promote the production of t-PA, which activates plasminogen into active plasmin to hydrolyze fibrin. Oral administration of natto or nattokinase effectively enhances the release of an endogenous plasminogen activator in animal models and in human subjects (17).
Microbial fibrinolytic enzymes from food-grade microorganisms have the potential to be developed as additives for functional foods and as drugs to prevent or cure cardiovascular diseases. Tempeh is an Indonesian fermented soybean food that can be made from a variety of raw materials. Gembus is a variety of tempeh. Gembus is different from tempeh in that it is made from the solid soybean waste of tofu (18). Previous work indicates that Bacillus pumilus 2.g can be isolated from gembus and has high proteolytic and fibrinolytic activities (19). In this paper, a fibrinolytic enzyme was purified from the supernatant of a B. pumilus 2.g culture, and the properties of the partially purified enzyme were studied.
MATERIALS AND METHODS
Bacterial strains and culture conditions
To compare media, B. pumilus 2.g was grown in Luria-Bertani broth (LB; Becton, Dickinson and Company, Sparks, MD, USA), tryptic soy broth (TSB), nutrient broth (NB), and brain heart infusion (BHI) at 37°C with vigorous shaking.
Assay of fibrinolytic activity
The fibrinolytic activities of the supernatants of B. pumilus 2.g cultures was determined using the fibrin plate method (20). Briefly, 7 mL of 0.3% (w/v) fibrinogen (MP Biomedicals, Santa Ana, CA, USA) solution in 1 M phosphate-buffered saline (PBS) was mixed with an equal volume of 2% (w/v) agarose solution and 0.1 mL of thrombin solution (100 NIH units/mL; MP Biomedicals) in a petri dish. The petri dish was left at room temperature for 1 h to allow a fibrin clot layer to form, a glass capillary tube was used to make a hole in the fibrin plate. Next, 20 μL of sample was dropped into the hole, and the plate was incubated at 37°C for 8 h. The size of the clear zone that formed was converted into plasmin units (U) by comparison to zones formed by known quantities of plasmin. Protein concentration was determined by the Bradford method (21) using bovine serum albumin (BSA) as the standard. All measurements were performed in triplicate and the average values are shown.
Partial purification of fibrinolytic enzymes
Fibrinolytic enzymes secreted by B. pumilus 2.g were purified from NB cultures that had been incubated for 72 h. The supernatant was isolated by centrifugation (12,000 g, 10 min, 4°C), filtered through a disposable filter unit (0.45 μm; Sarstedt AG & Co., Nümbrecht, Germany) and subjected to ammonium sulfate precipitation (80% saturation, w/v) overnight at 4°C. The precipitate was resuspended in buffer A (20 mM Tris-HCl, pH 7.0) and then dialyzed against 20 volumes of the same buffer for 24 h at 4°C with four buffer changes. After dialysis, the sample was freeze-dried and resuspended in buffer A. The resuspended sample was loaded onto a 2.5×8 cm CM-Sephadex column (Amersham Pharmacia Biotech AB, Uppsala, Sweden), and proteins were eluted by sequential application of 6×100 mL of buffer A containing increasing concentration of NaCl; NaCl concentrations increased from 0 M to 1 M, in a stepwise manner (0.2 M increments). The fibrinolytic activity of each fraction was measured with the fibrin plate method and fractions with fibrinolytic activity were pooled. Pooled samples were dialyzed against buffer A, lyophilized, and then resuspended in 20 mL of buffer A.
Hydrophobic interaction chromatography with Phenyl Sepharose 6 Fast Flow resin (Amersham Pharmacia Biotech AB) was used for further purification of fibrinolytic enzymes. Briefly, each sample was loaded onto a column (2.5×12 cm) that had been pre-equilibrated with buffer A containing 1 M (NH4)2SO4. Proteins were eluted by sequential application of 50 mL of buffer A containing decreasing concentration of (NH4)2SO4; (NH4)2SO4 concentrations decreased from 1 M to 0 M in a stepwise manner (0.2 M decrements). The protein content and fibrinolytic activity of each fraction were measured. Fractions with activity were pooled, dialyzed against buffer A, and lyophilized. Sodium dedocyl sulfate (SDS)-PAGE was conducted by Laemmli’s method (22), and the fibrin zymography was done as previously described (20). For fibrin zymography, the separating gel solution (12%, w/v) was prepared in the presence of fibrinogen (0.02%, w/v) and 100 μL of thrombin (10 NIH units/mL).
Properties of the partially purified fibrinolytic enzyme
To determine the effect of pH on the fibrinolytic enzyme activity, a 0.02 μg sample of partially purified fibrinolytic enzyme was incubated in either 50 mM citrate-NaOH buffer (pH 3.0~4.0), 50 mM sodium phosphate buffer (pH 5.0~6.0), 50 mM Tris-HCl (pH 7.0~8.0), or 50 mM glycine-NaOH buffer (pH 9.0~10.0) for 2 h at 37°C. Following incubation, the fibrinolytic enzyme activity of each mixture was measured by the fibrin plate method.
For thermal stability measurements, 0.02 μg of partially purified fibrinolytic enzymes were suspended in 50 mM Tris-HCl buffer (pH 7.0) and incubated in a water bath for 30 min at varying temperatures (37~80°C). After incubation at each temperature, the fibrinolytic enzyme activity of each mixture was measured by the fibrin plate method.
To determine the effect of metal ions and inhibitors on the activity of the partially purified fibrinolytic enzyme, 0.02 μg samples were incubated in Tris-HCl buffers (pH 7.0) containing 5 mM metal ions (i.e., KCl, MgCl2, CaCl2, CuSO4, MnCl2, or ZnCl2) or 1 mM inhibitors [i.e., phenylmethylsulfonyl fluoride (PMSF), SDS, ethylenediaminetetraacetic acid (EDTA), cantharidic acid, pepstatin A, bestatin hydrochloride, or E-64] for 30 min at 37°C.
The hydrolysis of fibrinogen by the partially purified fibrinolytic enzyme was measured. A total of 2 mg of fibrinogen was mixed with 0.02 μg of partially purified fibrinolytic enzyme in 500 μL of 20 mM Tris-HCl (pH 7.0), and the mixture was incubated at 37°C for up to 12 h. At each interval, 20 μL of sample was removed, mixed with 5×SDS sample buffer, boiled for 5 min, and then analyzed by SDS-PAGE using a 15% acrylamide gel.
Amidolytic activities
Amidolytic activity was determined according to the method of Jo et al. (23). Briefly, a 500 μL mixture containing 50 μL of 10 mM substrate, 10 μL of enzyme (10 μg), and 440 μL of 50 mM Tris-HCl buffer (pH 7.0) was incubated at 37°C for 10 min. Then, 500 μL of citrate-NaOH buffer (pH 3.0) was added to stop the reaction. The resulting mixture was immedietely placed on ice and the centrifuged at 12,000 g for 5 min. The OD410 nm of the supernatant was measured, and the degree of hydrolysis was calculated from the absorbance values and the molar extinction coefficient value of p-nitroanilide (p-NA) (8,800 M−1cm−1).
RESULTS AND DISCUSSION
Fibrinolytic activity of Bacillus pumilus 2.g
Previously, bacilli strains with fibrinolytic activities were isolated from gembus prepared by traditional methods in Semarang, Central Java, Indonesia (18,19). Among the isolated strains, the 2.g strain had the highest fibrinolytic activity. The strain was identified as B. pumilus by 16S rRNA gene sequencing (97% homology), and confirmed by API 50 CHB kit (bioMérieux SA, Marcy-l’Étoile, France) with apiweb™ software (99.7% identity). Among the microorganisms that produce fibrinolytic enzymes, bacilli from Asian traditional fermented foods are the most important (9–12,14). B. subtilis, B. amyloliquefaciens, and B. licheniformis are the most common bacilli species isolated from fermented foods, and the fibrinolytic enzymes found in these species have been reported (9,11,12). However, to the best of our knowledge, the fibrinolytic enzyme present in B. pumilus has not been reported. The isolation of diverse bacilli species and the characterization of their fibrinolytic enzymes are important for understanding the fibrinolytic capacities of these organisms and for developing functional foods and therapeutic agents for the prevention of cardiovascular diseases.
Growth and fibrinolytic activity of B. pumilus 2.g
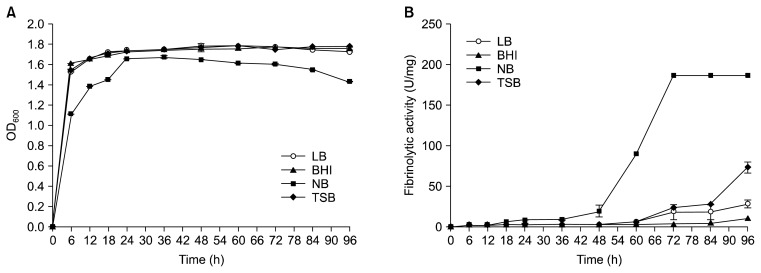
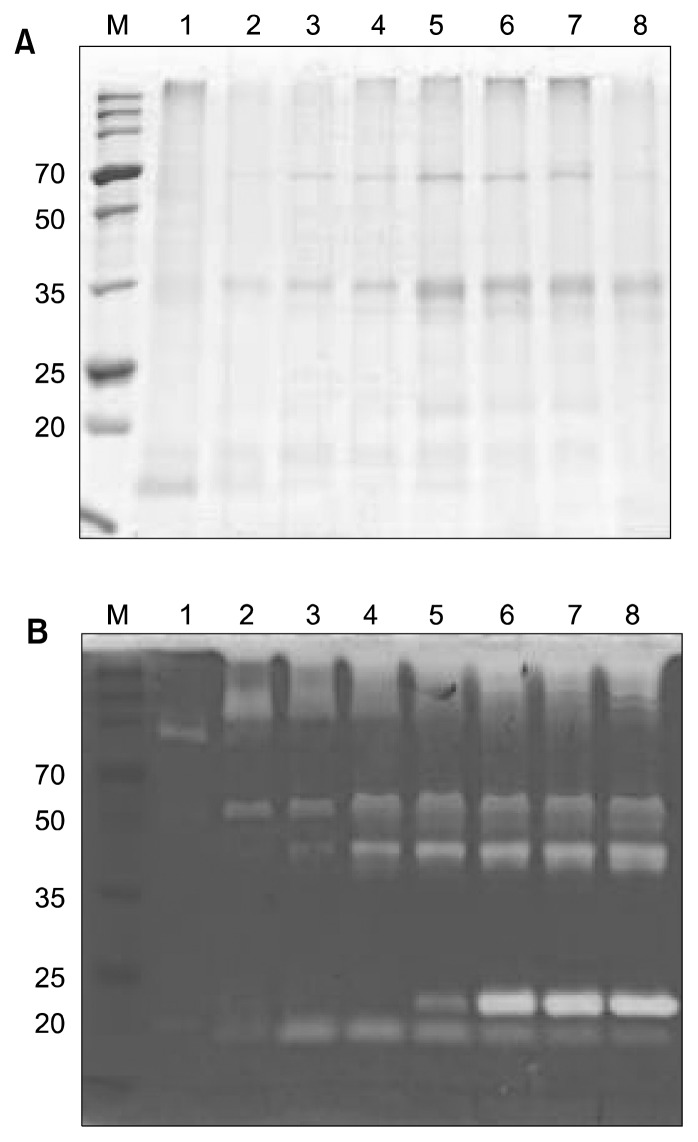
The effects of four different media on the growth and fibrinolytic activity of B. pumilus 2.g were compared (Fig. 1). Each medium was inoculated with an overnight culture (2%, v/v) and incubated for up to 96 h at 37°C with shaking. SDS-PAGE and zymography revealed that the culture in NB had the highest fibrinolytic activity (187 U/mg protein) at 72 h, and the fibrinolytic activity of this culture was stable until 96 h (Fig. 2).
Fig. 1.
Changes in the growth (A) and fibrinolytic activities (B) of B. pumilus 2.g cultured in different growth media.
Fig. 2.
SDS-PAGE (A) and fibrin zymography (B) of culture supernatant from B. pumilus 2.g incubated in NB medium. Lane M, broad-range size marker (Dokdo-Mark™, ElpisBiotech, Daejon, Korea); lanes 1~8: culture supernatant from 12 h (1), 24 h (2), 36 h (3), 48 h (4), 60 h (5), 72 h (6), 84 h (7), and 96 h (8) of incubation.
The highest fibrinolytic activity values were observed in the stationary phase culture. Cultures in LB, BHI, and TSB had much lower fibrinolytic activities, i.e., 29 U/mg protein for the culture in LB (96 h), 11 U/mg protein for the culture in BHI (96 h), and 74 U/mg protein for the culture in TSB (96 h). Previous work indicates that TSB is the best medium and NB is the second best medium for a 50 h incubation of B. amyloliquefaciens CH51 (11). In addition, LB is the best medium for the incubation of B. licheniformis CH3-17 (12). As these results indicate, the best medium for the measurement of fibrinolytic activity is variable, depending upon the specific organism of interest. In addition, growth environment greatly affects the fibrinolytic enzyme yield of a given organism.
Partial purification of a fibrinolytic enzyme
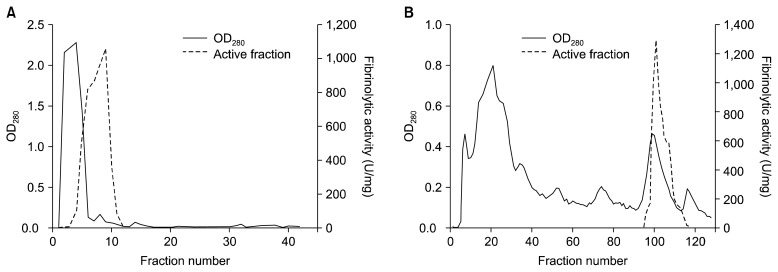
A fibrinolytic enzyme produced by B. pumilus 2.g was partially purified from 3 L of culture supernatant. The results are summarized in Table 1. After the Phenyl-Sepharose column chromatography step, the final purification fold was 16.0, and the yield was 25%. The partially purified enzyme was analyzed by SDS-PAGE and zymography. The results of these analyses confirmed that most (Fig. 3), but not all (Fig. 4A), of the other proteins were removed by the purification process. Fibrin zymography of the purified sample revealed a single band with a molecular mass of 20 kDa. Previous studies have reported different molecular masses for different fibrinolytic enzymes: 29 kDa for B. subtilis natto B-12 (9), 28.2 kDa for Bacillus sp. CK 11-4 (10), 27 kDa for B. licheniformis CH3-17 (12), 28 kDa for B. amyloliquefaciens DC-4 (16), 37 kDa for B. licheniformis KJ-31 (24), 41 kDa for Bacillus sp. KA38 (25), and 35 kDa for Streptomyces sp. CS684 (26).
Table 1.
Summary of the steps performed to partially purify a fibrinolytic enzyme from B. pumilus 2.g
| Total activity (U) | Total protein (mg) | Specific activity (U/mg) | Fold | Yield (%) | |
|---|---|---|---|---|---|
| Supernatant | 13,873.0 | 153.5 | 90.4 | 1 | 100 |
| Ammonium sulfate precipitation | 11,936.4 | 59.7 | 200.1 | 2.2 | 86 |
| CM-Sephadex | 8,252.7 | 16.1 | 512.2 | 5.7 | 59 |
| Phenyl Sepharose 6-FF | 3,421.4 | 2.4 | 1,442.3 | 16.0 | 25 |
Fig. 3.
Elution profile of a fibrinolytic enzyme from B. pumilus 2.g through a CM-Sephadex column (A) and a Phenyl Sepharose 6-FF column (B).
Fig. 4.
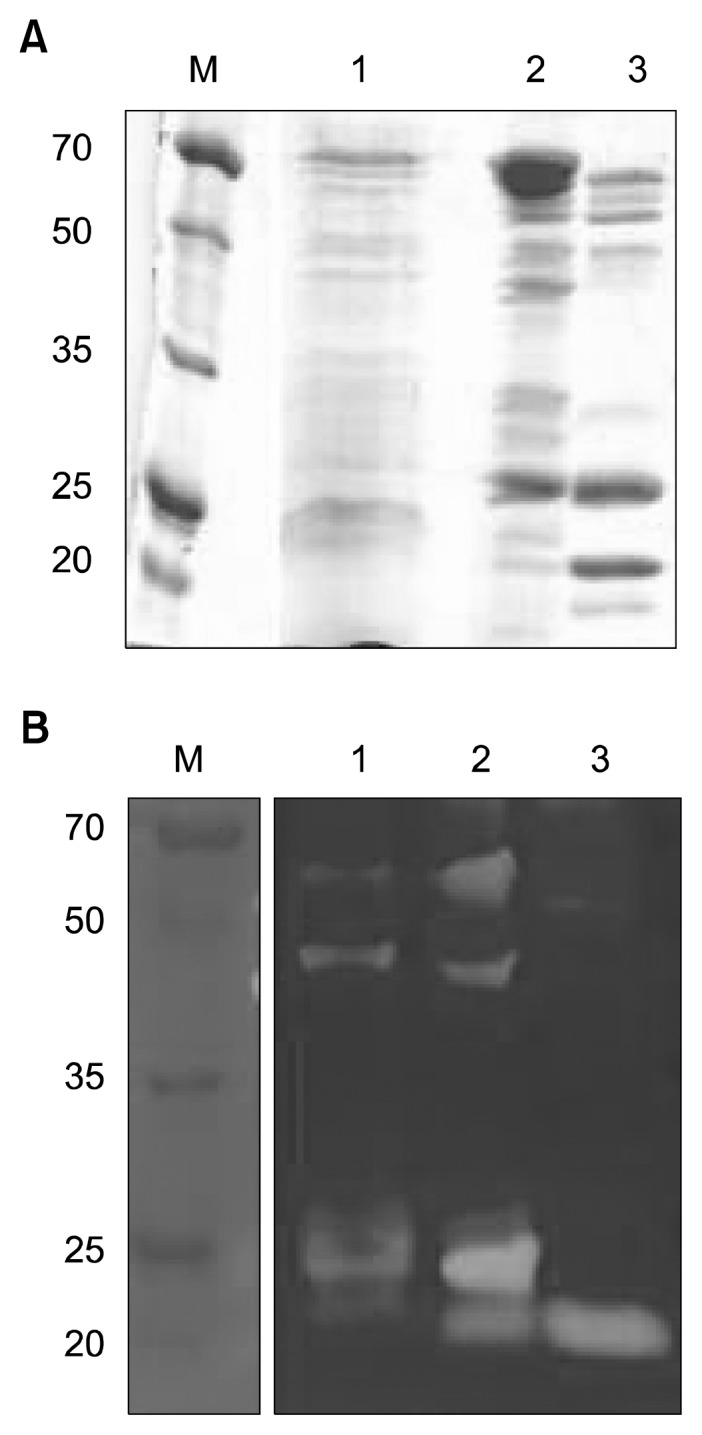
SDS-PAGE (A) and fibrin zymography (B) of protein samples at different purification stages. Lane M, size marker (Dokdo-Mark™); lanes 1~3: sample after 80% ammonium sulfate precipitation (1), sample after CM-Sephadex purification (2), sample after Phenyl Sepharose 6-FF purification (3).
Properties of a partially purified enzyme
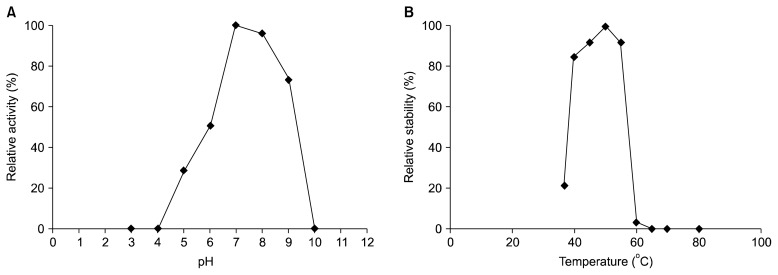
The activity of partially purified fibrinolytic enzyme from B. pumilus 2.g was highest at pH 7.0 (Fig. 5A). The enzyme was not stable under acidic conditions but was relatively stable at basic pHs (pH 7.0~9.0). No fibrinolytic activity was detected at pH 10. At pH 7.0, the purified enzyme was stable at temperature up to 50°C, but fibrinolytic activity rapidly decreased at temperatures greater than or equal to 60°C (Fig. 5B). The results of this study indicate that the partially purified enzyme is not thermotolerant. Thus, products containing this enzyme should not be subjected to heat treatment above 50°C.
Fig. 5.
Effect of pH (A) and temperature (B) on the stability of a partially purified fibrinolytic enzyme from B. pumilus 2.g.
The activity of the partially purified enzyme from B. pumilus 2.g was reduced with exposure to K+ (87.39%), Mn2+ (80.64%), and Zn2+ (89.77%) but slightly increased with exposure to Mg2+ (105.70%) and Ca2+ (102.85%). Cu2+ caused the strongest inhibition (100% inhibition) (Table 2). Copper (Cu) is one of the heavy metals that has a high affinity for organic compounds, including serine, glycine and cycloserine. Cu can bind with sulfhydryl groups, which causes the enzymes containing this group to become inactive. The effect of metal ions on the fibrinolytic activity depends on the origin of the enzyme. For example, the activity of AprE5-41 from B. amyloliquefaciens MJ5-41, a strain isolated from meju, is sligthly reduced by K+ and Mg2+ but is increased by Ca2+ (23). In addition, Nattokinase from B. subtilis YJ1 is completely inactivated by Zn2+ and severely inhibited by Cu2+ (27).
Table 2.
Effect of metal ions and inhibitors on the activity of a partially purified fibrinolytic enzyme from B. pumilus 2.g
| Metal ions (5 mM)/Inhibitors (1 mM) | Relative activity (%) |
|---|---|
| None | 100 |
| KCl | 87.39±3.40 |
| MgCl2 | 105.70±4.03 |
| CaCl2 | 102.85±4.03 |
| CuSO4 | 0 |
| MnCl2 | 80.64±3.03 |
| ZnCl2 | 89.77±3.42 |
| PMSF | 30.72±1.17 |
| SDS | 0 |
| EDTA | 0 |
| Cantharidic acid | 47.91±1.83 |
| Pepstatin A | 78.45±0.07 |
| Bestatin hydrochloric | 76.44±2.91 |
| E-64 | 89.77±3.42 |
In this study, the partially purified enzyme from B. pumilus 2.g was completely inhibited by 1 mM PMSF and 1 mM EDTA. PMSF is known to sulphonate the essential serine residue in the active site of a protease, resulting in a total loss of enzyme activity (28). Several different types of inhibitors were examined in this study, including cantharidic acid (an inhibitor of protein phosphatases), pepstatin A (an inhibitor of acid proteases), bestatin hydrochloride (an inhibitor of aminopeptidases), and E-64 (an inhibitor of cysteine proteases). All of the inhibitors tested inhibited the partially purified enzyme from B. pumilus 2.g to some degree. These results suggest that the purified enzyme is a serine protease.
Amidolytic activities
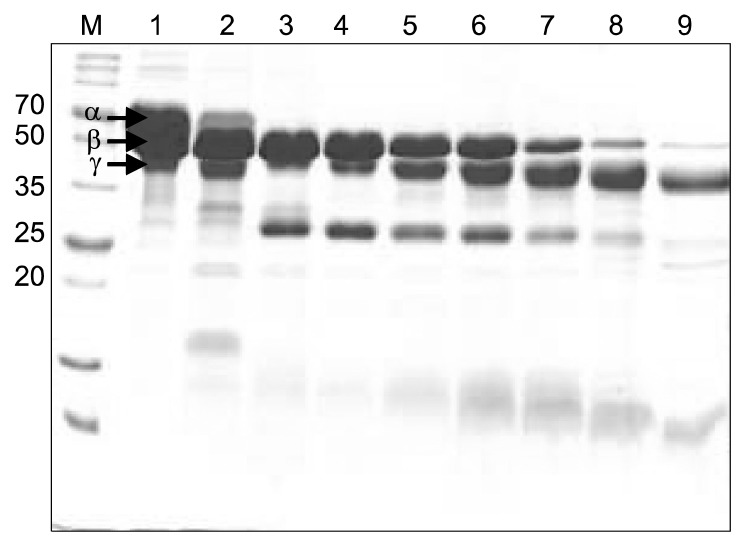
Among the synthetic substrates tested, N-Succinyl-Ala-Ala-Pro-Phe-pNA, a substrate for subtilisin and chymotrypsin, was the most efficient (Table 3). The fibrinogen hydrolysis assay revealed that the partially purified enzyme from B. pumilus 2.g degraded the α chain of fibrinogen in 10 min and the β chain of fibrinogen in 4 h but could not degrade the γ chain of fibrinogen, even after 12 h (Fig. 6). This degradation pattern is similar to that of AprE3-17, the major fibrinolytic protease of B. licheniformis CH3-17 (12), and AprE5-41, the major fibrinolytic pro-tease of B. amyloliquefaciens MJ5-41 (23), but different from that of a fibrinolytic enzyme from B. subtilis, which degrades the β chain first (29). The degradation pattern of fibrinogen could vary by fibrinolytic enzyme, and thus each enzyme should be individually checked.
Table 3.
Amidolytic activity of a partially purified fibrinolytic enzyme from B. pumilus 2.g
| Synthetic protease substrate (10 mM) | Substrate hydrolysis (mM/min/mg) |
|---|---|
| N-Succinyl-Ala-Ala-Pro-Phe-pNA | 61.34 |
| N-Benzoyl-Phe-Val-Arg-pNA hydrochloride | 4.41 |
| N-Benzoyl-Pro-Val-Arg-pNA hydrochloride | 0.24 |
| N-(p-tosyl)-Gly-Pro-Lys 4-nitroanilide acetate salt | 0.88 |
pNA: p-nitroanilide.
Fig. 6.
Fibrinogen hydrolysis by a partially purified enzyme from B. pumilus 2.g. Lane M, size marker (Dokdo-Mark™); lane 1: control (no enzyme treatment); lanes 2~9: fibrinogen after treatment with purified enzyme for 0 min (2), 10 min (3), 30 min (4), 1 h (5), 2 h (6),4 h (7), 8 h (8), and 12 h (9).
Bacillus strains with desirable properties, especially for the pH stability and temperature stability, could be used for the production of functional foods or medicines to treat cardiovascular diseases in the near future. The results of this study demonstrate that B. pumilus 2.g may be used as a novel fibrinolytic enzyme source.
ACKNOWLEDGEMENTS
This work was supported by the Directorate General of Higher Education, Ministry of Education and Culture, Republic of Indonesia and Diponegoro University.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.WHO. Global status report on noncommunicable diseases 2010. World Health Organization; Geneva, Switzerland: 2011. pp. 9–31. [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voet D, Voet JG. Biochemistry. 2nd ed. John Wiley and Sons; NY, USA: 1990. pp. 1087–1095. [Google Scholar]
- 4.Dobrovolsky AB, Titaeva EV. The fibrinolysis system: regulation of activity and physiologic functions of its main components. Biochemistry (Mosc) 2002;67:99–108. doi: 10.1023/a:1013960416302. [DOI] [PubMed] [Google Scholar]
- 5.Blann AD, Landray MJ, Lip GYH. ABC of antithrombotic therapy: an overview of antithrombotic therapy. BMJ. 2002;325:762. doi: 10.1136/bmj.325.7367.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turpie AGG, Chin BSP, Lip GYH. Venous thromboembolism: treatment strategies. BMJ. 2002;325:948. doi: 10.1136/bmj.325.7370.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumi H, Hamada H, Tsushima H, Mihara H, Muraki H. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia. 1987;15:1110–1111. doi: 10.1007/BF01956052. [DOI] [PubMed] [Google Scholar]
- 8.Fujita M, Nomura K, Hong K, Ito Y, Asada A, Nishimuro S. Purification and characterization of a strong fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto, a popular soybean fermented food in Japan. Biochem Biophys Res Commun. 1993;197:1340–1347. doi: 10.1006/bbrc.1993.2624. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Du M, Zheng D, Kong F, Zu G, Feng Y. Purification and characterization of nattokinase from Bacillus subtilis Natto B-12. J Agric Food Chem. 2009;57:9722–9729. doi: 10.1021/jf901861v. [DOI] [PubMed] [Google Scholar]
- 10.Sumi H, Nakajima N, Yatagai C. A unique strong fibrinolytic enzyme (katsuwokinase) in skipjack “Shiokara,” a Japanese traditional fermented food. Comp Biochem Physiol B Biochem Mol Biol. 1995;112:543–547. doi: 10.1016/0305-0491(95)00100-x. [DOI] [PubMed] [Google Scholar]
- 11.Kim WK, Choi KH, Kim YT, Park HH, Choi JY, Lee YS, Oh HI, Kwon IB, Lee SY. Purification and characterization of a fibrinolytic enzyme produced from Bacillus sp. strain CK 11-4 screened from Chungkook-Jang. Appl Environ Microbiol. 1996;62:2482–2488. doi: 10.1128/aem.62.7.2482-2488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim GM, Lee AR, Lee KW, Park JY, Chun J, Cha J, Song YS, Kim JH. Characterization of a 27 kDa fibrinolytic enzyme from Bacillus amyloliquefaciens CH51 isolated from Cheonggukjang. J Microbiol Biotechnol. 2009;19:997–1004. doi: 10.4014/jmb.0811.600. [DOI] [PubMed] [Google Scholar]
- 13.Jo HD, Kwon GH, Park JY, Cha J, Song YS, Kim JH. Cloning and overexpression of aprE3-17 encoding the major fibrinolytic protease of Bacillus licheniformis CH 3–17. Biotechnol Bioprocess Eng. 2011;16:352–359. [Google Scholar]
- 14.Kim SH, Choi NS. Purification and characterization of subtilisin DJ-4 secreted by Bacillus sp. strain DJ-4 screened from Doen-Jang. Biosci Biotechnol Biochem. 2000;64:1722–1725. doi: 10.1271/bbb.64.1722. [DOI] [PubMed] [Google Scholar]
- 15.Wong AHK, Mine Y. Novel fibrinolytic enzyme in fermented shrimp paste, a traditional Asian fermented seasoning. J Agric Food Chem. 2004;52:980–986. doi: 10.1021/jf034535y. [DOI] [PubMed] [Google Scholar]
- 16.Peng Y, Huang Q, Zhang RH, Zhang YZ. Purification and characterization of a fibrinolytic enzyme produced by Bacillus emyloliquefaciens DC-4 screened from Douchi, a traditional Chinese soybean food. Comp Biochem Physiol B Biochem Mol Biol. 2003;134:45–52. doi: 10.1016/s1096-4959(02)00183-5. [DOI] [PubMed] [Google Scholar]
- 17.Sumi H, Hamada H, Nakanishi K, Hiratani H. Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Acta Haematol. 1990;84:139–143. doi: 10.1159/000205051. [DOI] [PubMed] [Google Scholar]
- 18.Kuswanto KR. Industrialization of Tempe production. In: Steinkraus KH, editor. Industrialization of Indigenous Fermented Foods, Revised and Expanded. CRC Press; Boca Raton, FL, USA: 2004. pp. 587–635. [Google Scholar]
- 19.Afifah DN, Sulchan M, Syah D, Yanti Suhartono MT. Proteolytic and fibrinolytic activities of several microorganisms screened from Red Oncom and Gembus, Indonesian fermented soybean cakes. Abstract No. 1.1 presented at 4th Annual International Symposium on Wellness, Healthy Lifestyle and Nutrition; Yogyakarta, Indonesia. 2013. [Google Scholar]
- 20.Jeong SJ, Kwon GH, Chun JY, Kim JS, Park CS, Kwon DY, Kim JH. Cloning of fibrinolytic enzyme gene from Bacillus subtilis isolated from cheonggukjang and its expression in protease-deficient Bacillus subtilis strains. J Microbiol Biotechnol. 2007;17:1018–1023. [PubMed] [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:234–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Jo HD, Lee HA, Jeong SJ, Kim JH. Purification and characterization of a major fibrinolytic enzyme from Bacillus amyloliquefaciens MJ5-41 isolated from meju. J Microbiol Biotechnol. 2011;21:1166–1173. doi: 10.4014/jmb.1106.06008. [DOI] [PubMed] [Google Scholar]
- 24.Hwang KJ, Choi KH, Kim MJ, Park CS, Cha JH. Purification and characterization of a new fibrinolytic enzyme of Bacillus licheniformis KJ-31, isolated from Korean traditional Jeot-gal. J Microbiol Biotechnol. 2007;17:1469–1476. [PubMed] [Google Scholar]
- 25.Kim HK, Kim GT, Kim DK, Choi WA, Park SH, Jeong YK, Kong IS. Purification and characterization of a novel fibrinolytic enzyme from Bacillus sp. KA38 originated from fermented fish. J Ferment Bioeng. 1997;84:307–312. [Google Scholar]
- 26.Simkhada JR, Mander P, Cho SS, Yoo JC. A novel fibrinolytic protease from Streptomyces sp. CS684. Process Biochem. 2010;45:88–93. [Google Scholar]
- 27.Yin LJ, Lin HH, Jiang ST. Bioproperties of potent nattokinase from Bacillus subtilis YJ1. J Agric Food Chem. 2010;58:5737–5742. doi: 10.1021/jf100290h. [DOI] [PubMed] [Google Scholar]
- 28.Gold AM, Fahrney D. Sulfonyl fluorides as inhibitors of esterases. II. Formation and reactions of phenylmethanesulfonyl α-chymotrypsin. Biochemistry. 1964;3:783–791. doi: 10.1021/bi00894a009. [DOI] [PubMed] [Google Scholar]
- 29.Kim SB, Lee DW, Cheigh CI, Choe EA, Lee SJ, Hong YH, Choi HJ, Pyun YR. Purification and characterization of a fibrinolytic subtilisin-like protease of Bacillus subtilis TP-6 from an Indonesian fermented soybeand, Tempeh. J Ind Microbiol Biotechnol. 2006;33:436–444. doi: 10.1007/s10295-006-0085-4. [DOI] [PubMed] [Google Scholar]