Abstract
The discovery of bioactive compounds in foods has changed the dietary lifestyle of many people. Cyperus esculentus (tigernut) is highly underutilized in Africa, yet tigernut extract is highly profitable in Europe. This study aims to add value to tigernut extract by revealing its health benefits and food value. In this study, tigernut tubers were germinated or roasted and the extracts were combined with Moringa oleifera extract (MOE) or Hibiscus sabdariffa extract (HSE) and spiced with ginger to produce functional drinks. The drinks were evaluated for physicochemical characteristics, sensory parameters, and antioxidant potentials. The total phenolic content of each beverage was measured by the Folin-Ciocalteu method, and the antioxidant activity of each beverage was determined by the 2,2-diphenyl-1-picrylhydrazyl and 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid assays. The beverages from the germinated tigernut extracts had the highest titratable acidity and the lowest pH, while beverages containing the roasted tigernut extract had the highest ∘Brix. Germination and roasting significantly enhanced the total phenolic content of the drinks. The beverage containing HSE and germinated tigernut extract had a total phenolic content of 45.67 mg/100 mL gallic acid equivalents, which was significantly higher than the total phenolic content of all other samples. The DPPH inhibition activity of the beverages prepared with germinated tigernut extracts was significantly higher than the DPPH inhibition activity of the beverages prepared with fresh tigernut extract. The taste and overall acceptability of drinks containing the roasted tigernut extract were preferred, while the color and appearance of drinks with the germinated samples were preferred. Roasting or germinating tigernuts before extraction and addition of MOE or HSE extracts is another way to add value and enhance the utilization of tigernuts.
Keywords: tigernut, Moringa oleifera, Hibiscus sabdariffa, beverages, antioxidant
INTRODUCTION
Food insecurity is still prevalent in many developing nations of the world. In many of these developing nations, people live on less than US $2/day and feed predominantly on starchy foods that are devoid of essential vitamins and minerals. While all hungry people are food insecure, not all food insecure people are hungry (1). Poverty and deprivation, among other factors, contribute to food insecurity. Increasing awareness of the potency and encouraging the consumption of many underutilized crops may help these nations achieve food security.
Cyperus esculentus (tigernut) is an edible perennial weedy plant that primarily grows in the tropics and in the Mediterranean region. The milky extract of tigernut is consumed by humans in the raw form, and the by-product is usually discarded or used as animal feed (2). Tigernut is reported to be useful for enhancing blood circulation, preventing heart disease, and reducing the risk of colon cancer (3,4). In Spain, the milky extract of tigernut (i.e., “horchata de chufa”), a non-alcoholic beverage, has an annual economic impact of 60 million Euro (2,5). With this increase in consumer acceptability, the popularity of tigernut extract has spread beyond the boundaries of Spain to other European countries and the United States (6).
Tigernut tubers are available in large quantities in Sub-Saharan Africa. However, there is no record of the economic value of tigernuts because they are grossly underutilized. The development of functional beverages containing grossly underutilized crops has been encouraged, as it will increase the economic value of the crops and provide health benefits to consumers (2).
The antioxidant properties of Hibiscus sabdariffa have prompted an increase in the consumption of this plant in Sub-Saharan Africa (7). Hibiscus serves as a major source of anthocyanin (8), which has antihypertensive and anti-inflammatory effects (9). Nigeria produces 138,000 metric tons of hibiscus calyces annually, with only moderate value addition.
Moringa oleifera is a plant that has gained popularity because of its antioxidant potential and its therapeutic effect against several ailments (10). The leaves, seeds, flowers, bark, roots, and seed kernels of Moringa oleifera have been used for dietary purposes, pharmaceutical and cosmetic uses, and as an organic water purifier (11,12).
Ginger (Zingiber officinale) is a plant that has been used as a spice in food preparation and, more recently, for its antimicrobial and antioxidant properties (7,13).
As the middle class of most developing countries grows, the awareness of the impact of nutrition on health will continue to increase, government policies advocating the use of and addition of value to underutilized crops will continue to be passed, and there will be a continued need to develop affordable healthy drinks. The objective of the present research is to develop value-added functional drinks using tigernut and ginger extracts mixed with Hibiscus sabdariffa extract (HSE) or Moringa oleifera (MOE) extracts. The effect of various processing methods on the antioxidative potential, nutritional composition, and sensory properties of the drinks were examined.
MATERIALS AND METHODS
Chemicals and sample collection and preparation
The Folin-Ciocalteu’s phenol reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis-3-ethylbenzothia-zoline-6-sulphonic acid (ABTS), gallic acid, and butylated hydroxyl anisole (BHA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All reagents and chemicals used were of analytical grade. Tigernuts, ginger, and Hibiscus sabdariffa were purchased from a market in Akure South Local Government, Ondo State, Nigeria. The fresh ginger were washed, peeled, and grated before drying at 60°C for 5 h. The dried ginger were then milled to a powder and sieved. The hibiscus flowers were rinsed for 30 sec, drained, and oven dried at 55°C for 3 h. Dried flowers were then milled to a powder and sieved. Moringa oleifera leaves were harvested fresh from a tree near the Teaching and Research Farm of the Federal University of Technology Akure campus. The leaves were sorted to remove the yellow and brown leaves, which would have altered the color of the final product. The sorted leaves were then washed, blanched at 45°C for 30 sec, oven dried at 50°C for 5 h, and then blended to a powder.
Preparation of tigernut ‘milk’
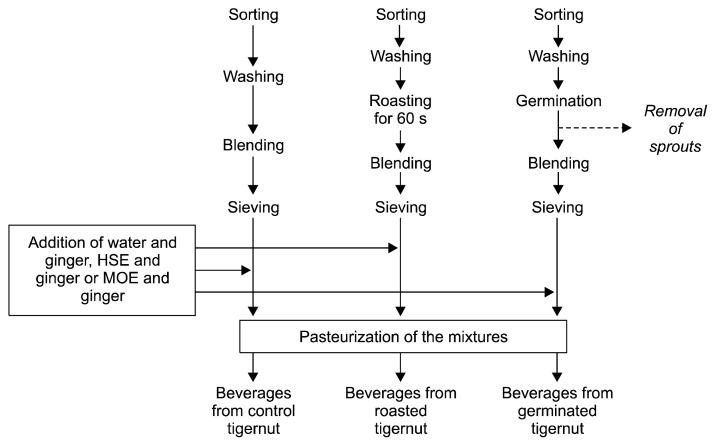
The cleaned, fresh tigernut tubers were divided into three groups. One group was used as a control, one group was germinated before extraction, and one group was roasted before extraction. The tubers assigned to the germinated group were spread on a clean tray, covered with moistened cotton wool, and kept in growth chamber at 25°C with a 14 h:10 h light: dark cycle until the mean sprout height was 0.5 cm. When the target sprout height was reached, the sprouts were removed from the growth chamber and processed as shown in Fig. 1. The tubers assigned to the roasted group were spread on a frying pan kept on a low-burning blue flame and roasted for 60 sec. After roasting, the samples were processed as shown in Fig. 1.
Fig. 1.
The flow chart for the production of tigernut extract beverages mixed with ginger, Hibiscus sabdariffa, or Moringa oleifera extracts.
Preparation of tigernut extract only (TEO), tigernut-water-ginger (TWG), tigernut-hibiscus-ginger (THG), and tigernut-moringa-ginger (TMG) beverages
Ginger, hibiscus, and moringa solutions were prepared by adding 2 g of powdered ginger, powdered hibiscus flowers, or powdered moringa to three clean bottles containing 100 mL of 25°C water. Each mixture was vigorously shaken for 30 s, allowed to stand for 30 s, and then filtered through a sieve with muslin cloth.
The beverages were made by combining the aforementioned solutions with tigernut milk extract in a 70:20:10 ratio. For example, the TWG beverage was prepared by combining 10 mL of the filtered ginger solution, 20 mL of water, and 70 mL of tigernut milk extract. To prepare the THG and TMG beverages, the water was replaced with HSE or MOE, respectively. The TEO beverage contained only the tigernut extract.
Determination of physicochemical parameters
The pH of each beverage was determined with a Mettler Toledo MP220 pH meter (Mettler Toledo, Greifensee, Switzerland). The refraction index of each beverage was measured with a handheld refractometer model RX 1000 (Atago Co. Ltd., Tokyo, Japan) and recorded as ∘Brix. The titratable acidity of the beverages was determined by titration with 0.1 M NaOH and phenol-phthalein indicator.
Determination of total phenolic content
The total phenolic content of the beverages was determined with Folin-Ciocalteu reagent according to the method of Singleton et al. (14) with slight modification as described by Genovese et al. (15). A 25-μL aliquot of each beverage was mixed with 0.25 mL of Folin-Ciocalteu reagent and incubated at room temperature. After 5 min, 0.25 mL of 10% sodium bicarbonate (Na2CO3) solution was added, and the mixture was allowed to stand in the dark for 30 min at 37°C. The absorbance of the incubated mixture was measured at 750 nm using a JENWAY UV-visible spectrophotometer (JENWAY Inc., Staffordshire, UK). A standard curve was prepared and used to translate the measured absorbance values to gallic acid equivalents (GAE).
Determination of DPPH radical scavenging activity
The free radical scavenging activity of each beverage was determined as described by Gyamfi et al. (16). A 2-mL aliquot of each sample was mixed with 2 mL of 0.1 mM methanolic DPPH solution, vortexed vigorously, and then incubated in the dark for 30 min at room temperature. The absorbance of the reaction mixture at 517 nm was determined with a JENWAY UV-visible spectrophotometer (JENWAY Inc.). The radical scavenging abilities of the beverages were calculated as the percentage of DPPH free radicals inhibited by each sample. A 50 g/mL solution of BHA at was used as control. The percent DPPH inhibition was calculated with the following equation:
Determination of ABTS radical scavenging activity
The ABTS radical scavenging activity of each beverage was measured according to the method of Re et al. (17) with slight modifications. This assay is based on the ability of the beverages to scavenge ABTS radical cations. The ABTS stock solution was prepared by reacting a 7 mM ABTS aqueous solution with 2.45 mM K2S2O8 in the dark for 12~16 h. The working solution was then prepared by diluting the radical stock solution in ethanol until its absorbance at 734 nm was 0.70±0.02. Aliquots (0.1 mL) of each beverage were mixed with 2.9 mL of diluted ABTS radical working solution and incubated at room temperature for 15 min. The absorbance of the incubated mixture was measured at 734 nm in a UV-visible spectrophotometer (JENWAY Inc.). A 50 g/mL solution of BHA was used as control. The ABTS radical scavenging activity of each beverage is provided as the percentage of ABTS radicals scavenged. The following equation was used to calculate the ABTS radical scavenging activity of each beverage:
CIE color analysis
An aliquot of each beverage was placed in a cuvette and the color measured with a CM-700d spectrophotometer (Konica Minolta Sensing, Inc., Tokyo, Japan). The spectrophotometer was calibrated against a white plate. The L*, a*, and b* values were read from the spectrophotometer.
Sensory evaluation
A sensory evaluation of each beverage was conducted. The sensory panel consisted of ten adults who were familiar with and experienced in drinking tigernut, Hibiscus sabdariffa, and Moringa oleifera extracts. Panelists were seated in booths with proper illumination and asked to judge each beverage on four attributes: taste, color, appearance, and overall acceptability. Nine-point hedonic scales where 1=‘dislike extremely’ and 9=‘like extremely’ were used for sensory evaluation.
Statistical analysis
Unless stated otherwise, all analyses were carried out in triplicate, and the differences between means were determined by one-way ANOVA. Sample means were separated using Duncan’s multiple range test with SPSS version 17 (SPSS Inc., Chicago, IL, USA), and considered statistically significant if P<0.05.
RESULTS AND DISCUSSION
Physicochemical properties of the tigernut beverages
The ∘Brix of the tigernut beverages ranged from 11.23± 0.03 to 14.27±0.02. The beverages prepared from the germinated tigernut extracts had the lowest ∘Brix, while those from the roasted samples had the highest ∘Brix (Table 1). This finding might be the result of an increase in the concentration of the roasted tigernut extracts, as roasting causes a decrease in the moisture content of nuts. However, the addition of H2O, HSE, or MOE to the various extracts did not result in significant changes to the ∘Brix of the beverages tested.
Table 1.
The physicochemical parameters of beverages containing processed tigernut extracts
| Samples | ∘Brix | pH | TA1) |
|---|---|---|---|
| Fresh | |||
| TEO | 12.56±0.03b | 5.46±0.02ab | 1.00±0.01b |
| TWG | 12.24±0.05b | 5.42±0.01b | 0.97±0.02b |
| THG | 12.18±0.03b | 5.52±0.01a | 1.19±0.01b |
| TMG | 12.12±0.04b | 5.56±0.01a | 1.05±0.02b |
| Germinated | |||
| TEO | 11.61±0.01c | 4.47±0.01c | 1.41±0.03a |
| TWG | 11.57±0.02c | 4.46±0.02c | 1.39±0.07a |
| THG | 11.62±0.08c | 4.48±0.01c | 1.53±0.03a |
| TMG | 11.23±0.03c | 4.50±0.01c | 1.44±0.02a |
| Roasted | |||
| TEO | 14.27±0.02a | 5.41±0.02b | 0.93±0.02bc |
| TWG | 14.21±0.11a | 5.35±0.01b | 0.88±0.05c |
| THG | 14.12±0.04a | 5.45±0.01ab | 0.94±0.03bc |
| TMG | 14.18±0.05a | 5.51±0.02a | 0.91±0.02bc |
Titratable acidity (g/100 mL).
Values are mean±SD (n=3). Within each column, values not sharing the same superscript letter are significantly different from one another (P<0.05) by Duncan’s multiple range test. TEO, tigernut extract only; TWG, tigernut/water/ginger; THG, tigernut/hibiscus extract/ginger; TMG, tigernut/moringa extract/ ginger.
The pH of the tigernut beverages ranged from 4.46± 0.02 to 5.56±0.01. The pH values of the germinated tigernut extracts were significantly lower than the pH values of the fresh and roasted tigernut extracts. High pH values have been attributed to poor tigernut extract storage and keeping quality (2). The titratable acidity, which is a measure of the ‘acid-taste’ of the beverages, ranged from 0.88±0.05 to 1.53±0.03 g/100 mL. The beverages made from the roasted extract had significantly lower titratable acidities than the beverages made from the germinated extract (Table 1). This indicates that the extract from the roasted tigernuts has less ‘acid-taste’.
Antioxidant capacities of the tigernut beverages
Two different assays were used to analyze the antioxidant capacity of the tigernut beverages: the DPPH assay and the ABTS assay. Two methods were used to determine antioxidant capacity because this type of assay is subjective, with each assay only providing an estimate of the antioxidant capacity. In the DPPH assay, radicals in the reaction medium are scavenged, resulting in a color change from purple to yellow, thus leading to a decrease in absorbance. This color change is an indication of the hydrogen donation ability of the sample. The degree of solution discoloration is an indication of the scavenging efficiency of the sample beverage (18).
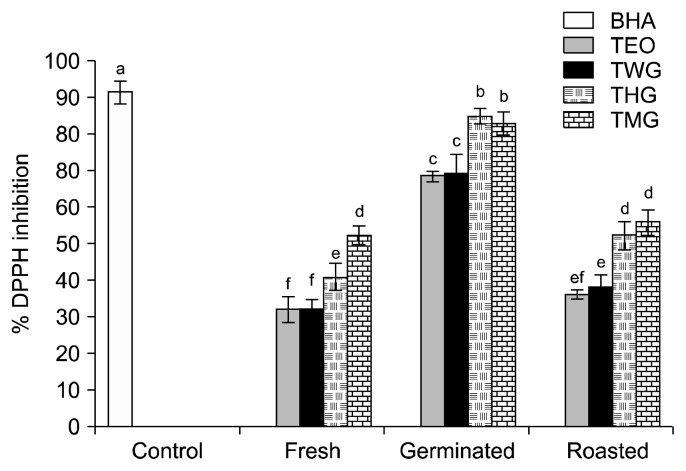
The DPPH inhibition capacity of the beverages tested ranged from 32.33±2.52% to 85.00±2.00%. There was no significant difference between the DPPH inhibition capacity of the TEO beverages and the TWG beverages prepared from fresh, germinated, or roasted tigernuts (Fig. 2). The TEO and TWG beverages prepared with germinated tigernut extracts had significantly higher DPPH inhibition capacities than the TEO and TWG beverages prepared with fresh or roasted tigernut extracts. The addition of HSE or MOE to the germinated tigernut beverages further enhanced the DPPH inhibition capacity of the beverages to 85.00±2.00% and 83.00±3.00%, respectively. These values are slightly lower than the DPPH inhibition capacity measured for the BHA used as control (Fig. 2). Germination has been reported to enhance the antioxidant capacity of peas and Lupinus albus (19,20). The exceptionally high DPPH inhibition capacity of beverages containing germinated tigernut extract and HSE or MOE might be due to a synergistic effect of the antioxidant capacity of tigernut extract with the antioxidant capacity of HSE or MOE. Previously, we reported that cocoa beverages containing HSE have a high antioxidant capacity (7).
Fig. 2.
The percentage DPPH inhibition of beverages prepared with processed tigernut extract only (TEO), tigernut extract mixed with water and spiced with ginger (TWG), tigernut extract mixed with Hibiscus sabdariffa extract and spiced with ginger (THG), and tigernut extract mixed with Moringa oleifera extract and spiced with ginger (TMG). Values are mean±SD (n=3). For all bars, means with different letters (a–f) are significantly different (P<0.05) by Duncan’s multiple range test.
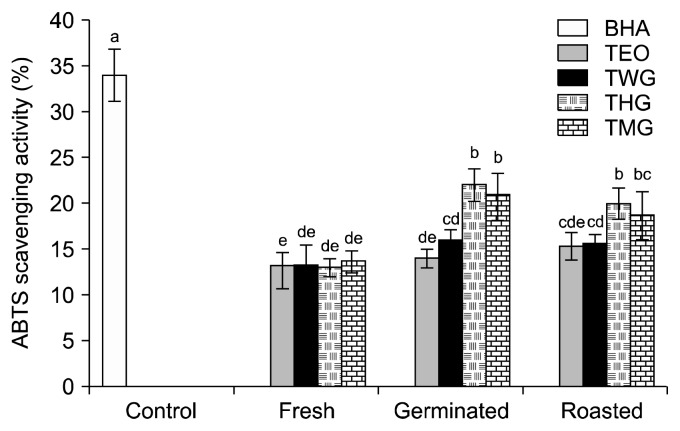
Food industries and agricultural researchers frequently use the ABTS assay to measure the antioxidant potential of foods and crops because the ABTS radical cation is very reactive towards most antioxidants, including phenolics, thiols, and vitamin C (21). In the ABTS assay, the ABTS radical cation, a blue-green chromophore with maximum absorption wavelengths of 645 nm, 734 nm, and 815 nm, is converted to its colorless neutral form to indicate the presence of an antioxidant (17). The ABTS scavenging activities of the beverages tested in this study ranged from 13.00±1.00% to 22.00±1.73% (Fig. 3). For all processing methods, there were no significant differences between the scavenging activities of the TEO and TWG beverages. The addition of MOE or HSE did not affect the scavenging activities of the beverages containing the fresh tigernut extract (Fig. 3). However, the scavenging activities of the beverages containing the germinated tigernut extract and the roasted tigernut extract were significantly higher than the scavenging activities of the beverages containing the fresh tigernut extract, especially with the addition of HSE. The antioxidant capacities of all the beverages measured in this study were higher than the previously reported antioxidant capacities of caramel containing soft drinks (22,23), yet many people, especially in Africa, consume such drinks as status symbol to the detriment of their health. Consuming a diet rich in antioxidants helps reduce the risk of many common chronic diseases (24).
Fig. 3.
The percentage ABTS scavenging activities of beverages prepared with processed tigernut extract only (TEO), tigernut extract mixed with water and spiced with ginger (TWG), tigernut extract mixed with Hibiscus sabdariffa extract and spiced with ginger (THG), and tigernut extract mixed with Moringa oleifera extract and spiced with ginger (TMG). Values are mean±SD (n=3). For all bars, means with different letters (a–e) are significantly different (P<0.05) by Duncan’s multiple range test.
Phenolic content of the tigernut beverages
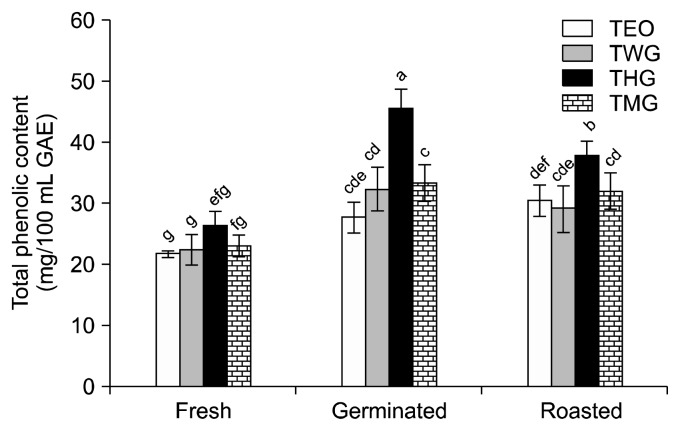
Polyphenols are plant secondary metabolites that are abundant in plant-based foods and beverages. The phenolic contents of the beverage mixes tested in this study ranged from 21.67±0.57 mg/100 mL GAE to 45.67± 2.08 mg/100 mL GAE. The beverage prepared from fresh TEO had the lowest phenolic content, which was not significantly different from that of the TWG beverage made with fresh tigernut extract (Fig. 4). The addition of HSE and MOE to the beverages containing fresh tigernut extract increased the phenolic contents of the beverages, but not to a significant level. Germination and roasting the tigernuts prior to extraction significantly increased the phenolic content of the resulting beverages (Fig. 4). There was no significant difference between the phenolic contents of the TEO and TMG beverages prepared with germinated or roasted tigernut extracts. However, the THG beverage containing germinated tigernut extract had a significantly higher phenolic content than the drinks prepared with roasted tigernut extracts.
Fig. 4.
The total phenolic content of beverages prepared with processed tigernut extract only (TEO), tigernut extract mixed with water and spiced with ginger (TWG), tigernut extract mixed with Hibiscus sabdariffa extract and spiced with ginger (THG), and tigernut extract mixed with Moringa oleifera extract and spiced with ginger (TMG). Values are mean±SD (n=3). For all bars, means with different letters (a–g) are significantly different (P<0.05) by Duncan’s multiple range test.
Researchers are interested in plant phenolics because of their antioxidant potential and ability to prevent degenerative diseases (25). The consumption of plants laden with phytochemicals is reported to have a positive regulatory effect in humans. Phenolics have the ability to scavenge free radicals, which would otherwise build up in the body due to an imbalance between the antioxidant system of the body and the formation of reactive oxygen species (26).
Consumer preference of the tigernut beverages
In a previous study by Sanful (27), forty panelists evaluated the sensory characteristics of roasted and non-roasted tigernut beverages and reported that roasted nuts were preferred because of the taste. In the present study, beverages prepared with roasted tigernut extract scored lowest in color and appearance only (Table 2). The CIE L*, a*, and b* color analysis revealed that beverages prepared with the roasted tigernut extract had the lowest L* value, which is indicative of a deviation from a bright color to a slightly dark color (Table 3). All the samples containing HSE had very low L* values, while the beverages containing fresh tigernut extract had higher L* values.
Table 2.
The sensory parameters of beverages containing processed tigernut extracts
| Samples | Parameters scored | |||
|---|---|---|---|---|
|
| ||||
| Taste | Color | Appearance | OA | |
| Fresh | ||||
| TEO | 7.1±0.2bc | 7.4±0.2a | 7.4±0.3a | 7.6±0.3ab |
| TWG | 6.2±0.1c | 7.1±0.1a | 7.1±0.1a | 7.5±0.3ab |
| THG | 5.2±0.2d | 6.3±0.2b | 6.6±0.2ab | 6.9±0.1b |
| TMG | 5.5±0.2d | 6.6±0.1b | 6.9±0.1ab | 7.3±0.1ab |
| Germinated | ||||
| TEO | 6.5±0.1c | 7.6±0.2a | 7.5±0.1a | 7.6±0.2ab |
| TWG | 5.6±0.1d | 7.6±0.2a | 7.5±0.2a | 6.9±0.1bc |
| THG | 4.9±0.1d | 7.1±0.1a | 6.9±0.2ab | 6.4±0.2c |
| TMG | 5.2±0.2d | 7.4±0.2a | 7.2±0.2a | 6.7±0.2bc |
| Roasted | ||||
| TEO | 9.2±0.2a | 7.2±0.1a | 6.7±0.1ab | 8.4±0.2a |
| TWG | 8.2±0.2b | 6.1±0.1b | 6.8±0.2ab | 8.1±0.1a |
| THG | 7.2±0.1bc | 5.4±0.2c | 6.2±0.2b | 7.4±0.2ab |
| TMG | 7.7±0.1b | 5.8±0.2bc | 6.6±0.1ab | 7.8±0.1ab |
Values are mean±SD (n=10).
Within each column, values not sharing the same superscript letter are significantly different from one another (P<0.05) by Duncan’s multiple range test.
OA: overall acceptability.
TEO, tigernut extract only; TWG, tigernut/water/ginger; THG, tigernut/hibiscus extract/ginger; TMG, tigernut/moringa extract/ginger.
Table 3.
CIE L*, a*, and b* values for beverages containing processed tigernut extracts
| Sample | L* (lightness) | a* (redness) | b* (yellowness) |
|---|---|---|---|
| Fresh | |||
| TEO | 77.21±0.22a | 0.99±0.03c | 5.67±0.05a |
| TWG | 76.53±0.14a | 1.01±0.01c | 5.21±0.02a |
| THG | 40.47±0.21d | 7.51±0.08a | 4.36±0.05b |
| TMG | 64.32±0.17b | 2.41±0.04b | 2.07±0.03c |
| Germinated | |||
| TEO | 76.36±0.12a | 1.06±0.01c | 5.11±0.06a |
| TWG | 75.81±0.17a | 1.14±0.03c | 5.02±0.04a |
| THG | 42.08±0.26d | 6.91±0.08a | 4.32±0.02b |
| TMG | 61.11±0.31b | 2.14±0.04b | 1.99±0.03c |
| Roasted | |||
| TEO | 68.48±0.31b | 1.09±0.02c | 5.36±0.04a |
| TWG | 67.14±0.24b | 1.11±0.02c | 5.41±0.04a |
| THG | 36.42±0.16e | 8.21±0.07a | 4.44±0.04b |
| TMG | 55.29±0.21c | 2.09±0.03b | 2.01±0.03c |
Values are mean±SD (n=3). Within each column, values not sharing the same superscript letter are significantly different from one another (P<0.05) by Duncan’s multiple range test. TEO, tigernut extract only; TWG, tigernut/water/ginger; THG, tigernut/hibiscus extract/ginger; TMG, tigernut/moringa extract/ ginger.
The results of the titratable acidity determination indicated that the beverages containing roasted tigernut extract had a less ‘acid taste’, which may be why these beverages were generally preferred in taste and overall acceptability. The tigernut beverages containing HSE generally scored the lowest in all the assessments. This might be due to the deep purple color of the HSE, which results in a light purple colored drink that, when combined with the milky color of tigernut extract, slightly deviates from the expected color of milky drinks.
CONCLUSION
People, especially those in African settings, choose drinks based on many factors, including religion, social gathering, gender, age, and disposition (i.e., intention to drive automobile after drinking or not). It is only in the case of sickness that health impact is considered when selecting beverages. To attenuate the buildup of oxidative stress in the body, attention needs to be given to plant-based foods, especially those that are grossly underutilized. Thus, the development of value-added functional drink products that quench thirst and improve health will enhance food security. Functional beverages containing tigernut extracts mixed with HSE or MOE are laden with antioxidative potential. The consumption of these beverages should be encouraged.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.FAO. Food Security Information for Action Practical Guides. 2008. [accessed on April 10th 2014]. Available from http://www.fao.org/docrep/013/al936e/al936e00.pdf.
- 2.Sanchez-Zapata E, Fernandez-Lopez J, Perez-Alvarez JA. Tiger nut (Cyperus esculentus) commercialization: health aspects, composition, properties and food applications. Compr Rev Food Sci Food Saf. 2012;11:366–377. [Google Scholar]
- 3.Chukwuma ER, Obioma N, Cristopher OI. The phytochemical composition and some biochemical effects of Nigerian tigernut (Cyperus esculentus L.) tuber. Pak J Nutr. 2010;9:709–715. [Google Scholar]
- 4.Adejuyitan JA. Tigernut processing: its food uses and health benefits. Am J Food Technol. 2011;6:197–201. [Google Scholar]
- 5.Mosquera LA, Sims CA, Bates RP, O’Keefe SF. Flavor and stability of “horchata de chufas”. J Food Sci. 1996;61:856–861. [Google Scholar]
- 6.Rubert J, Sebastià N, Soriano JM, Soler C, Mañes J. One-year monitoring of aflatoxins and ochratoxin A in tiger-nuts and their beverages. Food Chem. 2011;127:822–826. doi: 10.1016/j.foodchem.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Awe FB, Fagbemi TN, Ifesan BOT, Badejo AA. Antioxidant properties of cold and hot water extracts of cocoa, Hibiscus flower extract, and ginger beverage blends. Food Res Int. 2013;52:490–495. [Google Scholar]
- 8.Segura-Carretero A, Puertas-Mejía MA, Cortacero-Ramírez S, Beltrán R, Alonso-Villaverde C, Joven J, Dinelli G, Fernández-Gutiérrez A. Selective extraction, separation, and identification of anthocyanins from Hibiscus sabdariffa L. using solid phase extraction-capillary electrophoresis-mass spectrometry (time-of-flight/ion trap) Electrophoresis. 2008;29:2852–2861. doi: 10.1002/elps.200700819. [DOI] [PubMed] [Google Scholar]
- 9.Beltrán-Debón R, Alonso-Villaverde C, Aragonès G, Rodríguez-Medina I, Rull A, Micol V, Segura-Carretero A, Fernández-Gutiérrez A, Camps J, Joven J. The aqueous extract of Hibiscus sabdariffa calices modulates the production of monocyte chemoattractant protein-1 in humans. Phytomedicine. 2010;17:186–191. doi: 10.1016/j.phymed.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem. 2003;51:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- 11.Anwar F, Latif S, Ashraf M, Gilani AH. Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- 12.Beltrán-Heredia J, Sánchez-Martín J. Improvement of water treatment pilot plant with Moringa oleifera extract as flocculant agent. Environ Technol. 2009;30:525–534. doi: 10.1080/09593330902831176. [DOI] [PubMed] [Google Scholar]
- 13.Ghasemzadeh A, Jaafar HZE, Rahmat A. Antioxidant activities, total phenolics and flavonoids content in two varieties of malaysia young ginger (Zingiber officinale Roscoe) Molecules. 2010;15:4324–4333. doi: 10.3390/molecules15064324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 15.Genovese MI, Da Silva Pinto M, De Souza Schmidt Gonçalves AE, Lajolo FM. Bioactive compounds and antioxidant capacity of exotic fruits and commercial frozen pulps from Brazil. Food Sci Technol Int. 2008;14:207–214. [Google Scholar]
- 16.Gyamfi MA, Yonamine M, Aniya Y. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. Gen Pharmacol. 1999;32:661–667. doi: 10.1016/s0306-3623(98)00238-9. [DOI] [PubMed] [Google Scholar]
- 17.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez-Moreno C. Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci Technol Int. 2002;8:121–137. [Google Scholar]
- 19.López-Amorós ML, Hernández T, Estrella I. Effect of germination on legume phenolic compounds and their antioxidant activity. J Food Compos Anal. 2006;19:277–283. [Google Scholar]
- 20.Frias J, Miranda ML, Doblado R, Vidal-Valverde C. Effect of germination and fermentation on the antioxidant vitamin content and antioxidant capacity of Lupinus albus L. var. Multolupa. Food Chem. 2005;92:211–220. [Google Scholar]
- 21.Walker RB, Everette JD. Comparative reaction rates of various antioxidants with ABTS radical cation. J Agric Food Chem. 2009;57:1156–1161. doi: 10.1021/jf8026765. [DOI] [PubMed] [Google Scholar]
- 22.Atawodi SE, Pfundstein B, Haubner R, Spiegelhalder B, Bartsch H, Owen RW. Content of polyphenolic compounds in the Nigerian stimulants Cola nitida ssp. alba, Cola nitida ssp. rubra A. Chev, and Cola acuminata Schott & Endl and their antioxidant capacity. J Agric Food Chem. 2007;55:9824–9828. doi: 10.1021/jf0721038. [DOI] [PubMed] [Google Scholar]
- 23.Brenna OV, Ceppi ELM, Giovanelli G. Antioxidant capacity of some caramel-containing soft drinks. Food Chem. 2009;115:119–123. [Google Scholar]
- 24.Szajdek A, Borowska EJ. Bioactive compounds and health-promoting properties of berry fruits: a review. Plant Foods Hum Nutr. 2008;63:147–156. doi: 10.1007/s11130-008-0097-5. [DOI] [PubMed] [Google Scholar]
- 25.Roginsky V. Chain-breaking antioxidant activity of natural polyphenols as determined during the chain oxidation of methyl linoleate in Triton X-100 micelles. Arch Biochem Biophys. 2003;414:261–270. doi: 10.1016/s0003-9861(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 26.Savikin K, Zdunić G, Janković T, Tasić S, Menković N, Stević T, Dordević B. Phenolic content and radical scavenging capacity of berries and related jams from certificated area in Serbia. Plant Foods Hum Nutr. 2009;64:212–217. doi: 10.1007/s11130-009-0123-2. [DOI] [PubMed] [Google Scholar]
- 27.Sanful RE. Production and sensory evaluation of tigernut beverages. Pak J Nutr. 2009;8:688–690. [Google Scholar]