SUMMARY
Advanced variant detection in genes underlying risk of sudden unexpected death in epilepsy (SUDEP) can uncover extensive epistatic complexity and improve diagnostic accuracy of epilepsy related mortality. However, the sensitivity and clinical utility of diagnostic panels based solely on established cardiac arrhythmia genes in the molecular autopsy of SUDEP is unknown.
We applied the established clinical diagnostic panels, followed by sequencing and a high density copy number variant (CNV) detection array of an additional 253 related ion channel subunit genes to analyze the overall genomic variation in a SUDEP of the three year old proband with severe myoclonic epilepsy of infancy (SMEI).
We uncovered complex combinations of single nucleotide polymorphisms and CNVs in genes expressed in both neuro-cardiac and respiratory control pathways, including SCN1A, KCNA1, RYR3, and HTR2C.
Our findings demonstrate the importance of comprehensive high resolution variant analysis in the assessment of personally relevant SUDEP risk. In this case, the combination of de novo SNPs and CNVs in the SCN1A and KCNA1 genes respectively is suspected to be the principal risk factor for both epilepsy and premature death. However, consideration of the overall biologically relevant variant complexity with its extensive functional epistatic interactions reveals potential personal risk more accurately.
Keywords: SUDEP, SMEI, Epileptic Encephalopathy, Dravet Syndrome, Gene, Risk, Molecular Autopsy
INTRODUCTION
Children with epileptic encephalopathy and uncontrolled seizures are at increased risk for sudden unexpected death in epilepsy (SUDEP).1 Yet, the clinical risk factors do not provide a pathogenic mechanism, nor are they strongly predictive of the individual mortality hazard.
Ion channel genes modulating cardiac, autonomic, and respiratory functions are prime molecular risk factors for SUDEP. The causative mechanistic link between epilepsy, arrhythmias, sudden death, and the most common LQT gene, the potassium channel KCNQ1, was originally demonstrated in transgenic mice2 and subsequently clinically validated. 3
Since many ion channel genes critical for the regulation of neurocardiac and neurorespiratory pacemaking are also expressed within brain networks underlying epilepsy, potential number of novel SUDEP candidate genes extends beyond the cardiac LQT genes.4 For example, the voltage gated potassium channels KCNA is coexpressed in brain and vagus nerve and Kcna1 null mice have seizures, cardiac arrhythmias, vagal hyperexcitability, and die prematurely. 5, 6 Similarly, the voltage gated sodium channel SCN1A is dually expressed in the brain and the cardiac sinoatrial node and ventricular myocytes.7 Scn1a deficient mice also show autonomic instability and seizure-driven vagal activation preceding sudden death7 paralleling the clinical observations in children with SCN1A mutations and severe myoclonic epilepsy of infancy (SMEI).8 However, most SMEI patients do not die suddenly, suggesting the modulating influence of other candidates in the genetic background, beginning with ion channels themselves.
We identified a SUDEP patient who displayed multiple established clinical-pathological risk factors for SUDEP, including pharmacoresistant epileptic encephalopathy of the SMEI spectrum9, recurrent peri-ictal respiratory compromise, and a suspected cardioautonomic clinical phenotype. In order to comprehensively assess the SUDEP risk embedded within this SMEI phenotype, we designed and performed an extensive postmortem search for deleterious variants in candidate ion channel subunit genes regulating excitability within neural cardiorespiratory regulatory pathways.
METHODS
The 11 months old patient and his parents were recruited into the IRB-approved Ion Channels in Epilepsy Project at Baylor College of Medicine.10 Genomic DNA prepared from blood lymphocytes was submitted for commercial diagnostic exome sequencing in five LQT genes; KCNQ1, SCN5A, KCNH2, KCNE2, ANK2 (Transgenomics), whole genome copy number variants (CNV) analysis at the Medical Genetics Laboratory at Baylor College of Medicine, exome sequencing of 237 ion channel genes10, and screening on a custom designed Ion Channel Comparative Hybridization (ICCH) 4 × 44K microarray (Agilent Technologies, Santa Clara, CA, USA).11 (See Supplemental information for detailed methods).
RESULTS
Index Case Clinical Report
The proband was a healthy, full term Latin American male born to a G1P1 mother. At four months of age, the child developed a prolonged, afebrile hemiclonic seizure that subsided spontaneously but was followed by cessation of respiration. CPR was administered by a family member and the child promptly and fully recovered. General physical and neurological examinations, a head CT and an electroencephalogram (EEG) were normal, and treatment was deferred. Within a month he started experiencing weekly, treatment resistant hemiclonic seizures involving either side. Serial electroencephalograms and brain MRI studies remained unremarkable. Karyotyping confirmed a normal male chromosomal pattern. Routine serum and CSF studies were repeatedly normal and a comprehensive diagnostic work-up for inborn metabolic errors was non-contributory. His development remained normal. Detailed family history was positive for migraine headaches in the mother. An episode of elevated temperature of 100.8 F triggered the first generalized tonic-clonic seizure at 9 months. Treatment resistant daily myoclonic jerks associated with loss of tone began at 11 months of age and the EEG showed epileptiform bursts of fronto-centrally dominant generalized 2–3 Hz abortive spike and slow wave activity. The monthly, prolonged partial seizures were associated with cyanosis and frequent secondary generalization. By 18 months, global developmental delay became evident and the clinical evolution led to the diagnosis of SMEI.12 A cardiac murmur was noted during a follow-up visit. The routine EKG was unremarkable, but he was referred to a cardiologist for further evaluation. The proband was 3 years and 3 months old and in his usual state of health when he was found cyanotic and unresponsive in bed. Full autopsy showed only pulmonary congestion, a frequent finding in sudden death.13 SUDEP was confirmed as the official cause of death.
Integrated Genomic Analysis
Our initial search centered on the five principal LQTS genes. It showed inherited nsSNPs of unknown clinical significance in KCNH2 (LQT2), SCN5A (LQT3) and KCNE1 (LQT5) (TABLE 1A) and in the context of the LQT genotype (Supplemental TABLE 1), it failed to reveal a plausible molecular diagnosis. We next evaluated the channel variant profile (channotype) of the proband through parallel Sanger sequencing of 237 ion channel genes.10 This step confirmed the previously detected LQTS polymorphisms and additionally uncovered a maternally transmitted heterozygous ryanodine receptor 2 (RYR2) nsSNP Q2958R (rs34967813) that has been previously reported in association with catecholaminergic polymorphic ventricular tachycardia (CPVT)14 (TABLE 1A)(Supplemental FIGURE 1A).
Table 1A.
Nonsynonomous single nucleotide polymorphisms in candidate genes for SUDEP in the proband (IE124) compared to parental profiles
| Cardiac LQT Gene SNP Sequencing (detected missense mutation given with gene dosage(het=1; homo=2)
| |||||||
|---|---|---|---|---|---|---|---|
| Syndrome | GENE | PROTEIN | Polyphen | SIFT | IE124p (dbSNP) | IE125m | IE126f |
| LQT2 | KCNH2 | hERG/Kv11.1 | Tolerated | Benign | K897T (2) (rs1805123) | K897T (2) | K897T (1) |
| LQT3 | SCN5A | Nav1.5 | Tolerated | Benign | H558R (1)(rs1805124) | H558R (1) | -- |
| LQT5 | KCNE1 | MinK | Tolerated | Benign | S38G (1)(rs1805127) | S38G (2) | -- |
| CPVT | RYR2 | RyR2 | - | Probably damaging | Q2958R (1) (rs34967813) | Q2958R (1) | -- |
| Human Epilepsy Gene Sequencing (detected missense mutation given with gene dosage (het=1; homo=2)
| |||||||
|---|---|---|---|---|---|---|---|
| Syndrome | GENE | PROTEIN | Polyphen | SIFT | IE124p (dbSNP) | IE125m | IE126f |
| ADNFLE | CHRNA2 | nAChRα2 | Tolerated | Benign | T125A (1)(rs891398) | T125A (1) | T125A (2) |
| IGE | CLCN2 | CLC-2 | Tolerated | Benign | T668S (1) (rs9820367) | T668S (1) | T668S (2) |
| DEND | KCNJ11 | Kir6.2 | Tolerated | Benign | K23E (1); (rs5219) | --; | K23E (2); |
| V337I (1)(rs5215) | -- | V337I (2) | |||||
| Dravet/SMEI/GEFS+ | SCN1A | Nav1.1 | Tolerated; Deleterious | Benign; Probably damaging (benign)* | A1067T (1) (rs2298771); A1783V (1) (rs121917980) | A1067T (1); -- | A1067T (1); -- |
| Respiratory Serotonin Receptor Gene Sequencing (detected missense mutation given with gene dosage (het=1; homo=2)
| |||||||
|---|---|---|---|---|---|---|---|
| Syndrome | GENE | PROTEIN | Polyphen | SIFT | IE124p (dbSNP) | IE125m | IE126f |
| N/A | HTR3C | 5-HT3C | Tolerated | Benign | G405A (1)(rs6807362) | G405A (2) | -- |
| N/A | HTR3D | 5-HT3D | Tolerated; Tolerated | Benign; Possibly damaging (benign)* | G36A (2) (rs6443930); R260H (2) (rs6789754) | G36A (1); R260H (2) | G36A (1); R260H (1) |
The known epilepsy genes, SCN1A, KCNA1, and SCN8A have also been implicated in SUDEP.5, 7, 15–17 Proband channotype analysis10 revealed an inherited common polymorphism A1067T and a de novo nsSNP, A1783V in SCN1A (TABLE 1A; Supplemental FIGURE 1B) previously found in SMEI (http://www.molgen.vib-ua.be/SCN1AMutations/Home). This predicted deleterious de novo mutation in our case suggests a contribution to the epileptic encephalopathy phenotype, yet its influence on the lethality is uncertain. We also uncovered a paternally inherited, novel, nsSNP, C1288Y, in the RYR3 gene that is preferentially expressed in hippocampus and smooth muscle cells of the pulmonary artery18, 19 and the animal models support its role in learning, cognition, and in hypoxia-induced pulmonary vasoconstriction. Thus a dysfunctional RYR3 channel could contribute to the cognitive impairment and respiratory compromise of our patient and targeted RYR3 analysis in SMEI cohorts will be essential to validate this assumption.
Given the clinical history of recurrent, seizure-related apnea, we also analyzed genetic variation in all 18 of the known 5-HT ligand gated ion channels (HTR1A-F, HTR2A-C, HTR3A-E, HTR4, HTR5A, HTR6, and HTR7) and found three inherited nsSNPs (TABLE 1A) of which only the R260H variant is predicted to be possibly damaging by SIFT.
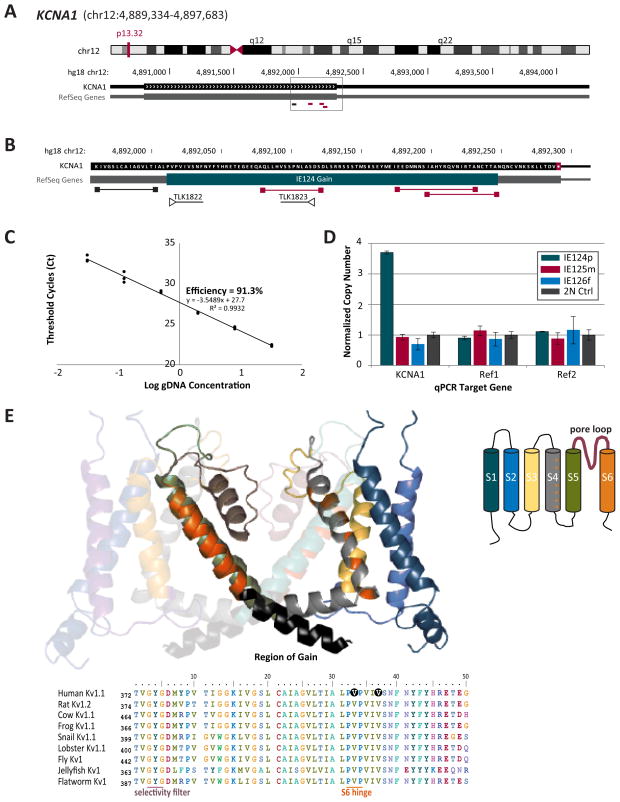
Considering the modulating role of genetic background on clinical phenotype, we also examined the whole genome for structural gene rearrangements. The clinical aCGH screen identified eight inherited autosomal copy number changes in the proband, such as the paternally inherited duplication in SLC6A10P, a gene recently implicated in autistic spectrum disorder20, and the recurrent deletion at 15q11.2 which was previously found in excess in children with congenital heart defects21(TABLE 1B). Since all eight CNVs were inherited and their pathogenic relevance to epilepsy or SUDEP was uncertain we applied our custom high resolution custom designed Ion Channel Comparative Hybridization (ICCH) Array which has minimal detection threshold of 50 bp and an ultra-dense coverage across the exome of 253 ion channel genes, their structurally related family members, and known accessory subunits. Eleven novel duplications in nine known SUDEP genes were confirmed by qPCR (TABLE 1B). Duplication size ranged from to 60 to 3059bp. Four CNVs were de novo. Two rearrangements were independent gains in RYR2, and one was a duplication in GABRG3. They were restricted to introns. The single coding de novo CNV, confirmed by qPCR was at the 3′ end of exon 2 in KCNA1 (FIGURE 2A, B, C), a gene encoding the Kv1.1 pore forming alpha subunit whose loss of function causes severe epilepsy and SUDEP in animal models 5 (FIGURE 2C). Normalization with two reference genes revealed that the proband harbored five extra copies of this exonic region as compared to the diploid genomes of both parents (FIGURE 2D). This gain has a direct impact on the protein coding sequence of the KCNA1 gene. It extends from the highly conserved proline hinge motif (Pro-X-Pro) to the end of the S6 transmembrane helix of the Kv1.1 subunit (FIGURE 2E). The PVP motif in this membrane spanning helix forms a flexible hinge in the transmembrane domain and is directly involved in channel function. Mutations in this region have previously been shown to cause epilepsy (V408T)22, and premature C-terminal truncation or deletion of the Kv1.1 gene leads to aberrant protein expression resulting in epilepsy, ataxia, megalencephaly and SUDEP in mice.23 The repeated gain of this transmembrane helix in the Kv1.1 subunit is likely to impact protein packing and lipid membrane insertion, and thus is an attractive candidate mechanism for Kv1.1 dysfunction contributing to both the seizure and SUDEP phenotype of the proband.
DISCUSSION
As the list of validated risk genes for SUDEP expands beyond those currently linked to cardiac-related mortality, robust diagnostic platforms must be developed for optimal assessment of integrated genetic risk.
Here we show that constructing the genetic variation risk profile for SUDEP benefits from complementary, comprehensive, candidate ion channel gene focused detection platforms. Both single base pair substitutions and architectural defects contribute to the risk of epilepsy and SUDEP as evidenced by the discovery of two biologically plausible pathogenic de novo variants in known SUDEP candidates, SCN1A and KCNA1. Mutations in both genes play a critical role in autonomic destabilization described in clinical reports16, 24 and experimental models of SUDEP 5, 7, and likely contributed to lethality in our patient. Yet, the co-occurrence of epileptic encephalopathy, ictal apnea, suspected cardiac compromise, and SUDEP in this patient may not be explained solely by the molecular mechanisms elucidated through the SCN1A and KCNA1 models 5, 7, but may also reflect the compound effect of these mutations together with the transmitted nsSNPs and CNVs of the cardiac arrhythmia and serotonin receptor genes, RYR3 gene variant, and the 15q11.2 region variant associated with structural heart defects. Since clinical phenotypes reflect the pattern of both the individually unique (de novo) and inherited ion channel variants10 (Supplemental FIGURE 2), resolving the full genetic context is essential for accurate assessment of risk. The integration of ion channel exome sequencing, high resolution ion channel specific CNV survey, and subsequent analysis of 54 candidate SUDEP genes in the neuro-cardiac-respiratory network in this case shows the need for multi-scale channel-based risk prediction for SUDEP.
We present the first comprehensive genomic interrogation of ion channel candidate gene pathways to dissect and personalize SUDEP risk prediction in pediatric epilepsy patients. This case harbored combination of de novo SNPs and CNVs in the SCN1A and KCNA1 genes potentially acting as the principal risk factors for premature death. The larger complexity of the risk load was revealed by additional inherited structural rearrangements and missense polymorphisms within the clinically evident neuro-cardiac and respiratory pathways. As we continue to refine our understanding of the specific biological pathways and genetic risk factors leading to SUDEP, comprehensive assessment of genomic variation in cardiac and respiratory networks using detailed gene profiling can enhance predictive value of gene testing in the routine neurological care of individuals with epilepsy.
Supplementary Material
Figure 1. A de novo gain in the human epilepsy and SUDEP gene KCNA1 was identified in the proband.
A. Chromosomal location of the human KCNA1 gene using Hg18 as the reference genome. The region of the detected genomic gain is in the grey box with the probe positions located beneath the coding exon. B. Higher magnification view of the 3′ end of the KCNA1 gene showing the region of the gain relative to the ICCH comparative hybridization microarray probes. The qPCR primers TLK1822 and TLK1823 used to validate the CNV are shown where primer TLK1823 overlaps with the CGH probe. C. Sybr green standard curve shown against known concentrations of human gDNA to establish qPCR assay efficiency (91.3%). All qPCR assays underwent optimization and efficiency analysis prior to validation experiments in proband gDNA. D. Quantification of the gain in the KCNA1 gene showed 5 additional copies of this region in the proband and not in either parent. Normalization of genomic copy number was performed using two reference genes which are known to be free of copy number variants and compared to a normal diploid control. E. Homology model of the human Kv1.1 ion channel subunit showing two opposing subunits in the tetrameric channel. The ribbon is colored the same as the 6TM schematic diagram top right for orientation. The S4 voltage sensor is grey with the positively charged arginine and lysine residues shown in red. The S6 domain is shown in red and the region of gain is in black. The S6 PVP hinge sequence is highly conserved from jellyfish to man. The residues V404 and V408 are shown in black where amino acid substitutions at these positions cause epilepsy, ataxia and myokymia.
Table 1B.
Copy number variation in SUDEP proband (IE124) compared to parental profiles using a clinical diagnostic microarray and a custom high density ion channel comparative hybridization array.
| Clinical aCGH Diagnostic Array (BCM Molecular Diagnostics Core - Director Dr. Ankita Patel)
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CNV# | Chromosome | CytoBand |
Start Position (Hg19) |
End Position (Hg19) |
Gain/Loss |
Number of Probes |
Length of CNV |
Known CNV* |
Number of Genes |
RefSeq (HUGO) Gene Names |
In Proband (IE124) |
In Mother (IE125) |
In Father (IE126) |
| 1 | chr1 | p21.1 | 104012051 | 104012498 | Loss | 8 | 447 | N(1,2,4) | y | n | y | ||
| 2 | chr4 | q12 | 57746415 | 57988228 | Gain | 9 | 241813 | N(1,2,3) | y | n | y | ||
| 3 | chr8 | p11.23 | 39369942 | 39499498 | Gain | 8 | 129556 | N(1,2,3) | 2 | ADAM5P, ADAM3A | y | y | y |
| 4 | chr10 | q11.22 | 46384979 | 46506801 | Gain | 4 | 121822 | N(1,2,3,4) | 3 | SYT15,GPRIN2,PPYR1 | y | y | y |
| 5 | chr14 | q11.2 | 21609644 | 22028409 | Loss | 14 | 418765 | N(1,2,3) | y | y | y | ||
| 6 | chr14 | q11.2 | 18864561 | 19459230 | Gain | 3 | 594669 | N(1,2,3) | 6 | P704P, OR4Q3, OR4M1, OR4N2, OR4K2, OR4K5 | y | y | y |
| 7 | chr15 | q11.2 | 19108763 | 19464920 | Loss | 113 | 356157 | N(1,2,3,4) | 3 | LOC646214, CXADRP2, POTEB | y | y | y |
| 8 | chr16 | p11.2 | 32481308 | 33528443 | Gain | 106 | 1047135 | N(1,2,3,4) | 5 | ZNF267, HERC2P4, LOC729355, TP53TG3, SLC6A10P | y | n | y |
| Ion Channel Comparative Hybridization (ICCH) Custom High Density aCGH Array (Translational Neurogenetics in Epilepsy Laboratory - Director Dr. Alica Goldman)
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CNV# | Chromosome | CytoBand |
Start Position (Hg18) |
End Position (Hg18) |
Gain/Loss |
Number of Probes |
Length of CNV |
Known CNV* |
Number of Genes |
RefSeq (HUGO) Gene Names |
In Proband (IE124) |
In Mother (IE125) |
In Father (IE126) |
| 1 | chr1 | q43 | 235879084 | 235879144 | Gain | 1 | 60 | N | 1 | RYR2 (intron 49/50) NM_001035.2 | y | n | n |
| 2 | chr1 | q43 | 236028304 | 236028634 | Gain | 3 | 330 | N(1,2) | 1 | RYR2 (intron 97/98) NM_001035.2 | y | n | n |
| 3 | chr1 | q43 | 19864621 | 19864666 | Gain | 2 | 45 | N | 1 | HTR6 (exon 1 5′UTR) NM_000871.1 | y | y | n |
| 4 | chr7 | q36.1 | 150285908 | 150285968 | Gain | 1 | 60 | N(1,2) | 1 | KCNH2 (intron 4/5) iso1 NM_000238.3 | y | n | y |
| 5 | chr12 | p13.33 | 2117163 | 2123823 | Gain | 3 | 6660 | N(1,2) | 1 | CACNA1C (intron 2/3) iso1 NM_199460.2 | y | n | y |
| 6 | chr12 | P13.32 | 4892174 | 4892251 | Gain | 2 | 77 | N | 1 | KCNA1 (exon 2): NM_000217.2 | y | n | n |
| 7 | chr15 | q12 | 24568642 | 24569014 | Gain | 3 | 372 | N(1) | 1 | GABRB3 (exon 2–3) iso1 NM_000814.5 | y | y | y |
| 8 | chr15 | q12 | 25052658 | 25055717 | Gain | 2 | 3059 | N(2) | 1 | GABRG3 (intron 3/4) iso1 NM_033223.4 | y | n | n |
| 9 | chr19 | P13.13 | 13178788 | 13179169 | Gain | 3 | 381 | N | 1 | CACNA1A (exon 47 3′UTR) iso1 NM_000068.3 | y | y | y |
| 10 | chr19 | p13.13 | 13478014 | 13478059 | Gain | 1 | 45 | N(2) | 1 | CACNA1A (exon 1) NM_000068.3 | y | Y | y |
| 11 | chr19 | p13.3 | 565234 | 565928 | Gain | 5 | 694 | N(1,2) | 1 | HCN2 (intron 3/4) NM_001194.3 | y | y | y |
Known aberrations Toronto DGV (1 = region has reported gain; 2 = region has reported loss; 3 = region has reported indels; 4 = region has reported inversions)
Acknowledgments
Funding: Supported by National Institute of Neurological Disorders and Stroke grants NS067013 and NS067013S (A.M.G.), NS049130, and NS076916 (J.L.N.), T32NS043124-09 (VCB); CURE; Fiorito Foundation and the Emma Bursick Memorial Fund Q2 (A.M.G.); the Blue Bird Circle Foundation (J.L.N.).
The authors wish to extend special acknowledgment to Dr. Benjamin Salisbury for his expert advice and assistance with the genetic screening of the samples on the Familion long QT gene panel.
Footnotes
Disclosures: J.R.L. is a paid consultant for Athena Diagnostics, has stock ownership in 23 and Me and Ion Torrent Systems, and is a co-inventor on multiple United States and European patents related to molecular diagnostics for inherited neuropathies, eye diseases and bacterial genomic fingerprinting. The Department of Molecular and Human Genetics at Baylor College of Medicine derives revenue from the chromosomal microarray analysis (CMA) and clinical exome sequencing offered in the Medical Genetics Laboratory (MGL; http://www.bcm.edu/geneticlabs/). The remaining authors have no conflicts of interest.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
AUTHORS’ CONTRIBUTION:
Klassen, T.L.: Design of high density ion channel array, analysis of sequence and copy number variant data, genetic data validation, preparation of the manuscript, figures, and tables, manuscript revisions, and final approval of the submitted manuscript.
Bomben, V.C.: Analysis of the sequence and copy number variant data, critical revision of the manuscript, final approval of the submitted manuscript.
Patel, A.: Analysis of samples on the diagnostic copy number variant array, validation of the copy number variants, critical revision of the manuscript, final approval of the submitted manuscript.
Drabek, J.: Analysis of samples on the copy number variant arrays, validation of the single nucleotide polymorphisms and copy number variants, critical revision of the manuscript, final approval of the submitted manuscript.
Chen, T.T.: Analysis of the ion channel based genetic variation, functional assessment of the identified variants, preparation of the manuscript, and critical revision of the manuscript, final approval of the submitted manuscript.
Gu, W.: Conceptual design of the copy number variant array experiments, analysis of samples on the copy number variant arrays, data sorting and analysis, critical revision of the manuscript, final approval of the submitted manuscript.
Zhang, F.: Conceptual design of the copy number variant array experiments, analysis of samples on the copy number variant arrays, data sorting and analysis, critical revision of the manuscript, final approval of the submitted manuscript.
Chapman, K.: Family recruitment, clinical data acquisition, diagnostic classification, critical revision of the manuscript, final approval of the submitted manuscript.
Lupski, J.R.: Leadership and expert guidance in the conceptual design and development of the targeted ion channel array, oversight and guidance in copy number array data acquisition and analysis, critical revision of the manuscript, final approval of the submitted manuscript.
Noebels, J.L.: Leadership and expert guidance in the conceptual design and development of the targeted ion channel array, acquisition of the ion channel exome data and commercial diagnostic long QT panel data, oversight and guidance in clinical-genetic data analysis, critical revisions of the manuscript, final approval of the submitted manuscript.
Goldman, A.M.: Conceptual design of the study, conceptual design and development of the targeted ion channel array, acquisition of the ion channel exome data and commercial diagnostic long QT panel data, clinical-genetic data analysis, data validation, preparation of the manuscript, and final approval of the submitted manuscript.
Contributor Information
Tara L. Klassen, Faculty of Pharmaceutical Sciences, The University of British Columbia, Vancouver, BC, Canada
Valerie C. Bomben, Department of Neurology, Baylor College of Medicine, Houston, TX, USA
Ankita Patel, Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, TX, USA
Janice Drabek, Department of Neurology, Baylor College of Medicine, Houston, TX, USA
Tim T. Chen, Faculty of Pharmaceutical Sciences, The University of British Columbia, Vancouver, BC, Canada
Wenli Gu, Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, TX, USA
Feng Zhang, School of Life Sciences, Fudan University, Shanghai, China
Kevin Chapman, Department of Neurology, University of Colorado, CO, USA
James R. Lupski, Department of Molecular and Human Genetics and Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA
Jeffrey L. Noebels, Department of Neurology and Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, TX, USA
A.M. Goldman, Department of Neurology, Baylor College of Medicine, Houston, TX, USA
References
- 1.Sillanpaa M, Shinnar S. Long-term mortality in childhood-onset epilepsy. N Engl J Med. 2010;363:2522–9. doi: 10.1056/NEJMoa0911610. [DOI] [PubMed] [Google Scholar]
- 2.Goldman AM, Glasscock E, Yoo J, Chen TT, Klassen TL, Noebels JL. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Sci Transl Med. 2009;1:2ra6. doi: 10.1126/scitranslmed.3000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson JN, Hofman N, Haglund CM, Cascino GD, Wilde AA, Ackerman MJ. Identification of a possible pathogenic link between congenital long QT syndrome and epilepsy. Neurology. 2009;72:224–31. doi: 10.1212/01.wnl.0000335760.02995.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerrone M, Priori SG. Genetics of sudden death: focus on inherited channelopathies. European heart journal. 2011;32:2109–18. doi: 10.1093/eurheartj/ehr082. [DOI] [PubMed] [Google Scholar]
- 5.Glasscock E, Yoo JW, Chen TT, Klassen TL, Noebels JL. Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. J Neurosci. 2010;30:5167–75. doi: 10.1523/JNEUROSCI.5591-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glasscock E, Qian J, Kole MJ, Noebels JL. Transcompartmental reversal of single fibre hyperexcitability in juxtaparanodal Kv1.1-deficient vagus nerve axons by activation of nodal KCNQ channels. The Journal of physiology. 2012;590:3913–26. doi: 10.1113/jphysiol.2012.235606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalume F, Westenbroek RE, Cheah CS, et al. Sudden unexpected death in a mouse model of Dravet syndrome. J Clin Invest. 2013;123:1798–808. doi: 10.1172/JCI66220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delogu AB, Spinelli A, Battaglia D, et al. Electrical and autonomic cardiac function in patients with Dravet syndrome. Epilepsia. 2011;52 (Suppl 2):55–8. doi: 10.1111/j.1528-1167.2011.03003.x. [DOI] [PubMed] [Google Scholar]
- 9.Harkin LA, McMahon JM, Iona X, et al. The spectrum of SCN1A-related infantile epileptic encephalopathies. Brain. 2007;130:843–52. doi: 10.1093/brain/awm002. [DOI] [PubMed] [Google Scholar]
- 10.Klassen T, Davis C, Goldman A, et al. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell. 2011;145:1036–48. doi: 10.1016/j.cell.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klassen TL, Drabek J, Tomson T, et al. Visual automated fluorescence electrophoresis provides simultaneous quality, quantity, and molecular weight spectra for genomic DNA from archived neonatal blood spots. The Journal of molecular diagnostics: JMD. 2013;15:283–90. doi: 10.1016/j.jmoldx.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerrini R, Oguni H. Borderline Dravet syndrome: a useful diagnostic category? Epilepsia. 2011;52 (Suppl 2):10–2. doi: 10.1111/j.1528-1167.2011.02995.x. [DOI] [PubMed] [Google Scholar]
- 13.Surges R, Thijs RD, Tan HL, Sander JW. Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms. Nature reviews Neurology. 2009;5:492–504. doi: 10.1038/nrneurol.2009.118. [DOI] [PubMed] [Google Scholar]
- 14.Tiso N, Stephan DA, Nava A, et al. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2) Hum Mol Genet. 2001;10:189–94. doi: 10.1093/hmg/10.3.189. [DOI] [PubMed] [Google Scholar]
- 15.Cheah CS, Yu FH, Westenbroek RE, et al. Specific deletion of NaV1.1 sodium channels in inhibitory interneurons causes seizures and premature death in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A. 2012;109:14646–51. doi: 10.1073/pnas.1211591109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Gal F, Korff CM, Monso-Hinard C, et al. A case of SUDEP in a patient with Dravet syndrome with SCN1A mutation. Epilepsia. 2010;51:1915–8. doi: 10.1111/j.1528-1167.2010.02691.x. [DOI] [PubMed] [Google Scholar]
- 17.Veeramah KR, O’Brien JE, Meisler MH, et al. De novo pathogenic SCN8A mutation identified by whole-genome sequencing of a family quartet affected by infantile epileptic encephalopathy and SUDEP. Am J Hum Genet. 2012;90:502–10. doi: 10.1016/j.ajhg.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balschun D, Wolfer DP, Bertocchini F, et al. Deletion of the ryanodine receptor type 3 (RyR3) impairs forms of synaptic plasticity and spatial learning. The EMBO journal. 1999;18:5264–73. doi: 10.1093/emboj/18.19.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng YM, Wang QS, Liu QH, Rathore R, Yadav V, Wang YX. Heterogeneous gene expression and functional activity of ryanodine receptors in resistance and conduit pulmonary as well as mesenteric artery smooth muscle cells. Journal of vascular research. 2008;45:469–79. doi: 10.1159/000127438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bayou N, M’Rad R, Belhaj A, et al. The creatine transporter gene paralogous at 16p11.2 is expressed in human brain. Comparative and functional genomics. 2008:609684. doi: 10.1155/2008/609684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soemedi R, Wilson IJ, Bentham J, et al. Contribution of global rare copy-number variants to the risk of sporadic congenital heart disease. Am J Hum Genet. 2012;91:489–501. doi: 10.1016/j.ajhg.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lerche H, Jurkat-Rott K, Lehmann-Horn F. Ion channels and epilepsy. Am J Med Genet. 2001;106:146–59. doi: 10.1002/ajmg.1582. [DOI] [PubMed] [Google Scholar]
- 23.Petersson S, Persson AS, Johansen JE, et al. Truncation of the Shaker-like voltage-gated potassium channel, Kv1.1, causes megencephaly. Eur J Neurosci. 2003;18:3231–40. doi: 10.1111/j.1460-9568.2003.03044.x. [DOI] [PubMed] [Google Scholar]
- 24.Hindocha N, Nashef L, Elmslie F, et al. Two cases of sudden unexpected death in epilepsy in a GEFS+ family with an SCN1A mutation. Epilepsia. 2008;49:360–5. doi: 10.1111/j.1528-1167.2007.01439_2.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.