Abstract
Autotrophic CO2 fixation is the most important biotransformation process in the biosphere. Research focusing on the diversity and distribution of relevant autotrophs is significant to our comprehension of the biosphere. In this study, a draft genome of a bacterium from candidate phylum SBR1093 was reconstructed with the metagenome of an industrial activated sludge. Based on comparative genomics, this autotrophy may occur via a newly discovered carbon fixation path, the hydroxypropionate-hydroxybutyrate (HPHB) cycle, which was demonstrated in a previous work to be uniquely possessed by some genera from Archaea. This bacterium possesses all of the thirteen enzymes required for the HPHB cycle; these enzymes share 30∼50% identity with those in the autotrophic species of Archaea that undergo the HPHB cycle and 30∼80% identity with the corresponding enzymes of the mixotrophic species within Bradyrhizobiaceae. Thus, this bacterium might have an autotrophic growth mode in certain conditions. A phylogenetic analysis based on the 16S rRNA gene reveals that the phylotypes within candidate phylum SBR1093 are primarily clustered into 5 clades with a shallow branching pattern. This bacterium is clustered with phylotypes from organically contaminated environments, implying a demand for organics in heterotrophic metabolism. Considering the types of regulators, such as FnR, Fur, and ArsR, this bacterium might be a facultative aerobic mixotroph with potential multi-antibiotic and heavy metal resistances. This is the first report on Bacteria that may perform potential carbon fixation via the HPHB cycle, thus may expand our knowledge of the distribution and importance of the HPHB cycle in the biosphere.
Introduction
As the most diverse and abundant cellular life forms in the biosphere, microorganisms play key roles in nearly all biogeochemical processes. However, most microorganisms are not available in pure cultures and can only be detected with culture-independent molecular surveys, which greatly inhibits our comprehension of their roles in ecological and biogeochemical processes. The genomic sequencing of these microorganisms is significant in the construction of blueprints for evolutionary and metabolic diversity [1]. With advances in next generation sequencing (NGS) and bioinformatics, draft genomes of uncultured bacteria can be reconstructed from various complex environmental samples via single-cell genome sequencing [2] or genome binning [3]. Therefore, metabolic deductions and evolutionary analyses can be performed based on the reconstructed genomes and comparative genomics [4], which may greatly expand our understanding of microbial metabolism and its potential role in ecology and biogeochemistry.
SBR 1093 was established as a candidate phylum using several 16S rRNA gene clones in phosphate-removing activated sludge from a sequencing batch reactor [5] that was supplied with sodium acetate for phosphate removal. Thereafter, they were continuously detected in an industrial wastewater treatment system receiving low-molecular-weight organic acids and short-chain alcohols [6], activated sludge from coking wastewater treatment, chlorinated hydrocarbon-contaminated soil and hydrocarbon-contaminated soil [7]. All of these environments were associated with short-chain fatty acids, which implied that the bacteria within this candidate phylum may proliferate effectively with short-chain fatty acids. In addition to the contaminated environment, 16S rRNA clones within candidate phylum SBR1093 were also detected in samples from ocean environments, such as ocean crust from the East Pacific Rise [8], polymetallic nodules and the surrounding sediments, oceanic surface sediment [9], sponges [10], etc. Considering these specific niches, deficiency of light, O2 and organics, the most probable metabolism for these bacteria may be chemoautotrophy rather than heterotrophy. This is consistent with a report on a stalactite microbial community found in a desert cave [11] in which SBR1093-like 16S rRNA gene sequences comprised up to 10% of the total bacterial 16S rRNA gene sequences. Thus far, the metabolism of bacteria within candidate phylum SBR1093 remains elusive because there are no available pure cultures or enrichments from experiments or genomes. Because their abundance in the known microbial community is very low (less than 1% [11]), the metabolism of SBR1093 in these artificial and biogeochemical processes is difficult to deduce. Therefore, genome binning using the metagenome of a microbial community enriched with a member from this phylum could shed light on its metabolic properties and ecological functions.
As opposed to microbial communities in municipal wastewater treatment plants, which are fed with a mixture of natural organics and dominated by bacteria within Proteobacteria, Bacteroidetes, Actinobacteria, etc. [12], those in industrial wastewater treatment plants show unique populations in each plant [13]. Shaped by the specific substrates and physical-chemical conditions, microbial communities in industrial wastewater treatment plants are often enriched with uncultured microorganisms with specific metabolisms [14], and their metabolisms are associated with the biotransformation and biodegradation of specific substrates. Considering their relatively high abundance in these systems, draft genomes of the dominant populations could be reconstructed via the genome binning of the metagenome [15], [16] in an attempt to elucidate their physiological and ecological functions in the microbial community (as well as their taxonomy) [17]. Based on a survey of 454 pyrosequencing for the microbial community pyrosequencings in industrial-activated sludge (data not shown here), a bacterium of candidate phylum SBR1093 was enriched in a full industrial wastewater treatment plant (WWTP), which fed with morpholine distilling-wastewater and performed an alternating anoxic/aerobic process. The objective of this study is to reconstruct the draft genome of a bacterium from candidate phylum SBR1093 with the metagenome of activated sludge from this WWTP. This may shed light on its taxonomic identity, metabolic properties and ecological role, thus be helpful in determining potential conditions for its cultivation and isolation.
Materials and Methods
Sample collection and DNA extraction
Activated sludge samples were collected from a local industrial wastewater treatment plant: two samples from anoxic and aerobic tanks, respectively, which were fed with morpholine distilling-wastewater and that operated in alternate anoxic/aerobic processes (There is no specific permission required for the collection of sludge samples. This sampling site is located at the Shanghai Industrial Park, N31.01, E121.41, and the field studies did not involve endangered or protected species.). The collected samples were fixed onsite with absolute ethanol at a volume ratio of 1∶1, and then transported in an icebox and stored at −20°C prior to DNA extraction. For the DNA extraction, the microbial cells in the samples were collected after centrifugation and washed twice with phosphate-buffered solution (PBS). The DNA extraction was performed according to the protocol of the FastDNA SPIN Kit for soil (Qbiogene Inc., CA, USA), which was verified as the most suitable method to extract DNA from the activated sludge samples [18].
Metagenomic sequencing
With the extracted DNA, libraries with insert sizes of 200 bp and 800 bp were constructed for each sample according to the manufacturer’s instructions (Illumina, San Diego, USA). Then, the metagenomic sequencing was performed with an Illumina HiSeq 2000 Platform (Illumina, San Diego, USA) using the 101 bp paired-end (PE101) strategy (BGI, Shenzhen, China). With a 2/5 Illumina sequencing run for the 200-bp library and a 1/5 sequencing run for the 800-bp library, approximately 101 and 35 million sequencing reads (100 bp) were obtained, respectively. Raw reads containing any ambiguous bases or those with an average quality score lower than 20 were removed prior to the following analysis.
De novo assembly
The filtered reads were imported into the CLC genomic workbench (version 4.9), and the paired-end sequences were used for the following de novo assembly in the CLC genomic workbench. The K parameter (k-mer size) was set to 51 (half of the PE sequencing length) during the assembly. Only contigs longer than 500 bp were output as well as the corresponding mapping reads for further analysis. More than 50% of the reads were assembled into contigs >500 bp (98,505 contigs), with a maximum length of 349,894 bp. As a test to examine the potential errors in the assembly, the coverage consistence of the assembled contigs was checked according to the previous report [15].
Genome binning
Genome binning was performed according to the previous work [19], based on a plot of coverage and GC ratio of contigs, including PE-tracking and reassembly, which was further refined with Metacluster 4.0 [20]. Then, the integrity and redundancy of the binning draft genome were assessed via the comparison of essential single copy genes (ESCGs) of most organisms in the domain Bacteria [15], [19].
Gene annotation and comparison
Open reading frames (ORFs) were predicted online with MetaGeneMark [21], and the deduced amino acid (AA) sequences were obtained for BLASTp against the NCBI nr database (released on July 18, 2013) with an E-value of 10−5 and minimum alignment of 50 AA, respectively [22]. The results were taxonomically assigned with the lowest common ancestor (LCA) algorithm and functionally annotated based on KEGG using MEGAN 4.0 [23]. A Pfam search with an E-value of 10−5 was performed based on the Hidden Markol Model and against PfamA database version 26.0 [24], which could also be used for the comparison of gene clusters and verification of MEGAN annotation.
Results and Discussion
Genome binning and completeness assessment
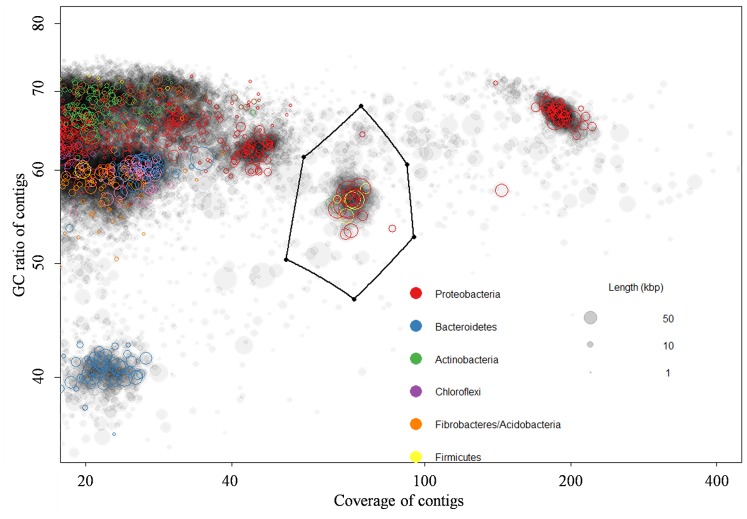
A draft genome containing 94 scaffolds with a total size of 3,099,643 bp with GC contents of 56.4% was reconstructed (Figure 1). According to the prediction of MetaGeneMark, 3,228 ORFs were presented and 3,037 were in full length with the sole initiator and terminator, implying that the de novo assembly was accurately performed and that only the ORFs at the ends of the contigs were incomplete. Considering the functional assignment, 2,532 ORFs shared a mean similarity of 51.2% with the known enzymes in the nr-database (released on July 18, 2013), which is nearly at full align length (with a mean cover ratio of 0.87). All of the 40 universally occurring clusters of orthologous groups (COGs) [25] and tRNAs for all 20 amino acids are presented in this draft genome (Table S1 in File S1), which implies that it is near completeness. Additionally, based on the Hidden Markov Model (HMM) search, 102 unique ESCGs were found in this draft genome (Table S2 in File S1), indicating a completeness >96% [26]. Among the four suspected repetitive ESCGs, TIGR00436 and PF00750 are not always a single copy gene [19], and TIGR02350 is hit with the duplicate genes located within contig_838, implying no contamination from other bacteria. The only suspected duplicate ESCGs (PF00162) are distributed in different contigs and in best hit on the NCBI web to sequences in heterotrophic Anoxybacillus sp. (YP002316858) and autotrophic Nitrososphaera sp. (YP006862459), respectively. These two sequences are assigned as glyceraldehyde 3-phosphate dehydrogenase and triosephosphate isomerase, which are responsible for glycolysis and gluconeogenesis, respectively, and thus may coexist in an autotrophic or mixotrophic bacterium (consistent with the following metabolism analysis). In summary, this draft genome has no verifiable contamination from other bacteria.
Figure 1. Genome binning of the dominant population with a plot of assembly contigs (based on coverage versus GC ratio).
The circles represent the contigs with the size of the square root of their length. Clusters of contigs with similar color present potential genome bins, and contigs cluster with a coverage of approximately 80 (enclosed with black line) were collected for genome binning in this study.
Phylogenetic and biogeographic characterization
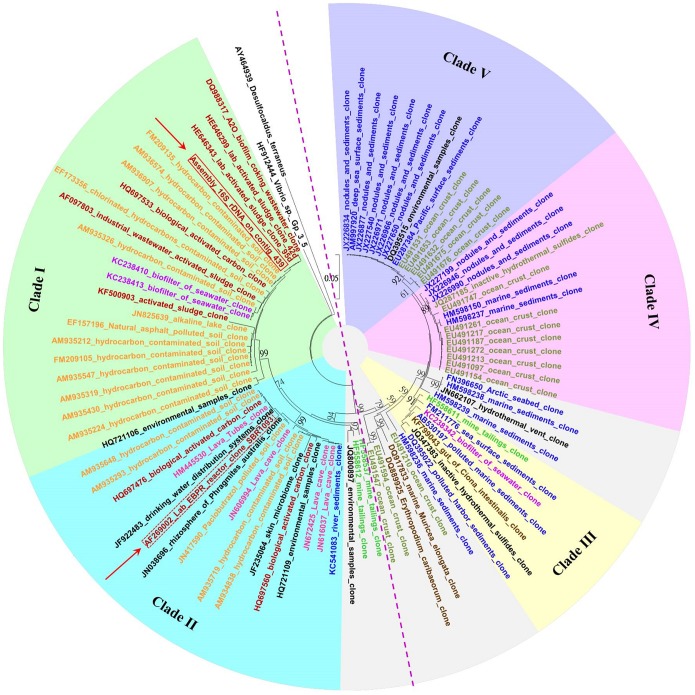
This draft genome contains a complete rRNA operon (16S, 5S and 23S, 3,379–8,941 bp) on contig_439, and the 16S rRNA gene (1,567 bp, 6,992–8,558 bp) is used for the taxonomic identification with BLASTn against NCBI and the Greengenes 16S rRNA gene database (released at May, 2013). The genome shares only 85.9% similarity with the 16S rRNA gene of pure culture Vibrio sp. Gp-3–5.1 (HF912444) but approximately 94.9% similarity with the first nominated SBR1093 sequence (AF269002) and 99.9% with the uncultured bacterium (HE646343). Therefore, this draft genome should represent a bacterium from candidate phylum SBR1093, named as SBR1093 HKSP. The neighbor-joining and maximum-likelihood phylogenetic tree of the 16S rRNA gene in this bacterium and strains from relative phyla revealed that the candidate phylum SBR1093 represented by this bacterium is close to Proteobacteria (Figure S1 in File S1). Additionally, as shown in the phylogenetic tree of this 16S rRNA gene and the reference sequences (Figure 2), phylotypes from candidate phylum SBR1093 are primarily divided into two subdivisions, terrestrial and marine. They are further clustered into 5 clades within which SBR1093 HKSP belongs to clade I. The shallow branching pattern within this phylum (the largest distance between these phylotypes is less than 0.12) implies that bacteria within SBR1093 are recently radiated [27]. It should be noted that some sequences named SBR1093 (GQ348518, 350258, 350948, AY907765, EF573230) are clustered with Vibrio sp. (HF912444) and should be excluded from this candidate phylum. Clades within this candidate phylum are distinguished clearly according to their biogeographic distributions, which are differed from dissolve oxygen, salinity, pH, as well as organic nutrients. Bacteria in clades I and II are primarily found in terrestrial environments associated with fertile organics, whereas those in clade IV and V are primarily found in barren marine environments. The 16S rRNA gene of this draft genome SBR1093 HKSP is clustered with phylotypes found in the activated sludge or contaminated environment (with similarity >99.9%), conditions with plenty of organics, thus implying the potential of organic metabolism (see details below).
Figure 2. Phylogeny of phylotypes affiliated with the candidate phylum SBR1093.
This phylogenetic tree is constructed with 16S rRNA gene sequences based on the neighbor-joining method with Jukes and Cantor distances. The main clades with nodes supported by a bootstrap value of >50% are labeled and marked with different background colors (Clade I green, Clade II blue, Clade III yellow, Clade IV pink and Clade V purple). The phylotypes derived from different sources are labeled with the following: dark red, activated sludge; orange, soil; blue, sediments; dark green, ocean crust; pink, lava; purple, seawater; green, mine tailings; brown, marine organisms; black, others. The phylotype SRB1093 HKSP obtained in this study is enclosed with a solid red line, whereas the first reported phylotype is enclosed with a red dashed line. The scale bar represents 0.05 nucleotide substitutions per site.
In addition to the taxonomy of the 16S rRNA gene, proteins encoding in the genome may be another important resource in determining the phylogenetic position of an unknown bacterium. With the results of BLASTp checked against those in the nr-database, MEGAN may be used to classify these proteins using an LCA algorithm. Therefore, the ancestry of this bacterium can be speculated according to the phylogenetic relationship of proteins encoding in this draft genome. Of the 2,532 proteins in SBR1093 HKSP that have homologs with proteins in the nr-database, the largest section is clustered within the phylum level of Proteobacteria (n = 549), which is followed by Firmicutes (n = 70), Cyanobacteria (n = 53) and Bacteroidetes (n = 42) (Figure S2 in File S1). On the genus level, the most hits (n = 104) belong to Geobacter in Deltaproteobacteria, and the rests are evenly distributed among more than 50 genera. Therefore, candidate phylum SBR1093 represented by this bacterium may be close to Proteobacteria and Firmicutes but nonetheless separate from them, which is consistent with the taxonomy analysis of the 16S rRNA gene.
Bacterial morphology and cell wall type
This SBR1093 bacterium might be rod-shape, for there is a complete set of genes for rod shape proteins identified in this draft genome (gene_1559–1563). Considering with the cell wall type, a complete set of genes responsible for peptidoglycan biosynthesis (gene_1066–1072) and outer membrane lipoprotein encoding (gene_41, 44, 411 and 2121) are identified, which are only present in Gram-negative bacteria, suggesting this bacterium might be Gram negative. During the life cycle, the bacterium may form spores in adverse conditions and germinate in more suitable conditions, because there is a complete set of genes for spore formation, maturation and germination (gene_1062, 1522 and 265).
Primary metabolism
According to the KEGG annotation in MEGAN, approximately half of the predicted proteins (1,153 of the total 2,329) belong to metabolism, including 268 for carbohydrate metabolism, 223 for amino acid metabolism and 76 for lipid metabolism. The genes responsible for glycolysis/gluconeogenesis and the citrate cycle are complete in this draft genome, as well as a set for oxidative phosphorylation (Table S3 in File S1), indicating that this bacterium should be an aerobic heterotroph. However, two genes encoding the CRP/FNR family regulator and five genes for the Fur family regulators were also identified in this draft genome, indicating the potential anoxic metabolism of this bacterium. Therefore, it appears to be a facultative aerobic bacterium. This is consistent with the conditions of the anoxic/aerobic process from which these samples were collected, as well as the biogeographic distribution of the phylotypes from candidate phylum SBR1093. Additionally, full genes responsible for the upstream metabolism of glycolysis, such as the metabolism of glycogen/starch and cellulose, are also identified in this draft genome, but there were no genes related to the uptake of glucose or metabolism of sucrose, maltose or xylose. Therefore, this bacterium may use glycolysis only for the catabolism of self-producing sugar rather than external sugar.
Based on the genetic analysis, this bacterium may reduce nitrate via an assimilation path and nitrite via an assimilation or dissimilation path (Figure S3 in File S1), which indirectly verifies the facultative aerobic metabolism. Although most of the amino acid can be synthetized with the assimilated ammonia, no genes for asparagine synthesis were detected, implying the necessity for asparagine supplementation in the enrichment of this bacterium. This is consistent with the branched-chain amino acid ABC transporters. Additionally, only genes encoding sulfite reductase exist in this draft genome (none for sulfate reductase), indicating that this bacterium may survive in niches with relatively high redox potential rather than strictly anaerobic conditions. This is also consistent with the conditions in which the microbial community enriched this bacterium (i.e., cycling between the anoxic and aerobic tanks).
Regarding the utilization of the substrates, microbes enriched in a specific condition may adapt to this environment and interact closely with their habitat through metabolic reactions [28]. Because this bacterium is enriched in an anoxic/aerobic process and is fed with morpholine distilling wastewater, it may be involved in the metabolism of the main organics in this wastewater. However, the organics in this influent are primarily in the form of morpholine (or its derivatives), which is reported to decompose into glyoxylate and glycolate only by some species of Mycobacterium containing a complex of cytochrome p450, ferredoxin and ferredoxin reductase [29]. Based on the genetic analysis, there is no p450 gene in this draft genome. Therefore, this bacterium may not be active in the ring-opening of morpholine and is more likely involved in the metabolism of the intermediates of morpholine decomposition such as glycolate and glyoxylate. This is consistent with multiple genes responsible for the conversion of glycolate (gene_1890, 1891, 2884, 2885, 2887) and glyoxylate (gene_55), as well as the fusion protein responsible for the glyoxylate shunt (Table S3 in File S1, gene_720), which combines the glyoxylate with acetyl-CoA for the synthesis of malate.
Identification of the carbon fixation pathway
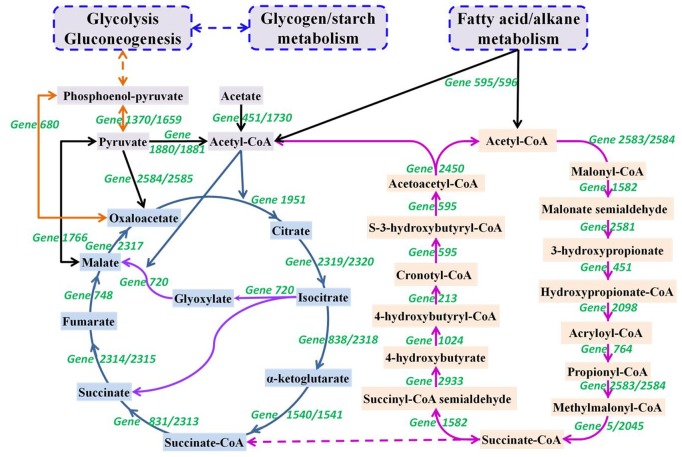
Interestingly, this draft genome possesses genes encoding phosphoenolpyruvate (PEP), acetyl-CoA and propionyl-CoA carboxylase, which catalyze carbon fixation, indicating that this bacterium might have autotrophic metabolism potential. Further comparative genetics reveal that this draft genome contains all of the 13 enzymes required for the hydroxypropionate–hydroxybutyrate (HPHB) cycle (Figure 3), a carbon fixation pathway possessed only by Archaea [30]. The enzymes in this draft genome share 30∼50% amino acid identity with those involved in the HPHB cycle in Metallosphaera sedula, a typical strain of Archaea that undergoes the HPHB cycle (Table 1). In this carbon fixation pathway, two molecules CO2 are assimilated in form of bicarbonate with the carboxylation of a bifunctional biotin-dependent acetyl-CoA/propionyl-CoA carboxylase, and the produced acetyl-CoA is transferred to the citrate cycle for the synthesis of the amino acids and fatty acids or alternatively glycogen via gluconeogenesis. For other carbon fixation pathways, some key enzymes are absent from this draft genome (Figure S4 in File S1), such as pyruvate synthase (EC:1.2.7.1) for the 3-dicarboxylate-hydroxybutyrate (DCHP) pathway, ATP-citric lyase (EC:2.3.3.8) and 2-oxoglutarate synthase (EC:1.2.7.3) for the reductive citric acid cycle pathway, NADP+ dependent formate dehydrogenase (EC:1.2.1.43) and formyltetrahydrofolate synthetase (EC:6.3.4.3) for the reductive acetyl-CoA pathway, malyl-CoA lyase (EC:4.1.3.24) for the 3-hydroxypropionate cycle pathway, and ribulose-bisphosphate carboxylase (EC:4.1.1.39) for the reductive pentose phosphate cycle pathway [31]. Therefore, SBR1093 HKSP may have autotrophic metabolism via the HPHB carbon fixation pathway, whereas the phosphoenolpyruvate carboxylase may function to maintain the balance of intermediates within the citrate cycle [32]. Additionally, enzymes employed for this carbon fixation pathway in Archaea are oxygen-tolerant, which is also consistent with the living conditions of this bacterium.
Figure 3. Putative metabolic pathway of SBR1093 HKSP (based on the genetic analysis).
Carbon fixation with the HPHB cycle is used for the biomass synthesis via the transfer of acetyl-CoA to the citrate cycle or gluconeogenesis, and the genes responsible for each step are marked with green words. Intermediates connected with colored lines represent different metabolic pathways.
Table 1. Genes responsible for the HPHB cycle in the identified genomes and their identity with those in SBR1093.
| SBR1093 HKSP | Functional enzymes | Ac. hospitalis | M. sedula | S. tokodaii | N. gargensis | Af. felis | B. sp. STM3843 | O.carboxidovorans | |||||||
| Acc. No. | Identity | Acc. No. | Identity | Acc. No. | Identity | Acc. No. | Identity | Acc. No. | Identity | Acc. No. | Identity | Acc. No. | Identity | ||
| gene_764 | ACR | 58889 | 32% | 91508 | 31% | 78610 | 32% | 63583 | 37% | 19219 | 37% | 39404 | 35% | 31729 | 36% |
| gene_2584 | ACC | 59288 | 44% | 90248 | 45% | 76481 | 45% | 60794 | 51% | 19087 | 53% | 33415 | 51% | 32993 | 52% |
| gene_2098 | HPCD | 58277 | 45% | 92065 | 47% | 77478 | 44% | 62785 | 46% | 19777 | 41% | 36654 | 42% | 32328 | 35% |
| gene_2045 | MCE | 59386 | 40% | 90738 | 38% | 76441 | 44% | 63084 | 43% | 18664 | 42% | 33379 | 38% | 33030 | 42% |
| gene_2581 | MSR | 59445 | 41% | 91505 | 41% | 78088 | 43% | 61994 | 39% | 21469 | 44% | 35006 | 54% | 34115 | 43% |
| gene_5 | MCM | 59385 | 47% | 90737 | 47% | 76440 | 48% | 63082 | 48% | // | // | 36638 | 63% | // | // |
| gene_1024 | HBCS | 58189 | 29% | 91107 | 31% | 77036 | 30% | 62874 | 25% | 20234 | 36% | 37419 | 37% | 31206 | 37% |
| gene_451 | HPCS | 58189 | 48% | 91435 | 53% | 78009 | 52% | 62874 | 54% | 20930 | 57% | 37438 | 59% | 31167 | 55% |
| gene_2450 | ACK | 59345 | 41% | 90755 | 43% | 76400 | 41% | 62339 | 28% | 21237 | 59% | 33768 | 59% | 31369 | 58% |
| gene_1582 | MCR | 58545 | 28% | 91779 | 26% | 77174 | 29% | 60718 | 31% | 20991 | 55% | 38304 | 55% | 31211 | 55% |
| gene_595 | CCH | 59445 | 24% | 90500 | 29% | 75917 | 30% | 61994 | 23% | 21942 | 27% | 35636 | 29% | 33680 | 27% |
| gene_213 | HBCD | 59205 | 30% | 91403 | 31% | 77631 | 30% | 64012 | 34% | 19793 | 82% | 33076 | 80% | 32741 | 82% |
| gene_2933 | SSR | 59446 | 31% | 91506 | 30% | 78341 | 29% | 63668 | 37% | 19498 | 32% | 38465 | 35% | 32182 | 30% |
The identified genomes are downloaded from NCBI FTP with the accession names Acidianus hospitalis W1 uid66875 (Ac. hospitalis), Metallosphaera sedula DSM 5348 uid58717 (M. sedula), Sulfolobus tokodaii 7 uid57807 (S. tokodaii), Candidatus Nitrososphaera gargensis Ga9 2 uid176707 (N. gargensis), Afipia felis ATCC 53690 uid179396 (Af. felis), Bradyrhizobium STM 3843 uid80711 (B. sp. STM3843), Oligotropha carboxidovorans OM5 uid72795 (O. carboxidovorans). The enzymes in bold are bifunctional enzymes in the HPHB pathway, and columns filled with//indicate that there were no hit enzymes in the genome. M. sedula, S. tokodaii and Ac. hospitalis are members of Sulfolobales and have the potential for autotrophic metabolism via the reported HPHB pathway, whereas B. sp. STM3843, Af. felis and O. carboxidovorans are members from Bradyrhizobiaceae with facultative autotrophic metabolism via an unknown carbon fixation pathway. The accession number for Acidianus hospitalis should have ‘YP0044’ before the presented numbers, and Metallosphaera should have ‘YP0011’, Sulfolobus ‘NP3’, Nitrososphaera ‘YP0068’, Afipia ‘ZP114’, Bradyrhizobium ‘ZP0943’, and Oligotropha ‘YP0046’.
Abbreviations for functional enzymes: Acryloyl-CoA reductase (ACR); Acetyl/propionyl-CoA carboxylase (ACC); 3-Hydroxypropionyl-CoA dehydratase (HPCD); Methylmalonyl-CoA epimerase (MCE); Malonic semialdehyde reductase (MSR); Methylmalonyl-CoA mutase (MCM); 4-Hydroxybutyrate-CoA ligase (HBCS); 3-Hydroxypropionate-CoA ligase (HPCS); Acetoacetyl-CoA β-ketothiolase (ACK); Succinyl/Malonyl-CoA reductase (MCR); Crotonyl-CoA hydratase (CCH); 4-Hydroxybutyryl-CoA dehydratase (HBCD); Succinic semialdehyde reductase (SSR).
However, microbes possessing the HPHB pathway may evolve as facultative autotrophs rather than obligate autotrophs because this carbon fixation pathway is the most energy-consuming pathway, requiring nine ATP equivalents for one pyruvate [33]. Organisms harboring this carbon fixation pathway may proliferate via a heterotrophic rather than autotrophic pathway as long as there are available organic substrates, which is the nutritional strategy of microbes [34]. For example, although many species within Sulfolobales are described as autotrophs or mixotrophs via this carbon fixation pathway, some strains deposited in the public culture collection could lose their autotrophic ability after continuous laboratory cultivation in nutrient-rich media [33]. According to the above discussion, SBR1093 HKSP may possess potential of both autotrophic and heterotrophic metabolisms, thus should be a mixotroph. This is consistent with the enriching conditions, as well as the habitats of strains in the same taxonomic clade. Mixotrophy has been demonstrated to be a widespread and important phenomenon in the biosphere [35], which combines the traits of autotrophs and heterotrophs to utilize both inorganic and organic resources to enable the host survive in oligotrophic or transient environments. In this study, SBR1093 HKSP is enriched in an anoxic/aerobic process, which cycled microbes continuously from feast to fast according to anoxic and aerobic conditions. The microorganisms thus adapt to the transient environment and strive for the dominant position in this microbial community.
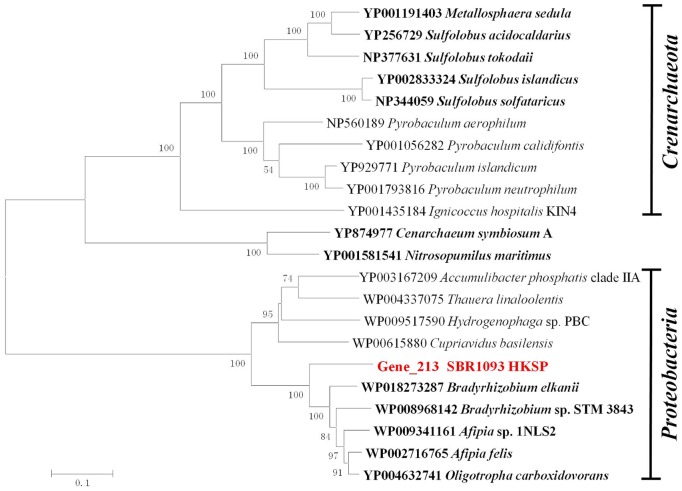
Additionally, in this draft genome, a key enzyme for this HPHB pathway, 4-hydroxybutyryl-CoA dehydratase (gene_213), is diverged from those in Archaea possessed the HPHB pathway, but clustered with facultative autotrophic bacteria such as Bradyrhizobium and Afipia within Bradyrhizobiaceae (Figure 4). This suggests that this gene is unlikely to be horizontally obtained across a recent domain for SBR1093. A further comparison analysis reveals that in addition to the identified autotrophic Archaea, all of the 13 enzymes involved in the HPHB pathway are also identified in some species within Bradyrhizobiaceae (Table 1), implying the wide distribution of the HPHB pathway in the biosphere and the potential importance of carbon fixation in soils and oceans. A phylogenetic analysis based on concatenated amino acid sequences, that are responsible for the transfer of succinyl-CoA to acetoacetyl-CoA and shared by DCHP & HPHB pathway, also reveals that this bacterium is closer to bacteria within Bradyrhizobiaceae than Archaea (those responsible for the transfer of crotonyl-CoA to acetoacetyl-CoA are not included because they are fusion proteins of enoyl-CoA hydratase and hydroxybutyryl-CoA dehydrogenase in bacteria but in reverse order in Archaea). Therefore, this bacterium may have a nutrition pattern similar to that of bacteria within Bradyrhizobiaceae.
Figure 4. Phylogenetic tree of 4-hydroxybutyryl-CoA dehydratase proteins.
The 4-hydroxybutyryl-CoA dehydratases in this draft genome and others in identified autotrophic Achaea were retrieved from NCBI to build the phylogenetic tree. The hosts of these marked (in bold) are suspected autotrophic microbes that undergo the HPHB cycle. The number in front of the taxonomy presents the accession number in NCBI. The tree topography and evolutionary distances are given via the neighbor-joining method with a Poisson correction. The numbers at the nodes indicate the percentage bootstrap values for the clade of this group in 1,000 replications. The scale bar represents a difference of 0.1 substitutions per site.
Compared with the oligotrophic niches in which Archaea reside, Bradyrhizobiaceae bacteria are often found in fertile habitats rich in nutrients. Bacteria within Bradyrhizobiaceae often appear as heterotrophs rather than autotrophs, although most can grow chemolithoautotrophically using hydrogen, thiosulfate or sulfide as an electron donor [36]. Similarly, the bacterium SBR1093 HKSP is enriched in an industrial WWTP with an influent chemical oxygen demand (COD) concentration of up to 7,000 mg L−1 and ammonia concentration up to 100 mg L−1; thus, it may also be a facultative heterotroph or obligate mixotroph.
Antibiotics and heavy metal resistance
It is also interesting to note that this bacterium may have multidrug resistance, such as penicillin, tetracycline, methylenomycin A, chloramphenicol and some macrolide-specific drugs, because abundance of genes associated with drug resistance and efflux transporters are identified in the draft genome. For example, 13 genes encoding a putative drug exporter in the RND superfamily may catalyze antibiotics efflux via an H+ antiport mechanism (Table S4 in File S1), and 19 genes encoding MFS transporter may have antibiotic resistance potential.
Additionally, a complete set gene associated with arsenate reduction and transport has also been identified in this draft genome, as well as 12 genes encoding the ArsR family transcriptional regulator, which may repress the expression of operons linked to stress of di- and multivalent heavy metal ions [37]. Therefore, a variety of heavy metal resistances and transporter genes can be expected in the genome, including the resistance genes for copper and mercury and transport genes for Cu2+ and chromate (Table S4 in File S1). The variety resistance to a variety of heavy metals is consistent with the relevant geographical distribution, ocean crust, marine sediment, contaminated soil and activated sludge.
Conclusions
In summary, a draft genome of uncultivated bacteria from candidate phylum SBR1093 was reconstructed with the metagenome of a microbial community from a full-scale industrial wastewater treatment plant. According to the phylogenetic analysis, this bacterium belongs to clade I of candidate phylum SBR1093, which is associated with a contaminated environment and indicates the demand of organics for metabolism. Genome analysis indicates that the bacterium SBR1093 in this phylum may grow autotrophically via the HPHB cycle, a carbon fixation pathway recently found only in some genera from Archaea. Enzymes in this draft genome involved in carbon fixation are diverged from those in Archaea but share obvious homology to those found in Bradyrhizobiaceae. Therefore, this indicates that this bacterium may be a mixotroph. So far, all of the metabolic properties of this SBR1093 HKSP are deduced only based on the genomic analysis and comparative genomics. Further understanding of the ecological role of this candidate phylum will be obtained through its effective enrichment in the laboratory and the investigation on pure culture.
Supporting Information
Supporting Figures and Tables. Figure S1. Phylogenetic tree of sequences of SBR1093 and the reference sequences from representative phyla. This phylogenetic tree is constructed with 16S rRNA gene sequences based on the maximum-likelihood method with Jukes and Cantor distances. Only bootstrap value of >50% is labeled and the phylotypes of SRB1093 are marked with red in bold. The scale bar represents 0.1 nucleotide substitutions per site. Figure S2. Taxonomy of proteins in this SBR1093 HKSP. Proteins are converted with the predicted gene, and performed BLASTp against NCBI nr database (released at July 18, 2013). Therefore, it is imported into Megan for taxonomic classification. Figure S3. Putative nitrogen metabolic pathway of this SBR1093 HKSP (Adapted from KEGG). Figure S4. Suspected carbon fixation pathway possessed by this SBR1093 HKSP (Adapted from KEGG). Columns with solid fill indicate these enzymes are identified in this draft genome, which are connected with colorful lines and arrows and each color represents a type of carbon fixation pathway. Only the red one are full filled, which represents the HPHB cycle. Table S1. Universally occurring clusters of orthologous groups and tRNAs for amino acids identified in this draft genome. Table S2. List of ESCGs identified in this draft genome and their taxonomy. Table S3. Suspected genes in this draft genome that involving in the primary metabolism. Table S4. Suspected genes in this draft genome that involving in the antibiotics and heavy metal resistance or export.
(PDF)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Data are deposited on web of MG-RAST with accession number 4539207.3.
Funding Statement
This research work is financed by the Research Grants Council of Hong Kong (GRF7190/12E) and National Natural Science Foundation of China (51108262). Dr. Wang would thank Hong Kong Scholar Program for financial support (XJ2012030), and Dr. Guo would thank the University of Hong Kong (HKU) for the postdoctoral fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rinke C, Schwientek P, Sczyrba A, Ivanova NN, Anderson IJ, et al. (2013) Insights into the phylogeny and coding potential of microbial dark matter. Nature 499: 431–437. [DOI] [PubMed] [Google Scholar]
- 2. Lasken RS (2012) Genomic sequencing of uncultured microorganisms from single cells. Nat Rev Microbiol 10(9): 631–640. [DOI] [PubMed] [Google Scholar]
- 3. Tyson GW, Chapman J, Hugenholtz P, Allen EE, Ram RJ, et al. (2004) Community structure and metabolism through reconstruction of microbial genomes from the environment. Nature 428: 37–43. [DOI] [PubMed] [Google Scholar]
- 4. Flowers JJ, He SM, Malfatti S, del Rio TG, Tringe SG, et al. (2013) Comparative genomics of two Candidatus Accumulibacter' clades performing biological phosphorus removal. ISME J 7: 2301–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bond PL, Hugenholtz P, Keller J, Blackall LL (1995) Bacterial Community Structures of phosphate-removing and non-phosphate-removing activated sludges from sequencing batch reactors. Appl Environ Microbiol 61(5): 1910–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Layton AC, Karanth PN, Lajoie CA, Meyers AJ, Gregory IR, et al. (2000) Quantification of Hyphomicrobium populations in activated sludge from an Industrial wastewater treatment system as determined by 16S rRNA analysis. Appl Environ Microbiol 66(3): 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Militon C, Boucher D, Vachelard C, Perchet G, Barra V, et al. (2010) Bacterial community changes during bioremediation of aliphatic hydrocarbon- contaminated soil. FEMS Microbiol Ecol 74: 669–681. [DOI] [PubMed] [Google Scholar]
- 8. Santelli CM, Orcutt BN, Banning E, Bach W, Moyer CL, et al. (2008) Abundance and diversity of microbial life in ocean crust. Nature 453: 653–656. [DOI] [PubMed] [Google Scholar]
- 9. Li H, Yu Y, Luo W, Zeng Y, Chen B (2009) Bacterial diversity in surface sediments from the Pacific Arctic Ocean. Extremophiles 13(2): 233–246. [DOI] [PubMed] [Google Scholar]
- 10. Schmitt S, Tsai P, Bell J, Fromont J, Ilan M, et al. (2012) Assessing the complex sponge microbiota: core, variable and species-specific bacterial communities in marine sponges. ISME J 6: 564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ortiz-Ortiz M (2012) Kartchner caverns: habitat scale community diversity and function in a carbonate cave. Thesis. The University of Arizona. 65: 99. [Google Scholar]
- 12. Zhang T, Shao MF, Ye L (2012) 454 Pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. ISME J 6: 1137–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ju F, Xia Y, Guo F, Wang ZP, Zhang T (2014) Taxonomic relatedness shapes bacterial assembly in activated sludge of globally distributed wastewater treatment plants. Environmental Microbiology DOI: –10.1111/1462–2920.12355 [DOI] [PubMed] [Google Scholar]
- 14. Werner JJ, Knights D, Garcia ML, Scalfone NB, Smith S, et al. (2010) Bacterial community structures are unique and resilient in full-scale bioenergy systems. PNAS 108(10): 4158–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hess M, Sczyrba A, Egan R, Kim TW, Chokhawala H, et al. (2011) Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science 331: 463–467. [DOI] [PubMed] [Google Scholar]
- 16. Sharon I, Banfield JF (2013) Genomes from metagenomics. Science 342: 1057–1058. [DOI] [PubMed] [Google Scholar]
- 17. Strous M, Pelletier E, Mangenot S, Rattei T, Lehner A, et al. (2006) Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 440: 790–794. [DOI] [PubMed] [Google Scholar]
- 18. Guo F, Zhang T (2013) Biases during DNA extraction of activated sludge samples revealed by high throughput sequencing. Appl Microbiol Biotechnol 97: 4607–4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Albertsen M, Hugenholtz P, Skarshewski A, Nielsen KL, Tyson GW, et al. (2013) Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat Biotechnol 31: 533–538. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Leung HCM, Yiu S, Chin FYL (2012) MetaCluster 4.0: a novel binning algorithm for NGS reads and huge number of species. J Comput Biol 19: 241–249. [DOI] [PubMed] [Google Scholar]
- 21. Zhu W, Lomsadze A, Borodovsky M (2010) Ab initio gene identification in metagenomic sequences. Nucleic Acids Res 38(12): e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong MT, Zhang D, Li J, Hui RK, Tun HM, et al. (2013) Towards a metagenomic understanding on enhanced biomethane production from waste activated sludge after pH 10 pretreatment. Biotechnol Biofuels 6(1): 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu K, Zhang T (2013) Construction of customized sub-databases from NCBI-nr database for rapid annotation of huge metagenomic datasets using a combined BLAST and MEGAN Approach. PLoS ONE 8(4): e59831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sonnhammer EL, Eddy SR, Birney E, Bateman A, Durbin R (1998) Pfam: multiple sequence alignments and HMM-profiles of protein domains. Nucleic Acids Res 26(1): 320–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stark M, Berger SA, Stamatakis A, von Mering C (2010) MLTreeMap-accurate Maximum Likelihood placement of environmental DNA sequences into taxonomic and functional reference phylogenies. BMC Genomics 11: 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dupont CL, Rusch DB, Yooseph S, Lombardo MJ, Richter RA, et al. (2012) Genomic insights to SAR86, an abundant and uncultivated marine bacterial lineage. ISME J 6: 1186–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hunt DE, David LA, Gevers D, Preheim SP, Alm EJ, et al. (2008) Resource partitioning and sympatric differentiation among closely related Bacterioplankton . 320: 1080–1085. [DOI] [PubMed] [Google Scholar]
- 28. Finlay B, Clark K (1999) Ubiquitous dispersal of microbial species. Nature 400: 828–828. [Google Scholar]
- 29. Trigui MPS, Truffaut N, Thomas D, Poupin P (2004) Molecular cloning, nucleotide sequencing and expression of genes encoding a cytochrome P450 system involved in secondary amine utilization in Mycobacterium sp. strain RP1. Res Microbiol 155: 1–9. [DOI] [PubMed] [Google Scholar]
- 30. Ramos-Vera WH, Berg IA, Fuchs G (2009) Autotrophic carbon dioxide assimilation in Thermoproteales revisited. J Bacteriol 191: 4286–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berg IA (2011) Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl Environ Microbiol 77(6): 1925–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dunn MF (2011) Anaplerotic function of phosphoenolpyruvate carboxylase in Bradyrhizobium japonicum USDA110. Curr Microbiol 62: 1782–1788. [DOI] [PubMed] [Google Scholar]
- 33. Berg IA, Ramos-Vera WH, Petri A, Huber H, Fuchs G (2010) Study of the distribution of autotrophic CO2 fixation cycles in Crenarchaeota . Microbiology 156: 256–269. [DOI] [PubMed] [Google Scholar]
- 34. Tittel J, Bissinger V, Zippel B, Gaedke U, Bell E, et al. (2003) Mixotrophs combine resource use to outcompete specialists: Implications for aquatic food webs. PNAS 100(22): 12776–12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zubkov MV, Tarran GA (2008) High bacterivory by the smallest phytoplankton in the North Atlantic Ocean. Nature 455: 224–227. [DOI] [PubMed] [Google Scholar]
- 36. Fuhrmann S, Ferner M, Jeffke T, Henne A, Gottschalk G, et al. (2003) Complete nucleotide sequence of the circular megaplasmid pHCG3 of Oligotropha carboxidovorans: function in the chemolithoautotrophic utilization of CO, H2 and CO2 . Gene 322: 67–75. [DOI] [PubMed] [Google Scholar]
- 37. Busenlehner LS, Pennella MA, Giedroc DP (2003) The SmtB/ArsR family of metalloregulatory transcriptional repressors: structural insights into prokaryotic metal resistance. FEMS Microbiol Rev 17: 131–143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figures and Tables. Figure S1. Phylogenetic tree of sequences of SBR1093 and the reference sequences from representative phyla. This phylogenetic tree is constructed with 16S rRNA gene sequences based on the maximum-likelihood method with Jukes and Cantor distances. Only bootstrap value of >50% is labeled and the phylotypes of SRB1093 are marked with red in bold. The scale bar represents 0.1 nucleotide substitutions per site. Figure S2. Taxonomy of proteins in this SBR1093 HKSP. Proteins are converted with the predicted gene, and performed BLASTp against NCBI nr database (released at July 18, 2013). Therefore, it is imported into Megan for taxonomic classification. Figure S3. Putative nitrogen metabolic pathway of this SBR1093 HKSP (Adapted from KEGG). Figure S4. Suspected carbon fixation pathway possessed by this SBR1093 HKSP (Adapted from KEGG). Columns with solid fill indicate these enzymes are identified in this draft genome, which are connected with colorful lines and arrows and each color represents a type of carbon fixation pathway. Only the red one are full filled, which represents the HPHB cycle. Table S1. Universally occurring clusters of orthologous groups and tRNAs for amino acids identified in this draft genome. Table S2. List of ESCGs identified in this draft genome and their taxonomy. Table S3. Suspected genes in this draft genome that involving in the primary metabolism. Table S4. Suspected genes in this draft genome that involving in the antibiotics and heavy metal resistance or export.
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Data are deposited on web of MG-RAST with accession number 4539207.3.