Abstract
Graft-versus-host disease (GVHD) represents a major hurdle impeding the efficacy of allogeneic bone marrow transplantation (BMT). Bortezomib is a proteasome inhibitor that was recently approved for treatment of myeloma. We found that bortezomib potently inhibited in vitro mixed lymphocyte responses and promoted the apoptosis of alloreactive T cells. Bortezomib given at the time of allogeneic BMT in mice resulted in significant protection from acute GVHD. Reductions in GVHD-associated parameters and biological evidence of proteasome inhibition were observed with this regimen but with no adverse effects on long-term donor reconstitution. Assessment of graft-versus-tumor responses in advanced leukemia-bearing mice demonstrated that only the combination of allogeneic BMT and T cells with bortezomib promoted significant increases in survival. Increased cytotoxic T cell killing of the tumor was also observed. Thus, the combination of proteasome inhibition with selective immune attack can markedly increase the efficacy of BMT in cancer.
The clinical use of allogeneic bone marrow transplantation (BMT) for cancer treatment is seriously hampered by the occurrence of graft-versus-host disease (GVHD). For the most part, efforts to reduce the incidence of GVHD have also diminished graft-versus-tumor (GVT) responses with increased tumor relapse, suggesting that these two processes are intimately linked (1). GVHD is an immune-mediated disease in which donor T cells recognize and attack the genetically disparate recipient. It has a complex pathophysiology, ultimately involving many different cell types at the final effector stage and it affects multiple organs (2–4). Cytokines, in particular, inflammatory cytokines produced by the T cells and other immune cells, have been shown to be critical for GVHD generation and play an important role in fueling the entire process (5–12). The conditioning of the recipient by irradiation or chemotherapy contributes to the production of inflammatory cytokines and has a dramatic impact on GVHD pathophysiology (13). The transcription factor NF-κB has been demonstrated to play a pivotal role in cytokine signaling and the generation of cell-mediated immune responses in numerous models (14–16). In addition, the proteasome has been shown to play critical roles in T cell activation, proliferation, and apoptosis (17), in part, because of blockade of NF-κB activation (15, 18).
The proteasome inhibitor bortezomib (Velcade, formerly PS-341) has been demonstrated to exert numerous biological effects that include blocking the activation of NF-κB (19). Bortezomib, a boronic acid dipeptide derivative, is a member of a class of antitumor compounds and has recently been approved as a single agent for the therapy of multiple myeloma because of its direct growth-inhibitory and apoptotic effects on this cancer (20, 21). Bortezomib has also been shown to have antitumor effects either alone or in combination with chemotherapeutic agents against numerous other tumors (22–24). We have recently demonstrated that bortezomib can sensitize both murine solid tumor and leukemia cell lines to apoptosis by tumor necrosis factor-related apoptosis-inducing ligand (25). Thus, bortezomib is a potentially attractive candidate for administration after BMT because of its direct antitumor effects and its ability to sensitize tumor cells to immune-mediated death pathways, in addition to its potential ability to inhibit alloreactive T cell expansion during GVHD.
The results presented in this study demonstrate that systemic proteasome inhibition can significantly inhibit acute lethal GVHD and preserve GVT responses in advanced tumor-bearing mice after allogeneic BMT with no observed adverse effects on myeloid recovery and donor chimerism.
Materials and Methods
Animals. Female BALB/c (H2d) and C57BL/6 (B6, H2b) mice were purchased from the Animal Production Area of the National Cancer Institute (Frederick, MD). Mice were between 8 and 13 weeks of age at the start of the experiments.
Cell Lines and Reagents. C1498 (H2b) murine leukemia cell line was maintained as described (25). The proteasome inhibitor bortezomib was kindly provided by Millennium Pharmaceuticals (Cambridge, MA).
In Vivo Studies. B6 mice received myeloablative doses of total body irradiation from a 137Cesium source, based on previously determined irradiation studies at each of the two animal facilities where experiments were performed (900–950 cGy at the National Cancer Institute; 1,300 cGy at the University of Nevada, Reno). Irradiation was followed by the infusion of 1.5 × 107 BALB/c bone marrow cells i.v. with or without BALB/c splenocytes (SCs; 2–4 × 107 cells i.v.) as a source of allogeneic T cells or 1.5 × 107 carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled purified splenic T cells. Mice then received Dulbecco's PBS, or bortezomib in PBS with a dose range of 10–20 μg from days 0 through +3 post-BMT, with comparable results. Mice received sulfamethoxazole/trimethoprim oral suspension USP (Alpharma USPD, Baltimore) in their drinking water beginning 7 days before the transplant.
In the GVT studies, C1498 tumor cells (2 × 105 cells) were injected i.v. into B6 mice 10 days before lethal total body irradiation. BMT was performed as described above. Mice were monitored and weighed weekly. Moribund mice were killed, and organs were subjected to pathological examination to determine the cause of death. All experiments were performed at least three times with 8–11 mice per group.
In Vitro Mixed Lymphocyte Reaction (MLR) Cultures. To prepare responder BALB/c T cells, lymph node cells were enriched by passage through a goat anti-mouse and goat anti-rat-Ig-coated column (Cedarlane Laboratories). Stimulator cells were prepared from single-cell suspensions of spleens from B6 mice that were anti-Thy 1.2 mAb (clone 30H-12) and anti-NK1.1 mAb (clone PK136) plus rabbit complement-treated and irradiated (30 Gy). Responders and stimulators were cultured at a final concentration of 0.5 × 106 per ml, pulsed with tritiated thymidine (1 μCi per well) (Amersham Pharmacia Life Sciences) 16–18 h before harvesting and counted in the absence of scintillation fluid on a β-plate reader (Packard). Five individual wells were analyzed per data point.
CFSE Labeling. Purified T cells were labeled with 1–2.5 μM CFSE (Molecular Probes) according to the manufacturer's instructions.
Flow Cytometry. Leukocytes were labeled as described (26). In brief, 106 cells were labeled with antibody for two- or three-color flow cytometry by using CFSE, FITC, phycoerythrin, PerCP, or biotin-(along with SA-PerCP) conjugated mAbs purchased from BD Biosciences (San Diego). Nonspecific binding was corrected with isotype-matched controls. All results were obtained by using a either a FACSCalibur or a FACScan (Becton Dickinson).
Cytokine Analysis. Serum levels of cytokines were determined by multiplex analysis based on a Luminex (Austin, TX) by using mouse cytokine-specific bead sets and standards according to the manufacturer's instructions (R & D Systems).
Cytotoxicity Assay. For the generation of cytotoxic T cells (CTLs), BALB/c mice were immunized with 107 irradiated (100 Gy) C1498 cells by i.p. injection, twice a week for 2 weeks, and spleens were harvested 2 weeks later. CTLs were restimulated with irradiated C1498 for 5 days in vitro. Two thousand viable C1498 cells were then incubated with CTL, at different effector-to-target ratios with or without 4 nM bortezomib for 36–45 h. The level of cytotoxicity was determined by annexin V binding and propidium iodide incorporation and clonogeneic tumor cell growth in semisolid media as described (27).
Measurement of 20S Proteasome Inhibition. Tissue homogenates were prepared from whole spleens of treated animals. Kinetic measurement of proteasome activity has been described (28, 29). Details and calculation for specific activity are available in Supporting Text, which is published as supporting information on the PNAS web site.
Electromobility Shift Assays. Preparation of nuclear and cytoplasmic extracts and subsequent electromobility shift assays were performed as described (30). Details are described in Supporting Text.
Statistics. Survival data were plotted by the Kaplan–Meier method and analyzed by the log-rank test. In vitro assays were analyzed by ANOVA and the unpaired Student t test as indicated. A P value of <0.05 was considered significant.
Results
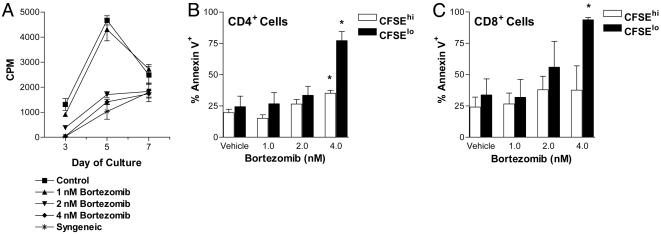
Bortezomib Can Inhibit MLRs in Vitro. Bortezomib has been demonstrated to potently inhibit proteasome activity both in vitro and in vivo. It also blocks degradation of IκB, leading to impaired NF-κB activation (21), an important factor in both cytokine responsiveness and maturation of functional cell-mediated immune responses. Therefore, we assessed the effects of bortezomib on alloreactive MLR responses in vitro. The results demonstrate that bortezomib is capable of significantly inhibiting proliferation of alloreactive T cells in a dose-dependent manner (Fig. 1A). In an MLR assay, assessment of cell division by CSFE dye dilution and annexin V binding as a marker of apoptosis demonstrated that significant increases in apoptosis of alloreactive CD4+ and CD8+ T cells occurred on day 6 in the presence of 4 nM bortezomib (Fig. 1 B and C). Apoptotic cells were predominately in the CSFElo population, suggesting that the proliferating T cells were primarily targeted. These results indicate that bortezomib is capable of potently suppressing T cell responses, in part, through promotion of apoptosis of activated T cells. Reductions in proliferative capability, as indicated by decreases in the number of CFSElo cells with increasing amounts of bortezomib, were also observed (data not shown). Thus, bortezomib was capable of both inhibiting cell proliferation and selectively inducing apoptosis in the activated alloreactive T cell population in vitro.
Fig. 1.
Proliferating, and not resting, T cells are highly sensitive to bortezomib-mediated cytotoxicity. Allogeneic T cell responses are inhibited by bortezomib in vitro. (A–C) Proliferation and induction of apoptosis of alloreactive T cells in a MLR. (A) Alloreactive T cell proliferation was significantly decreased at days 3 and 5 (P < 0.001) in the presence of 2 nM (▾) and 4 nM (♦) but not 1 nM (▴) bortezomib. (B) Proportionally greater increase in annexin V binding on proliferating (CFSElo) compared with nonalloreactive (CFSEhi) CD4+ T cells with exposure to 4 nM bortezomib. (C) Proportionally greater increase in cell surface expression of annexin V on proliferating (CFSElo) compared with nonalloreactive (CFSEhi) CD8+ T cells with exposure to 2 and 4 nM bortezomib. *, significant differences of annexin V binding on bortezomib-exposed T cells compared with vehicle control (P < 0.05). The combined results of two independent experiments presented in B and C.
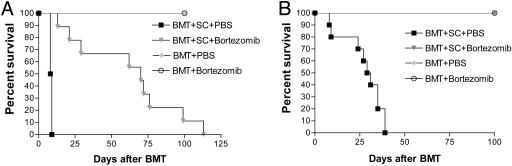
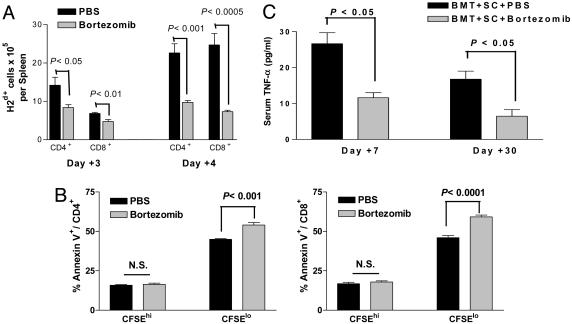
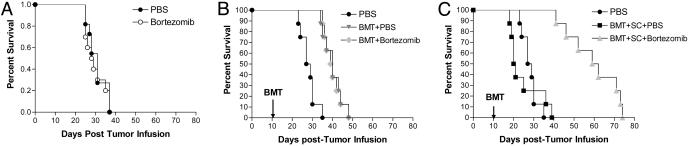
Bortezomib Administration Protects Mice from Acute Lethal GVHD. Because bortezomib significantly suppressed alloreactivity in vitro, we next assessed its effects in vivo by using a murine model of acute lethal GVHD. C57BL/6 mice received a myeloablative dose of irradiation followed with allogeneic bone marrow cells and SCs from full MHC major and minor antigen disparate BALB/c donors. With this regimen, all mice succumbed to severe acute GVHD affecting the gut and liver within 10 days (Fig. 2A). However, when bortezomib (10 μg per dose) was administered daily on days 0 through +3 post-BMT, significant (P < 0.0001) increases in survival were observed. In this model, all mice eventually succumbed to acute GVHD although at a much later time point after bortezomib treatment (median, day +70 post-BMT). The level of protection from GVHD by bortezomib was even greater (100% survivors, day +100 post-BMT) when the number of donor splenocytes was decreased to reduce the severity of the GVHD in which all control mice succumbed within 40 days (Fig. 2B). In these experiments, the administration of bortezomib resulted in 100% long-term survival. These results demonstrate that early administration of bortezomib is capable of preventing the occurrence of acute lethal GVHD after allogeneic BMT in mice. Similar protection from GVHD was obtained when the dose of bortezomib was changed to 20 μg given at day 0 and +2 post-BMT (data not shown). The administration of bortezomib did not significantly affect either platelet or white blood cell recovery or long-term donor chimerism after allogeneic BMT (Fig. 7, which is published as supporting information on the PNAS web site). When the mice were assessed for the effects on donor T cell engraftment and proliferation 3 and 4 days after BMT, it was found that administration of bortezomib resulted in significantly less donor CD4+ and CD8+ cell engraftment (Fig. 3A) due to decreases in proliferating T cells (data not shown) with a significantly (P < 0.001) higher proportion of annexin V binding on this population, indicating increased apoptosis of alloreactive T cells (Fig. 3B) similar to the effects observed in vitro (Fig. 1 B and C). Donor T cell chimerism was also not adversely affected by bortezomib treatment. Mice that received BMT and donor splenocytes and either bortezomib or control had >90% donor T cells at day 15 and >99% donor T cells at day 30 after induction of moderate GVHD. Serum tumor necrosis factor α (TNF-α) levels assessed after BMT indicated that administration of bortezomib resulted in significantly (P < 0.05) reduced TNF-α levels at both day 7, during the period of T cell activation and expansion, and day 30 (Fig. 3C) after BMT, correlating with reduced GVHD in treated animals. Thus, the protection from acute lethal GVHD is associated with an initial reduction of donor T cell engraftment, increased alloreactive T cell apoptosis, and reductions in systemic TNF-α after bortezomib treatment.
Fig. 2.
Protection of mice from acute lethal GVHD with bortezomib administration. (A) Bortezomib administration delays GVHD mortality in an aggressive model of acute lethal GVHD. B6 (H-2b) recipients of 15 million BALB/c (H-2d) bone marrow and 40 million spleen cells were treated with 10 μg per dose of bortezomib or vehicle control (PBS) daily from day 0 through day +3 post-BMT. Significant increases in survival were observed in bortezomib-treated animals (▾) compared with PBS-treated mice (▪)(P < 0.0001). (B) Bortezomib administration protects mice from GVHD mortality in a moderately aggressive model of acute lethal GVHD. B6 (H-2b) recipients of 15 million BALB/c (H-2d) bone marrow and 25 million spleen cells were treated with 10 μg per dose of bortezomib or vehicle control (PBS) daily from day 0 through +2 post-BMT. Significant increases in survival were observed in bortezomib-treated animals (▾) compared with PBS-treated mice (▾) (P < 0.0001). Results from one of three independent experiments are presented for A and B. Each experiment consists of 8–10 mice per treatment group in GVHD induction arms and 3–4 mice in BMT control arms.
Fig. 3.
Bortezomib administration reduces donor-derived T cell expansion after BMT. B6 (H-2b) recipients of 15 million BALB/c (H-2d) T cell-depleted bone marrow and 15 million CFSE-labeled purified T cells were treated with bortezomib or vehicle control (PBS) daily on day 0 and day +2 post-BMT. Each treatment group consists of three mice per group. Representative data from one of two independent experiments are presented. (A) Significant reductions (Student's t test; P < 0.05) in donor-derived CD4+ and CD8+ splenocytes were observed on days +3 and +4 post-BMT of bortezomib-treated mice. (B) Significant increases in annexin V binding on proliferating (CFSElo) but not on nonalloreactive (CFSEhi) CD4+ and CD8+ T cells in spleens from bortezomib-treated mice on day +3 post-BMT. (C) B6 (H-2b) recipients of 15 million BALB/c (H-2d) bone marrow and 20 million spleen cells were treated with bortezomib or vehicle control (PBS) daily from day 0 through day +2 post-BMT. Mice were bled on days +7 and +30 after BMT. Serum was analyzed for TNF-α as described in Materials and Methods. Significant reductions (Student's t test; P < 0.05) in serum TNF-α were observed at both time points in bortezomib-treated mice. Representative data from one of two independent experiments are presented.
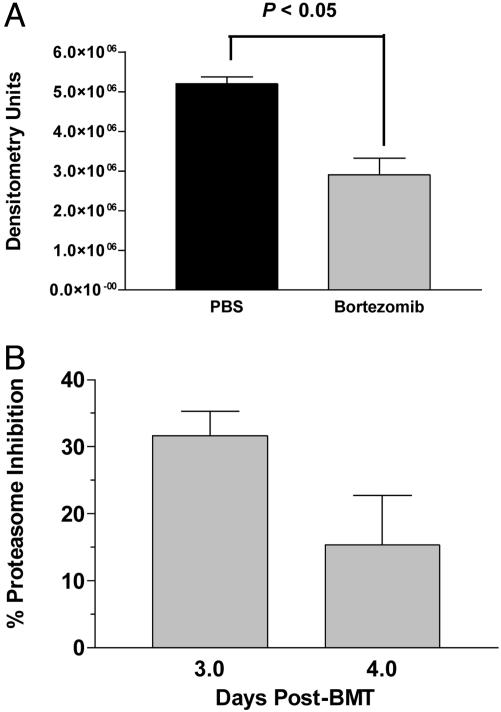
Mechanism of Bortezomib Action in Vivo. Bortezomib has been shown previously to affect the activation and nuclear translocation of NF-κB by blocking the function of the 20S proteasome. We next determined whether these mechanisms were involved in the in vivo activity of bortezomib after BMT. The activity of NF-κB in nuclear lysates was assessed by electromobility shift assays on splenocytes recovered day + 4 post-BMT. By day +4 a 45% reduction had occurred in activity of NF-κB in the nucleus of splenocytes from mice treated with bortezomib compared with PBS (Fig. 4A; P < 0.05). Mice were killed on days +3 and +4 post-BMT when the enzymatic activity of the 20S proteasome in spleen cells was quantified. A 31% reduction in 20S proteasome activity was observed in the splenocytes of mice after treatment with bortezomib on day +3, which decreased to 15% by day +4 post-BMT (Fig. 4B). This finding suggests that systemic administration of bortezomib at this dose and regimen results in reduction of function of the 20S proteasome and also leads to diminished nuclear activity of NF-κB in vivo.
Fig. 4.
Proteasome inhibition and nuclear NF-κB activity after treatment with bortezomib in vivo. B6 mice were transplanted with BALB/c bone marrow and 25 million splenocytes as described in Materials and Methods and treated on days 0 and +2 with 20 μg of bortezomib or PBS. Spleens from two to three mice per treatment group were collected on days +3 and +4 for measurement of 20S proteasome inhibition and day +4 for nuclear NF-κB activity analysis. (A) Significant reductions in nuclear NF-κB activity in bortezomib-treated mice were measured by electromobility shift assay. (B) Inhibition of chymotrypic activity in splenic cell homogenates of bortezomib-treated mice was compared with samples from PBS-treated mice.
Bortezomib Preserves GVT Responses after Allogeneic BMT in Advanced Tumor-Bearing Mice. Although preventing GVHD is an important step in improving the efficacy of BMT, it is imperative to also evaluate effects on GVT, because many agents that curtail GVHD also suppress GVT responses. Because bortezomib can directly mediate antitumor effects in vitro and in vivo, it seemed likely that antitumor responses might be maintained after allogeneic BMT and bortezomib treatment. The C1498 murine acute myeloid leukemia line was found to need higher amounts (10–20 nM) of bortezomib to directly inhibit growth in vitro (25) compared with ranges affecting MLR responses (3–4 nM) (Fig. 1 A). We first treated advanced-tumor-bearing mice with bortezomib alone at a dose range that was found to inhibit GVHD (15 μg per dose). When the mice were treated 10 days after receiving the tumor, no protective effects of bortezomib alone were observed in these advanced-tumor-bearing recipients (Fig. 5A). Modest increases in survival were observed when mice received allogeneic BMT alone (P < 0.005; Fig. 5B) and the addition of bortezomib administration did not result in significant increases in survival compared with mice that received an allogeneic BMT alone by using this dose and regimen (Fig. 5B). When the tumor-bearing mice received allogeneic BMT with donor splenocytes, all mice rapidly succumbed to GVHD (Fig. 5C). Only the tumor-bearing mice that received an allogeneic BMT with donor splenocytes and bortezomib demonstrated significant increases in survival (P < 0.0001; Fig. 5C). In agreement with the GVHD data, mice receiving these higher numbers of spleen cells and bortezomib eventually succumbed to GVHD rather than tumor, indicating that significant GVT effects had been obtained. When mice were given lower numbers of spleen cells with bortezomib, no significant antitumor effects were observed as the mice all succumbed to tumor because of the extensive tumor burden at the start of the treatment (data not shown). These results demonstrate that bortezomib can protect from GVHD and preserve GVT effects after allogeneic BMT in advanced tumor-bearing mice.
Fig. 5.
GVT activity is preserved with bortezomib administration after BMT. (A–C) B6 (H-2b) mice received 2 × 105 syngeneic C1498 cells on day 0. Effects of bortezomib administration on tumor survival were determined in various models. Mice received 15 μg per dose bortezomib or vehicle control (PBS) daily on days +11 and +13 after tumor injection. Results from one of three independent experiments are presented. Each experiment consists of 8–11 mice per treatment group. (A) No difference in survival was observed in nontransplanted mice. (B) Some groups were irradiated on day +10 after tumor cell injection, followed by injection of 15 million BALB/c (H-2d) bone marrow cells on day +11. Allogeneic BMT provided significant protection in tumor survival (P < 0.005) that was not changed by bortezomib administration. (C) In the same representative experiment as B, some groups additionally received 35 million BALB/c spleen cells. Mice that received vehicle control (PBS) injections succumbed to GVHD-associated morbidity. Mice that received bortezomib (▴) survived significantly longer than either untreated tumor-bearing mice (•; P < 0.0001) or mice that received a BMT with spleen cell and vehicle control treatment (▪; P < 0.0001).
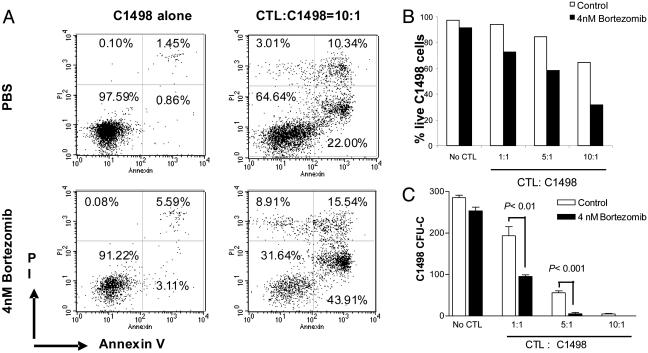
It has been shown that bortezomib can synergize with tumor necrosis factor-related apoptosis-inducing ligand in the killing of tumor cells (25); therefore, we explored the possibility that the T cells capable of mediating GVT in vivo could also mediate increased killing of the tumor after bortezomib administration. In these studies, C1498 leukemia cells were coincubated with allogeneic CTL in the presence or absence of bortezomib in vitro. The data show that the addition of bortezomib, at dose levels that do not directly affect tumor cell viability, does indeed result in increased CTL killing of tumor cells in vitro (Fig. 6). Significant increases in tumor cell apoptosis, decreases in total viable leukemia cells recovered (Fig. 6 A and B), and decreases in clongeneic leukemia cell growth (Fig. 6C) were observed when bortezomib was cocultured with CTL and the tumor. This result represents another potential pathway by which bortezomib can promote GVT effects in vivo, i.e., by sensitizing tumor cells to immune-mediated destruction. Therefore, the administration of bortezomib early after BMT can maintain or augment the GVT response and protect from GVHD, indicating that these two biological responses are influenced to differing degrees after this treatment.
Fig. 6.
Bortezomib enhances CTL killing of allogeneic tumor cells. BALB/c (H2d) mice were primed with irradiated C1498 cells (H2b) as described in Materials and Methods. Spleen cells were then harvested and restimulated with irradiated C1498 cells in vitro at a ratio of 20:1 for 5 days. CTL were collected and cultured with C1498 (H2b) in the presence or absence of 4 nM bortezomib. Tumor cells were analyzed by flow cytometry and clonogeneic growth. (A and B) C1498 cells were gated on based on H2b expression and forward- and side-scatter properties; the gated cells were analyzed for annexin V binding and propidium iodide incorporation at the end of 36-h incubation. (A) Representative dot plots. (B) Decreased numbers of live C1498 tumor cells (annexin V and propidium iodide negative). (C) Clonogeneic C1498 growth was determined after treatment with CTL in the presence or absence of bortezomib for 45 h. Representative data from one of two independent experiments are presented.
Discussion
Dissociation of GVHD and GVT remains of paramount importance in improving the efficacy of BMT. The data presented here demonstrate that proteasome inhibition by using bortezomib can markedly prevent acute lethal GVHD yet still retain GVT responses in advanced tumor-bearing mice. These results suggest that bortezomib may be of significant value for removal of the minimal residual disease that can exist after BMT. That bortezomib has also been shown by us and others to affect solid tumors as well would suggest that the combined use of proteasome inhibition with bortezomib and allogeneic BMT may be of use in the therapy of both blood-borne and solid cancers.
Proteasome inhibition by bortezomib has the potential to provide numerous antitumor effects, both direct and indirect. Although many effects of bortezomib on numerous neoplastic cells have been presumed to be due to blockade of NF-κB, the leukemia cell line used in our previous studies has been shown to resist the inhibitory effects of bortezomib on NF-κB (25). This finding suggests that even greater antitumor effects could be anticipated on tumor cells that are additionally sensitive to the blockade of this pathway. Promotion of GVT responses were observed only when both bone marrow and SCs were administered. Therefore, in this model, it is likely that the major role of bortezomib is to potentiate immune-mediated tumor clearance rather than directly inhibit tumor cell growth.
We recently reported that bortezomib can sensitize tumor cells to tumor necrosis factor-related apoptosis-inducing ligand-mediated killing, in part, because of cellular reductions in cellular FLICE inhibitory protein (25). This finding would also suggest that other cytolytic pathways (i.e., Fas ligand/Fas and TNF/TNFR1) may be enhanced after exposure of tumor cells to bortezomib. Because bortezomib can sensitize the tumor cells to T cell-mediated killing, the cell-mediated GVT effects that follow BMT, or with delayed lymphocyte infusion, may not only be spared but actually enhanced after bortezomib administration.
The ability of bortezomib to prevent GVHD was predicated on its administration immediately after BMT. Although no adverse effects or toxicities were observed by using this regimen, later administration of bortezomib while acute GVHD was ongoing resulted in marked acceleration of the disease (K.S., L.A.W., B.R.B., T.J.S., and W.J.M., unpublished data), suggesting that proteasome inhibition can be used for the prevention but not necessarily for the treatment of GVHD. It is possible that later administration of bortezomib can result in sensitization of the target organs in GVHD to cell-mediated injury, much as the tumor is sensitized. Numerous reports have indicated that the same agents (IFN-γ, IL-2, and IL-12) that can prevent GVHD if given early can also increase GVHD if administered later when GVHD is actively ongoing (31–33).
The mechanism by which proteasome inhibition by bortezomib prevents GVHD is not definitively known, although a likely candidate would be inhibition of the NF-κB pathway. This transcription factor has been demonstrated to be critical for cytokine responsiveness and development of cell-mediated responses (15, 34). The suppression of GVHD may be due, in part, to a reduction of cytokine production concurrent with the induction of apoptosis of the alloreactive T cells. The reductions in serum TNF-α levels would also suggest that a blunting of the cytokine production may be occurring. The demonstration that increased apoptosis of alloreactive T cells can occur after exposure to bortezomib suggests that this may also be an important contributing mechanism. It is possible that bortezomib rapidly induces the preferential deletion of the very-high-affinity alloreactive T cells (as suggested by the in vitro MLR and in vivo CD4+ and CD8+ cell engraftment data), thus allowing for the expansion of the remaining T cells that maintain GVT responses yet have a reduced potential for promoting GVHD. Although it will be of interest to compare the efficacy of agents specific for blockade of NF-κB with bortezomib, it may be difficult to definitively delineate the mechanism(s) of action of bortezomib on GVHD protection and GVT promotion because of the differing efficiencies of these agents in blocking NF-κB in vivo.
In summary, the data presented here demonstrate that proteasome inhibition with bortezomib may be of significant use in preventing GVHD and preserving or possibly even promoting GVT responses in cancer. The models in which advanced tumor-bearing mice were used suggests that this therapeutic approach may be of use to remove the minimal residual disease that can exist after extensive conditioning by selectively predisposing the tumor cells to immune-mediated attack. Further, proteasome inhibition may also be of significant use for the suppression of T cell responses after solid organ transplantation or during autoimmune diseases.
Supplementary Material
Acknowledgments
We thank Dr. Ruth Gault for assisting in the preparation of the manuscript and helpful discussions and Millennium Pharmaceuticals for providing bortezomib. This work was supported in part by National Institutes of Health Grants RO1 CA102282, R01 AI 34495, 2 R37 HL56067, and R01 CA72669 and by funds from the National Cancer Institute, National Institutes of Health, Contract N01-CO-12400.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GVHD, graft-versus-host disease; BMT, bone marrow transplantation; GVT, graft-versus-tumor; CFSE, carboxyfluorescein diacetate succinimidyl ester; SC, splenocyte; MLR, mixed lymphocyte reaction; CTL, cytotoxic T cell; TNF-α, tumor necrosis factor α.
References
- 1.Ferrara, J. L., Deeg, H. & Burakoff, S. J. (1997) Graft-versus-Host Disease (Marcel Decker, New York).
- 2.Graubert, T. A., Russell, J. H. & Ley, T. J. (1996) Blood 87, 1232–1237. [PubMed] [Google Scholar]
- 3.Murphy, G. F., Whitaker, D., Sprent, J. & Korngold, R. (1991) Am. J. Pathol. 138, 983–990. [PMC free article] [PubMed] [Google Scholar]
- 4.Nestel, F. P., Greene, R. N., Kichian, K., Ponka, P. & Lapp, W. S. (2000) Blood 96, 1836–1843. [PubMed] [Google Scholar]
- 5.Welniak, L. A., Blazar, B. R., Wiltrout, R. H., Anver, M. R. & Murphy, W. J. (2001) Transplant. Proc. 33, 1752–1753. [DOI] [PubMed] [Google Scholar]
- 6.Murphy, W. J., Welniak, L. A., Taub, D. D., Wiltrout, R. H., Taylor, P. A., Vallera, D. A., Kopf, M., Young, H., Longo, D. L. & Blazar, B. R. (1998) J. Clin. Invest. 102, 1742–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korngold, R., Marini, J. C., de Baca, M. E., Murphy, G. F. & Giles-Komar, J. (2003) Biol. Blood Marrow Transplant. 9, 292–303. [DOI] [PubMed] [Google Scholar]
- 8.Hill, G. R., Teshima, T., Rebel, V. I., Krijanovski, O. I., Cooke, K. R., Brinson, Y. S. & Ferrara, J. L. (2000) J. Immunol. 164, 656–663. [DOI] [PubMed] [Google Scholar]
- 9.Reddy, P., Teshima, T., Kukuruga, M., Ordemann, R., Liu, C., Lowler, K. & Ferrara, J. L. (2001) J. Exp. Med. 194, 1433–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill, G. R., Teshima, T., Gerbitz, A., Pan, L., Cooke, K. R., Brinson, Y. S., Crawford, J. M. & Ferrara, J. L. (1999) J. Clin. Invest. 104, 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abhyankar, S., Gilliland, D. G. & Ferrara, J. L. (1993) Transplantation 56, 1518–1523. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy, P. L., Jr., Abhyankar, S., Neben, S., Newman, G., Sieff, C., Thompson, R. C., Burakoff, S. J. & Ferrara, J. L. (1991) Blood 78, 1915–1918. [PubMed] [Google Scholar]
- 13.Xun, C. Q., Thompson, J. S., Jennings, C. D., Brown, S. A. & Widmer, M. B. (1994) Blood 83, 2360–2367. [PubMed] [Google Scholar]
- 14.Barnes, P. J. & Karin, M. (1997) N. Engl. J. Med. 336, 1066–1071. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh, S., May, M. J. & Kopp, E. B. (1998) Annu. Rev. Immunol. 16, 225–260. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin, A. S., Jr. (1996) Annu. Rev. Immunol. 14, 649–683. [DOI] [PubMed] [Google Scholar]
- 17.Wang, X., Luo, H., Chen, H., Duguid, W. & Wu, J. (1998) J. Immunol. 160, 788–801. [PubMed] [Google Scholar]
- 18.Finn, P. W., Stone, J. R., Boothby, M. R. & Perkins, D. L. (2001) J. Immunol. 167, 5994–6001. [DOI] [PubMed] [Google Scholar]
- 19.Sunwoo, J. B., Chen, Z., Dong, G., Yeh, N., Crowl Bancroft, C., Sausville, E., Adams, J., Elliott, P. & Van Waes, C. (2001) Clin. Cancer Res. 7, 1419–1428. [PubMed] [Google Scholar]
- 20.Richardson, P. G., Hideshima, T. & Anderson, K. C. (2003) Cancer Control 10, 361–369. [DOI] [PubMed] [Google Scholar]
- 21.Hideshima, T., Richardson, P., Chauhan, D., Palombella, V. J., Elliott, P. J., Adams, J. & Anderson, K. C. (2001) Cancer Res. 61, 3071–3076. [PubMed] [Google Scholar]
- 22.Mitsiades, N., Mitsiades, C. S., Richardson, P. G., Poulaki, V., Tai, Y. T., Chauhan, D., Fanourakis, G., Gu, X., Bailey, C., Joseph, M., et al. (2003) Blood 101, 2377–2380. [DOI] [PubMed] [Google Scholar]
- 23.McBride, W. H., Iwamoto, K. S., Syljuasen, R., Pervan, M. & Pajonk, F. (2003) Oncogene 22, 5755–5773. [DOI] [PubMed] [Google Scholar]
- 24.Bold, R. J., Virudachalam, S. & McConkey, D. J. (2001) J. Surg. Res. 100, 11–17. [DOI] [PubMed] [Google Scholar]
- 25.Sayers, T. J., Brooks, A. D., Koh, C. Y., Ma, W., Seki, N., Raziuddin, A., Blazar, B. R., Zhang, X., Elliott, P. J. & Murphy, W. J. (2003) Blood 102, 303–310. [DOI] [PubMed] [Google Scholar]
- 26.Wigginton, J. M., Komschlies, K. L., Back, T. C., Franco, J. L., Brunda, M. J. & Wiltrout, R. H. (1996) J. Natl. Cancer Inst. 88, 38–43. [DOI] [PubMed] [Google Scholar]
- 27.Koh, C. Y., Blazar, B. R., George, T., Welniak, L. A., Capitini, C. M., Raziuddin, A., Murphy, W. J. & Bennett, M. (2001) Blood 97, 3132–3137. [DOI] [PubMed] [Google Scholar]
- 28.Adams, J., Palombella, V. J., Sausville, E. A., Johnson, J., Destree, A., Lazarus, D. D., Maas, J., Pien, C. S., Prakash, S. & Elliott, P. J. (1999) Cancer Res. 59, 2615–2622. [PubMed] [Google Scholar]
- 29.Lightcap, E. S., McCormack, T. A., Pien, C. S., Chau, V., Adams, J. & Elliott, P. J. (2000) Clin. Chem. 46, 673–683. [PubMed] [Google Scholar]
- 30.Mayo, M. W., Norris, J. L. & Baldwin, A. S. (2001) Methods Enzymol. 333, 73–87. [DOI] [PubMed] [Google Scholar]
- 31.Sykes, M., Romick, M. L., Hoyles, K. A. & Sachs, D. H. (1990) J. Exp. Med. 171, 645–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sykes, M., Szot, G. L., Nguyen, P. L. & Pearson, D. A. (1995) Blood 86, 2429–2438. [PubMed] [Google Scholar]
- 33.Sykes, M., Pearson, D. A., Taylor, P. A., Szot, G. L., Goldman, S. J. & Blazar, B. R. (1999) Biol. Blood Marrow Transplant 5, 277–284. [DOI] [PubMed] [Google Scholar]
- 34.Baeuerle, P. A. & Baltimore, D. (1996) Cell 87, 13–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.