Abstract
The gastric pathogen Helicobacter pylori is one of the most genetically diverse of bacterial species. Much of its diversity stems from frequent mutation and recombination, preferential transmission within families and local communities, and selection during persistent gastric mucosal infection. MLST of seven housekeeping genes had identified multiple distinct H. pylori populations, including three from Africa: hpNEAfrica, hpAfrica1 and hpAfrica2, which consists of three subpopulations (hspWAfrica, hspCAfrica and hspSAfrica). Most detailed H. pylori population analyses have used strains from non-African countries, despite Africa's high importance in the emergence and evolution of humans and their pathogens. Our concatenated sequences from seven H. pylori housekeeping genes from 44 Gambian patients (MLST) identified 42 distinct sequence types (or haplotypes), and no clustering with age or disease. STRUCTURE analysis of the sequence data indicated that Gambian H. pylori strains belong to the hspWAfrica subpopulation of hpAfrica1, in accord with Gambia's West African location. Despite Gambia's history of invasion and colonisation by Europeans and North Africans during the last millennium, no traces of Ancestral Europe1 (AE1) population carried by those people were found. Instead, admixture of 17% from Ancestral Europe2 (AE2) was detected in Gambian strains; this population predominates in Nilo-Saharan speakers of North-East Africa, and might have been derived from admixture of hpNEAfrica strains these people carried when they migrated across the Sahara during the Holocene humid period 6,000–9,000 years ago. Alternatively, shared AE2 ancestry might have resulted from shared ancestral polymorphisms already present in the common ancestor of sister populations hpAfrica1 and hpNEAfrica.
Introduction
H. pylori is a genetically diverse Gram negative micro-aerophilic bacterial species that chronically infects some half of all humans worldwide [1]. It is implicated in chronic gastritis, gastroduodenal ulcers and gastric cancer [2], [3] and also increases the risk of infection by diarrheal pathogens [4], which may lead to infant malnutrition with growth faltering [5] in low income societies. Despite this, most infections are benign, and some may even be beneficial [6], [7]. The risk of infection resulting in overt disease is likely to be determined by H. pylori genotype in combination with other variables such as human genotype and physiology, nutrition and environmental factors.
H. pylori is usually acquired in childhood [8] and can persist for life unless eradicated by antibiotics [9]. A prevalence of ≥80% is typical in developing nations [10]–[13], but has fallen dramatically in industrialized countries during the last century (currently, in the range of 20%), probably due to major improvements in hygiene and sanitation [14]. Transmission is predominantly intrafamilial with a low risk of adult infection in industrialized countries [15], [16], whereas transmission within the local community is frequent in developing countries, with new infections often occurring in adults as well as in children [17].
Independent H. pylori isolates typically differ by some 2% or more in DNA sequence, which allows different strains to be distinguished readily by the arbitrarily primed PCR (RAPD) method, wherein each strain yields a characteristic pattern of DNA fragments different from those of nearly all other independent strains [18], or by the sequencing of one or more housekeeping genes. As an extension of this approach, multilocus sequence typing (MLST; typically sequencing seven such genes) soon provided early indications that different sets of H. pylori genotypes predominated in different human populations [19], [20]. Although high sequence variability confounded the use of MLST in H. pylori, more sophisticated nucleotide analyses of DNA sequences have since provided more refined demonstrations both of major geographic or human population differences among H. pylori populations and also admixture often linked to human migrations [21], [22]. This great diversity within and between populations can be ascribed to H. pylori's high rates of mutation and recombination [23], coupled with its having chronically infected humans for many thousands of years, its transmission being predominantly within families or local communities, and the tendency of any person's gastric mucosal infection to persist for decades if not treated with antimicrobials. This epidemiologic pattern resulted in the isolation of populations from each other by distance, and allowed mutational divergence by random genetic drift, and selection for locally adapted genotypes. The sequences of housekeeping genes of strains from many parts of the world identified three African H. pylori populations, which may be of particular importance to the Gambian strain analyses presented here, designated hpAfrica1, hpAfrica2 and hpNEAfrica [21], [22], [24]. Four further populations, hpEurope, hpEAsia, hpAsia2 and hpSahul, were detected in the rest of the world. More focussed analyses distinguished three hpAfrica1 subpopulations, designated hspWAfrica (Western and North-Western Africa), hspCAfrica (Cameroon) and hspSAfrica (South Africa), and two hpNEAfrica subpopulations - hspEastNEAfrica and hspCentralNEAfrica [25]. The distribution of the various hpAfrica1 subpopulations may reflect the expansion of the Bantu people throughout subSaharan Africa from an ancestral homeland in or near present day Nigeria/Cameroon during the last 4000 years. In contrast the hspEastNEAfrica and hspCentralNEAfrica subpopulations found in Algeria and northern Nigeria are thought to reflect migrations of Nilo-Saharan people through what were then central Saharan wetlands during the Holocene humid period some 6,000–9,000 years ago [25].
The Gambia is a small country, some 47 Km wide and 338 Km long, embracing the west-flowing Gambia River in the most western part of Africa, bordered by Senegal to the North, South and East and the Atlantic Ocean to the West. Gambian contacts with Europeans were mostly with the Portuguese and French beginning in the 15th century, and then with the British in the 17th century and lasting until independence from British colonial rule in 1965. The great majority of present-day Gambians are indigenous West Africans, predominantly of the Mandinka, Wollof and Fulani linguistic groups that are also abundant in nearby countries of Senegal, Guinea Bissau, Guinea and Mali. Most Gambians are Muslim, reflecting conversion of residents by Arab traders from North Africa who crossed the Sahara beginning in the 8th century. Given the tendency of H. pylori populations to reflect human host migrations, and since the Gambia was also a major source of slaves taken to the Americas and Europe for several centuries until the slave trade ended in the 1800s, it is possible that Gambian strains contributed significantly to H. pylori's gene pool in The Americas and Europe. Indeed, a suggestion of West African admixture in European H. pylori had emerged in our earlier study of a novel regulatory gene-linked insertion-deletion polymorphism (indel) in Spanish vs. Gambian H. pylori strains [26].
It is with this background that we sequenced the MLST genes of H. pylori strains from ethnic West African adults and children in The Gambia. These strains are expected to be broadly representative of H. pylori of much of West Africa, a relatively unstudied population. Furthermore, given the historical contact with Europeans and North Africans, we may also detect traces of the ancestral European nucleotides carried by these people within our Gambian sample.
Materials and Methods
Ethics statement
Ethical approval for this study was provided by The Gambia Government/MRC joint ethics committee and the NIH Division of Microbiology Infectious Diseases (DMID) International Review Board, USA (NIH number DMID 06-0053; MRC Unit, The Gambia IRB registration number: IRB00003943 and Federal Wide Assurance number: FWA 00006873).
Patients
Gastric biopsies were collected from patients referred for endoscopy at the Medical Research Council Unit (MRC), The Gambia, for routine clinical investigations of symptoms attributable to gastroduodenal disease. All patients who agreed to join this study provided written informed consent; in addition, for children aged less than 18 years, biopsies were obtained after written informed parental consent. The patients belonged to the following ethnic groups: Mandinka (19), Wollof (9), Jola (6), Fulani (5), Sarahule (4), Serere (1).
Biopsies
Endoscopes were cleaned and sterilized with Cidex (Johnson and Johnson Co) after each use according to standard care at the MRC Unit, The Gambia (SOP-CLS-001). Biopsies from the gastric antrum and corpus of study participants were immediately placed in 1 ml Brain-Heart Infusion (BHI) broth containing 20% glycerol and transported in ice to the laboratory for culture or stored at −70°C until used.
Bacterial culture
Biopsies were spread on the surface of selective Columbia-Blood agar, incubated at 37°C in a microaerobic atmosphere and processed as previously described [27]. Single H. pylori colonies were isolated and confluent growth derived by spreading cells from them was harvested and preserved in BHI broth containing 20% glycerol and stored at −70°C until analysed.
Sample choice for MLST
Samples were chosen according to successful subculture of individual H. pylori colonies. One or more single colonies were isolated from each of 44 patients and used for these analyses. The 44 patients (23 male and 21 female) ranged in age from 18 months to 72 years (mean 32 years) and had the following clinical manifestations: normal endoscopic appearance (29), gastric erosions (6), gastric ulcer (3), and oesophageal ulcer (1). Five of these patients were malnourished children with enteropathy (ages 18–31 months, mean 19 months). Thirty three patients (75%) were from the Greater Banjul Area (GBA) and 11 (25%) were from rural villages. To investigate genetic heterogeneity within the same stomach, multiple single colonies from each of two patients with normal gastroduodenal appearance by endoscopy were tested by MLST: seven colonies (4 antrum and 3 corpus) from a 14 year old subject; and 11 single colonies (6 antrum and 5 corpus) from a 72 year old subject.
Genomic DNA extraction
Genomic DNA was prepared from confluent H. pylori growth using a commercial kit (Qiagen DNA Mini kit, UK) [27].
PCR to detect cagA
PCR was performed to detect the cagA virulence gene using previously described primers and methods [27], and PCR products were detected by electrophoresis in a 1.5% agarose gel with ethidium bromide, and band visualization using Gel Doc 2000 (Bio-Rad laboratories, Milan, Italy).
Housekeeping gene sequencing and MLST
The seven standard MLST genes (atpA, efp, mutY, ppa, trpC, ureI and yphC) were amplified and sequenced as detailed in http://pubmlst.org/Helicobacter. The PCR products were sequenced using an ABI Prism 3130X DNA sequencer (Applied Biosystems, USA). Consensus sequences were generated and assembled using DNAstar programme (Lasergene, USA, Version 7). The sequences obtained were submitted to the H. pylori MLST database (http://pubmlst.org/Helicobacter) for allele and sequence type identification. Concatenated sequences were aligned and imported into MEGA version 5. Evolutionary history was inferred using the neighbor-joining tree reconstruction method [28]. The percentage of replicate trees in which the associated taxa clustered together in a bootstrap test of 2000 replicates is depicted beside each branch.
Nucleotide analyses
Calculation of the ratio of non-synonymous to synonymous changes (dN/dS) was done with the START2 (Sequence Type Analysis and Recombinational Tests Version 2) tool, which uses the method of Nei and Gojobori to estimate parameters [29].
Analyses of population structure
To determine the relatedness of Gambian H. pylori to previously studied strains from elsewhere, 246 strains representing H. pylori's global diversity were selected from the public MLST data base (Table 1). This data set included all strains previously published from West Africa [22], [25], [30]. Reconstruction of a global phylogeny was carried out using neighbor-joining as described above. In addition, two Bayesian population cluster analyses were performed using STRUCTURE V2.3.4 software [21]. First, the no-admixture model was used to determine the overall structure of the Gambian sequence data with respect to predefined modern populations. Then, the linkage model was used to ascertain ancestral components within our Gambian data set. In the unlikely event that Gambian H. pylori represent a new population, we set our values for K at greater than the known number of modern and ancestral H. pylori populations. We carried out three independent runs at 2≤K≤10 for the no-admixture model and three independent runs at 2≤K≤8 for the linkage model. Each run comprised 50,000 iterations, the first half of which were discarded as burnin.
Table 1. H. pylori strains and populations selected for comparison with 46 Gambian strains.
| Country | hpAfrica1 | hpNEAfrica | hpEurope | hpEAsia | hpAfrica2 | hpSahul | hpAsia2 |
| Senegal | 73 | 0 | 0 | 0 | 0 | 0 | 0 |
| Burkina Faso | 12 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cameroon | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Morocco | 5 | 0 | 2 | 0 | 0 | 0 | 0 |
| Algeria | 1 | 3 | 2 | 0 | 0 | 0 | 0 |
| South Africa | 8 | 0 | 0 | 0 | 16 | 0 | 0 |
| Nigeria | 0 | 8 | 0 | 0 | 0 | 0 | 0 |
| Ethiopia | 0 | 7 | 0 | 0 | 0 | 0 | 0 |
| Somalia | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Egypt | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| Spain | 0 | 0 | 33 | 0 | 0 | 0 | 0 |
| Finland | 0 | 0 | 9 | 0 | 0 | 0 | 0 |
| Estonia | 0 | 0 | 11 | 0 | 0 | 0 | 0 |
| India | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Bangladesh | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Malaysia | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Papua New Guinea | 0 | 0 | 0 | 0 | 0 | 6 | 0 |
| Australia | 0 | 0 | 0 | 0 | 0 | 6 | 0 |
| Japan | 0 | 0 | 0 | 24 | 0 | 0 | 0 |
| Totals | 104 | 20 | 60 | 24 | 16 | 12 | 10 |
Results
Allelic frequency and nucleotide analyses
DNAs from H. pylori strains from 44 Gambians (one strain/patient in 43 cases; three strains/patient in one case) yielded 42 unique MLST sequence types based on concatenated DNA sequences of seven housekeeping gene loci. Four pairs of strains yielded identical MLSTs. One pair was from consecutive unrelated patients whose biopsies were obtained on the same day. The other three pairs were also from unrelated patients who were biopsied between one week and two years apart. These exceptions aside, most alleles of individual genes occurred only once among the 46 strains, although identical alleles were found in 11 to 16 strains, depending on the gene. Except for the four pairs of strains that were identical at all loci (noted above), no pair of strains identical at one locus was identical at another of the seven loci tested (data not shown). The mean nucleotide diversity in the 7 genes was 2.9% (Table 2). The gene trpC had most strains with identical alleles (16 alleles); allele 1774 of mutY was most frequent (5 occurrences; 10.9%); the most diverse gene was trpC (mean nucleotide level diversity 4.6%); and the least diverse was ureI (1.2%, Table 2). No deletions or insertions were found in any of the analysed gene fragments.
Table 2. Genetic diversity of 46 Gambian isolates.
| Locus | Dn | Ds | dn/ds | Diversity % |
| atpA | 0.0006 | 0.1009 | 0.006 | 2.3 |
| efp | 0.0005 | 0.1059 | 0.0046 | 2.2 |
| mutY | 0.0098 | 0.1822 | 0.0536 | 4.5 |
| ppa | 0.0116 | 0.042 | 0.2761 | 2.1 |
| trpC | 0.0187 | 0.1513 | 0.1238 | 4.6 |
| ureI | 0.0052 | 0.0365 | 0.1428 | 1.2 |
| yphC | 0.0092 | 0.0924 | 0.0995 | 3.4 |
| overall | 0.0079 | 0.1016 | 0.1009 | 2.9 |
dS and dn are the average number of synonymous substitutions per synonymous site and non-synonymous substitutions per non-synonymous site, respectively.
Analyses of selection
A nucleotide substitution in a coding region results either in a change or no change in the protein's amino acid sequence (non-synonymous (N), synonymous (S), respectively). The dN/dS ratio in a population reflects genetic drift and selection operating on individual genes. All dN/dS values in the seven housekeeping genes from Gambian H. pylori strains were close to zero (Table 2), indicating intense selection to maintain functions and amino acid sequences of the encoded proteins. This is expected for genes whose encoded proteins act within bacterial cells and provide important housekeeping functions.
Phylogenetic analysis of Gambian strains, relative to those from elsewhere
A phylogenetic tree reconstructed using concatenated sequences of the seven housekeeping genes (Figure 1) provided no evidence of association of particular clusters (clades) of strains with variables such as age of participant at time of endoscopy, endoscopic diagnosis, sex or district of residence within the Gambia. However, cagA + strains seemed to cluster separately from cagA - (Figure 2).
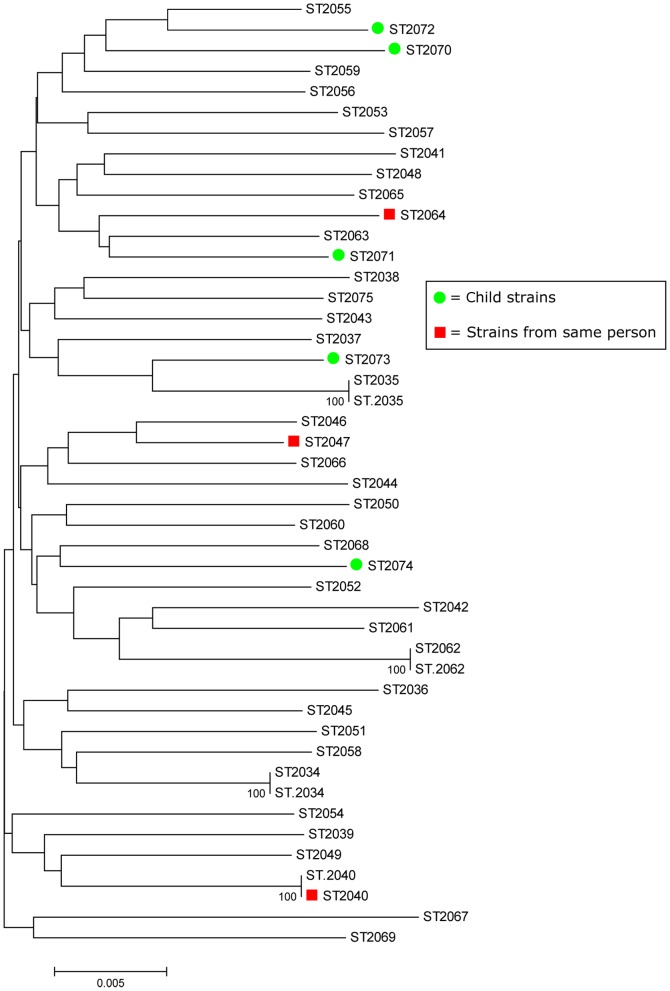
Figure 1. Evolutionary relationship among H. pylori strains isolated from The Gambia.
Evolutionary history was inferred from concatenated sequences of the seven MLST housekeeping gene fragments (3406 bp) from 46 Gambian H. pylori using the neighbor-joining method. The analyses were conducted in MEGA5. The five strains from young children are identified with green circles. There was one subject with three different MLST types shown in red squares.
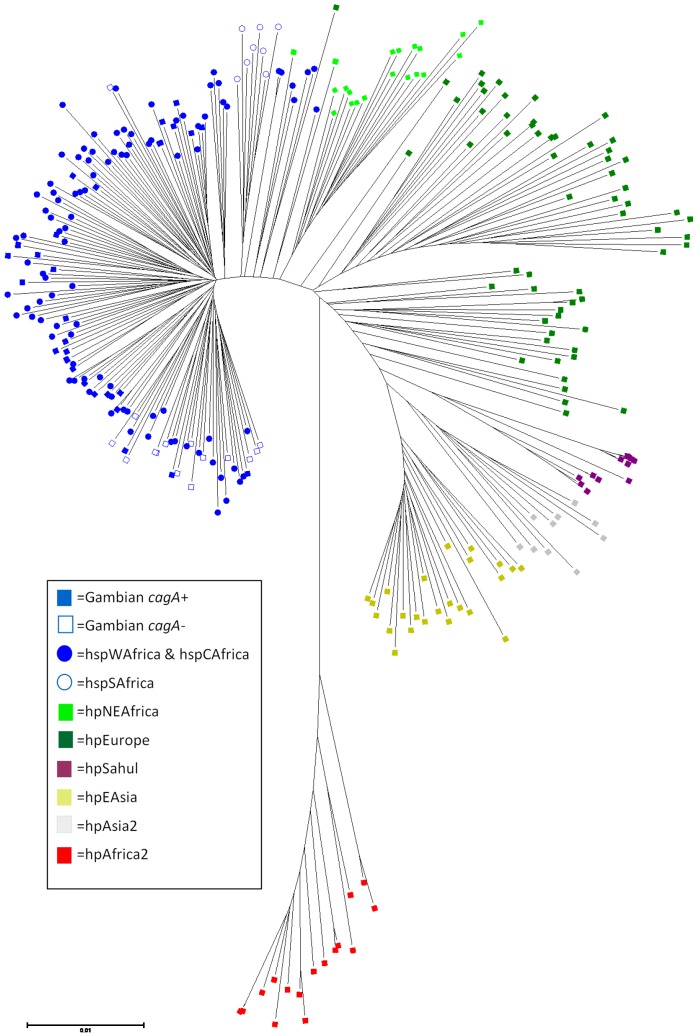
Figure 2. Evolutionary relationships among a global sample of H. pylori strains.
The neighbor-joining tree was calculated from concatenated sequences of 246 globally representative H. pylori strains downloaded from MLST website (Http://pubmlst.org/Helicobacter) plus the 46 isolates studied here. Strains were colour-coded by population as follows: blue, hpAfrica1; light green, hpNEAfrica; dark green, hpEurope; grey, hpAsia2; purple, hpSahul; olive, hpEastAsia; red, hpAfrica2.
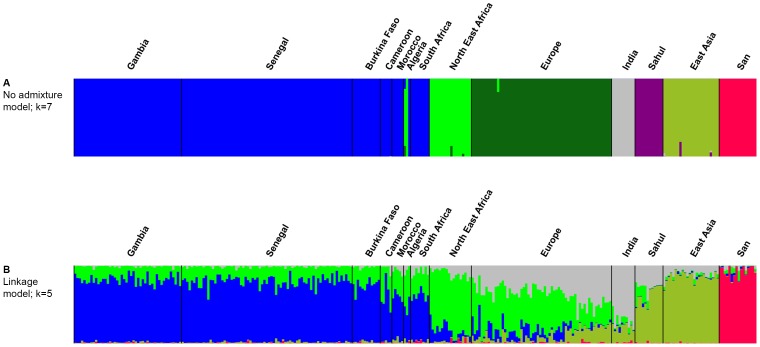
Gambian strain sequences were also compared with sequences from 246 H. pylori strains selected from other informative human populations (African, European, Australian and Asian; Table 1) using neighbor-joining and Bayesian cluster analysis with both the no-admixture and linkage models of STRUCTURE. We found that all Gambian strains clustered within hpAfrica1 and were intermingled with strains from Senegal and Burkina Faso. hpAfrica1 strains formed a separate clade that was sister to strains from hpNEAfrica (Figure 2), with other European, Australian and Asian populations more distantly related. Under the no-admixture model, the number of populations (K) with the highest likelihood was seven, which corresponds to what is already known about the global structure of this bacterial species. Almost all Gambian isolates formed a homogeneous group together with other hpAfrica1 strains (Figure 3A). However, the linkage model showed that Gambian strains were more similar in their ancestral nucleotide composition to hspWAfrica strains from Senegal (Gambia's immediate neighbour) and Burkina Faso (population 3), whereas the North-East African ancestral component (ancestral Europe 2) was more apparent among those hpAfrica1 strains from more distant communities in Cameroon, Morocco, Algeria and South Africa (Figure 3B).
Figure 3. STRUCTURE analysis of 46 Gambian strains in relation to a representative sample of the global diversity in H. pylori.
Gambian H. pylori were compared with strains from other previously assigned populations using no-admixture model (3A) and the linkage model (3B). Population are colour coded according to Figure 2. Each line represents an isolate and colours indicate its inferred modern (3A) or ancestral population(s) (3B).
Phenotypic heterogeneity of H. pylori in a single host
Bacterial colonies that differed markedly in morphology, seen among H. pylori cultured from biopsies from two patients with normal endoscopic gastroduodenal tract appearance (14 and 72 years of age), were used to test for H. pylori DNA level heterogeneity in individual hosts, perhaps equivalent to that seen previously in a European with a mixed cagA + and cagA - infection [31]. Seven colonies representative of the different morphologies were analysed from the 14 year old and 11 such colonies were analysed from the 72 year old. Three different MLST types were identified among the isolates from the first (14 year old) patient (MLST types 2040 in all four colonies from antrum, and 2047 and 2064 from one and two colonies, respectively, from the corpus; Figure 1). Each of these three MLST types was different from the two others in all seven gene loci tested. In the second (72 year old) patient, all 11 colonies (6 antrum, 5 corpus) were of the same MLST type. In neither case did the different colony morphologies that first encouraged analysis of multiple isolates from these two patients correspond to different MLST types.
Discussion
Most detailed H. pylori population analyses to date have used strains from non-African countries, despite Africa's great importance for the emergence and evolution of humans and pathogens such as H. pylori. Here we sequenced the housekeeping genes of strains from The Gambia, one of the most detailed studies to date of a West African H. pylori population. These Gambian strains exhibited high nucleotide sequence diversity (mean, 2.9%), much as in isolates from other geographic regions [24], [32], with no obvious clustering of MLST types in particular age or disease groups. Our sequence data showed that Gambian H. pylori strains belong to the hpAfrica1 population and added greater resolution to the geographic distribution of this population in Africa. Comparison of ancestral nucleotides with those available from other African countries indicated some geographic differentiation, even within West Africa, as did a recent complementary analysis of many strains from Dakar, Senegal [30].
STRUCTURE analyses indicated that the contribution of ancestral nucleotides from other populations, mainly AE2 (which originated in North East Africa [22], to strains circulating in The Gambia was about 17%, which is the average of 3 independent linkage model runs at K = 5. These analyses also showed that Gambian strains are closely related to each other and belong to the hpAfrica1 population (Figures 3). The proportion of AE2 sequences in the Gambian strains is similar to, although marginally higher than, that found in strains from Senegal (19%) and Burkina Faso (23%), and much lower than in any other African country studied to date. This suggests a history of limited admixture (recombination) with strains from elsewhere, as also noted by others [30].
In the case of Gambian H. pylori, recombination from other non-hpAfrica1 strains could possibly have occurred after contact with Europeans or North Africans. However, the population (hpEurope) carried by Europeans is itself a hybrid (recombinant), consisting of roughly equal contributions from AE1 and AE2, originating in Central Asia and North-East Africa, respectively [30], [33]. Since no trace of AE1 was found in our sample, we conclude that the source of the AE2 nucleotides in the Gambia is unlikely to have resulted from hpEurope strains following European colonization of African lands during the last six centuries. This also sheds light on the nature of the centuries-long contact between Gambians and Europeans/North Africans. In South Africa, hpEurope was found in the stomachs of indigenous Africans from several ethnicities [33], pointing to a more extensive association between colonizing Europeans and local populations than in the Gambia, where Europeans never made up a significant part of the total population.
Therefore, we propose that the Gambian H. pylori's AE2 nucleotides were derived from contact with Nilo-Saharan speakers, perhaps when these people migrated westwards from the Nile valley across the Sahara during the Holocene humid period, 6,000–9,000 or more years ago [25]. In accord with this, hpNEAfrica sequences have also been detected in strains from Cameroon [25], northern (predominantly Muslim) Nigeria [22] and Algeria [22] (Figure 3B). Alternatively, since hpAfrica1 and hpNEAfrica are sister phylogenetic groups (Figure 2), the observed AE2 ancestry among Gambian, Senegalese and Burkina Faso strains could also be attributed to background linkage disequilibrium, where ancestral nucleotides were already present in the common ancestor of both populations.
Identical MLST types
We found four pairs of isolates with identical MLST types. Three of these pairs were from people whose gastric biopsies were obtained between one week and two years apart and processed in the laboratory on different dates. They thus are likely to reflect occasional carriage of closely related strains by unrelated members of a community. We also suggest this explanation for the one matched pair from patients who had gastric biopsies obtained consecutively on the same day, since our endoscopes were rigorously cleaned and washed after each use (see Materials and Methods).
Although none of the strain pairs with identical MLSTs were from persons from the same village or with the same family names, further study will be needed to learn if these people had ever lived in the same extended family compound, village or district, or had some other connection, vs. if such identical MLST types reflect some other factor such as The Gambia's small size and easy hospitality to strangers, and/or frequent community transmission of H. pylori in developing country settings [17].
Given the lack of obvious connection between these paired strains, genome-wide analyses of their patterns of micro-sequence divergence vs. conservation could also be highly informative, especially in genes for secreted proteins involved in host interaction and more subject to diversifying selection.
Heterogeneity of H pylori strains within one stomach
Among isolates from the two patients tested for possible H. pylori heterogeneity, all colonies from one patient were identical by MLST whilst the other patient had three distinct MLST types, consistent with other findings [15], [16], [31]. The sequence types of these three strains were different in all seven of the gene loci scored, thereby suggesting co-infection by unrelated strains [27], [34].
Our study indicated that Gambian H. pylori are not particularly clonal, in accord with patterns seen in other non-African populations. Since the MLST types of strains from young children were intermingled with those from adults, there may also not be any special strain type uniquely able to initiate infection in naive infant stomachs. We also note that our Gambian strains showed more genetic similarity with strains from Senegal and Burkina Faso (both countries in far Western Africa) than from elsewhere, reflecting again geographic partitioning of H. pylori.
Acknowledgments
We are grateful to the members of the endoscopy team, clinical services and microbiology department of MRC unit, The Gambia and to all patients and parents who made this study possible.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This study was funded by MRC unit, The Gambia and US National Institutes of Health (NIH) grants RO3-AI061308, R21-AI078237 and R21-AI088337. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Blaser M (1998) Helicobacter pylori and gastric diseases. BMJ 316: 1507–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergman M, Del Prete G, van Kooyk Y, Appelmelk B (2006) Helicobacter pylori phase variation, immune modulation and gastric autoimmunity. Nature Reviews Microbiology 4: 151–159. [DOI] [PubMed] [Google Scholar]
- 3. Isaacson G, Du MQ (2005) Gastrointestinal lymphoma: where morphology meets molecular biology. Journal of Pathology 205: 255–274. [DOI] [PubMed] [Google Scholar]
- 4. Passaro DJ, Taylor DN, Meza R, Cabrera L, Gilman RH, et al. (2001) Acute Helicobacter pylori Infection Is Followed by an Increase in Diarrheal Disease Among Peruvian Children. Pediatrics 8: e87. [DOI] [PubMed] [Google Scholar]
- 5. Thomas JE, Dale A, Bunn JEG, Harding M, Coward WA, et al. (2004) Early Helicobacter pylori colonisation: the association with growth faltering in The Gambia. Arch Dis Child 89: 1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cover TL, Blaser MJ (2009) Helicobacter pylori in health and disease. Gastroenterology 136: 1863–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perry S, de Jong BC, Solnick JV, de la Luz Sanchez M, Yang S, et al. (2010) Infection with Helicobacter pylori is associated with protection against tuberculosis. PLoS One 5: e8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weyermann M, Rothenbacher D, Brenner H (2009) Acquisition of Helicobacter pylori infection in early childhood: independent contributions of infected mothers, fathers, and siblings. Am J Gastroenterol 104: 182–189. [DOI] [PubMed] [Google Scholar]
- 9. Vakil N, Zullo A, Ricci C, Hassan C, Vaira D (2008) Duplicate Breath Testing To Confirm Eradication of Helicobacter pylori: Incremental Benefit and Cost in 419 Patients. Alimentary Pharmacology & Therapeutics 28: 1304–1308. [DOI] [PubMed] [Google Scholar]
- 10. Campbell DI, Warren BF, Thomas JE, Figura N, Telford JL, et al. (2001) The African enigma: low prevalence of gastric atrophy, high prevalence of chronic inflammation in West African adults and children. Helicobacter 6: 263–267. [DOI] [PubMed] [Google Scholar]
- 11. Everhart JE, Kruszon-Moran D, Perez-Perez GI, Tralka TS, McQuillan G (2000) Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis 181: 1359–1363. [DOI] [PubMed] [Google Scholar]
- 12. Jemilohun AC, Otegbayo JA, Ola SO, Oluwasola OA, Akere A (2010) Prevalence of Helicobacter pylori among Nigerian patients with dyspepsia in Ibadan. Pan Afr Med J 6 186: 18. [PMC free article] [PubMed] [Google Scholar]
- 13. Thomas JE, Dale A, Harding M, Coward WA, Cole TJ, et al. (1999) Helicobacter pylori colonization in early life. Pediatr Res 45: 218–223. [DOI] [PubMed] [Google Scholar]
- 14. Chong VH, Lim KC, Rajendran N (2008) Prevalence of active Helicobacter pylori infection among patients referred for endoscopy in Brunei Darussalam. Singapore Med J 49: 42–46. [PubMed] [Google Scholar]
- 15. Raymond J, Thiberge JM, Chevalier C, Kalach N, Bergeret M, et al. (2004) Genetic and transmission analysis of Helicobacter pylori strains within a family. Emerg infect Dis 10: 1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schwarz S, Morelli G, Kusecek B, Manica A, Balloux F, et al. (2008) Horizontal versus Familial Transmission of Helicobacter pylori . PLoS Pathogens 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herrera PM, Mendez M, Velapatiño B, Santivañez L, Balqui J, et al. (2008) DNA-level diversity and relatedness of Helicobacter pylori strains in shantytown families in Peru and transmission in a developing-country setting. J Clin Microbiol 46: 3912–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alm RA, Trust TJ (1999) Analysis of the genetic diversity of Helicobacter pylori: the tale of two genomes. J Mol Med 77: 834–846. [DOI] [PubMed] [Google Scholar]
- 19. Achtman M, Azuma T, Berg DE, Ito Y, Morelli G, et al. (1999) Recombination and clonal groupings within Helicobacter pylori from different geographical regions. Molecular Microbiology 32: 459–470. [DOI] [PubMed] [Google Scholar]
- 20. Israel DA, Salama N, Krishna U, Rieger UM, Atherton JC, et al. (2001) Helicobacter pylori genetic diversity within the gastric niche of a single host. Proc Nat Acad Science USA 98: 14625–14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Falush D, Wirth T, Linz B, Pritchard JK, Stephens M, et al. (2003) Traces of human migrations in Helicobacter pylori populations. Science 299: 1582–1585. [DOI] [PubMed] [Google Scholar]
- 22. Linz B, Balloux F, Moodley Y, Manica A, Liu H, et al. (2007) An African origin for the intimate association between humans and Helicobacter pylori . Nature 445: 915–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suerbaum S, Smith MJ, Bapumia K, Morelli G, Smith NH, et al. (1998) Free recombination within Helicobacter pylori . Proc Natl Acad Sci USA 95: 12619–12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moodley Y, Linz B, Yamaoka Y, Windsor HM, Breurec S, et al. (2009) The peopling of the Pacific from a bacterial perspective. Science 323: 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nell S, Eibach D, Montano V, Maady A, Nkwescheu A, et al. (2013) Recent acquisition of Helicobacter pylori by Baka pygmies. PLoS Genet 9: e1003775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McNulty SL, Mole BM, Dailidiene D, Segal S, Ally R, et al. (2004) Novel 180- and 480-Base-Pair Insertions in African and African-American Strains of Helicobacter pylori . J Clin Microbiol 42: 5658–5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Secka O, Antonio M, Tapgun M, Berg DE, Bottomley C, et al. (2011) PCR-based genotyping of Helicobacter pylori of Gambian children and adults directly from biopsy specimens and bacterial cultures. Gut Pathog 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saitou N, Nei M (1987) The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4: 406–425. [DOI] [PubMed] [Google Scholar]
- 29. Jolley KA, Feil EJ, Chan MS, Maiden MC (2001) Sequence type analysis and recombinational tests (START). Bioinformatics 17: 1230–1231. [DOI] [PubMed] [Google Scholar]
- 30. Linz B, Vololonantenainab CR, Seck A, Carod JF, Dia D, et al. (2014) Population genetic structure and isolation by distance of Helicobacter pylori in Senegal and Madagascar. PLoS One 9: e87355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kersulyte D, Chalkauskas H, Berg DE (1999) Emergence of recombinant strains of Helicobacter pylori during human infection. Mol Microbiol 31: 31–43. [DOI] [PubMed] [Google Scholar]
- 32. Breurec S, Guillard B, Hem S, Brisse S, Dieye FB, et al. (2011) Evolutionary History of Helicobacter pylori Sequences Reflect Past Human Migrations in Southeast Asia. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moodley Y, Linz B, Bond RP, Nieuwoudt M, Soodyall H, et al. (2012) Age of the association between Helicobacter pylori and man. PLoS pathog 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Akada JK, Ogura K, Dailidiene D, Dailide G, Cheverud JM, et al. (2003) Helicobacter pylori tissue tropism: mouse-colonizing strains can target different gastric niches. Microbiology 149: 1901–1909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.