Abstract
High concentrations of glucose induce β cell production of IL-1β, leading to impaired β cell function and apoptosis in human pancreatic islets. IL-1 receptor antagonist (IL-1Ra) is a naturally occurring antagonist of IL-1β and protects cultured human islets from glucotoxicity. Therefore, the balance of IL-1β and IL-1Ra may play a crucial role in the pathogenesis of diabetes. In the present study, we observed expression of IL-1Ra in human pancreatic β cells of nondiabetic individuals, which was decreased in tissue sections of type 2 diabetic patients. In vitro, chronic exposure of human islets to leptin, a hormone secreted by adipocytes, decreased β cell production of IL-1Ra and induced IL-1β release from the islet preparation, leading to impaired β cell function, caspase-3 activation, and apoptosis. Exogenous addition of IL-1Ra protected cultured human islets from the deleterious effects of leptin. Antagonizing IL-1Ra by introduction of small interfering RNA to IL-1Ra into human islets led to caspase-3 activation, DNA fragmentation, and impaired β cell function. Moreover, siIL-1Ra enhanced glucose-induced β cell apoptosis. These findings demonstrate expression of IL-1Ra in the human β cell, providing localized protection against leptin- and glucose-induced islet IL-1β.
Amajor factor determining the amount of insulin that can be secreted is β cell mass, which increases during obesity (1, 2). In individuals who lose the ability to produce sufficient quantities of insulin to maintain normoglycemia in the face of insulin resistance, type 2 diabetes mellitus manifests (3). Increasing evidence suggests that not only β cell function but also a progressive decrease in β cell mass contributes to this (2). The deficit of β cell mass in type 2 diabetes seems to be due in major part to increased β cell apoptosis (2, 4). Possible mediators of the process of β cell destruction include increased serum concentrations of cell nutrients. Indeed, increased free fatty acid (FFA) levels per se are known to be toxic for β cells, leading to the concept of lipotoxicity (5–10). However, not all obese individuals or pretype 2 diabetes patients exhibit dyslipidemia. Thus, lipotoxicity may play an important role in the process of β cell destruction but probably does not act alone. Elevated glucose concentrations induce β cell apoptosis in cultured islets from diabetes-prone Psammomys obesus, an animal model of type 2 diabetes (4), in human islets (11–14), and at higher concentrations also in rodent islets (9, 15). In human islets, glucose-induced β cell apoptosis and dysfunction are mediated by β cell production and secretion of IL-1β (13). Probably already in the pretype 2 diabetic stage, insulin resistance diminishes glucose uptake, resulting in transient postprandial hyperglycemic excursions. However, it is unlikely that transient hyperglycemia is sufficient to cause progression from obesity to diabetes.
Leptin is expressed primarily in the adipose tissue, and this peptide hormone plays a major role in interorgan signaling emanating from the adipocyte (16). β cells express leptin receptors, and leptin inhibits insulin secretion in vivo and in vitro (17–23). In rodent islets, leptin induces β cell proliferation and protects from FFA-induced β cell apoptosis (24–27). However, the effect of leptin on human β cell apoptosis is not known.
IL-1 receptor antagonist (IL-1Ra) is an antiinflammatory cytokine and naturally occurring antagonist of IL-1α and -β (28–30). Exogenous recombinant human (rh) IL-1Ra protects cultured human islets from glucose-induced IL-1β-mediated β cell apoptosis and improves β cell function (13). Interestingly, leptin induces secretion of IL-1Ra in monocytes (31). However, expression and regulation of IL-1Ra in human islets have not been investigated.
Therefore, we studied IL-1Ra in human pancreatic β cells of nondiabetic and diabetic patients and identified its regulation by leptin. Effects of leptin on IL-1β production and on human β cell function and survival, partly mediated by changes in IL-1Ra expression, were also explored.
Methods
Islet Isolation and Cell Culture. Islets were isolated from pancreases of 17 organ donors at the University of Geneva Medical Center. For short-term in vitro studies, the islets were cultured in nonadherent dishes, and for long-term studies, on extracellular matrix-coated plates derived from bovine corneal endothelial cells (Novamed, Jerusalem). Islets were cultured in CMRL 1066 medium containing 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% FCS (Invitrogen), hereafter referred to as culture medium. In some experiments, islets were cultured with rh leptin (PeproTech, London), rh IL-1Ra (R & D Systems) streptozotocin, or staurosporine (Sigma). Human islets from four isolations were purified into single β cells and cultured as described (32, 33); rat islets were isolated and cultured in RPMI medium (Invitrogen) as described (9); and human blood monocytes from four healthy individuals were isolated and cultured as described (13).
RNA Interference. RNAs of 21 nucleotides, designed to target human IL-1Ra (5′-AUCUGCAGAGGCCUCCGCAtt-3′/5′-UGCGGAGGCCUCUGCAGAUtt-3′), and scrambled small interference RNA (siRNA) were synthesized by Ambion (Austin, TX). siRNA was transfected by using SiPortAmine and transfection efficiency estimated with cy3-labeled siRNA by using the Silence siRNA Labeling Kit (Ambion).
Detection of IL-1Ra- and IL-1β-Expressing Cells. Pancreases from necropsies and cultured islets were processed as described (13). Cultured cells, pancreas, and islet sections were double-labeled for IL-1Ra or -1β and insulin, CD68, glucagon, or somatostatin by incubation with biotinylated goat anti-IL-1Ra or mouse anti-IL-1β antibodies (R & D Systems). Detection was performed by using cy3-(Jackson ImmunoResearch) or cy2-(Transduction Laboratories, Lexington, KY) conjugated streptavidin. Subsequently, specimens were incubated with guinea pig (DAKO) or mouse (Sigma) anti-insulin, rabbit anti-somatostatin (DAKO), rabbit anti-glucagon (DAKO), or mouse anti-CD68 (BioSource International, Nivelles, Belgium) antibodies, followed by incubation with FITC-conjugated rabbit anti-guinea pig (DAKO), cy3-conjugated donkey anti-mouse, cy3-conjugated donkey anti-rabbit, or cy2-conjugated donkey anti-mouse antibodies (Jackson ImmunoResearch). The specificity of the IL-1Ra antibody was assessed by an absorption test. For mRNA in situ hybridization of IL-1Ra, DNA templates were generated by PCR with incorporation of a T3 or a T7 promoter into the antisense or sense primer. The following primers were used: T3 5′-CCAATTAACCCTCACTAAAGGGACTGAGGACCAGCCATTGAG-3′ and T7 5′-CCGTAATACGACTCACTATAGGGCAGGTGGAATGAGGGAGGAAG-3′.
Electron Microscopy. Cultured human islets were fixed in 2.5% paraformaldehyde/0.1% glutaraldehyde/0.01% picric acid and dehydrated in graded ethanol before embedding in LR white (Polysciences). Ultrathin sections were treated with 50 mM gelatin in PBS/0.2 g of BSA, incubated with guinea pig anti-porcine insulin (DAKO), followed by incubation with anti-guinea pig IgG conjugated with gold-5 nm (Amersham Pharmacia). Sections were incubated with biotinylated goat anti-IL-1Ra antibody as described, followed by incubation with streptavidin-gold-15 nm (Amersham Pharmacia) and counterstaining with uranyl acetate.
Western Blot Analysis. Islets and proteins were separated as described (13, 34). Nitrocellulose membranes were incubated with biotinylated goat anti-IL-1Ra, mouse anticaspase 3 (Pharmingen), phospho-Thr-202, Tyr-204-ERK1 (ERK, extracellular signal-regulated kinase) (p-ERK1/2), phospho-Stat3 (Tyr 705) (Cell Signaling Technology, Beverly, MA), and phospho-Jak2 (Upstate Biotechnology, Lake Placid, NY) or mouse antiactin (C-2; Santa Cruz Biotechnology) antibodies. Thereafter, membranes were incubated with horseradish-peroxidase-linked anti-mouse IgG (Santa Cruz Biotechnology) or streptavidin (Jackson ImmunoResearch). Between incubations, membranes were stripped with 280 μl of 2-mercaptoethanol/5mlof0.5MTris·HCl (pH 6.8)/10% SDS and then washed in Tris-buffered saline containing 0.1% Tween 20.
RNA Extraction and RT-PCR. Total RNA extraction and quantitative PCR were performed as described (13). The following primers were used: 5′-ACTGAGGACCAGCCATTGAG-3′/5′-AGGTGGAATGAGGGAGGAAG-3′ (human IL-1Ra) and 5′-AAGCTGATGGCCCTAAACAG-3′/5-′AGGTGCATCGTGCACATAAG-3′ (human IL-1β) 5′-CCAGAAACGTTTGAGCATCT-3′/5′-CAAAAGCACACCACTCTCTC-3′ and 5′-GAAGGAGTGGGAAAACCAAAG-3′/5′-CCACCATATGTTAACTCTCAG-3′ [human leptin long (hOB-RI) and short form (hOB-Rs) form (35)]. For stantardization, α-tubulin (5′-AGAGTCGCGCTGTAAGAAGC-3′/5′-TGGTCTTGTCACTTGGCATC-3′) and GAPDH (5′-AACAGCGACACCCACTCCTC-3′/5′-GGAGGGGAGATTCAGTGTGGT-3′) were used and showed similar results.
β Cell Apoptosis. Cells were double stained by the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) technique and for insulin as described (4, 13, 14). In parallel, apoptosis was confirmed by detection of caspase 3 activation as described above.
Cytokine Release. Cytokine release was evaluated by using human IL-1β and IL-1Ra ELISA kits (R & D Systems).
Insulin Release and Content. For acute insulin release in response to glucose, islets were washed and preincubated (30 min) in Krebs–Ringer bicarbonate buffer (KRB) containing 3.3 mM glucose and 0.5% BSA. KRB was then replaced by KRB 3.3 mM glucose for 1 h (basal), followed by an additional 1 h in KRB 16.7 mM glucose. Islets were extracted with 0.18 M HCl in 70% ethanol for determination of insulin content. Insulin was determined by a human insulin RIA kit (CIS Biointernational, Gif-sur-Yvette, France).
Triglyceride Content of Islets. Islets were resuspended in 50 μl of 2 mM NaCl/20 mM EDTA/50 mM sodium phosphate buffer (pH 7.4) and sonicated for 1 min. Thereafter, 25 μl of homogenate was mixed with 25 μl of tert-butyl alcohol and 12.5 μl of Triton X-100/methyl alcohol mixture (1:1 by volume) to allow for extraction of lipids. Triglycerides were assayed as described (36).
Evaluation and Statistical Analysis. Samples were evaluated in a randomized manner by a single investigator (K.M.) blinded to the treatment conditions as described (13, 14). Data were analyzed by Student's t test or by analysis of variance with a Bonferroni correction for multiple group comparisons.
Results
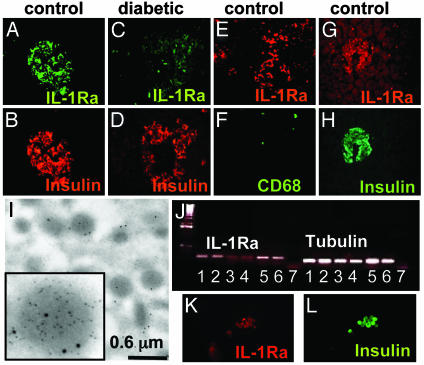
IL-1Ra Is Expressed by Human Pancreatic β Cells and Down-Regulated in Type 2 Diabetic Patients. Immunodetection of human pancreatic sections from nondiabetic patients revealed the presence of IL-1Ra localized in the β cells (Fig. 1 A and B). Additionally, some non-β cells expressed IL-1Ra and were identified as resident macrophages (CD68 positive; Fig. 1 E and F). No specific staining for IL-1Ra was observed in α and δ cells, as assessed by double immunostaining with anti-IL-1Ra and antiglucagon or antisomatostatin antibodies, respectively (data not shown). IL-1Ra was also undetectable in the exocrine tissue. Expression of IL-1Ra by the β cell itself was confirmed by electron microscopy of sections from isolated human islets (Fig. 1I), the presence of IL-1Ra mRNA transcripts in β cells of nondiabetic patients (Fig. 1 G and H), and by RT-PCR and double-immunofluorescence of purified human β cells (Fig. 1 J–L). Next we studied expression of IL-1Ra in pancreas sections from six poorly controlled type 2 diabetic patients, all with documented fasting plasma glucose >8 mM. In most specimens, IL-1Ra protein expression was decreased as compared to the nondiabetic controls (for representative images, see Fig. 1 C and D).
Fig. 1.
IL-1Ra is expressed by human islets and down-regulated in type 2 diabetes. Double immunostaining for IL-1Ra in green (A and C) or red (E) and insulin in red (B and D) or CD68 in green (F) in tissue sections of pancreases from a nondiabetic patient without (A, B, E, and F) and from a patient with type 2 diabetes (C and D). In situ hybridization for IL-1Ra mRNA in red (G) double immunostained for insulin in green (H) in tissue sections of pancreas from a nondiabetic patient. (I) Electron microscopy of secretory granules within a β cell in cultured human islets double gold-immunolabeled for insulin (small particles) and IL-1Ra (large particles). (J) RT-PCR analysis of IL-1Ra expression by purified human β cells (lanes 1 and 2), cultured human islets (lanes 3 and 4), human blood monocytes (lanes 5 and 6), and water (negative control; lane 7). One of four experiments from four donors is shown. Double immunostaining for IL-1Ra in red (K) and insulin in green (L) in purified human β cells.
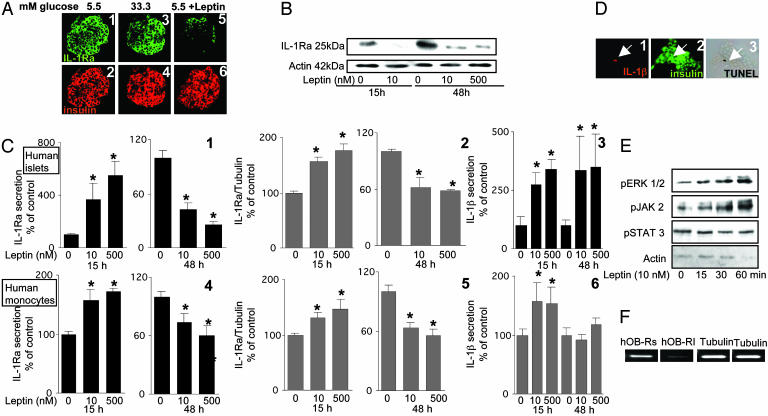
Leptin Decreases β Cell Production of IL-1Ra and Induces IL-1β Release in Human Islets. Because glucose induces β cell production of IL-1β (13), the hypothesis that a high concentration of glucose may also be responsible for the decreased expression of IL-1Ra in islets of type 2 diabetic patients was tested in vitro in primary cultures of human islets. Exposure of islets to 33.3 mM glucose for 48 h in fact failed to affect IL-1Ra protein expression (Fig. 2A 1–4), IL-1Ra release (data not shown) or mRNA expression (Fig. 3A), as compared to controls at 5.5 mM glucose. Because leptin regulates IL-1Ra secretion in monocytes (31), it was postulated that it, rather than glucose, may be the regulator of IL-1Ra in β cells. Indeed, exposure of cultured human islets to 10 nM leptin for 48 h decreased IL-1Ra expression in most β cells, as determined by double immunostaining (Fig. 2A5 and 6). Western blot analysis of IL-1Ra in human islets revealed that leptin already decreased IL-1Ra protein levels after 15 h of exposure (Fig. 2B). Interestingly, IL-1Ra released into the culture medium and IL-1Ra mRNA were increased after short-term exposure to 10–500 nM leptin for 15 h, but prolonged exposure for 48 h resulted in a marked decrease (Fig. 2C1 and 2). A similar dual effect was observed in peripheral blood monocytes that were used as control (Fig. 2C 4 and 5).
Fig. 2.
Leptin induces phosphorylation of ERK1/2 and JAK2, decreases β cell production of IL-1Ra, and induces IL-1β release in human islets. (A) Double immunostaining for IL-1Ra in green (1, 3, and 5) and insulin in red (2, 4, and 6) in sections of cultured human islets exposed for 48 h to 5.5 mM glucose alone (1 and 2), 33.3 mM glucose (3 and 4), or 5.5 mM glucose and 10 nM leptin (5 and 6). (B) Immunoblotting of IL-1Ra and actin. Human islets cultured with and without 10 or 500 nM leptin were analyzed after 15 or 48 h of incubation. One of three experiments from three donors is shown. (C) Secretion of IL-1Ra (1 and 4) and RT-PCR detection and quantification of IL-1Ra mRNA expression (2 and 5) and secretion of IL-1β (3 and 6). Supernatants and total RNA were obtained from human islets (1–3) and human blood monocytes (4–6) cultured for 15 and 48 h in the presence of medium alone or 10 or 500 nM leptin. Data were collected from three tubes per treatment of five separate experiments from five islets donors. Results are means ± SE of percentage relative to control incubations (100%, in absolute values IL-1Ra release from islets: 152.0 ± 18.3 and 594.7 ± 24.5 pg/20islets per 2 ml after 15 and 48 h of culture, respectively, and from monocytes: 48.4 ± 1.7 and 96.4 ± 1.8 ng/ml after 15 and 48 h of culture, respectively. In absolute values for IL-1β release from islets: 0.28 ± 0.07 and 0.79 ± 0.13 pg/20 islets per 2 ml after 15 and 48 h of culture, respectively, and from monocytes: 332.0 ± 42.4 and 456.4 ± 88.6 pg/ml after 15 and 48 h of culture, respectively). *, P < 0.01 compared to controls. (D) Triple immunostaining for IL-1β in red (1), insulin in green (2), and DNA fragmentation by TUNEL assay in black (3). Human islets were cultured for 4 days at 5.5 mM glucose with 10 nM leptin. The arrows mark a β cell stained positive for IL-1β, insulin, and the TUNEL reaction. (E) Effects of 10 nM leptin on the time course of ERK1/2, JAK2, and STAT3 phosphorylation in cultured human islets. Lysates were subjected to Western blotting for activated ERK 1/2 (pERK1/2), JAK 2 (pJAK2), and STAT 3 (pSTAT3). One of three experiments from three donors is shown. (F) RT-PCR analysis for expression of the human long (hOB-Rl) and short forms (hOB-Rs) of the leptin receptor and tubulin (control) by cultured human islets.
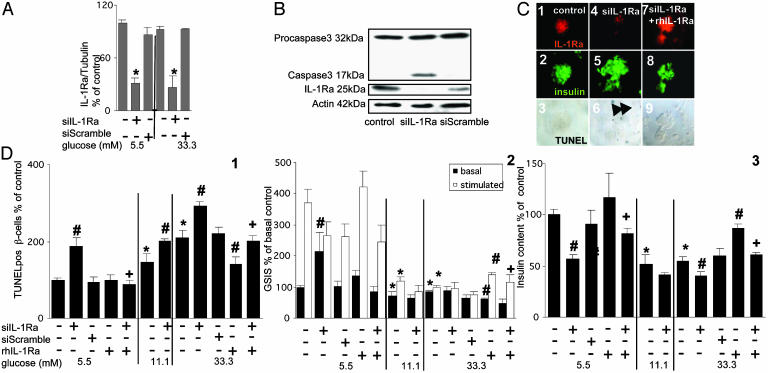
Fig. 3.
Antagonizing IL-1Ra by siIL-1Ra induces β cell apoptosis and impairs β cell function. (A) RT-PCR detection and quantification of IL-1Ra mRNA expression. Total RNA was isolated from human islets cultured for 48 h in 5.5 or 33.3 mM glucose alone or after transfection with 50 nM siIL-1Ra or with 50 nM of a scrambled RNA sequence (siScramble) with or without addition of 500 ng/ml exogenous rh IL-1Ra. Results are presented as means ± SE of five independent experiments from five donors. *, P < 0.001 compared to control islets at the same glucose concentration. (B) Immunoblotting of procaspase 3, activated caspase 3, IL-1Ra, and actin. Human islets were cultured for 48 h in medium containing 5.5 mM glucose alone or after transfection with 50 nM siIL-1Ra or with 50 nM siScramble. The antibodies were blotted on the same membrane after stripping. One of four experiments from four donors is shown. (C and D) Human islets were cultured for 4 days in 5.5, 11.1, and 33.3 mM glucose alone or after transfection with 50 nM siIL-1Ra or 50 nM siScramble with or without addition of 500 ng/ml exogenous rh IL-1Ra. (C) Triple immunostaining for IL-1Ra in red (1, 4, and 7), insulin in green (2, 5, and 8), and DNA fragmentation by TUNEL assay in black (3, 6, and 9) in islets exposed to 5.5 mM glucose alone (1–3), with siIL-1Ra (4–6), or with siIL-1Ra and rh IL-1Ra (7–9). The arrows mark β cell nuclei stained positive for the TUNEL reaction. (D)(1) Results are means ± SE of percentage of TUNEL-positive β cells relative to control incubations at 5.5 mM glucose alone (100%, in absolute value: 0.33 ± 0.03% TUNEL-positive β cells). The mean number of islets scored from each donor was 44 (range 24–80) for each treatment condition. Islets were isolated from five organ donors. (2) Basal and glucose-stimulated insulin secretion (GSIS) during successive 1-h incubations at 3.3 (basal) and 16.7 (stimulated) mM glucose after the 4-day culture period at different glucose concentrations. (3) Insulin content. Results are means ± SE relative to 5.5 mM glucose alone (100%, in absolute values: 0.53 ± 0.08 and 7.9 ± 0.7 pmol/islet for basal insulin secretion and cell content, respectively). Data presented are means of three experiments from three separate donors. In each experiment, the data were collected from three plates per treatment. #, P < 0.05 compared with control islets at same glucose concentration; *, P < 0.05 compared with islets at 5.5 mM glucose alone; +, P < 0.05 compared to siIL-1Ra transfected islets alone at same glucose concentration.
IL-1Ra action depends on its antagonism toward IL-1β. Therefore, we also studied the regulation of IL-1β by leptin. Similarly to the regulation of IL-1Ra, exposure of human islets to leptin for 15 h increased IL-1β release into the culture medium (Fig. 2C3). In contrast to the leptin-induced decrease of IL-1Ra release after 48 h, IL-1β release remained increased after 48 h of exposure. Thus, the modulation by leptin of IL-1Ra and IL-1β secretion slightly favored the balance for the protective IL-1Ra after 15 h, whereas prolonged exposure to leptin strongly shifted the balance toward IL-1β (543 and 752 IL-1Ra:IL-1β ratio in control vs. 868 and 34 in 500 nM leptin-treated islets after 15 and 48 h, respectively). Moreover, exposure of peripheral blood monocytes to leptin for 15 h increased IL-1β release, whereas prolonged exposure for 48 h did not significantly affect IL-1β release (Fig. 2C6). Quantitative RT-PCR measurements revealed that islet expression of IL-1β mRNA was not significantly changed by leptin (not shown). However, exposure of cultured human islets to 10 nM leptin for 48 h did increase IL-1β immunostaining but this was limited to TUNEL-positive β cells only, as determined by triple immunostaining of islets with anti-IL-1β and anti-insulin antibodies and by the TUNEL assay (Fig. 2D). To demonstrate the presence of functional leptin receptors, activation of major leptin signal transducers was analyzed. Exposure of human islets to 10 nM leptin induced time-dependent phosphorylation of ERK1/2 and Janus tyrosine kinase (JAK)2, whereas signal transducers and activators of transcription (STAT)3 activity remained unchanged (Fig. 2E). We also confirmed (18) the presence of both the short and long forms of the leptin receptor (Fig. 2F). Finally, the effects of leptin on human and rat islet triglyceride content were compared. Exposure of human islets to 10 nM leptin for 48 h decreased islet triglyceride content by 23.1 ± 9.41% compared to control at 5.5 mM glucose alone, whereas in rat islets, the decrease of triglyceride content was by 58.18 ± 22.79% compared to control at 11.1 mM glucose.
Endogenously Produced IL-1Ra Is a Survival Factor of β Cells and Preserves β Cell Function. We next studied the functional role of IL-1Ra in human β cells by means of siRNA. Two days after exposure of human islets to siRNA to IL-1Ra (siIL-1Ra), endogenous IL-1Ra RNA expression decreased by 69.4 ± 6.5% as compared to islets incubated at 5.5 mM glucose alone, whereas scrambled siRNA had no such effect (Fig. 3A).
Transfection efficiency of human islets with siRNA was evaluated with a Cy3-labeled siRNA. Analysis of the islets showed a transfection efficiency of ≈70% (data not shown). This is in accordance with the level of decrease of IL-1Ra RNA by siIL-1Ra and could thus indicate a complete antagonism in cells expressing siRNA.
The decrease in IL-1Ra expression evoked by siIL-1Ra was similar at low (5.5 mM) and high (33.3 mM) glucose concentrations (Fig. 3A). siIL-1Ra also dramatically decreased IL-1Ra protein expression, inducing an apoptotic process as demonstrated by caspase 3 activation (Fig. 3B) and β cell DNA fragmentation (TUNEL-positive nuclei, Fig. 3 C and D1). Addition of exogenous rh IL-1Ra prevented the effect of siIL-1Ra on DNA fragmentation, proving the specificity of the RNA interference (Fig. 3 C and D1). After blockade of endogenous IL-1Ra by siIL-1Ra, exogenous rh IL-1Ra restored anti-IL-1Ra immunobinding of β cells and uncovered binding to non-β cells (Fig. 3C7). This is probably secondary to rh IL-1Ra binding to the IL-1 receptor, which remains on the cell during the fixation and is detected by anti-IL-1Ra.
Exposure of cultured human islets to elevated glucose concentrations for 4 days increased the number of β cells displaying DNA fragmentation (Fig. 3D1). This glucose effect was enhanced by coincubation with siIL-1Ra, whereas exogenous rh IL-1Ra protected the β cells from apoptosis induced by 33.3 mM glucose alone and partially from apoptosis induced by 33.3 mM glucose and siIL-1Ra.
Next, the role of endogenous IL-1Ra on β cell function was evaluated. Antagonizing IL-1Ra by siRNA dramatically decreased the ratio of acute to basal glucose-stimulated insulin release from human islets previously maintained at 5.5 mM glucose during the 48-h incubation (Fig. 3D2). Coincubation with exogenous rh IL-1Ra restored glucose stimulation. Exposure of human islets for 4 days to 11.1 mM glucose severely blunted acute (1 h) glucose-stimulated insulin release, which was totally lost in the presence of siIL-1Ra at 11.1 or 33.3 mM glucose or by 33.3 mM alone. Exogenous rh IL-1Ra partially prevented those effects. Insulin content of islets exposed to siIL-1Ra and/or high glucose concentrations was decreased compared to control (5.5 mM glucose) and partially restored by rh IL-1Ra (Fig. 3D3)
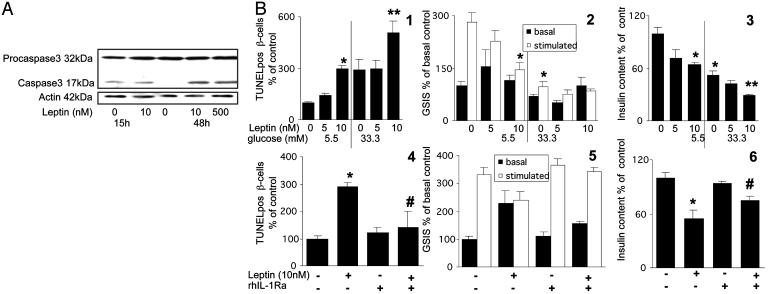
Leptin Induces β Cell Apoptosis and Impairs β Cell Function via IL-1β Signaling. Exposure of cultured human islets to 10 nM leptin for 4 days increased the number of TUNEL-positive β cells (Fig. 4B1). Moreover, leptin enhanced 33.3 mM glucose-induced apoptosis (Fig. 4B1). To further demonstrate an apoptotic process, caspase 3 activation was investigated. Cleaved caspase 3 of control human islets was detectable after 15 h of culture, probably secondary to the stress of the isolation procedure, and disappeared after 48 h (Fig. 4A). Exposure of human islets to leptin for 15 h did not change the baseline cleaved caspase 3 level, but an increase became apparent after 48 h (Fig. 4A). Moreover, exposure to leptin for 4 days decreased chronic insulin secretion during the culture period by 41.7% (P < 0.01, not shown), impaired glucose-stimulated insulin secretion, and decreased insulin content at 5.5 mM glucose, an effect that was additive to the deleterious effect of high glucose (Fig. 4B 2 and 3). To show that leptin-induced DNA fragmentation, impaired β cell function and decreased insulin content are mediated by the dysbalance between IL-1β and IL-1Ra, human islets were coincubated with leptin and exogenous rh IL-1Ra. In human islets exposed to leptin, addition of rh IL-1Ra prevented leptin-induced β cell apoptosis and restored β cell function and insulin content (Fig. 4B4–6). To demonstrate the specificity of the inhibition of rh IL-1Ra in leptin-induced apoptosis, cell death was triggered by streptozotocin and staurosporine. Streptozotocin causes damage to DNA either by alkylation or by the generation of NO and staurosporine is an inhibitor of protein kinases. Exposure of human islets to 1 mM streptozotocin and 2 μM staurosporine induced a 2.4- and 3.7-fold increase in TUNEL-positive β-cells, respectively (P < 0.05). The presence of rh IL-1Ra failed to prevent those effects. Moreover, no IL-1β-positive cells were observed under these conditions (data not shown).
Fig. 4.
Leptin induces β cell apoptosis and impairs β cell function via IL-1β signaling. (A) Immunoblotting of procaspase 3, activated caspase 3, and actin. Human islets were cultured for 15 and 48 h in 5.5 mM glucose alone or with 10 or 500 nM leptin. One of four experiments from four donors is shown. (B) Human islets were cultured on extracellular matrix-coated dishes for 4 days at 5.5 and 33.3 mM glucose with or without 5 and 10 nM leptin or with addition of 500 ng/ml exogenous rh IL-1Ra. (1 and 4) Results are means ± SE of percentage of TUNEL-positive β cells relative to 5.5 mM glucose alone [100%, in absolute value: 0.35 ± 0.04% (1) or 0.37 ± 0.06% (4) TUNEL-positive β cells]. The mean number of islets scored from each donor was 30 (range 21–45) for each treatment condition. Islets were isolated from five organ donors. (2 and 5) Basal and glucose-stimulated insulin secretion (GSIS) denote the amount secreted during successive 1-h incubations at 3.3 (basal) and 16.7 (stimulated) mM glucose after the 4-day culture period. (3 and 6) Insulin content. Results are means ± SE relative to control incubations at 5.5 mM glucose alone [100%, in absolute values: 0.70 ± 0.11 and 10.9 ± 0.7 pmol per islet (2 and 3) and 0.43 ± 0.10 and 7.5 ± 0.7 pmol per islet (5 and 6) for basal insulin secretion and cell content, respectively]. Data presented are means of three experiments from three separate donors. In each experiment, the data were collected from three plates per treatment. *, P < 0.01 compared to islets at 5.5 mM glucose alone; **, P < 0.01 compared to islets at 33.3 mM glucose alone; P < 0.05 compared to leptin-treated islets alone.
Discussion
Chronically elevated blood glucose levels impair the function of β cells in the pancreas (37, 38). Studying the underlying mechanisms, we have previously observed that exposure of cultured islets to elevated glucose levels leads to β cell production and release of IL-1β, followed by impaired β cell function and death (13). We now show that leptin induces IL-1β release from human islets in culture while decreasing β cell expression of IL-1Ra. The resulting increase in the IL-1β/IL-1Ra ratio, impaired β cell function, and enhanced β cell apoptosis. The deleterious effects of leptin can be alleviated by supplementation of exogenous IL-1Ra.
This study shows previously undescribed expression of IL-1Ra by β cells. This is particularly intriguing in the context of an endocrine organ not primarily belonging to the immune system. In addition to previous findings that showed β cell expression of IL-1β (13, 39), the present observation provides further support for the role of local inflammatory mediators in the pathophysiology of diabetes (40).
Serum concentrations of IL-1Ra seem to be influenced by adipose tissue, which is a source of IL-1Ra, and circulating IL-1Ra levels are increased during obesity (41). Interestingly, patients with type 2 diabetes have significantly lower IL-1Ra concentrations than nondiabetic patients (42). Moreover, the possible crucial role of inflammatory cytokines in the pathogenesis of type 2 diabetes is underscored by a recent prospective study. Spranger et al. (43) observed that individuals with elevated levels of IL-1β and -6 have a significantly increased risk of developing type 2 diabetes. Isolated elevation of IL-6 alone, however, did not modify the risk of developing diabetes. Whether changes in circulating cytokines are physiologically relevant in the face of locally produced inflammatory mediators remains unknown. In any event, the ratio between a cytokine and its antagonist(s) (in the present example, IL-1Ra:IL-1β) is considered a major determinant for its biological efficacy and thus for its role in physiological or pathophysiological situations.
The concentrations of leptin used in our study are in good agreement with numerous other in vitro studies (23, 44), and the lowest concentration used (5 nM) is in the upper range measured in obese people (45). Regarding the effect of IL-1Ra, we used much higher concentrations than those found in the circulation in vivo. However, based on our observation of production of IL-1Ra by β cells themselves, circulating concentrations are difficult to compare with local ones possibly responsible for paracrine/autocrine effects.
Exposure of human β cells to leptin induced an initial increase in IL-1Ra mRNA expression and release after 15 h, whereas longer exposure for 48 h resulted in their inhibition. By contrast, leptin decreased IL-1Ra islet content already after 15 h of exposure, and this effect was maintained over 48 h. Probably part of the increase in IL-1Ra released shortly after exposure to leptin reflects a depletion of IL-1Ra produced by β cells. However, the molecular mechanisms of the duality of leptin-induced changes in IL-1Ra mRNA expression are unclear. They are reminiscent of the duality of the effects induced by other modulators of β cell turnover, e.g., glucose and FFA (9, 10). Each time, short-term exposure seems beneficial and prolonged exposure, toxic.
Antagonizing endogenous β cell-IL-1Ra by siRNA led to impaired β cell function and apoptosis. This is not a nonspecific effect of siRNA, because scrambled siRNA was not toxic. It is likely that a certain amount of IL-1Ra is necessary for the survival of cultured human β cells. IL-1β, which is present in the supernatant of cultured human islets even in basal conditions (5.5 mM glucose) (13), is perhaps ineffective as long as IL-1Ra is expressed, becoming deleterious as soon as the balance changes in its favor. In vivo, it is conceivable that IL-1Ra protects β cells from other sources of IL-1β, e.g., the innate immune system (40), and that β cells become particularly vulnerable if leptin decreases its expression.
The cellular source of leptin-induced IL-1β secretion from human islets is not clear. Triple immunostaining for IL-1β, insulin, and the TUNEL assay of islets exposed to leptin revealed only TUNEL-positive β cells producing IL-1β. Because rh IL-1Ra blocked leptin-induced apoptosis, one may assume that at least some part of leptin-induced IL-1β production by human islets in culture precedes the apoptotic process and must therefore originate from non-β cells. Macrophages contained within isolated islets would be logical candidates, because leptin induces the secretion of IL-1β in peripheral blood monocytes, as shown (31), and confirmed in the present study. On the other hand, whereas it is true that leptin did increase IL-1β, the physiological relevance of this remains to be examined. Indeed, it is suggested that the leptin-induced decrease in IL-1Ra would be sufficient to shift the IL-1Ra:IL-1β ratio toward activation of the IL-1 receptor. The situation regarding leptin is thus quite different from the previously documented stimulation by high glucose of IL-1β production by human β cells (13).
In contrast to the present study, the antiapoptotic effects of leptin have been convincingly shown in rodent islets (26). Furthermore, the present in vitro study of human islets contradicts in vivo studies in rats pointing to protective effects of leptin in the development of diabetes. Indeed, normal rats made chronically hyperleptinemic develop hypoglycemia, whereas β cells remain morphologically unchanged (46). In humans, plasma leptin levels are mainly attributed to the extent of s.c. obesity rather than to visceral obesity (47, 48), yet individuals with visceral obesity are at higher risk of developing diabetes. Finally, progression from obesity to diabetes takes years to develop, in contrast to present data showing leptin-induced β cell apoptosis within days.
Conclusion This study demonstrates that human β cells can produce IL-1Ra that can in turn protect against the deleterious impact on β cell function and survival of IL-1β secreted by human islets. Increasing the ratio between IL-1β and its antagonist IL-1Ra via leptin or glucose, results in an increase in β cell apoptosis and impaired glucose-stimulated insulin secretion.
Acknowledgments
We thank C. A. Meier for his support and comments. This work was supported by the Swiss National Science Foundation, by a European Foundation for the Study of Diabetes/Johnson & Johnson Research Award, and by the Juvenile Diabetes Research Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: FFA, free fatty acid; IL-1Ra, IL-1 receptor antagonist; rh IL-1Ra, recombinant human IL-1Ra; siRNA, small interfering RNA; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling; siIL-1Ra, siRNA to IL-1Ra; ERK, extracellular signal-regulated kinase; JAK, Janus tyrosine kinase; STAT, signal transducers and activators of transcription.
References
- 1.Bonner-Weir, S. (2000) J. Mol. Endocrinol. 24, 297–302. [DOI] [PubMed] [Google Scholar]
- 2.Butler, A. E., Janson, J., Bonner-Weir, S., Ritzel, R., Rizza, R. A. & Butler, P. C. (2003) Diabetes 52, 102–110. [DOI] [PubMed] [Google Scholar]
- 3.Cerasi, E. (1995) Diabetologia 38, 992–997. [DOI] [PubMed] [Google Scholar]
- 4.Donath, M. Y., Gross, D. J., Cerasi, E. & Kaiser, N. (1999) Diabetes 48, 738–744. [DOI] [PubMed] [Google Scholar]
- 5.Leroith, D. (2002) Am. J. Med. 113 Suppl 6a, 3s–11s. [DOI] [PubMed] [Google Scholar]
- 6.McGarry, J. D. & Dobbins, R. L. (1999) Diabetologia 42, 128–138. [DOI] [PubMed] [Google Scholar]
- 7.Randle, P. J., Garland, P. B., Newsholme, E. A. & Hales, C. N. (1965) Ann. N. Y. Acad. Sci. 131, 324–333. [DOI] [PubMed] [Google Scholar]
- 8.Unger, R. H. (1995) Diabetes 44, 863–870. [DOI] [PubMed] [Google Scholar]
- 9.Maedler, K., Spinas, G. A., Dyntar, D., Moritz, W., Kaiser, N. & Donath, M. Y. (2001) Diabetes 50, 69–76. [DOI] [PubMed] [Google Scholar]
- 10.Maedler, K., Oberholzer, J., Bucher, P., Spinas, G. A. & Donath, M. Y. (2003) Diabetes 52, 726–733. [DOI] [PubMed] [Google Scholar]
- 11.Maedler, K., Spinas, G. A., Lehmann, R., Sergeev, P., Weber, M., Fontana, A., Kaiser, N. & Donath, M. Y. (2001) Diabetes 50, 1683–1690. [DOI] [PubMed] [Google Scholar]
- 12.Federici, M., Hribal, M., Perego, L., Ranalli, M., Caradonna, Z., Perego, C., Usellini, L., Nano, R., Bonini, P., Bertuzzi, F., et al. (2001) Diabetes 50, 1290–1301. [DOI] [PubMed] [Google Scholar]
- 13.Maedler, K., Sergeev, P., Ris, F., Oberholzer, J., Joller-Jemelka, H. I., Spinas, G. A., Kaiser, N., Halban, P. A. & Donath, M. Y. (2002) J. Clin. Invest. 110, 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maedler, K., Fontana, A., Ris, F., Sergeev, P., Toso, C., Oberholzer, J., Lehmann, R., Bachmann, F., Tasinato, A., Spinas, G. A., Halban, P. A. & Donath, M. Y. (2002) Proc. Natl. Acad. Sci. USA 99, 8236–8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Efanova, I. B., Zaitsev, S. V., Zhivotovsky, B., Kohler, M., Efendic, S., Orrenius, S. & Berggren, P. O. (1998) J. Biol. Chem. 273, 33501–33507. [DOI] [PubMed] [Google Scholar]
- 16.Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L. & Friedman, J. M. (1994) Nature 372, 425–432. [DOI] [PubMed] [Google Scholar]
- 17.Campfield, L. A., Smith, F. J., Guisez, Y., Devos, R. & Burn, P. (1995) Science 269, 546–549. [DOI] [PubMed] [Google Scholar]
- 18.Seufert, J., Kieffer, T. J., Leech, C. A., Holz, G. G., Moritz, W., Ricordi, C. & Habener, J. F. (1999) J. Clin. Endocrinol. Metab. 84, 670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emilsson, V., Liu, Y. L., Cawthorne, M. A., Morton, N. M. & Davenport, M. (1997) Diabetes 46, 313–316. [DOI] [PubMed] [Google Scholar]
- 20.Kieffer, T. J., Heller, R. S. & Habener, J. F. (1996) Biochem. Biophys. Res. Commun. 224, 522–527. [DOI] [PubMed] [Google Scholar]
- 21.Roduit, R. & Thorens, B. (1997) FEBS Lett. 415, 179–182. [DOI] [PubMed] [Google Scholar]
- 22.Koyama, K., Chen, G., Lee, Y. & Unger, R. H. (1997) Am. J. Physiol. 273, E708–E713. [DOI] [PubMed] [Google Scholar]
- 23.Kulkarni, R. N., Wang, Z. L., Wang, R. M., Hurley, J. D., Smith, D. M., Ghatei, M. A., Withers, D. J., Gardiner, J. V., Bailey, C. J. & Bloom, S. R. (1997) J. Clin. Invest. 100, 2729–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Islam, M. S., Sjoholm, A. & Emilsson, V. (2000) Int. J. Obes. Relat. Metab. Disord. 24, 1246–1253. [DOI] [PubMed] [Google Scholar]
- 25.Okuya, S., Tanabe, K., Tanizawa, Y. & Oka, Y. (2001) Endocrinology 142, 4827–4830. [DOI] [PubMed] [Google Scholar]
- 26.Shimabukuro, M., Wang, M. Y., Zhou, Y. T., Newgard, C. B. & Unger, R. H. (1998) Proc. Natl. Acad. Sci. USA 95, 9558–9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanabe, K., Okuya, S., Tanizawa, Y., Matsutani, A. & Oka, Y. (1997) Biochem. Biophys. Res. Commun. 241, 765–768. [DOI] [PubMed] [Google Scholar]
- 28.Dinarello, C. A. (2000) N. Engl. J. Med. 343, 732–734. [DOI] [PubMed] [Google Scholar]
- 29.Seckinger, P., Williamson, K., Balavoine, J. F., Mach, B., Mazzei, G., Shaw, A. & Dayer, J. M. (1987) J. Immunol. 139, 1541–1545. [PubMed] [Google Scholar]
- 30.Arend, W. P. & Guthridge, C. J. (2000) Ann. Rheum. Dis. 59 Suppl 1, I60–I64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabay, C., Dreyer, M., Pellegrinelli, N., Chicheportiche, R. & Meier, C. A. (2001) J. Clin. Endocrinol. Metab. 86, 783–791. [DOI] [PubMed] [Google Scholar]
- 32.Meyer, K., Irminger, J. C., Moss, L. G., De Vargas, L. M., Oberholzer, J., Bosco, D., Morel, P. & Halban, P. A. (1998) Diabetes 47, 1974–1977. [DOI] [PubMed] [Google Scholar]
- 33.Bosco, D., Meda, P., Halban, P. A. & Rouiller, D. G. (2000) Diabetes 49, 233–243. [DOI] [PubMed] [Google Scholar]
- 34.Ehses, J. A., Pelech, S. L., Pederson, R. A. & McIntosh, C. H. (2002) J. Biol. Chem. 277, 37088–37097. [DOI] [PubMed] [Google Scholar]
- 35.Laud, K., Gourdou, I., Pessemesse, L., Peyrat, J. P. & Djiane, J. (2002) Mol. Cell Endocrinol. 188, 219–226. [DOI] [PubMed] [Google Scholar]
- 36.Danno, H., Jincho, Y., Budiyanto, S., Furukawa, Y. & Kimura, S. (1992) J. Nutr. Sci. Vitaminol. (Tokyo) 38, 517–521. [DOI] [PubMed] [Google Scholar]
- 37.Unger, R. H. & Grundy, S. (1985) Diabetologia 28, 119–121. [DOI] [PubMed] [Google Scholar]
- 38.Weir, G. C., Clore, E. T., Zmachinski, C. J. & Bonner-Weir, S. (1981) Diabetes 30, 590–595. [DOI] [PubMed] [Google Scholar]
- 39.Heitmeier, M. R., Arnush, M., Scarim, A. L. & Corbett, J. A. (2001) J. Biol. Chem. 276, 11151–11158. [DOI] [PubMed] [Google Scholar]
- 40.Donath, M. Y., Storling, J., Maedler, K. & Mandrup-Poulsen, T. (2003) J. Mol. Med. 81, 455–470. [DOI] [PubMed] [Google Scholar]
- 41.Meier, C. A., Bobbioni, E., Gabay, C., Assimacopoulos-Jeannet, F., Golay, A. & Dayer, J. M. (2002) J. Clin. Endocrinol. Metab. 87, 1184–1188. [DOI] [PubMed] [Google Scholar]
- 42.Marculescu, R., Endler, G., Schillinger, M., Iordanova, N., Exner, M., Hayden, E., Huber, K., Wagner, O. & Mannhalter, C. (2002) Diabetes 51, 3582–3585. [DOI] [PubMed] [Google Scholar]
- 43.Spranger, J., Kroke, A., Mohlig, M., Hoffmann, K., Bergmann, M. M., Ristow, M., Boeing, H. & Pfeiffer, A. F. (2003) Diabetes 52, 812–817. [DOI] [PubMed] [Google Scholar]
- 44.Bjorbaek, C., Buchholz, R. M., Davis, S. M., Bates, S. H., Pierroz, D. D., Gu, H., Neel, B. G., Myers, M. G., Jr., & Flier, J. S. (2001) J. Biol. Chem. 276, 4747–4755. [DOI] [PubMed] [Google Scholar]
- 45.De Marinis, L., Bianchi, A., Mancini, A., Gentilella, R., Perrelli, M., Giampietro, A., Porcelli, T., Tilaro, L., Fusco, A., Valle, D., et al. (2004) J. Clin. Endocrinol. Metab. 89, 174–180. [DOI] [PubMed] [Google Scholar]
- 46.Koyama, K., Chen, G., Wang, M. Y., Lee, Y., Shimabukuro, M., Newgard, C. B. & Unger, R. H. (1997) Diabetes 46, 1276–1280. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi, M., Funahashi, T., Shimomura, I., Miyaoka, K. & Matsuzawa, Y. (1996) Horm. Metab. Res. 28, 751–752. [DOI] [PubMed] [Google Scholar]
- 48.Van, H., V, Reynisdottir, S., Eriksson, P., Thorne, A., Hoffstedt, J., Lonnqvist, F. & Arner, P. (1998) Diabetes 47, 913–917. [DOI] [PubMed] [Google Scholar]