Abstract
Early-onset familial Alzheimer's disease is the most aggressive form of Alzheimer's, striking patients as early as their 30s; those patients typically carry mutations in presenilin-1 and presenilin-2. To investigate the coordinated functions of presenilin in the adult brain, we generated double knockout mice, in which both presenilins were deleted in the forebrain. We found that concurrent loss of presenilins in adulthood resulted in massive cortical shrinkage, atrophy of hippocampal molecular layers and corpus callosum, and enlargement of the lateral and third ventricles. We further revealed that deficiency of presenilins caused a series of biochemical alterations, including neuronal atrophy, astrogliosis, caspase-3-mediated apoptosis, and tau hyperphosphorylation. Thus, our study demonstrates that presenilins are essential for the ongoing maintenance of cortical structures and function.
Alzheimer's disease (AD) is a degenerative disease of the CNS (1), currently affecting >25 million people worldwide. Among various subtypes of AD, early-onset familial AD is known to be the most aggressive form, striking patients as early as their 20s or 30s (2, 3). The clinical symptoms of these patients include accelerated onset of memory loss and dementia and progressive impairment in problem solving, language, and other cognitive abilities. In late stages, patients exhibit greatly reduced physical function and are often incontinent, mute, and incapable of caring for themselves. The terminal brains of AD patients are usually hallmarked by accumulations of senile plaques and neurofibrillary tangles, selective shrinkage of cortical and hippocampal tissues, and enlargement of lateral and third ventricle volume (4–6).
Discovery of mutations in the presenilin-1 (PS1) and presenilin-2 (PS2) genes in patients with early-onset AD pathogenesis in the mid1990s marked the beginning of the molecular analysis of this devastating subform of the disease (3, 7–10). The PS1 gene encodes a transmembrane protein with γ-secretase activity important for the proteolytic processing of Notch, amyloid precursor protein, and several other proteins (11, 12). In addition, it interacts directly with proteins involved in apoptosis (13, 14), tau hyperphosphorylation (15), and neuronal cell adhesion (16). Genetic linkage analysis also revealed a second presenilin gene, PS2, located on chromosome 1 (17, 18). PS1 and PS2 share ≈60% homology in amino acid sequence and are expressed ubiquitously in a variety of tissues, including in the brain. In general, PS1 is expressed at a relatively higher level than PS2.
Currently, little information is available regarding the functional relationship and in vivo coordination between these two presenilin genes in the adult brain; it is also not clear whether those mutations exert deleterious effects through a gain-of-function or loss-of-function mechanism. Genetic manipulations of Alzheimer's genes represent a valuable approach to the study of the molecular basis underlying the disease process (19–21). Because the conventional knockout of PS1 resulted in prenatal death (22, 23), we and other researchers used Cre/loxP-mediated conditional knockout techniques to study the function of PS1 in the adult CNS (24, 25). We found that forebrain-specific PS1 knockout mice are mostly normal, except that they exhibit a reduced enrichment-induced neurogenesis in the dentate gyrus (24). These observations have led us to speculate that the minor phenotype in the forebrain-specific PS1 knockout may be due to the genetic compensation of the PS2 gene, a closely related homologue of PS1 (3).
Unlike the perinatal lethality of global PS1 knockout mice, PS2 knockout mice are viable, fertile, and exhibit no obvious phenotype (26). However, when PS2 knockout mice were crossed into conventional PS1 knockout mice, complete deletion of both PS1 and PS2 genes led to more severe embryonic lethality at embryonic day 9.5 (26), indicating that PS1 and PS2 are critical for normal embryonic development. Unfortunately, embryonic lethality of the conventional knockouts has also excluded the investigation of functional roles of presenilins in the adult brain. Here, we report that double deletion of presenilins in adulthood causes massive forebrain degeneration.
Materials and Methods
Production and Genotyping of PS1 and PS2 Double Knockout Mice (dKO). Conditional dKO were produced by crossing the forebrain-specific PS1 heterozygous knockout mice (24) with conventional PS2 heterozygous knockout mice (26). dKO (on the BCF background) carry the Cre transgene, homozygous floxed with PS1 and homozygous PS2–/–genes. Their sibling nontransgenic (no Cre) and heterozygous littermates (fPS1/+ and PS2+/+ or fPS1/+ and PS2+/–) served as controls. Genotyping of the floxed PS1 allele was conducted by both Southern blot and PCR methods, as reported (26, 27). For detailed procedures, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Behavioral and Physical Measurement. The mice used for the measurement of body weight and locomotion experiments were between 10 and 12 months old. The experimental protocol for novel object recognition task was the same as described (28, 29). Student's t test was used to determine genotype effects, and data were calculated as mean ± SEM.
Western Blot and Histology. Forebrain tissues, primarily cortex, and hippocampus were dissected from several mice per line and pooled for Western blot analysis. For histological analysis, brains were removed, fixed, and then sliced into 25-μm sections by using a cryostat. The stained sections were examined under an Olympus (Melville, NY) fluorescence microscope (BX-60) and collected by imagepro software (www.mediacy.com).
Results
Production and Basic Characterization of PS1 and PS2 dKO. We generated conditional dKO (Fig. 1 a and b) by crossing our previously produced forebrain excitatory neuron-specific PS1 knockout mice (24) with conventional PS2 knockout mice (26). These conditional dKO are viable, exhibit normal growth during the postnatal period, and mate normally. This is consistent with the fact that Cre/loxP-mediated knockout of the PS1 gene does not reach completion in the cortex until at least 6 months or older (24), and PS2 global knockout produced no obvious phenotype (26). By 10 months of age, our dKO started to show reduced body weight (Fig. 1c) and exhibited overt abnormal behaviors, exemplified by dichotomous distribution in the open-field locomotion test (Fig. 1d) and impaired performance in novel object recognition, a form of the visual memory test that assesses the structural and functional integrity of the cortex and hippocampus (28, 30) (Fig. 1e).
Fig. 1.
Production and basic characterization of conditional forebrain-specific dKO. (a) A general strategy for making PS1 and PS2 dKO. (b) PCR detection of Cre transgene, floxed PS1 alleles, and PS2–/–knockout alleles. The PCR band size (bp) is shown on the right. (c) dKO (at 10 months old) showed a significant reduction in their body weights. (d) The scatterplot shows that these dKO exhibited a dichotomous distribution in their locomotion during the open-field test (measured in total numbers of squares crossed in 3 min). (e) dKO were also severely impaired in performance in the 1-hr novel object recognition tests in comparison to recognition of new objects by the controls. Fifty percent represents the chance level performance. Data were calculated as mean ± SEM.
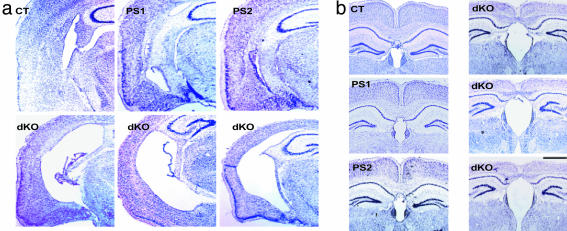
Forebrain Cortical Shrinkage in dKO Brains. To search for the neurological basis of these abnormalities, we used Nissl staining to examine the anatomy of mutant brains. We found that dKO exhibited several hallmark features of the degenerating brain restricted to forebrain areas (Fig. 2 a–c). First, dKO brains had greatly reduced cortical thickness. On average, the cortex in dKO is approximately only half the normal thickness (also see high magnification, Fig. 3 a and c). This is in stark contrast to the apparently normal cortical structure and thickness in mice carrying a single null mutation of PS1 or PS2 (Fig. 2d). At the higher magnification, it is further evident that the classic six layers in the cortex, typically seen in control mice, are no longer obvious in the dKO cortex (Fig. 3a). On the other hand, consistent with the forebrain-specific dKO of PS1 and PS2, the cerebellum in the dKO appears indistinguishable from control brains (Fig. 2c).
Fig. 2.
Forebrain degeneration in conditional dKO. Control mice (CT), conditional PS1 single knockout (PS1), or conventional PS2 single knockout (PS2) mice show normal brain morphology. (a–c) Representative coronal sections show forebrain-specific degeneration (a from the frontal and b from the middle section) but normal cerebellum (c). [Bar (a–c) = 1,000 μm.] (d) PS1 and PS2 dKO caused cortical degeneration. Representative brain sections from two individual control littermates (CT), conditional PS1 single knockout (PS1), and conventional PS2 single knockout (PS2). In contrast, the conditional dKO consistently exhibited drastic reduction in the thickness of the cortex. Representative sections from four different dKO are shown here. (Bar = 400 μm.)
Fig. 3.
Forebrain degeneration in the dKO is evident at higher magnification. (a) Cortical shrinkage caused by concurrent loss of both presenilins. Six layers in the control cortex (CT) are marked on the left; the molecular layers between the CA1 pyramidal cells and dentate gyrus granule cells are indicated by the arrow (ML). (Bar = 100 μm.) (b) Reduction in thickness of the CC in dKO at high magnification. (Bar = 50 μm.) (c) Measurement of cortical thickness in control and dKO. There is a significant difference between the controls and dKO (**, P < 0.01). (d) Histogram showing a significant difference in the thickness of hippocampal molecular layers between the controls and dKO (**, P < 0.01). (e). Reduction in thickness of CC in dKO brains (**, P < 0.01). Controls, n = 9; dKO, n = 7. Data were calculated as mean ± SEM. Student's t test was used to determine statistical significance.
Second, although the principal hippocampal neurons did not appear to overtly differ in terms of their numbers in comparison to that of controls, the thickness of the molecular layers between CA1 pyramidal cells and dentate gyrus was significantly reduced in the dKO (Fig. 3 a and d). Third, cortical degeneration is also evident in the reduction in thickness of the corpus callosum (CC). In fact, the double knockout CC is only about one-third that of controls (Fig. 3 b and e).
Enlargement of the Lateral and Third Ventricles in the dKO Brain. In addition to massive cortical and hippocampal shrinkage, we also found that dKO brains showed an immense enlargement of the lateral ventricles (Fig. 4a). At similar longitude axis sections, brains from dKO showed massive enlargement of the forebrain lateral ventricles in comparison to those of wild-type mice. Importantly, this ventricle enlargement was specific to the dKO genotype, because neither PS1 nor PS2 single knockout brains had such enlargements. From detailed examination of those sections, it seems ventricle enlargements were accompanied by a significant loss of surrounding brain tissue, including the external and internal capsules as well as the fimbria at the tip of the hippocampal CA3 region.
Fig. 4.
Dramatic enlargement of ventricles in dKO brains. (a) Concurrent loss of both presenilins leads to enlarged lateral ventricles in adult conditional dKO. Representative sections from three different individual dKO are shown here. (b) Concurrent loss of both PS1 and PS2 in adulthood also leads to enlargement of the third ventricle. Light microscopic images show representative sections from three different individual dKO brains. One representative section from control (CT), PS1, or PS2 single knockout is shown. (Bar = 1,000 μm.)
Similarly, the third ventricle in dKO brains also exhibited massive enlargement (Fig. 4b), which, in fact, was closely accompanied by dramatic reduction in the thickness of the CC bundle. Also, the enlargement of the third ventricle was specific to the dKO of PS1 and PS2, because PS1 or PS2 single knockout brains had indistinguishable ventricle volume in comparison to that of controls.
Our observations above suggest that the coordinated functions of PS1 and PS2 are essential for ensuring the maintenance of preestablished cortical architectures in the adult brain. The concurrent loss of both presenilins in the forebrain causes devastating degeneration in these areas.
Biochemical Signs of Neuronal Atrophy and Astrogliosis. To examine the molecular basis underlying the above neuropathology, we conducted a series of experiments using biochemical and immunohistological techniques. We asked whether PS1 and PS2 deficiency produced neuronal atrophy. We approached this question by examining the expression levels of well known neuronal markers. First, we used a specific antibody to assess the expression of microtubule-associated protein 2 (MAP2), a major neuronal marker for dendritic processes. Indeed, there is a significant decrease in MAP2 protein expression in conditional dKO brains compared with controls (Fig. 5a Left). The down-regulation of MAP2 expression is further illustrated by immunostaining of MAP2 in the cortical sections. Although control brain sections showed well organized dendritic processes projecting within the region, the dKO did not have such streaks and exhibited only fragmented signals (Fig. 5a Right).
Fig. 5.
Neuronal atrophy and astrogliosis in conditional dKO brains. (a) Expression of a typical neuronal maker, MAP2 protein, was significantly decreased in dKO forebrains (Western blot, Left). Immunostaining (Right) reveals the fragmented and lower expression of MAP2 in the cortex of dKO, in sharp contrast to obvious dendritic processes in the controls. (b) The expression level of the second neuronal marker, NeuN, was also specifically decreased in dKO brains [Western blot (Left) and immunostaining of cortex (Right)]. (c) Concurrent loss of both presenilins caused a significant increase in tau phosphorylation. AT8 antibody was used to probe hyperphosphorylation. (d) Excessive production of glial fibrillary acidic protein in the brains of dKO. The numbers to the right of each Western blot indicate the size of the protein. The same amount of proteins was loaded by examination of PSD95 expression and several other proteins (data not shown). (Bar = 20 μm.)
Neuronal atrophy was further confirmed by our examination of a second major neuronal marker, NeuN, a nuclear protein specifically expressed in the neurons. We found that the expression of NeuN was also greatly reduced in conditional dKO brains as measured by both Western blot (Fig. 5b Left) and immunostaining (Fig. 5b Right). Interestingly, we observed no significant decrease in either the NMDAR1 subunit or PSD95 level in the same dKO brains (data not shown). These results suggest that disabling of PS1 and PS2 functions in the adult brain has selectively resulted in neuronal atrophy with a pronounced reduction of neuronal markers.
It has been reported that PS1 mutations are associated with an elevated level of tau phosphorylation in the brain (31, 32), and presenilins may interact directly with tau and its kinase (15). However, there are somewhat conflicting reports on the role of presenilins in regulating tau phosphorylation (33). Therefore, we examined whether there was any change in tau phosphorylation in dKO brains. Indeed, our Western blot analysis revealed that significant tau hyperphosphorylation is mostly evident in dKO tissues (Fig. 5c Left), and this elevation in AT8-recognizing sites was also observed in immunostaining (Fig. 5c Left).
Reactive astrogliosis is known to serve as yet another feature of pathogenesis in AD brains (34). To assess whether the observed neuronal atrophy in the dKO may have triggered possible astrogliosis, we measured the expression level of glial fibrillary acidic protein (GFAP), a major sign of astrogliosis (35). We observed that the level of GFAP was dramatically elevated in the forebrains of the dKO (Fig. 5d Left). Similarly, immunostaining also showed much stronger GFAP staining in the astroglial cells (Fig. 5d Right). These biochemical and cellular assessments above all pointed to the existence of classic signs of neuronal atrophy and astrogliosis in dKO brains.
Activation and Alteration in Apoptosis Pathways. To further investigate the molecular and cellular basis underlying observed forebrain degeneration, we examined the occurrence of apoptosis in those dKO. Apoptosis is thought to be a factor contributing to neuronal degeneration in AD (7, 36, 37). In fact, presenilins appeared to interact with many proteins involved in apoptotic pathways (13, 38). Furthermore, it has been reported that presenilins could change the sensitivity of cultured cells to artificially induced apoptosis (13, 14, 38–42).
To determine whether in vivo cortical degeneration in dKO involved apoptosis, we used the terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL) staining method to stain brain sections. We observed a drastic increase in TUNEL-positive neurons in the forebrains of the double mutant mice (Fig. 6), in stark contrast to the nearly absent signals in control brains. We further used a set of biochemical assays to determine the alterations of apoptotic signaling in dKO brains. Because caspase-3 is a key down-stream protease essential for apoptosis (43), we examined whether the degenerating brains of the dKO contained any cleavage of caspase-3, a reliable sign of the activation of apoptosis. Our Western blot showed that dKO brains contain a significant amount of the cleaved caspase-3 (Fig. 6 Top Right). Interestingly, caspase-9, a protease involved in the upstream regulation of apoptosis, did not show any significant changes (data not shown), indicating that presenilin alteration seems to cause apoptosis by selective activation of caspase-3-dependent, but caspase-9-independent, mechanism (44).
Fig. 6.
Apoptosis in the conditional dKO brains. (a) Double labeling with NeuN and terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling staining identified some neurons in the dKO brain are undergoing apoptosis. (b) Biochemical measurements further revealed the activation of the caspase-3 pathway (an activated caspase-3 fragment is indicated by the arrow). Meanwhile, some known antiapoptotic factors, such as XIAP (X-linked inhibitor of apoptosis protein) and CIAP (cellular inhibitor of apoptosis protein), are also elevated in dKO brains. Interestingly, Bcl2 are no longer changed by this age. Arrows and numbers (on the left) indicate size of proteins. (Bar = 10 μm.)
It has been reported that antiapoptotic factors often tend to be up-regulated in Alzheimer's brains, apparently as a compensatory response to increased apoptosis. Because presenilins directly interact with Bcl-2, a key antiapoptotic regulator in mitochondria, we investigated whether the concurrent loss of PS1 and PS2 in the adult brain would alter the expression of Bcl-2. We observed there was no significant change in Bcl-2 protein level in dKO brains by the time the cortex reached the degenerative stage (10 months of age) (Fig. 6b). Interestingly, at 6 months of age, we saw a significant increase of Bcl-2 (data not shown), indicating apoptotic dysfunctions at earlier stages. However, when we checked for another major antiapoptotic regulator, X-linked inhibitor of apoptosis protein (XIAP), it was found there was still significant up-regulation of XIAP expression in degenerating dKO brains (Fig. 6b). Similarly, there was also an increase in cellular inhibitor of apoptosis protein (CIAP), the third antiapoptotic regulator. Therefore, our biochemical results suggest significant alterations of the apoptotic signaling process in dKO brains.
Discussion
By applying a conditional genetic strategy, we have produced viable dKO in which both PS1 and PS2 were deleted in the forebrain regions and then analyzed the genotypic consequences of concurrent loss of both presenilins in the adult brain. A major finding of our current study is that the coordinated functions between PS1 and PS2 are essential for ongoing maintenance of adult cortical structure and function in the brain. The second finding is that PS1 and PS2 proteins have synergistic or overlapping activities in vivo, despite the fact there is only 60% homology in amino acid sequence. Moreover, the deficiency of presenilins, or the “loss-of-function” mechanism, could contribute at least partially to the pathogenesis of some of the degenerative pathologies in early-onset AD patients carrying PS1 or PS2 mutations.
This is a particularly important issue, because nearly all mutations in the presenilin genes are missense mutations. The lack of mutations in causing frame-shift or truncation of the ORF has often led to the notion that a “gain-of-function” mechanism by presenilin mutations may be the leading explanation for the molecular pathogenesis of early-onset AD. Our evidence here suggests loss of function in presenilins is capable of causing brain degeneration. However, it is still possible that the gain-of-function mechanism can contribute to some aspects of disease pathogenesis. Nonetheless, the massive degenerations caused by the loss of presenilins raise a cautionary note for a current therapeutic strategy based on the blockade of presenilin activity.
From our previous work (24), Cre/loxP-mediated knockout in PS1 did not occur in the cortex in any significant way until 3 mo of age and only then gradually spread to cortical excitatory neurons by 6 mo of age or older. Indeed, at the age of 10 mo, the dKO showed reduced body weights and overt behavioral differences in the most basic tests, such as open-field locomotion and novel object recognition. Those overt physical and behavioral alterations clearly point to the existence of structural abnormalities in mutant brains. Therefore, we focused our analysis on histological and cellular characterizations in an effort to detect underlying abnormalities in brain structures.
From the series of histological examinations, we revealed that PS1 and PS2 genes are essential for maintaining adult neuronal structure in the mouse forebrain, and the loss of both presenilins disrupted normal intracellular signaling processes and resulted in massive shrinkage in cortical thickness and reduction in the CC bundles and the hippocampal molecular layer, as well as drastic enlargement in both the lateral and third ventricles.
Traditionally, investigations of pathologies of AD-like symptoms in transgenic mice have been mostly focused on the relationship between genetic mutations and formations of senile plaques and neuronal tangles. Cortical shrinkage and ventricle enlargement, two additional signs of terminal neurodegeneration in the aged brain, have not been investigated with the same intensity as for senile plaques and neuronal tangles. However, there is an increased effort in the study of the molecular and cellular mechanisms underlying cortical tissue loss. A recent study (35) reports that aberrant Cdk5 activation by p25 triggers pathological events leading to neurofibrillary tangles that are also accompanied by a loss in cortical volume. Intriguingly, there is no apparent change in ventricle volume in those transgenic mice. These findings, along with our observations, indicate the possible convergence or overlap in molecular mechanisms between the tau and presenilin signaling pathways. In support of this interpretation, Takashima et al. (15) showed that PS1 associates with glycogen synthase kinase-3β and its substrate tau (15). Indeed, our biochemical experiments demonstrated that dKO brains contained a significantly elevated tau hyperphosphorylation, indicating close presenilin–tau interactions in maintaining normal cortical structures. At present, it is clear neither how these two biochemical pathways interact and regulate each other nor how the alteration in one pathway affects the other. In addition, at the histological level, it will be interesting to examine the precise relationship between presenilin dysfunction and the formation of plaques and tangles. It would also be interesting to examine the interaction between presenilins and plaques.
At the biochemical and cellular levels, we have found a set of characteristic alterations, such as neuronal atrophy, astrogliosis, and aberrant neuronal processes, all of which can contribute to and accelerate neural tissue degeneration. Furthermore, the increased apoptosis in the dKO brain may also play a significant role in causing loss of forebrain tissues. In the literature, there is increasing evidence for the role of apoptosis in AD (7, 36, 37, 41). In fact, several studies report direct interaction between presenilin and apoptosis-related proteins (13, 14, 38, 39, 42). Our biochemical experiments have revealed a deficiency of presenilin-activated apoptosis by a selective activation of a caspase-3-dependent, but caspase-9-independent, mechanism. Furthermore, in the accompanying activation of apoptosis, we also observed that antiapoptotic factors, such as XIAP and CIAP, were up-regulated in dKO brains, indicating a compensatory response to increased apoptosis in those tissues. One future experiment will be to further establish the direct biochemical steps linking presenilins to caspase-3 activation (43).
Finally, because presenilins are known to interact with many other pathways, including amyloid precursor protein processing, Notch signaling, and neuronal cell adhesion (11, 12, 16), it will be very important in the future to systematically examine whether and how those signaling pathways may interact with or interfere in the absence of presenilin functions. Do some of those pathways engage in a parallel or a positive-feedback interactive loop, or both, to accelerate cortical degeneration? The exact sequence and relationship of those biochemical interactions will likely provide insight into AD pathogenesis as well as better strategies for the therapeutic intervention of AD.
Supplementary Material
Acknowledgments
We thank Vilma Zolynas and her staff for the maintenance of our mice and Noel Hunt for secretarial assistance. We also thank Drs. Miles Miller, Bryce Sopher, and George Martin for the wonderful collaborations on PS1 knockout; we also deeply appreciate the generosity of Drs. Dorit Donoviel and Alan Bernstein (Mount Sinai Hospital, Toronto) for providing PS2 knockout mice and the stimulating discussion with Sam Sisodia and Philip Wong. This research was supported in part by grants from the Keck Foundation, the National Institutes of Health (J.Z.T.), the Ministry of Science and Technology of China's “973 Preproject” and “973 Project,” and the Shanghai Municipal Administrations of Science and Technology. J.Z.T. is a Beckman, Burroughs Welcome Fund and a Keck Distinguished Scholar.
Abbreviations: PS1, presenilin-1; PS2, presenilin-2; AD, Alzheimer's disease; CC, corpus callosum; dKO, double knockout mice.
References
- 1.Alzheimer, A. (1907) Allg. Z. Psychiatr. Psych.-Gerichtl. Med. 64, 146–148. [Google Scholar]
- 2.Campion, D., Flaman, J. M., Brice, A., Hannequin, D., Dubois, B., Martin, C., Moreau, V., Charbonnier, F., Didierjean, O., Tardieu, S., et al. (1995) Hum. Mol. Genet. 4, 2373–2377. [DOI] [PubMed] [Google Scholar]
- 3.St. George-Hyslop, P. H. (2000). Sci. Am. 76–84. [DOI] [PubMed]
- 4.McKhann, G., Drachman, D., Folstein, M., Katzman, R. Price, D. & Stadlan, E. M. (1984) Neurology 34, 939–944. [DOI] [PubMed] [Google Scholar]
- 5.Roses, A. D. (1996) Curr. Opin. Neurobiol. 6, 644–650. [DOI] [PubMed] [Google Scholar]
- 6.Regeur, L. (2000) Eur. J. Neurol. 7, 47–54. [DOI] [PubMed] [Google Scholar]
- 7.Haass, C. (1997) Neuron 18, 687–690. [DOI] [PubMed] [Google Scholar]
- 8.Sherrington, R., Rogaev, E. I., Liang, Y., Rogaeva, E. A., Levesque, G., Ikeda, M., Chi, H., Lin, C., Li, G., Holman, K., et al. (1995) Nature 375, 754–760. [DOI] [PubMed] [Google Scholar]
- 9.Hardy, J. (1997) Trends Neurosci. 20, 154–159. [DOI] [PubMed] [Google Scholar]
- 10.Czech, C., Tremp, G. & Pradier, L. (2000) Prog. Neurobiol. 60, 363–384. [DOI] [PubMed] [Google Scholar]
- 11.Li, T., Ma, G., Cai, H., Price, D. L. & Wong, P. C. (2003) J. Neurosci. 23, 3271–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfe, M. S. (2002) Int. Immunopharmacol. 2, 1919–1929. [DOI] [PubMed] [Google Scholar]
- 13.Passer, B. J., Pellegrini, L, Vito, P, Ganjei, J. K. & D'Adamio, L. (1999) J. Biol. Chem. 274, 24007–24013. [DOI] [PubMed] [Google Scholar]
- 14.Alves da Costa, C., Mattson, M. P., Ancolio, K. & Checler, F. (2003) J. Biol. Chem. 278, 12064–12069. [DOI] [PubMed] [Google Scholar]
- 15.Takashima, A., Murayama, M., Murayama, O., Kohno, T., Honda, T., Yasutake, K., Nihonmatsu, N., Mercken, M., Yamaguchi, H., Sugihara, S., et al.(1998) Proc. Natl. Acad. Sci. USA 95, 9637–9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Annaert, W. G., Esselens, C., Baert V., Boeve, C., Snellings, Cupers, P., Craessaerts, K. & De Strooper, B. (2001) Neuron 3 2, 579–589. [DOI] [PubMed] [Google Scholar]
- 17.Rogaev, E. I., Sherrington, R., Rogaeva, E. A., Levesque, G., Ikeda, M., Liang, Y., Chi, H., Lin, C., Holman, K., Tsuda, T., et al. (1995) Nature 376, 775–778. [DOI] [PubMed] [Google Scholar]
- 18.Levy-Lahad, E., Wasco, W., Poorkaj, P., Romano, D. M., Oshima, J., Pettingell, W. H., Yu, C. E., Jondro, P. D., Schmidt, S. D., Wang, K., et al. (1995) Science 269, 973–977. [DOI] [PubMed] [Google Scholar]
- 19.Price, D. L. & Sisodia, S. (1998) Annu. Rev. Neurosci. 21, 479–505. [DOI] [PubMed] [Google Scholar]
- 20.Guenett, S. & Tanzi, R. E. (1999) Neurobiol. Aging 20, 201–211. [DOI] [PubMed] [Google Scholar]
- 21.Wong, P. C., Cai, H., Borchelt, D. R. & Price, D. L. (2002) Nat. Neurosci. 5, 633–639. [DOI] [PubMed] [Google Scholar]
- 22.Wong, P. C., Zheng, H., Chen, H., Becher, M. W., Sirinathsinghji, D. J,, Trumbauer, M. E., Chen, H. Y., Price, D. L., Van der Ploeg, L. H. & Sisodia, S. S. (1997) Nature 387, 288–292. [DOI] [PubMed] [Google Scholar]
- 23.Shen, J., Bronson, R. T., Chen, D. F., Xia, W., Selkoe, D. J. & Tonegawa, S. (1997) Cell 89, 629–639. [DOI] [PubMed] [Google Scholar]
- 24.Feng, R., Rampon, C., Tang, Y. P., Shrom, D., Kyin, M., Sopher, B., Miller, M. W., Ware, C. B., Martin, G. M., Kim, S. H., et al. (2001) Neuron 32, 911–926. [DOI] [PubMed] [Google Scholar]
- 25.Yu, H., Saura, C. A., Choi, S. Y., Sun, L. D., Yang, X., Handler, M., Kawarabayashi, T., Younkin, L., Fedeles, B., Wilson, M. A., et al. (2001) Neuron 31, 713–726. [DOI] [PubMed] [Google Scholar]
- 26.Donoviel, D. B., Hadjantonakis, A. K., Ikeda, M., Zheng, H., Hyslop. P. S. & Bernstein, A. (1999) Genes Dev. 13, 2801–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsien, J. Z., Chen, D. F., Gerber, D., Tom, C., Mercer, E. H., Anderson, D. J., Mayford, M., Kandel, E. R. & Tonegawa, S. (1996) Cell 87, 1317–1326. [DOI] [PubMed] [Google Scholar]
- 28.Tang, Y. P., Shimizu, E., Dubem G. R., Rampon, C., Kerchner, G. A., Zhuo, M., Liu, G. & Tsien, J. Z. (1999) Nature 401, 63–69. [DOI] [PubMed] [Google Scholar]
- 29.Rampon, C., Tang, Y. P., Goodhouse, J., Shimizu, E., Kyin, M. & Tsien, J. Z. (2000) Nat. Neurosci. 3, 238–244. [DOI] [PubMed] [Google Scholar]
- 30.Brown, M. W. & Aggleton, J. P. (2001) Nat. Rev. Neurosci. 2, 51–61. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd, C. E., Gregory, G. C., Vickers, J. C., Brooks, W. S., Kwok, J. B., Schofield, P. R., Kril, J. J. & Halliday, G. M. (2004) Neurobiol. Dis. 15, 115–119. [DOI] [PubMed] [Google Scholar]
- 32.Kurt, M. A., Davies, D. C., Kidd, M., Duff, K. & Howlett, D. R. (2003) Neurobiol. Dis. 14, 89–97. [DOI] [PubMed] [Google Scholar]
- 33.Irving, N. G. & Miller, C. C. (1997) Neurosci. Lett. 222, 71–74. [DOI] [PubMed] [Google Scholar]
- 34.Eng, L. F. & Ghirnikar, R. S. (1994) Brain Pathol. 4, 229–237. [DOI] [PubMed] [Google Scholar]
- 35.Cruz, J. C., Tseng, H. C., Goldman, J. A., Shih, H. & Tsai, L. H. (2003) Neuron 40, 471–483. [DOI] [PubMed] [Google Scholar]
- 36.Shimohama, S. (2000) Apoptosis 5, 9–16. [DOI] [PubMed] [Google Scholar]
- 37.Smale, G., Nichols, N. R, Brady, D. R. Finch, C. E. & Horton, W. E., Jr. (1995) Exp. Neurol. 133, 225–230. [DOI] [PubMed] [Google Scholar]
- 38.Wolozin, B., Alexander, P. & Palacino, J. (1998) Neurobiol. Aging 19, S23–S27. [DOI] [PubMed] [Google Scholar]
- 39.Xu, X., Shi, Y. C., Gao, W., Zhao, G., Agrawal, S., Chisolm, G. M., Sui, D. & Cui, M. Z. (2002) J. Biol. Chem. 277, 48913–48922. [DOI] [PubMed] [Google Scholar]
- 40.Zhou, Y., Zhang, W., Easton, R., Ray, J. W., Lampe, P., Jiang, Z., Brunkan, A. L., Goate, A., Johnson, E. M. & Wu, J. Y. (2002) Neurobiol. Dis. 9, 126–138. [DOI] [PubMed] [Google Scholar]
- 41.Popescu, B. O. & Ankarcrona, M. (2000) J. Cell Mol. Med. 4, 249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang, Z., Hartmann, H., Do, V. M., Abramowski, D., Sturchler-Pierrat, C., Staufenbiel, M., Sommer, B., van de Wetering, M., Clevers, H., Saftig, P., et al. (1998) Nature 395, 698–702. [DOI] [PubMed] [Google Scholar]
- 43.Wang, X. (2001) Sci. World J. 1, 49. [Google Scholar]
- 44.Kim, T. W., Pettinggell, W. H., Jung, Y. K., Kovacs, D. M. & Tanzi, R. E. (1997) Science 277, 373–376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.