Abstract
Given the recent emergence of chikungunya in the Americas, the accuracy of forecasting and prediction of chikungunya transmission potential in the U.S. requires urgent assessment. The La Reunion-associated sub-lineage of chikungunya (with a valine substitution in the envelope protein) was shown to increase viral fitness in the secondary vector, Ae. albopictus. Subsequently, a majority of experimental and modeling efforts focused on this combination of a sub-lineage of the East-Central-South African genotype (ECSA-V) – Ae. albopictus, despite the Asian genotype being the etiologic agent of recent chikungunya outbreaks world-wide. We explore a collection of data to investigate relative transmission efficiencies of the three major genotypes/sub-lineages of chikungunya and found difference in the extrinsic incubation periods to be largely overstated. However, there is strong evidence supporting the role of Ae. albopictus in the expansion of chikungunya that our R0 calculations cannot attribute to fitness increases in one vector over another. This suggests other ecological factors associated with the Ae. albopictus-ECSA-V cycle may drive transmission intensity differences. With the apparent bias in literature, however, we are less prepared to evaluate transmission where Ae. aegypti plays a significant role. Holistic investigations of CHIKV transmission cycle(s) will allow for more complete assessment of transmission risk in areas affected by either or both competent vectors.
Introduction
Public health threats from arboviruses have become increasingly problematic for the United States in the last decade. The summer of 2012 saw high transmission intensity of West Nile Virus (WNV), most notably in Dallas, TX [1]. Dengue virus (DENV) has been repeatedly introduced into South Texas, South Florida, and New York [2]–[5]. And while these DENV introductions may result in limited transmission, South Florida has significant transmission events in 2009 when DENV-1 established in Key West [6], [7]. In 2013, a genetically distinct DENV-1 was introduced separately into Martin County and resulted in sustained transmission over the summer months [8].
Even more recent is the emergence of chikungunya (CHIKV) in the Americas. First detected in St. Martin, it quickly spread throughout the Caribbean [7]. The number of cases rapidly grew to the tens of thousands over the course of a handful of months and numerous imported cases have been detected in the U.S. [9]. CHIKV is a relatively recent threat. First identified in Tanzania in the 1950s [10], it has not been as extensively studied as DENV, which shares a similar Ae. aegypti-driven transmission cycle. As such, the accuracy of forecasting and prediction of CHIKV emergence in the Americas requires urgent assessment.
After the outbreak of CHIKV on La Reunion Island in 2006 [11], a variant of the East-Central-South-African (ECSA) genotype was identified with an amino acid substitution on the envelope (E1) at position 226 (alanine to valine, sub-lineages ECSA-A and ECSA-V, respectively) and this mutation was shown to increase the virus’ fitness in a historically secondary vector, Ae. albopictus [12], [13]. This increase in efficiency manifested in a decrease in the extrinsic incubation period (EIP, the time it takes for a mosquito to become infectious after exposure via viremic bloodmeal) in this mosquito species, and was used to explain in part the apparent dominance of the ECSA genotype over the Asian genotype in subsequent outbreaks [14]–[17]. In some of these same epidemics, the E226A sub-lineage persisted in the areas where Ae. aegypti is predominant, indicating that the E226 V-albopictus combination could not completely displace the E226A-aegypti transmission pairing [15].
However, after this shift in transmission ecology and distribution, a large proportion of experimental and modeling efforts focused on the combination of ECSA-V in Ae. albopictus (details in Metadata Results below), despite the Asian genotype being the etiologic agent of recent CHIKV outbreaks in China, the Philippines, Indonesia and the Caribbean [7], [18], [19].
As there is no vaccine available for CHIKV, the focus of intervention efforts will necessarily be on prevention of introductions and emergence in the United States through vector control and avoidance campaigns. An influential component of forecasting or predictive capabilities is our understanding of the efficiency of CHIKV in the two vector species present in the United States, Ae. aegypti and Ae. albopictus without bias. We hypothesized that available, published data did not support the apparent assumption that an increase in fitness of ECSA-V in Ae. albopictus has been the driving factor of transmission differences. Thus, we explore here a collection of data comparing the relative efficiency of the two major genotypes (and sub-lineages of ECSA) of CHIKV in these mosquito species to determine whether there is a meaningful difference among these genetic variants. Detecting any such differences (or lack thereof) will better inform modeling capabilities (heavily biased towards ECSA-V:albopictus), and identify gaps in our CHIKV knowledge base, indicating avenues of future investigation.
Materials and Methods
We utilized Google Scholar and PubMed to search combinations of the following terms: chikungunya, models, transmission, prediction, and mathematical models, which returned 14 articles that directly consider the efficiency of the virus in the vector by explicitly modeling the extrinsic incubation period in CHIKV transmission [20]–[33]. Of these, 71.4% (10/14) focused exclusively on transmission based on the combination of Ae. albopictus and the ECSA-V sub-lineage either directly by informing parameters from experimental data, indirectly by parameterizing models based on the La Reunion outbreak of 2005-6, or both [20]–[26], [29]–[32]. Only one paper looked at CHIKV dynamics in both mosquito species [22] and two papers modeled CHIKV transmission dynamics in Ae. aegypti only [27], [28]. This again highlights the bias towards the La Reunion outbreak dynamics, which is understandable as it was this outbreak that generated the renewed interest in CHIKV.
Considering the bias in the model literature, we next conducted a search on Google Scholar and PubMed using various combinations of the following search terms: chikungunya, vector competence, Aedes aegypti, Aedes albopictus, extrinsic incubation period. The search returned over 130 articles. Some papers did not present data in absolute numeric (as opposed to only ratios), did not identify the day post exposure on which dissemination was evaluated, while others were not included because they utilized recombinant or otherwise genetically modified virus or hybrid mosquitoes, investigated co-infections within the mosquito (viral or parasitic), subjected mosquitoes to extreme temperatures, or investigated other routes of transmission (i.e. venereal). In addition, our exploration was restricted to the two genotypes of CHIKV responsible for the majority of outbreaks: the ECSA and the Asian genotypes for a total of 22 studies included [12], [13], [34]–[53]. These studies infected mosquitoes orally with virus titer ranging from 104.3–8 pfu/ml and incubation temperatures between 26 and 30C. Data was censored for those studies that fed titers between 105.5 and 107.3, but this did not affect distribution fits and thus all 22 studies were included here.
Comparison of viral efficiency in two vector species
Our model framework has been previously published [54] and we used this framework to calculate the basic reproductive number (R0) for each of the scenarios (1). Human infectiousness (viremia curve) and acquisition curves (representing the probability that a mosquito imbibes virus in the bloodmeal) were constructed using data from [55], [56] as in [54]. It is important to note that acquisition does not take into account phenomena that alter the down-stream process of vector competence (e.g., midgut barrier, salivary gland barrier, viral efficiency differences). It more accurately represents vertebrate competence, which we have chosen to hold constant here to focus on these potential viral efficiency differences in the vector. There is no study where mosquitoes have been allowed to feed on CHIK-infected, viremic individuals. Thus, we are assuming that the rate of acquisition is viral type-blind, based on the number of viral particles circulating in the bloodstream, and therefore not significantly different among arboviruses. While this is potentially a large assumption, altering the value of q (should more data provide specific estimates) would result in proportional changes to the R0 values for these combinations of mosquitoes and viral strains. Thus, the interpretation of differences would remain the same. We base our acquisition:viremia rate on DENV-1 from [56]. All calculations and curve fitting were performed in R version 3.0.1 (stats package).
The R0 formulation is given by:
where m = 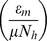 is the mosquito density (mosquitoes/person), a is the biting rate (number of times a female will bite/day), b
−1 is the average EIP of the virus in the mosquito (b is the rate parameter of the cumulative exponential distribution), µ
−1 is the average lifespan of the mosquito, qi is the acquisition of virus by the mosquito (dependent on viremia over interval i), and vi is the length of interval i in days.
is the mosquito density (mosquitoes/person), a is the biting rate (number of times a female will bite/day), b
−1 is the average EIP of the virus in the mosquito (b is the rate parameter of the cumulative exponential distribution), µ
−1 is the average lifespan of the mosquito, qi is the acquisition of virus by the mosquito (dependent on viremia over interval i), and vi is the length of interval i in days.
Model parameters are given in Table 1. To compare viral efficiency in the two species directly, all parameters were held constant except for b−1 and µ−1, which varied according to species of mosquito and genotype and sub-lineages of CHIKV, based on the metadata.
Table 1. Values, definitions and sources for parameters used in modeling efforts.
| Parameter (value) | Definition | References |
| a (.38) | Biting rate | Average for both spp. and held constant [22] |
| b−1 (variable) | Average extrinsicincubation period | Calculated from metadata |
| µ−1 (19.5 days) | Average mosquitolifespanfor Ae. aegypti and Ae. albopictus, | Average for both spp. and held constant [22] |
| m (1.9mosquitoes/human) | Mosquito density | [58] |
| v1–8 (1 day each) | Duration of eachInfectioussubclass (1–8) | [55] |
| q1 (9.07e-01) | Acquisition potentialrelative to viremia | Viremia from [55] and associated acquisition potential calculated from [56] |
| q2 (8.52e-01) | ||
| q3 (2.24e-01) | ||
| q4 (6.20e-02) | ||
| q5 (1.13e-02) | ||
| q6 (4.48e-03) | ||
| q7 (5.73e-03) | ||
| q8 (4.81e-03) |
Data points were divided by mosquito species (Ae. aegypti versus Ae. albopictus) and genotype of CHIKV (Asian versus ECSA). The ECSA genotype was further subdivided based on the mutation at the E226 position. Those strains that did not have the amino acid shift were coded as ECSA-A and those with the La Reunion amino acid shift were coded as ECSA-V.
We fit cumulative exponential distributions to the compiled meta-data by first averaging all points per day post exposure for each lineage-mosquito combination. Default parameterization of vector competence and EIP in models assumes the exponentially distributed EIP, which requires a single rate parameter [20]–[27], [29]–[33]. This rate parameter is the inverse of the average EIP 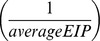 . We estimated specific rates (using the fitdist package and moment matching estimation) for each mosquito-genotype (and sub-lineages where applicable) combination and determined the average rate of transition from the exposed to the infectious class. We then employed a bootstrapping re-sampling (n = 5000 iterations) technique to obtain the 95% confidence intervals of these rates and transformed them to average EIP (in days) and the associated 95% CI.
. We estimated specific rates (using the fitdist package and moment matching estimation) for each mosquito-genotype (and sub-lineages where applicable) combination and determined the average rate of transition from the exposed to the infectious class. We then employed a bootstrapping re-sampling (n = 5000 iterations) technique to obtain the 95% confidence intervals of these rates and transformed them to average EIP (in days) and the associated 95% CI.
Results
Metadata exploration reveals bias in literature (Figures S1–S3)
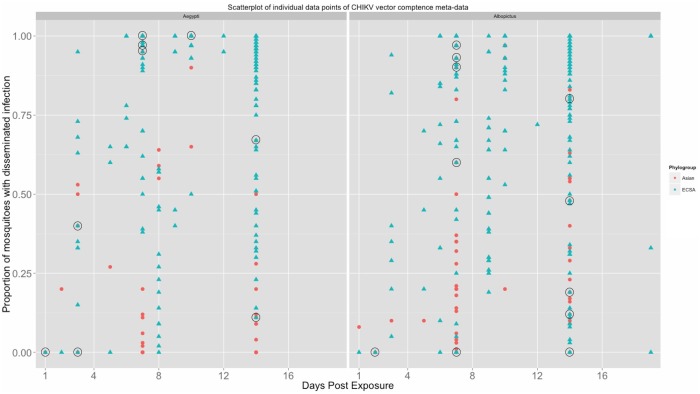
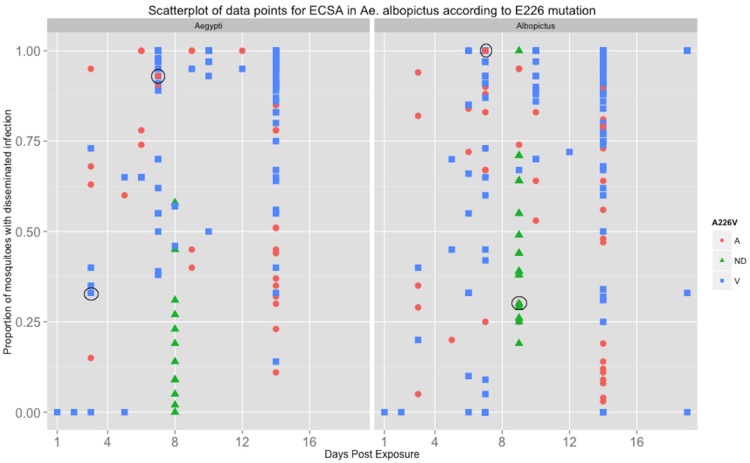
Of the vector competence studies, 22.7% looked at Ae. albopictus only, 18.2% looked at Ae. aegypti only, and 59.1% looked at both mosquito species (Figure S1). With regard to CHIKV genotype, 81.8% of studies investigated the ECSA genotype only while only 1 study looked at the Asian genotype only; however, 13.6% (n = 3) studies studied both genotypes (Figure S2). Sixteen studies (72.7%) investigated ECSA in Ae. aegypti (9/16 ECSA-V only, 2/16 ECSA-A only, 1 not determined, and 4/16 considered both ECSA-A and ECSA-V). Sixteen studies also delineated between ECSA-A and V in Ae. albopictus. Of these 16, 62.5% (10/16) investigated ECSA-V only (all since 2008), 25% considered both sub-lineages and 1 study looked at the ECSA-A sub-lineage only (with 1 study not determined) (Figure S3). Further demonstration the data bias towards the Ae. albopictus ECSA-V combination is displayed in the scatter plot (Figures 1 and 2) of all data points from the 22 papers. Figure 1 shows that the ECSA genotype in general has been more studied than the Asian genotype and Figure 2 shows that within the ECSA genotype, the ECSA-V sub-lineage has received the most attention in both mosquito species.
Figure 1. Scatterplot depicting the data points from mosquito infection experiments the Asian (red dots) versus ECSA (green triangles) genotype where circles indicate an overlap where both Asian and ECSA data point exists.
Figure 2. Scatterplot depicting the data points from mosquito infection experiments ECSA-A (red dots) versus ECSA-V (blue squares) with those data of non-determined lineage (green triangles).
Circles indicate where there is an overlap of at least two sub-lineages.
Also apparent in these figures is the lack of any clear delineation in viral fitness among the genotype/sub-lineages of CHIKV. The scatter along the y-axis (proportion of tested mosquitoes reported with a disseminated infection) does not support the supposition of a significant increase in fitness of the ECSA-V sub-lineage in Ae. albopictus, nor does it indicate there is a clear difference among any combination of genotype/sub-lineage and mosquito vector species.
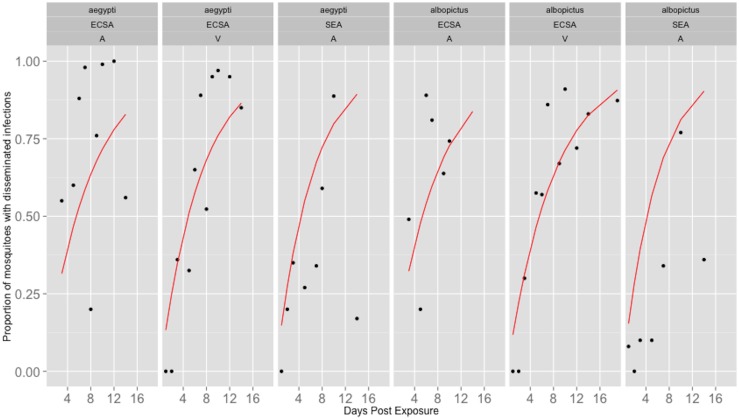
The daily average vector competence from the 22 studies was plotted against to the corresponding day post exposure (Figure 3). The estimated rates of movement from the exposed to infectious classes based on these fits and the confidence intervals from the bootstrap method are given in Table 2. In addition, the back-calculated average EIP and associated confidence intervals are also given in Table 2. These calculated average EIP values range between 5.9 and 8.2 days. Further, the EIP differences do not translate to large differences in epidemic potential, as measured by R0, which range from 7.81–8.49. These results indicate that differences in transmission potential are not significantly affected by the small differences in EIP and are given in Table 3.
Figure 3. The average proportion of mosquitoes with disseminated infections from all 23 studies (black dots) were used to fit the average rate of dissemination, estimated by the cumulative exponential distribution (red line).
Table 2. Estimates of the average rate of transition from Exposed class to Infectious class and corresponding EIP of each (sub) lineage:mosquito combination.
| genotype | Mosquito | Rate (b) (95% CI) | Avg. EIP (b−1) in days (95% CI)* |
| Asian | Ae. aegypti | 0.160 (0.088, 0.364) | 6.25 (2.75, 11.36) |
| ECSA-A | 0.122 (0.069, 0.261) | 8.20 (3.83, 14.50) | |
| ECSA-V | 0.143 (0.086, 0.295) | 6.99 (3.39, 11.63) | |
| Asian | Ae. albopictus | 0.167 (0.098, 0.415) | 5.99 (2.41, 10.20) |
| ECSA-A | 0.130 (0.069, 0.322) | 7.69 (3.11, 14.50) | |
| ECSA-V | 0.125 (0.075, 0.246) | 8.00 (4.07, 13.10) |
*As rate and EIP are inverses, the confidence intervals are also inverses. That is, the lower CI limit for the rate value is the upper CI limit for the EIP value.
Table 3. R0 values calculated based on differences in viral efficiency among (sub) lineage:mosquito combinations.
| Lineage:mosquito Combination | R0 |
| Asian:aegypti | 8.39 |
| ECSA-A:aegypti | 7.81 |
| ECSA-V:aegypti | 8.17 |
| Asian:albopictus | 8.49 |
| ECSA-A:albopictus | 7.96 |
| ECSA-V:albopictus | 7.87 |
Discussion
Published studies of CHIKV vector competence and EIP are skewed towards investigations of the ECSA genotype, which is not surprising given that 81.8% (20/23) of studies were published after the large outbreak on La Reunion [11], where that genotype of CHIKV was purported to have adapted to Ae. albopictus through the A226 V envelope mutation. The bias in subsequent literature is clear, and the assumption has been that Ae. albopictus and the ECSA-V sub-lineage were of primary concern because of the large scale of the La Reunion outbreak and the subsequent spread of the ECSA-V sub-lineage throughout the Indian Ocean and into Southeast Asia [57]. However, in December 2013, it was not this ECSA genotype, but a strain of the Asian genotype that was introduced into the Caribbean and quickly established [7]. For this reason, it is likely that the Asian genotype that poses the most immediate threat to the mainland United States. However, only 4 out of the 22 studies investigated the Asian lineage in either mosquito [35], [48], [50], [53]. Given the apparent insignificant difference in vector efficiency between Ae. aegypti and Ae. albopictus and the widespread distribution of Ae. albopictus in the U.S., the bias in data is potentially misleading.
As vector competence has become increasingly recognized as a dynamic, temporal process [58]–[61], we were interested to determine how many of the experimental studies took this approach when evaluating CHIKV vector competence and EIP. Of the 22 studies, 59.1% (13/22) of the studies assessed vector comp/EIP at a single time point, 18.2% (4/22) had 2 time points, and 22.7% (5/22) had 3 or more time points (Figure S4). If time post exposure was defined as “early” (a week or less) or “late” (anything more than 7 days post exposure), 9.1% (2/22) looked at early only, 77.3% (17/22) looked at late only, and 13.6% looked at both stages (3/22) (Figure S5). Multiple time points are critical when investigating the average rate of transition from exposed to infectious mosquitoes, used to predict and forecast transmission dynamics and potentially inform policy. We also recognize that vector competence varies among populations of mosquitoes of the same species, as demonstrated in [53]. However, we found no discernable pattern when we explored the data delineating on geographic (or ‘colony’, if applicable) origin of the mosquito used in each study (Figure S6). Likely the geographical differences in mosquito competence would be more useful for very spatially detailed model efforts, which our R0 calculations are not. If appropriate, more detailed hypotheses and analyses should, however, make effort to explore this phenomenon.
When we fit the available data as such (assuming an exponentially distributed EIP), we found no meaningful differences in the rates of mosquito transition from exposed to infectious among the genotype/lineage – mosquito species combinations. The ECSA-V:albopictus combination EIP averaged 8 days, which is one of the higher values. This is especially important as most modeling studies focus on this combination and assume a 1–3 day extrinsic incubation period within the mosquito, when in fact this experimental data represents the earliest dissemination point, not the average [21]–[26], [62]. This suggests that the epidemic potential resulting from direct calculations of R0 and the epidemic dynamics from these models may be overestimated as much as 26% (R0[1,3] day EIP = [10.54,9.6]). This would affect not only the estimations of emergence potential, but would likely also alter the estimation of the epidemic intensity and timing. Together, errors in these metrics would affect policy decisions such as resource allocation and timing of vector control measures.
However, the success of the ECSA-V:albopictus combination has been well documented [15]–[17], [63]–[65]. Recently, a study explored the difference in the indirect biting rates of the two vector species, Ae. aegypti and Ae. albopictus [66]. Unsurprisingly, the study found that in urban areas, Ae. aegypti landed on the collector almost 4 times more than Ae. albopictus, while in a suburban area there were no Ae. aegypti and the indirect biting rate of Ae. albopictus was over 15/human/day [66]. Many of the outbreaks of CHIKV have implicated the ECSA-V lineage and Ae. albopictus as the primary virus and vector, respectively. However, examination of the human population affected shows that in many of these outbreaks, the focus of transmission was in suburban areas or peri-rural areas such as rubber plantations [15], [16], [64]. Further, Ae. albopictus has been implicated as the primary vector in places where either it has an ecological-associated advantage over Ae. aegypti [63] or is the only competent Aedes species present, such as in more temperate climates [17]. Thus, it is not necessarily the viral dynamics in albopictus that have driven the CHIKV outbreaks so much as the interactions of the human and mosquito ecologies. For example, the mortality rates of the two mosquitoes species is purported to be different, as well as the biting rates [22], and although there are no consistent estimates of the densities of c0oincident populations of the two Aedes species, studies have shown that there is a large difference in the density of each species relative to the human population, often relying on larval counts or other indirect methods (i.e. landing rates). For Ae. aegypti, a mosquito density was reported and averaged to be 2.23/person (censoring extreme observations) from [67], and the density for Ae. albopictus ranged from.85 to 80/person [68]–[70]. All of these factors may influence the enhancing role of Ae. albopictus in CHIKV transmission, perhaps more so than the efficiency differences.
Of particular interest is the mortality rate of the mosquito, as it is critical to evaluating fitness differences [58], [61], [71], [72]. Here, we assumed an average lifespan of 19.5 days in our R0 calculations, which means that the ratio of lifespan to EIP is relatively large here. Thus, we briefly investigated the role of lifespan in the context of the differences in EIP, and the affect on corresponding disparities in R0. Since only the rate parameter b and µ would change in our calculations (we hold all other R0 parameter constant), we can look at the proportional change in 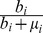 to determine how mortality rates µ−1 and these transition rates (EIP) from exposed to infectious classes modulate the differences in R0.
to determine how mortality rates µ−1 and these transition rates (EIP) from exposed to infectious classes modulate the differences in R0.
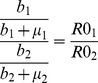 |
For example, the difference in EIP in the Asian:aegypti combination (EIP = 6.25 days) to the ECSA-V:albopictus combination (EIP = 8.00 days) corresponds to an approximate 7% greater R0 value for the Asian:aegypti combination. As the mortality rate of the mosquito is decreased (thus the ratio of lifespan to EIP gets closer to 1), the advantage of the Asian:aegypti combination also increases (comparatively 13% greater R0 value when µ−1 = 7.5 days). Indeed, for the R0 difference between the Asian:aegypti and ECSA-V:albopictus combinations to be less than 5%, the average lifespan of the mosquito(es) would need to be greater than 28.5 days. So while mosquito mortality is paramount to assessing differences in EIP and viral efficiency within the mosquito, likely the other ecological factors are more critical drivers of the transmission intensity attributed to the ECSA-V:albopictus combination.
Conclusions
The 2006 outbreak on La Reunion Island likely was the impetus for renewed and expanded interest in CHIKV. Subsequent discovery of the E226 mutation (A→V) and the reported increased efficiency in Ae. albopictus [12] lead to an apparent bias in the literature, experimental and theoretical alike. This compilation of available experimental CHIKV vector competence data indicates that there is a similar efficiency among the genotypes and sub-lineages in both mosquito species. Thus, the species of mosquito and differences in behavior, habitat, etc. (as parameterized by contact rate with humans) may be the most critical defining factors of transmission intensity. However, there are several apparent differences in the data and transmission model outputs. This prompts several observations:
Genotype (i.e. ECSA, Asian) is likely too coarse a characterization on which to accurately assess transmission intensity, as is the case where closely related DENV strains have been shown to have different transmission results [5].
Most models implicitly assume exponentially distributed EIP, which should be parameterized with the average EIP, not the earliest detection times (as in the case of ECSA-V:albopictus EIP of 1–3 days) [22]–[25].
Relatedly, the cumulative exponential parameterization of EIP is not the best distributional assumption [28], [58], [73] for modeling the transition of mosquitoes from exposed to infectious, as it might be that differences on the fringes of this distribution may be most important.
More focus should be placed on the context of transmission differences (ecological, i.e.), similar to the One Health initiative, which places an emphasis on the interaction of the environment in zoonotic disease transmission.
There is very strong evidence supporting the role of Ae. albopictus in the expansion of CHIKV, especially into more temperate and/or suburban areas [14]–[17]. The clear data bias in the literature, however, does not sufficiently account for the entirety of CHIKV transmission ecology, because there are few experimental or modeling efforts that address the Asian genotype and other sub-lineages in the primary vector Ae. aegypti. This means that we are less prepared to evaluate transmission where Ae. aegypti still play a significant role or where this species is implicated as the primary transmission vector [7]. Indeed, as Ae. aegypti has been implicated in the dengue introduction in Key West, FL and in transmission along the US-Mexican border of Texas [3], this is an issue directly speaking to the public health security of the southern United States.
In summary, it is imperative that experiments account for the utility of time-course data when describing a dynamic process such as vector competence and EIP identification. Models, on the other hand, would benefit from recognizing the context of the biological data and carefully vet the assumptions of parameters developed from these data. Multi-disciplinary efforts and better cross-discipline understanding are key to more accurate forecasts, better informed policy decisions, and ultimately a less at-risk populace.
Supporting Information
The number of vector competence data points (y-axis) for each study (x-axis). The papers are subdivided (colored bars) depending on whether the data was for Ae. aegypti or Ae. albopictus or both.
(TIFF)
The number of vector competence data points (y-axis) for each study (x-axis). Studies are divided (colored bars) depending on whether the data is for the Asian or ECSA genotype.
(TIFF)
The number of vector competence data points (y-axis) for each study (x-axis). The studies are divided (colored bars) based on whether the data corresponds to the ECSA-A (A), ECSA-V (V) sublineage of the ECSA genotype or if the dilineation in the ECSA genotype was not determined (ND).
(TIFF)
The number of vector competence data points (y-axis) for each study (x-axis) divided (colored bars) by the day on which the data point was assessed.
(TIFF)
The number of vector competence data points (y-axis) for each study (x-axis) divided (colored bars) depending on whether the dissemination determination was done during the early stage of infection (≤7 days post exposure) or late stage (>7 days).
(TIFF)
Scatterplot of data points when subdivided by CHIKV genotype (columns) and mosquito species (rows). Color denotes the continental or regional origin of the mosquito strains utilized (or denoted as ‘colony’ if applicable) and shape of the point denotes sub-lineage of ECSA (A or V) or ND (not-determined) if Asian genotype.
(TIFF)
Acknowledgments
The authors would like to further acknowledge Dr. Ann M. Powers, CDC NCEZID for her insights into chikungunya transmission and critical review of this manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Data are from PubMed indexed published papers which have been listed in the references and supplemental information.
Funding Statement
This study was funded by the National Institutes of Health/NIGMS U01GM097661. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Beasley DW, Barrett AD, Tesh RB (2013) Resurgence of West Nile neurologic disease in the United States in 2012: what happened? What needs to be done? Antiviral Res 99: 1–5. [DOI] [PubMed] [Google Scholar]
- 2. Brunkard JM, Robles Lopez JL, Ramirez J, Cifuentes E, Rothenberg SJ, et al. (2007) Dengue fever seroprevalence and risk factors, Texas-Mexico border, 2004. Emerg Infect Dis 13: 1477–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dengue hemorrhagic fever–U.S.-Mexico border, 2005. MMWR Morb Mortal Wkly Rep 56: 785–789. [PubMed] [Google Scholar]
- 4. Imported dengue–United States, 1999 and 2000. MMWR Morb Mortal Wkly Rep 51: 281–283. [PubMed] [Google Scholar]
- 5. Ritchie SA, Pyke AT, Hall-Mendelin S, Day A, Mores CN, et al. (2013) An explosive epidemic of DENV-3 in Cairns, Australia. PLoS One 8: e68137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease C, Prevention (2010) Locally acquired Dengue–Key West, Florida, 2009–2010. MMWR Morb Mortal Wkly Rep 59: 577–581. [PubMed] [Google Scholar]
- 7. Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, de Lamballerie X (2014) Chikungunya in the Americas. Lancet 383: 514. [DOI] [PubMed] [Google Scholar]
- 8.(2013) Florida Health.
- 9. Fischer M, Staples JE, Arboviral Diseases Branch NCfE, Zoonotic Infectious Diseases CDC (2014) Notes from the field: chikungunya virus spreads in the americas - Caribbean and South america, 2013–2014. MMWR Morb Mortal Wkly Rep 63: 500–501. [PMC free article] [PubMed] [Google Scholar]
- 10. Powers AM, Logue CH (2007) Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol 88: 2363–2377. [DOI] [PubMed] [Google Scholar]
- 11.Paquet C, Quatresous I, Solet JL, Sissoko D, Renault P, et al. (2006) Chikungunya outbreak in Reunion: epidemiology and surveillance, 2005 to early January 2006. Euro Surveill 11: E060202 060203. [DOI] [PubMed]
- 12. Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S (2007) A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog 3: e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dubrulle M, Mousson L, Moutailler S, Vazeille M, Failloux AB (2009) Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLoS One 4: e5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh P, Mittal V, Rizvi MA, Bhattacharya D, Chhabra M, et al. (2012) Northward movement of East Central South African genotype of Chikungunya virus causing an epidemic between 2006–2010 in India. J Infect Dev Ctries 6: 563–571. [DOI] [PubMed] [Google Scholar]
- 15. Hapuarachchi HC, Bandara KB, Sumanadasa SD, Hapugoda MD, Lai YL, et al. (2010) Re-emergence of Chikungunya virus in South-east Asia: virological evidence from Sri Lanka and Singapore. J Gen Virol 91: 1067–1076. [DOI] [PubMed] [Google Scholar]
- 16. Ng LC, Tan LK, Tan CH, Tan SS, Hapuarachchi HC, et al. (2009) Entomologic and virologic investigation of Chikungunya, Singapore. Emerg Infect Dis 15: 1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonilauri P, Bellini R, Calzolari M, Angelini R, Venturi L, et al. (2008) Chikungunya virus in Aedes albopictus, Italy. Emerg Infect Dis 14: 852–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Bortel W, Dorleans F, Rosine J, Blateau A, Rousset D, et al. (2014) Chikungunya outbreak in the Caribbean region, December 2013 to March 2014, and the significance for Europe. Euro Surveill 19. [DOI] [PubMed]
- 19.Nasci RS (2014) Movement of Chikungunya Virus into the Western Hemisphere. Emerging Infectious Diseases 20. [DOI] [PMC free article] [PubMed]
- 20. Yakob L, Clements AC (2013) A mathematical model of chikungunya dynamics and control: the major epidemic on Reunion Island. PLoS One 8: e57448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poletti P, Messeri G, Ajelli M, Vallorani R, Rizzo C, et al. (2011) Transmission potential of chikungunya virus and control measures: the case of Italy. PLoS One 6: e18860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manore CA, Hickmann KS, Xu S, Wearing HJ, Hyman JM (2014) Comparing dengue and chikungunya emergence and endemic transmission in A. aegypti and A. albopictus. J Theor Biol 356: 174–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dumont Y, Chiroleu F, Domerg C (2008) On a temporal model for the Chikungunya disease: modeling, theory and numerics. Math Biosci 213: 80–91. [DOI] [PubMed] [Google Scholar]
- 24. Dumont Y, Chiroleu F (2010) Vector control for the Chikungunya disease. Math Biosci Eng 7: 313–345. [DOI] [PubMed] [Google Scholar]
- 25. Dommar CJ, Lowe R, Robinson M, Rodo X (2014) An agent-based model driven by tropical rainfall to understand the spatio-temporal heterogeneity of a chikungunya outbreak. Acta Trop 129: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pongsumpun P (2010) Dynamical Transmission Model of Chikungunya in Thailand. World Academy of Science, Engineering and Technology 4: 969–973. [Google Scholar]
- 27. Pongsumpun P, Sangsawang S (2013) Local Stability Analysis for Age Structural Model of Chikungunya Disease. Journal of Basic and Applied Scientific Research 3: 302–312. [Google Scholar]
- 28. Massad E, Ma S, Burattini MN, Tun Y, Coutinho FA, et al. (2008) The risk of chikungunya fever in a dengue-endemic area. J Travel Med 15: 147–155. [DOI] [PubMed] [Google Scholar]
- 29. Ruiz-Moreno D, Vargas IS, Olson KE, Harrington LC (2012) Modeling dynamic introduction of Chikungunya virus in the United States. PLoS Negl Trop Dis 6: e1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Moor PP, Steffens FE (1970) A computer-simulated model of an arthropod-borne virus transmission cycle, with special reference to Chikungunya virus. Trans R Soc Trop Med Hyg 64: 927–934. [DOI] [PubMed] [Google Scholar]
- 31. Mecoli M, De Angelis V, Brailsford SC (2013) Using system dynamics to evaluate control strategies for mosquito-borne diseases spread by human travel. Computers & Operations Research 40: 2219–2228. [Google Scholar]
- 32. Bacaër N (2007) Approximation of the Basic Reproduction Number R 0 for Vector-Borne Diseases with a Periodic Vector Population. Bulletin of Mathematical Biology 69: 1067–1091. [DOI] [PubMed] [Google Scholar]
- 33. Boelle PY, Thomas G, Vergu E, Renault P, Valleron AJ, et al. (2008) Investigating transmission in a two-wave epidemic of Chikungunya fever, Reunion Island. Vector Borne Zoonotic Dis 8: 207–217. [DOI] [PubMed] [Google Scholar]
- 34. Tesh RB, Gubler DJ, Rosen L (1976) Variation among goegraphic strains of Aedes albopictus in susceptibility to infection with chikungunya virus. Am J Trop Med Hyg 25: 326–335. [DOI] [PubMed] [Google Scholar]
- 35. Turell MJ, Beaman JR, Tammariello RF (1992) Susceptibility of selected strains of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) to chikungunya virus. J Med Entomol 29: 49–53. [DOI] [PubMed] [Google Scholar]
- 36. Mourya DT, Gokhale MD, Malunjkar AS, Bhat HR, Banerjee K (1994) Inheritance of oral susceptibility of Aedes aegypti to Chikungunya virus. Am J Trop Med Hyg 51: 295–300. [DOI] [PubMed] [Google Scholar]
- 37. Vazeille M, Moutailler S, Coudrier D, Rousseaux C, Khun H, et al. (2007) Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS One 2: e1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reiskind MH, Pesko K, Westbrook CJ, Mores CN (2008) Susceptibility of Florida mosquitoes to infection with chikungunya virus. Am J Trop Med Hyg 78: 422–425. [PMC free article] [PubMed] [Google Scholar]
- 39. Vazeille M, Moutailler S, Pages F, Jarjaval F, Failloux AB (2008) Introduction of Aedes albopictus in Gabon: what consequences for dengue and chikungunya transmission? Trop Med Int Health 13: 1176–1179. [DOI] [PubMed] [Google Scholar]
- 40. Moutailler S, Barre H, Vazeille M, Failloux AB (2009) Recently introduced Aedes albopictus in Corsica is competent to Chikungunya virus and in a lesser extent to dengue virus. Trop Med Int Health 14: 1105–1109. [DOI] [PubMed] [Google Scholar]
- 41. Pesko K, Westbrook CJ, Mores CN, Lounibos LP, Reiskind MH (2009) Effects of infectious virus dose and bloodmeal delivery method on susceptibility of Aedes aegypti and Aedes albopictus to chikungunya virus. J Med Entomol 46: 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martin E, Moutailler S, Madec Y, Failloux AB (2010) Differential responses of the mosquito Aedes albopictus from the Indian Ocean region to two chikungunya isolates. BMC Ecol 10: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paupy C, Ollomo B, Kamgang B, Moutailler S, Rousset D, et al. (2010) Comparative role of Aedes albopictus and Aedes aegypti in the emergence of Dengue and Chikungunya in central Africa. Vector Borne Zoonotic Dis 10: 259–266. [DOI] [PubMed] [Google Scholar]
- 44. Talbalaghi A, Moutailler S, Vazeille M, Failloux AB (2010) Are Aedes albopictus or other mosquito species from northern Italy competent to sustain new arboviral outbreaks? Med Vet Entomol 24: 83–87. [DOI] [PubMed] [Google Scholar]
- 45. van den Hurk AF, Hall-Mendelin S, Pyke AT, Smith GA, Mackenzie JS (2010) Vector competence of Australian mosquitoes for chikungunya virus. Vector Borne Zoonotic Dis 10: 489–495. [DOI] [PubMed] [Google Scholar]
- 46. Girod R, Gaborit P, Marrama L, Etienne M, Ramdini C, et al. (2011) High susceptibility to Chikungunya virus of Aedes aegypti from the French West Indies and French Guiana. Trop Med Int Health 16: 134–139. [DOI] [PubMed] [Google Scholar]
- 47. Bellini R, Medici A, Calzolari M, Bonilauri P, Cavrini F, et al. (2012) Impact of Chikungunya virus on Aedes albopictus females and possibility of vertical transmission using the actors of the 2007 outbreak in Italy. PLoS One 7: e28360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dupont-Rouzeyrol M, Caro V, Guillaumot L, Vazeille M, D’Ortenzio E, et al. (2012) Chikungunya virus and the mosquito vector Aedes aegypti in New Caledonia (South Pacific Region). Vector Borne Zoonotic Dis 12: 1036–1041. [DOI] [PubMed] [Google Scholar]
- 49. McTighe SP, Vaidyanathan R (2012) Vector competence of Aedes albopictus from Virginia and Georgia for chikungunya virus isolated in the Comoros Islands, 2005. Vector Borne Zoonotic Dis 12: 867–871. [DOI] [PubMed] [Google Scholar]
- 50. Sam IC, Loong SK, Michael JC, Chua CL, Wan Sulaiman WY, et al. (2012) Genotypic and phenotypic characterization of Chikungunya virus of different genotypes from Malaysia. PLoS One 7: e50476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vazeille M, Yebakima A, Lourenco-de-Oliveira R, Andriamahefazafy B, Correira A, et al. (2013) Oral receptivity of Aedes aegypti from Cape Verde for yellow fever, dengue, and chikungunya viruses. Vector Borne Zoonotic Dis 13: 37–40. [DOI] [PubMed] [Google Scholar]
- 52. Vega-Rua A, Zouache K, Caro V, Diancourt L, Delaunay P, et al. (2013) High efficiency of temperate Aedes albopictus to transmit chikungunya and dengue viruses in the Southeast of France. PLoS One 8: e59716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vega-Rua A, Zouache K, Girod R, Failloux AB, Lourenco-de-Oliveira R (2014) High Level of Vector Competence of Aedes aegypti and Aedes albopictus from Ten American Countries as a Crucial Factor in the Spread of Chikungunya Virus. J Virol 88: 6294–6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Christofferson RC, Mores CN, Wearing HJ (2014) Characterizing the likelihood of dengue emergence and detection in naive populations. Parasit Vectors 7: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Appassakij H, Khuntikij P, Kemapunmanus M, Wutthanarungsan R, Silpapojakul K (2013) Viremic profiles in asymptomatic and symptomatic chikungunya fever: a blood transfusion threat? Transfusion 53: 2567–2574. [DOI] [PubMed] [Google Scholar]
- 56. Nguyet MN, Duong TH, Trung VT, Nguyen TH, Tran CN, et al. (2013) Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A 110: 9072–9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tsetsarkin KA, Chen R, Leal G, Forrester N, Higgs S, et al. (2011) Chikungunya virus emergence is constrained in Asia by lineage-specific adaptive landscapes. Proc Natl Acad Sci U S A 108: 7872–7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Christofferson RC, Mores CN (2011) Estimating the magnitude and direction of altered arbovirus transmission due to viral phenotype. PLoS One 6: e16298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chan M, Johansson MA (2012) The incubation periods of Dengue viruses. PLoS One 7: e50972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Smith DL, Dushoff J, McKenzie FE (2004) The risk of a mosquito-borne infection in a heterogeneous environment. PLoS Biol 2: e368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bellan SE (2010) The importance of age dependent mortality and the extrinsic incubation period in models of mosquito-borne disease transmission and control. PLoS One 5: e10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dumont Y, Tchuenche JM (2012) Mathematical studies on the sterile insect technique for the Chikungunya disease and Aedes albopictus. J Math Biol 65: 809–854. [DOI] [PubMed] [Google Scholar]
- 63. Delatte H, Paupy C, Dehecq JS, Thiria J, Failloux AB, et al. (2008) [Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: biology and control]. Parasite 15: 3–13. [DOI] [PubMed] [Google Scholar]
- 64. Kumar NP, Joseph R, Kamaraj T, Jambulingam P (2008) A226 V mutation in virus during the 2007 chikungunya outbreak in Kerala, India. J Gen Virol 89: 1945–1948. [DOI] [PubMed] [Google Scholar]
- 65. Pages F, Peyrefitte CN, Mve MT, Jarjaval F, Brisse S, et al. (2009) Aedes albopictus mosquito: the main vector of the 2007 Chikungunya outbreak in Gabon. PLoS One 4: e4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Casas Martinez M, Orozco Bonilla A, Munoz Reyes M, Ulloa Garcia A, Bond JG, et al. (2013) A new tent trap for monitoring the daily activity of Aedes aegypti and Aedes albopictus. J Vector Ecol 38: 277–288. [DOI] [PubMed] [Google Scholar]
- 67. Focks DA, Brenner RJ, Hayes J, Daniels E (2000) Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am J Trop Med Hyg 62: 11–18. [PubMed] [Google Scholar]
- 68. Effler PV, Pang L, Kitsutani P, Vorndam V, Nakata M, et al. (2005) Dengue fever, Hawaii, 2001–2002. Emerg Infect Dis 11: 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Carrieri M, Angelini P, Venturelli C, Maccagnani B, Bellini R (2012) Aedes albopictus (Diptera: Culicidae) population size survey in the 2007 chikungunya outbreak area in Italy. II: Estimating epidemic thresholds. J Med Entomol 49: 388–399. [DOI] [PubMed] [Google Scholar]
- 70. Oki M, Yamamoto T (2013) Simulation of the probable vector density that caused the Nagasaki dengue outbreak vectored by Aedes albopictus in 1942. Epidemiol Infect 141: 2612–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Paaijmans KP, Blanford S, Chan BH, Thomas MB (2012) Warmer temperatures reduce the vectorial capacity of malaria mosquitoes. Biol Lett 8: 465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Novoseltsev VN, Michalski AI, Novoseltseva JA, Yashin AI, Carey JR, et al. (2012) An age-structured extension to the vectorial capacity model. PLoS One 7: e39479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chowell G, Fuentes R, Olea A, Aguilera X, Nesse H, et al. (2013) The basic reproduction number R0 and effectiveness of reactive interventions during dengue epidemics: the 2002 dengue outbreak in Easter Island, Chile. Math Biosci Eng 10: 1455–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The number of vector competence data points (y-axis) for each study (x-axis). The papers are subdivided (colored bars) depending on whether the data was for Ae. aegypti or Ae. albopictus or both.
(TIFF)
The number of vector competence data points (y-axis) for each study (x-axis). Studies are divided (colored bars) depending on whether the data is for the Asian or ECSA genotype.
(TIFF)
The number of vector competence data points (y-axis) for each study (x-axis). The studies are divided (colored bars) based on whether the data corresponds to the ECSA-A (A), ECSA-V (V) sublineage of the ECSA genotype or if the dilineation in the ECSA genotype was not determined (ND).
(TIFF)
The number of vector competence data points (y-axis) for each study (x-axis) divided (colored bars) by the day on which the data point was assessed.
(TIFF)
The number of vector competence data points (y-axis) for each study (x-axis) divided (colored bars) depending on whether the dissemination determination was done during the early stage of infection (≤7 days post exposure) or late stage (>7 days).
(TIFF)
Scatterplot of data points when subdivided by CHIKV genotype (columns) and mosquito species (rows). Color denotes the continental or regional origin of the mosquito strains utilized (or denoted as ‘colony’ if applicable) and shape of the point denotes sub-lineage of ECSA (A or V) or ND (not-determined) if Asian genotype.
(TIFF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Data are from PubMed indexed published papers which have been listed in the references and supplemental information.