Abstract
The effects of the adaptive immune system on the cognitive performance and abnormal behaviors seen in mental disorders such as schizophrenia have never been documented. Here, we show that mice deprived of mature T cells manifested cognitive deficits and behavioral abnormalities, which were remediable by T cell restoration. T cell-based vaccination, using glatiramer acetate (copolymer-1, a weak agonist of numerous self-reactive T cells), can overcome the behavioral and cognitive abnormalities that accompany neurotransmitter imbalance induced by (+)dizocilpine maleate (MK-801) or amphetamine. The results, by suggesting that peripheral T cell deficit can lead to cognitive and behavioral impairment, highlight the importance of properly functioning adaptive immunity in the maintenance of mental activity and in coping with conditions leading to cognitive deficits. These findings point to critical factors likely to contribute to age- and AIDS-related dementias and might herald the development of a therapeutic vaccination for fighting off cognitive dysfunction and psychiatric conditions.
Mental disorders are now known to be characterized not only by behavioral abnormalities but also by somatic manifestations. In a number of psychiatric disorders, a neurodegenerative component has been identified. In schizophrenia, for example, there is loss of hippocampal volume and death of hippocampal neurons (1, 2), as well as anatomical and molecular abnormalities of excitatory neurons in the dorsolateral prefrontal cortex (3, 4). The etiology and pathogenesis of schizophrenia are still obscure although there is general agreement that genetic predisposition is a significant factor (5, 6). Symptoms of schizophrenia can be classified as positive, negative, and cognitive, by using standard rating scales such as the Positive and Negative Symptom Scale (7). Positive symptoms include hallucinations, agitation, and paranoia; negative symptoms reflect the loss of interpersonal drive and normal interest in the environment; and cognitive symptoms include conceptual disorganization and disorientation (7). Whereas the positive symptoms usually respond well to dopamine-receptor antagonists (which however have significant and devastating side effects such as induction of Parkinson's disease), the negative symptoms and cognitive deficits typically persist, resulting in chronic morbidity and poor long-term outcome (8).
Until quite recently, the body's principal adaptive responses to stressful stimuli were attributed to the hypothalamic-pituitary-adrenocortical axis (9). An association between the immune system and the cognitive performance, anxiety, and sensorimotor dysfunction seen in mental disorders or acute psychological stress has generally been considered unlikely or of little significance, despite a growing body of evidence suggesting that such an association not only exists, but might be an important consideration in the design of therapy (10). Immune abnormalities have been reported in patients with schizophrenia, and there have been numerous attempts to find a connection between schizophrenia and autoimmune disease. However, studies over the last 60 years aimed at identifying schizophrenia as an autoimmune disease have so far been unsuccessful (11).
A link between brain maintenance and peripheral adaptive immunity was suggested by recent findings that the ability to cope with neurodegenerative conditions depends on CD4+ T cells (12–14, 15). The beneficial effect of CD4+ T cells in fighting off mediators of degeneration was found to be specific to antigens residing in the site of the lesion. Naturally occurring CD4+CD25+ regulatory T cells (Treg) normally suppress the ability to spontaneously evoke this neuroprotective response, which is amenable to boosting by weakening of Treg (16) or by a well controlled vaccination with self-antigens or with weak agonists of self-antigens (17) such as Copaxone [copolymer-1 (Cop-1)].
In this study, we examined whether adaptive immunity plays a role in higher brain functions, both under normal conditions and in the abnormal situation generated by neurotransmitter imbalance. Here, we show that, in the absence of mature T cells, cognition in mice was impaired and could be restored by passive T cell transfer. Moreover, cognitive impairment and behavioral abnormalities caused by pharmacologically induced deficits were significantly counteracted by T cell-based vaccination with Cop-1.
Materials and Methods
Animals. Inbred adult male wild-type C57BL/6J and BALB/c/OLA mice, as well as BALB/c/OLA mice with severe combined immune deficiency (SCID) (due to RAG1/2 knockout) and nude mice (deficient in mature T cells), all 8–12 weeks old, were supplied by the Animal Breeding Center of The Weizmann Institute of Science.
Antigens. Copaxone (Cop-1) is supplied by Teva Pharmaceuticals (Petah Tikva, Israel).
Immunization. Each mouse was injected with a total of 100 μg of Cop-1 emulsified in an equal volume of complete Freund's adjuvant (CFA) containing 5 mg/ml mycobacteria H37 RA (Difco). The emulsion, in a total volume of 0.1 ml, was injected into the flank 1 week before the mouse was first injected with a psychotomimetic drug. Control mice were injected with an equal volume of PBS emulsified in CFA.
Drug Solutions. Fresh solutions of dizocilpine maleate (MK-801; Sigma–Aldrich) were prepared in physiological saline (0.9% NaCl in sterile distilled water) for each batch of mice. Physiological saline was also used as a vehicle for d-amphetamine sulfate (AMPH; Sigma). MK-801 (0.1 mg/kg) or AMPH (2.5 mg/kg) or saline was injected i.p. in a total volume of 5 ml per kg of body weight. Mice were injected with MK-801, AMPH, or vehicle 15 min before being subjected to behavioral tests.
Morris Water Maze (MWM) Behavioral Test. Spatial learning/memory was assessed by performance on a hippocampal-dependent visuo-spatial learning task in the MWM. Mice were given four trials per day, for four consecutive days, to find a hidden platform located 1.5 cm below the water surface in a pool 1.4 m in diameter. Within the testing room, only distal visuo-spatial cues were available to the mice for location of the submerged platform. The escape latency, i.e., the time required by the mouse to find and climb onto the platform, was recorded for up to 60 s. Each mouse was allowed to remain on the platform for 30 s, and was then moved from the maze to its home cage. If the mouse did not find the platform within 120 s, it was manually placed on the platform and returned to its home cage after 30 s. The inter-trial interval was 30 s. On day 5, the platform was removed from the pool, and each mouse was tested by a probe trial for 60 s. On days 6–7 the platform was placed at the opposite location, and the mouse was retrained in four sessions. Data were recorded by using an EthoVision automated tracking system (Noldus Information Technology, Wageningen, The Netherlands).
Prepulse Inhibition (PPI). All sessions for testing of PPI consisted of startle trials (pulse-alone), prepulse trials (prepulse plus pulse), and no-stimulus trials (no-stim). The pulse-alone trial consisted of a 40-ms, 120-dB pulse of broadband noise. Acoustic PPI was measured by prepulse plus pulse trials consisting of a 20-ms prepulse, 100-ms delay, and then a 40-ms, 120-dB startle pulse. The onset-to-onset interval was 120 ms. Acoustic prepulse intensities were 4, 8, 13, and 16 dB above the 65-dB background noise (i.e., 69, 73, 78, and 81 dB). The no-stim trial consisted of background noise only. The acoustic section of the test session began and ended with five presentations of the pulse-alone trial; in between, each acoustic or no-stim trial type was presented 10 times in a pseudorandom order. The average time between trials was 15 s (range, 12–30 s). After the mice were placed in the startle chambers, a 65-dB background noise was presented for a 5-min period of acclimation, and then throughout the test session.
PPI was calculated as a percentage score for each acoustic prepulse trial type: % PPI = 100 – {[(startle response for prepulse + pulse)/(startle response for pulse-alone)] × 100}. The magnitude of the acoustic startle response was calculated as the average response to all of the pulse-alone trials, excluding the first and last blocks of five pulse-alone trials each. For brevity, the main effects of prepulse intensity (which were always significant) are not discussed here. Data from the no-stim trials are not included in Results because the values obtained were negligible relative to values from trials containing startle stimuli.
Results
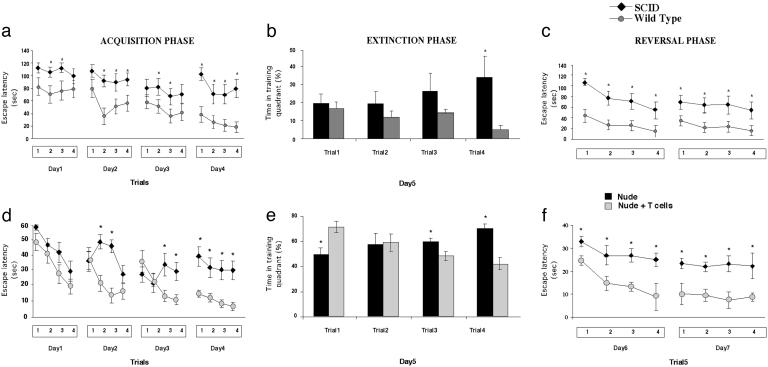
Cognitive Functions Are Impaired in the Absence of T Cells. To establish whether learning and memory processes are dependent on the integrity of the immune system, we compared the spatial learning/memory of wild-type and SCID BALB/c/OLA mice, by using the MWM, a hippocampal-dependent visuo-spatial learning/memory task. The SCID mice manifested significant impairment of spatial memory compared with their wild-type counterparts (Fig. 1 a–c). During the acquisition (Fig. 1a), extinction (Fig. 1b), and reversal (Fig. 1c) phases of the MWM task, mice devoid of adaptive immunity showed significantly increased latency in finding the hidden platform compared with wild-type mice (Fig. 1 a–c). Unlike the wild-type, the immune-deficient (SCID) mice failed to recall data from the previous day's training trial (Fig. 1 a and c). Moreover, the SCID mice started out at a lower level of performance than the wild-type, indicating that their general skills in carrying out the task were impaired, at least to some extent (Fig. 1a). The two groups did not differ in their performance of the visual platform task, or in a test in which distal cues were removed and the mice were tested once a day for 4 days, or in their swimming strategies, distance, or speed (data not shown).
Fig. 1.
Cognitive activity is affected by the integrity of the immune system. BALB/c/OLA wild type, nude, and SCID mice were monitored while attempting a spatial learning/memory task in the MWM. (a–c) Comparison of wild-type and SCID mice. During the acquisition (a), extinction (b), and reversal (c) phases of the task, SCID mice took significantly longer than wild-type mice to acquire the spatial learning needed (3-way ANOVA, repeated measures: groups, df (1,20), F = 23.0, P < 0.0001; trials, df (3,60), F = 10.995, P < 0.00001; days, df (1,60), F = 4.6, P < 0.006, for the acquisition phase; and groups, df (1,20), F = 7.9, P < 0.01; trials, df (3,60), F = 10.77, P < 0.00001; days, df (1,20), F = 34.4, P < 0.001, for the reversal phase). The presented results are from one of two experiments performed, with 10 mice per group in each experiment. (d–f) Comparison of nude mice with nude mice that were replenished with T cells 3 weeks before being tested on the MWM. During the acquisition (d), extinction (e), and reversal (f) phases of the task, nonreplenished nude mice took significantly longer to acquire spatial learning than nude mice replenished with T cells from naive wild-type mice (3-way ANOVA, repeated measures: groups, df (1,18), F = 32.3, P < 0.00001; trials, df (3,54), F = 10.1, P < 0.00001; days, df (3,54), F = 20.56, P < 0.00001, for the acquisition phase; and groups, df (1,18), F = 58.6, P < 0.00001; trials, df (3,54), F = 12.6, P < 0.00001; days, df (1,18), F = 19.2, P < 0.0004, for the reversal phase). The presented results are from one of two experiments performed, with 10 mice per group in each experiment.
It should be emphasized that, in the above set of experiments, we used SCID mice (deficient in both T cell and B cell responses) rather than nude mice (deficient only in mature T cells) to exclude differences that might be attributable to absence of fur (in nude mice) rather than to the mere differences in immune system activity. The immune deficiency of SCID mice, however, is a result of knockout of RAG1/2 genes. Because RAG1 is expressed in brains of normal mice (18) (although their functions there are still unknown), it was necessary to further substantiate our conclusion that the observed difference between the SCID and the wild-type mice was attributable to T cell immunity. We therefore compared nude mice replenished with a normal T cell population with nonreplenished nude mice (Fig. 1). We replenished nude mice with T cells from matched wild-type mice and tested them 3 weeks later on the MWM task (Fig. 1 d–f). During the acquisition and the reversal phases, nude mice replenished with T cells showed significantly shorter latency in finding the hidden platform than did nonreplenished nude mice. In the extinction phase, the replenished mice needed to spend significantly less time than the nonreplenished mice in the training quadrant for the trials. Moreover, the nonreplenished mice were significantly less able to recall data from the previous day's training trial and showed significantly slower rates of learning than those of their replenished counterparts (Fig. 1 d–f).
Cop-1 Vaccination Protects Against Cognitive Impairment Induced by Psychotomimetic Agents. The above results encouraged us to examine the possibility that, in mice with impaired cognitive functions caused for example by neurotransmitter imbalance, boosting of the relevant T cells (e.g., by T cell-based vaccination) might have a therapeutic effect.
MK-801 [an antagonist of the N-methyl-d-aspartate (NMDA) receptor channel] and AMPH (a blocker of dopamine re-uptake) act as psychotomimetic agents, inducing psychotic symptoms (19). In mice, such symptoms evidently simulate the cognitive impairment and behavioral abnormalities associated with schizophrenia (20). A number of authors have reported an MK-801-induced deficit in acquisition of spatial memory (21, 22) and nonspatial memory tasks (23). To examine whether T cell-based vaccination can overcome behavioral abnormalities and cognitive impairment resulting from neurotransmitter imbalance, we immunized mice with Cop-1. This synthetic antigen apparently acts as a weak agonist of a wide range of self-reactive T cells, thereby stimulating a response that is mediated by T cells that crossreact with CNS antigens and is needed to fight off neurodegenerative conditions (17). It should be emphasized that, unlike myelin-associated and other CNS-associated antigens, antigens (such as ovalbumin) that do not crossreact with CNS-specific proteins fail to accumulate in the healthy brain and have no protective effect (17, 24).
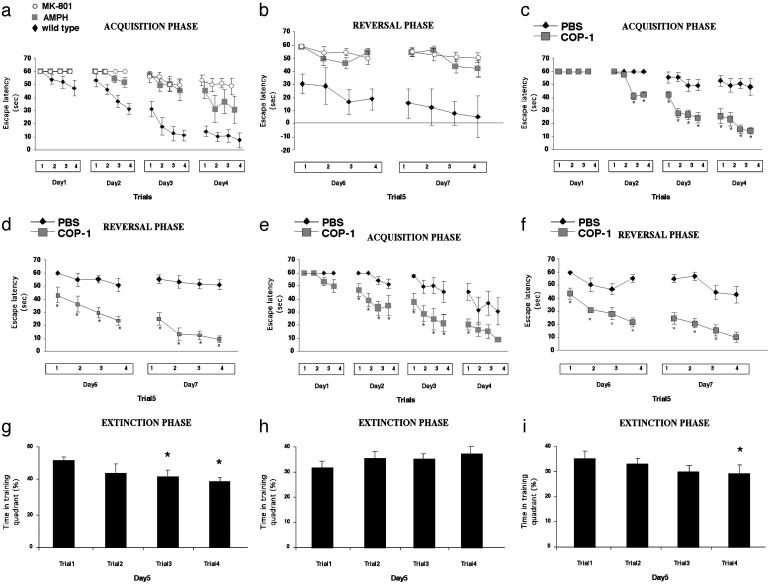
One week before receiving the psychotomimetic drug, C57BL/6J mice were immunized with Cop-1 emulsified in CFA or with PBS emulsified in CFA. Relative to behavior in naive normal mice, performance of a task requiring spatial learning and memory in the MWM was significantly impaired in mice injected with MK-801 or AMPH (Fig. 2 a and b), as indicated by their significantly higher escape latencies. During the acquisition (Fig. 2 c and e) and the reversal (Fig. 2 d and f) phases of the MWM task, PBS/CFA-injected mice that had received either of the psychotomimetic drugs took significantly longer than the corresponding Cop-1/CFA-vaccinated mice to acquire the spatial navigation task, if they were able to acquire it at all. In the extinction phase, naive normal mice showed a decrease over successive trials in the time spent in the training quadrant (Fig. 2g). Injection of MK-801 weakened this characteristic feature in mice immunized with PBS/CFA (Fig. 2h), but not in mice immunized with Cop-1/CFA (Fig. 2i). Similar results were obtained in amphetamine-injected mice (data not shown). The Cop-1/CFA-vaccinated mice injected with each of the psychotomimetic drugs learned to swim to the hidden platform and make use of it as a refuge by climbing onto it and remaining there, as indicated by decreasing latencies in successive trials. In contrast, when the corresponding PBS/CFA-injected mice encountered the hidden platform, they behaved in an abnormal and maladaptive way. Even when placed directly on the hidden platform after a trial in which they had failed to locate it, these mice quickly walked or jumped off and continued swimming in a haphazard and disorganized manner. In all of these tasks, the behavior of normal naive mice and of normal CFA/PBS-injected mice was identical (data not shown).
Fig. 2.
Effect of Cop-1 vaccination on the performance by C57BL/6J mice of a spatial learning/memory task in the MWM after injection of a psychotomimetic drug. Acquisition of the spatial learning task in the MWM took significantly longer in mice injected with MK-801 (0.1 mg/kg i.p.) or AMPH (2.5 mg/kg i.p.) than in naive (PBS-injected) C57BL/6J mice in the acquisition (a) and the reversal (b) phases. Immunization with Cop-1/CFA resulted in decreased escape latencies in acquisition and reversal phases after injection of MK-801 (c and d) or amphetamine (e and f). [MK-801 (c and d); 3-way ANOVA, repeated measures: groups, df (1,9), F = 56.6, P < 0.0001; trials, df (3,27), F = 54.0, P < 0.00001; days, df (3,27), F = 15.6, P < 0.00001, for the acquisition phase; and groups, df (1,9), F = 42.7, P < 0.0001; trials, df (3,27), F = 24.4, P < 0.00001; days, df (1,9), F = 7.9, P < 0.02, for the reversal phase; or AMPH (e and f); 3-way ANOVA, repeated measures: groups, df (1,10), F = 9.8, P < 0.01; trials, df (3,30), F = 29.9, P < 0.00001; days, df (1,30), F = 21.3, P < 0.00001, for the acquisition phase; and groups, df (1,10), F = 53.7, P < 0.00003; trials, df (3,30), F = 16.1, P < 0.00002; days, df (1,10), F = 5.0, P < 0.05 for the reversal phase]. The performance of mice immunized with Cop-1 did not differ significantly from normal behavior. (h) The decrease in time spent in a training quadrant, obtained in control mice, was abolished upon injection of MK-801 (g); however, mice immunized with Cop-1 behaved similarly to wild-type mice (i).
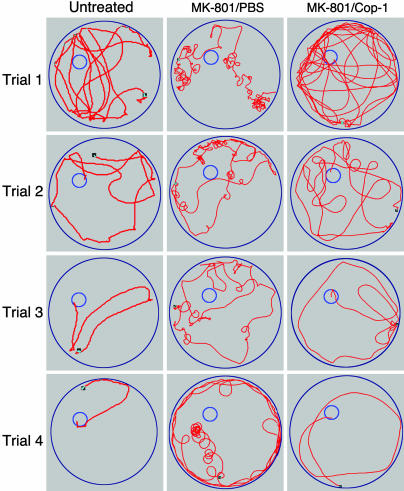
Fig. 3 depicts the behavior of naive normal mice and of MK-801-treated mice immunized with Cop-1/CFA or PBS/CFA, and shows that Cop-1/CFA-immunized mice, unlike the PBS/CFA immunized mice, adopted methodical swimming strategies similar to those seen in normal mice. Thus, Cop-1/CFA-vaccinated mice injected with a psychotomimetic drug learned to swim away from the wall to search for the platform in the inner part of the pool and to use the platform as a refuge when they found it. In contrast, the behavior of the PBS/CFA-treated mice injected with a psychotomimetic drug showed severe disturbances, including hyperactivity, swimming over the platform, and aimless swimming in circles. Their subsequent performance in the elevated-plus-maze task indicated, however, that the observed differences in spatial learning ability in the MWM between these two groups of mice was not caused by a difference in anxiety. Furthermore, in the social behavior test, the mice that were vaccinated with Cop-1 also showed better communicative behavior than the controls (data not shown).
Fig. 3.
Cop-1 counteracted MK-801-induced impairment of learning and memory. The figure depicts the swimming strategies of C57BL/6J mice that were immunized with Cop-1/CFA or PBS/CFA, and injected 1 week later with MK-801. Naive mice served as controls for performance in the MWM. On the second day after administration of the drug, performance was tested in four consecutive trials at 5-min intervals. The Cop-1-vaccinated mice, like the naive mice, learned to swim away from the wall to search for the platform in the inner 50% of the pool and to use the platform as a refuge when they found it. A significantly less efficient strategy was used by MK-801-injected mice immunized with vehicle.
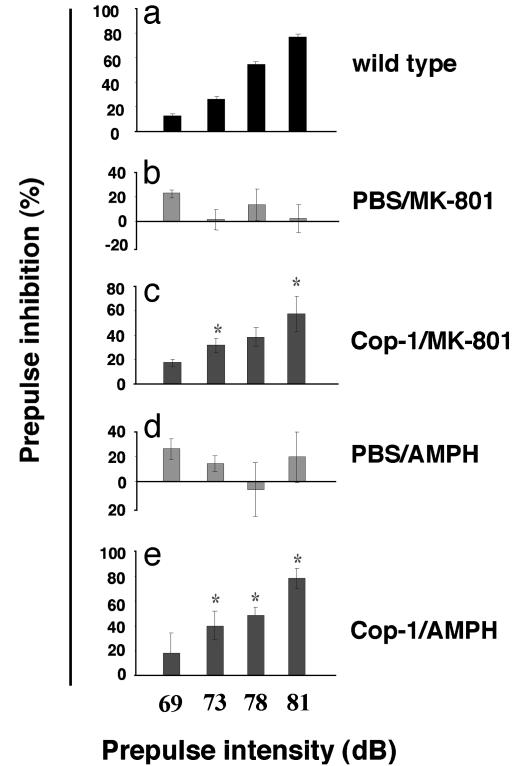
Cop-1 Vaccination Protects Against Sensorimotor Dysfunction Induced by Psychotomimetic Agents. The neurotransmitter imbalance induced by MK-801 or AMPH also causes sensorimotor dysfunction, another characteristic feature of patients with schizophrenia. Because (as shown above) T cell-based therapy counteracted the effect of these drugs on cognition, we assumed that other functions impaired by these drugs would be similarly affected. Sensorimotor gating can be assessed experimentally by drug-induced PPI of the acoustic startle response. One week before administration of MK-801 or AMPH, C57BL/6J mice were either inoculated with Cop-1/CFA or with PBS/CFA. The psychotomimetic drugs were injected 15 min before measurement of the PPI (25). As expected, PPI in untreated normal mice increased with increasing prepulse intensity (Fig. 4a) whereas, in mice injected with MK-801 (C57BL/6J) or AMPH (Fig. 4d) the PPI response was abnormal. Vaccination with Cop-1/CFA, but not with PBS/CFA, prevented the abnormal behavior induced by MK-801 (Fig. 4 b and c). Similar results were obtained with AMPH with (Fig. 4d) and without Cop-1 immunization (Fig. 4e). Thus, sensorimotor dysfunction induced by administration of each of the psychotomimetic agents was prevented or partially restored by vaccination with Cop-1/CFA.
Fig. 4.
Restoration of impaired prepulse inhibition of the acoustic startle response in C57BL/6J mice by Cop-1 immunization. C57BL/6J mice were immunized with Cop-1/CFA or with PBS/CFA. Naive mice were used as controls. One week later, the immunized mice were injected with MK-801 (0.1 mg/kg, i.p.; b and c) or with AMPH (2.5 mg/kg i.p.; d and c). The PPI of the acoustic startle response in control mice served as a baseline (a). Vehicle-immunized mice injected with MK-801 showed significantly disrupted PPI (b). In mice immunized with Cop-1/CFA, PPI was monotonically increased as a function of prepulse intensity (c; F(1,9) = 14.05, P < 0.005). Vehicle-immunized mice injected with AMPH also showed significantly disrupted PPI (d). In mice immunized with Cop-1/CFA, PPI was monotonically increased as a function of prepulse intensity (e; F(1,10) = 8.6, P < 0.015).
Cop-1-Reactive T Cells Produce BDNF upon Encountering Brain Proteins. Several research groups have reported that T cells reactive to Cop-1 home to sites of pathology in the CNS (17, 26), and that activated Cop-1-reactive T cells can produce BDNF (17, 26, 27). BDNF deficiency has been reported in patients with schizophrenia (28, 29); it is unclear, however, whether the deficiency is a cause or an effect, and whether BDNF-based treatment will be beneficial. Production of neurotrophic factors by T cells depends on the state of activation of the T cells (24). Production of neurotrophic factors by Cop-1-reactive T cells therefore evidently requires a local signal from resident antigen-presenting cells that these T cells can recognize. We therefore carried out an experiment in vitro to determine whether Cop-1-reactive T cells, on encountering CNS myelin, can produce BDNF. Table 1 shows that the production of BDNF by Cop-1-reactive T cells was increased when these T cells encountered not only their specific antigen (Cop-1) but also the CNS-related self-antigen MBP.
Table 1. Enzyme-linked immunosorbent assay of BDNF secreted by Cop-1-reactive T cells.
| Antigen | — | Cop-1 | MBP | OVA | Con A |
|---|---|---|---|---|---|
| pg/ml | 400 ± 45 | 1220 ± 100 | 1500 ± 150 | 440 ± 50 | 1100 ± 120 |
Cop-1-reactive T cells were cultured for 48 h with the indicated antigens in stimulation medium. T cell supernatants were collected and subjected to sandwich ELISA (see Supportng Text, which is published as supporting information on the PNAS web site). Compared with unstimulated T cells, secretion of BDNF by Cop-1-reactive T cells was significantly increased after stimulation of the T cells with a specific antigen (Cop-1) or with a self-antigen (MBP) that crossreacts with Cop-1. Values are mean values (pg/ml) ± SE (from three independent experiments) of the amounts of BDNF secreted by Cop-1-specific T cells in response to stimulation by various antigens. OVA, ovalbumin.
Discussion
Brain performance in immune-deficient animals has not been previously documented. Moreover, the influence of immune integrity on brain activity has not been considered. The results of this study show that integrity of the adaptive immune system plays a pivotal role in cognitive functions. Systemic immune deficiency resulted in cognitive impairment which could be reversed by replenishment with T cells. Vaccination with Cop-1, a synthetic low-affinity agonist of a wide range of self-reactive T cell clones, presumably by boosting the number of T cells capable of crossreacting with relevant CNS antigens, prevented drug-induced psychosis and reduced cognitive impairment.
The question of whether adaptive peripheral immunity affects emotional and cognitive states has never been addressed. It is interesting to note, however, that the cognitive dysfunction demonstrated in a mouse model of virally induced AIDS (30) can be interpreted along similar lines to our present findings. Experimental evidence strongly suggests that integrity of the peripheral immune system is a critical factor in determining the ability of rodents to cope with CNS damage, with those devoid of T cells showing the lowest potential for recovery (13, 31), and that in all cases the injured CNS can benefit from a well controlled autoimmunity (32). Boosting of the injury-induced T cell response with an antigen (e.g., Cop-1) that weakly crossreacts with self-antigens results in neuronal protection (17, 33–35).
The present results showed that another brain function critically affected by the integrity of the peripheral adaptive immune system is cognition. When tested in the MWM (on a task that generates stress), immune-deficient mice showed impaired cognitive function, which was prevented by restoration of immune system integrity. The observed impairment of cognitive activity, which occurred here when the immune system was intact but the brain was suffering from an imbalance in neurotransmitters, suggests that the peripheral immune system can contain the small fluctuations in neurotransmitter levels that occur daily and enable normal cognitive performance but is unable to cope with a pathological imbalance that causes cognitive impairment. In the latter situation, therefore, the relevant T cells need to be boosted. Boosting was achieved in this study by vaccination with Cop-1, which significantly prevented the cognitive impairment induced by psychotomimetic drugs. Both behavioral and cognitive abnormalities were observed in our mouse model within 15 min of administration of MK-801 or AMPA and were counteracted by the effects of the Cop-1 vaccination given 1 week earlier. Due to the rapid onset of symptoms that occurs in the experimental paradigm used here, the mice had to be vaccinated before psychosis was induced. It should be noted, however, that the vaccination is intended not only for preventive use, but also as a remedial therapy for chronic patients, in whom intervention at any stage of the disease should be beneficial. Therapeutic vaccination with Cop-1 was shown to be beneficial in rodent models of neurodegenerative conditions (16, 35, 36). Moreover, preliminary studies in an experimental model of psychosis with a wider window for therapeutic intervention have indeed shown that vaccination with Cop-1 after exposure of rats to psychotomimetic drugs was beneficial (unpublished observations).
The rapidity of the Cop-1-reactive T cells in counteracting the psychotic effects of the drugs suggests that T cells already elicited by the immunization were patrolling the healthy brain. It was recently shown that Cop-1 vaccination in healthy animals indeed leads to increased accumulation of T cells in the CNS and local production of BDNF (17, 37). It is also possible that, as a result of the early immunization, a significant number of Cop-1-reactive T cells circulate in the blood, and that they home to the relevant site only when it is under stress, caused for example by a pathological alteration in the brain level of dopamine (by injection of AMPH) or of glutamate (by injection of MK-801). Regardless of whether the effector T cells circulate in the blood or reside in the brain, the rapidity of the T cell response to neurotransmitter imbalance testifies to the importance of well functioning adaptive immunity in the daily maintenance of the brain (38). These results might also explain why, in the elderly population, who are known to suffer a disproportionate reduction in immunity (39) and increased metabolic and neurotransmitter imbalance in the brain, the incidence of dementia is increased (40).
In our view, it can be assumed that the primary function of the autoimmune T cells in patients with schizophrenia is to rectify the imbalance causing chemical abnormalities in the CNS. It is likely, however, that the spontaneous response of these T cells is not strong enough, or not of the required phenotype, or not of suitable antigenic specificity to do the job. In our mouse model, vaccination with a self-like antigen might be a way to boost T cells that can crossreact with brain-specific antigens, thus inducing the anti-psychotic effect.
It is possible that the beneficial effect of the T cells is mediated by their activation of resident nonneuronal cells in a way that allows homeostasis to be restored (42). Autoimmune T cells, when activated by the relevant antigen, can produce neurotrophic factors, among them BDNF (24). Cop-1, a drug approved by the Food and Drug Administration for the treatment of multiple sclerosis, has been shown experimentally to exert a significantly beneficial effect on neuronal survival after CNS injury in several animal models of neurodegenerative disorders (35, 36, 43). Systemically injected Cop-1-reactive T cells were recently shown to home to the brain tissue and stimulate local production of BDNF (37). Cop-1-reactive T cells, upon activation ex-vivo with a MBP, were shown in the present study to produce BDNF in large amounts. Cop-1-reactive T cells from human subjects have also been shown to produce BDNF (44).
Deficiency of BDNF has been observed in schizophrenic patients (28, 45). It is not known whether BDNF deficiency is a cause or an effect of the disease, and treatment of schizophrenic patients with BDNF has never been reported. Nevertheless, the finding that exogenous BDNF is neuroprotective in models of CNS trauma (46), coupled with the fact that neurodegeneration plays a significant role in the course of schizophrenia, suggests that Cop-1-reactive T cells by means of production of BDNF might be of benefit for schizophrenic patients. It should be noted, however, that the model used here, which is based on the use of psychotomimetic drugs whose psychotic effects are rapid and transient, might limit our ability to assess the relevance of BDNF in the alleviation of symptoms.
Psychological trauma, like physical insults to the CNS, can cause widespread, long-term changes in neurological and neurohormonal functioning, which seem to be related to morphological changes (47). It seems reasonable to assume that exacerbation of both the behavioral and the cognitive manifestations of schizophrenia over time can be correlated with the neurodegeneration occurring in certain regions of the brain (48). The present finding that Cop-1-reactive T cells in mice mediated the prevention of MK-801-induced or amphetamine-induced psychoses, which mimic in part the symptoms of schizophrenia in humans, coupled with the increasing recognition that neurodegeneration plays a role in schizophrenia and other mental diseases, suggests that the development of immune-based neuroprotection might provide a global remedy that addresses both positive and negative symptoms of schizophrenia and possibly also of other psychiatric conditions, including age-related and HIV-related dementias.
Supplementary Material
Acknowledgments
We thank S. R. Smith for editing the manuscript, A. Shapira for animal maintenance, and H. Avital for help with the graphic work. M.S. holds the Maurice and Ilse Katz Professorial Chair in Neuroimmunology. This work was supported by Proneuron Ltd., Industrial Park, Ness-Ziona, Israel.
Abbreviations: AMPH, d-amphetamine sulfate; BDNF, brain-derived neurotrophic factor; CFA, complete Freund's adjuvant; Cop-1, Copolymer-1; MBP, myelin basic protein; MK-801, (+)dizocilpine maleate; MWM, Morris water maze; PPI, prepulse inhibition; SCID, severe combined immune deficiency.
References
- 1.Lieberman, J., Chakos, M., Wu, H., Alvir, J., Hoffman, E., Robinson, D. & Bilder, R. (2001) Biol. Psychiatry 49, 487–499. [DOI] [PubMed] [Google Scholar]
- 2.Velakoulis, D., Pantelis, C., McGorry, P. D., Dudgeon, P., Brewer, W., Cook, M., Desmond, P., Bridle, N., Tierney, P., Murrie, V., et al. (1999) Arch. Gen. Psychiatry 56, 133–141. [DOI] [PubMed] [Google Scholar]
- 3.McCullumsmith, R. E. & Meador-Woodruff, J. H. (2002) Neuropsychopharmacology 26, 368–375. [DOI] [PubMed] [Google Scholar]
- 4.Lewis, D. A., Pierri, J. N., Volk, D. W., Melchitzky, D. S. & Woo, T. U. (1999) Biol. Psychiatry 46, 616–626. [DOI] [PubMed] [Google Scholar]
- 5.Brzustowicz, L. M., Hayter, J. E., Hodgkinson, K. A., Chow, E. W. & Bassett, A. S. (2002) Hum. Hered. 54, 199–209. [DOI] [PubMed] [Google Scholar]
- 6.Falkai, P., Schneider-Axmann, T., Honer, W. G., Vogeley, K., Schonell, H., Pfeiffer, U., Scherk, H., Block, W., Traber, F., Schild, H. H., et al. (2003) Eur. Arch. Psychiatry Clin. Neurosci. 253, 92–99. [DOI] [PubMed] [Google Scholar]
- 7.Javitt, D. C. (1999) Curr. Psychiatry Rep. 1, 25–30. [DOI] [PubMed] [Google Scholar]
- 8.Rummel, C., Hamann, J., Kissling, W. & Leucht, S. (2003) Cochrane Database Syst. Rev., CD004410. [DOI] [PMC free article] [PubMed]
- 9.de Kloet, R. E. (2003) Endocr. Regul. 37, 51–68. [PubMed] [Google Scholar]
- 10.Raison, C. L. & Miller, A. H. (2001) Semin. Clin. Neuropsychiatry 6, 277–294. [DOI] [PubMed] [Google Scholar]
- 11.Amital, H. & Shoenfeld, Y. (1993) Isr. J. Med. Sci. 29, 593–597. [PubMed] [Google Scholar]
- 12.Moalem, G., Leibowitz-Amit, R., Yoles, E., Mor, F., Cohen, I. R. & Schwartz, M. (1999) Nat. Med. 5, 49–55. [DOI] [PubMed] [Google Scholar]
- 13.Kipnis, J., Yoles, E., Schori, H., Hauben, E., Shaked, I. & Schwartz, M. (2001) J. Neurosci. 21, 4564–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoles, E., Hauben, E., Palgi, O., Agranov, E., Gothilf, A., Cohen, A., Kuchroo, V., Cohen, I. R., Weiner, H. & Schwartz, M. (2001) J. Neurosci. 21, 3740–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz, M. & Kipnis, J. (2001) Trends Mol. Med. 7, 252–258. [DOI] [PubMed] [Google Scholar]
- 16.Kipnis, J., Mizrahi, T., Hauben, E., Shaked, I., Shevach, E. & Schwartz, M. (2002) Proc. Natl. Acad. Sci. USA 99, 15620–15625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kipnis, J., Yoles, E., Porat, Z., Cohen, A., Mor, F., Sela, M., Cohen, I. R. & Schwartz, M. (2000) Proc. Natl. Acad. Sci. USA 97, 7446–7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, S. K., Wang, K. C., Hong, S. J., Chung, C. K., Lim, S. Y., Kim, Y. Y., Chi, J. G., Kim, C. J., Chung, Y. N., Kim, H. J. & Cho, B. K. (2003) Epilepsy Res. 56, 175–183. [DOI] [PubMed] [Google Scholar]
- 19.Lahti, A. C., Weiler, M. A., Tamara Michaelidis, B. A., Parwani, A. & Tamminga, C. A. (2001) Neuropsychopharmacology 25, 455–467. [DOI] [PubMed] [Google Scholar]
- 20.Tenn, C. C., Fletcher, P. J. & Kapur, S. (2003) Schizophr. Res. 64, 103–114. [DOI] [PubMed] [Google Scholar]
- 21.Whishaw, I. Q. & Auer, R. N. (1989) Psychopharmacology (Berl) 98, 500–507. [DOI] [PubMed] [Google Scholar]
- 22.Ahlander, M., Misane, I., Schott, P. A. & Ogren, S. O. (1999) Neuropsychopharmacology 21, 414–426. [DOI] [PubMed] [Google Scholar]
- 23.Griesbach, G. S., Hu, D. & Amsel, A. (1998) Behav. Brain. Res. 97, 29–38. [DOI] [PubMed] [Google Scholar]
- 24.Moalem, G., Gdalyahu, A., Shani, Y., Otten, U., Lazarovici, P., Cohen, I. R. & Schwartz, M. (2000) J. Autoimmun. 20, 6421–6430. [DOI] [PubMed] [Google Scholar]
- 25.Van den Buuse, M., Garner, B. & Koch, M. (2003) Curr. Mol. Med. 3, 459–471. [DOI] [PubMed] [Google Scholar]
- 26.Aharoni, R., Meshorer, A., Sela, M. & Arnon, R. (2002) J. Neuroimmunol. 126, 58–68. [DOI] [PubMed] [Google Scholar]
- 27.Kerschensteiner, M., Stadelmann, C., Dechant, G., Wekerle, H. & Hohlfeld, R. (2003) Ann. Neurol. 53, 292–304. [DOI] [PubMed] [Google Scholar]
- 28.Weickert, C. S., Hyde, T. M., Lipska, B. K., Herman, M. M., Weinberger, D. R. & Kleinman, J. E. (2003) Mol. Psychiatry 8, 592–610. [DOI] [PubMed] [Google Scholar]
- 29.Egan, M. F., Weinberger, D. R. & Lu, B. (2003) Am. J. Psychiatry 160, 1242. [DOI] [PubMed] [Google Scholar]
- 30.Iida, R., Yamada, K., Mamiya, T., Saito, K., Seishima, M. & Nabeshima, T. (1999) J. Neuroimmunol. 95, 65–72. [DOI] [PubMed] [Google Scholar]
- 31.Schori, H., Yoles, E. & Schwartz, M. (2001) J. Neuroimmunol. 119, 199–204. [DOI] [PubMed] [Google Scholar]
- 32.Wekerle, H. (2002) J. Infect. Dis. 186, Suppl. 2, S140–S144. [DOI] [PubMed] [Google Scholar]
- 33.Fisher, J., Levkovitch-Verbin, H., Schori, H., Yoles, E., Butovsky, O., Kaye, J. F., Ben-Nun, A. & Schwartz, M. (2001) J. Neurosci. 21, 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schori, H., Kipnis, J., Yoles, E., WoldeMussie, E., Ruiz, G., Wheeler, L. A. & Schwartz, M. (2001) Proc. Natl. Acad. Sci. USA 98, 3398–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bakalash, S., Kessler, A., Mizrahi, T., Nussenblatt, R. & Schwartz, M. (2003) Invest. Ophthalmol. Vis. Sci. 44, 3374–3381. [DOI] [PubMed] [Google Scholar]
- 36.Angelov, D. N., Waibel, S., Guntinas-Lichius, O., Lenzen, M., Neiss, W. F., Tomov, T. L., Yoles, E., Kipnis, J., Schori, H., Reuter, A., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 4790–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aharoni, R., Kayhan, B., Eilam, R., Sela, M. & Arnon, R. (2003) Proc. Natl. Acad. Sci. USA 100, 14157–14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kipnis, J. & Schwartz, M. (2002) Trends Mol. Med. 8, 319–323. [DOI] [PubMed] [Google Scholar]
- 39.Linton, P. J. & Dorshkind, K. (2004) Nat. Immunol. 5, 133–139. [DOI] [PubMed] [Google Scholar]
- 40.Wick, G., Berger, P., Jansen-Durr, P. & Grubeck-Loebenstein, B. (2003) Exp. Gerontol. 38, 13–25. [DOI] [PubMed] [Google Scholar]
- 41.Farber, N. B., Jiang, X. P., Heinkel, C. & Nemmers, B. (2002) Mol. Psychiatry 7, 726–733. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz, M., Shaked, I., Fisher, J., Mizrahi, T. & Schori, H. (2003) Trends Neurosci. 26, 297–302. [DOI] [PubMed] [Google Scholar]
- 43.Kipnis, J., Nevo, U., Panikashvili, D., Alexandrovich, A., Yoles, E., Akselrod, S., Shohami, E. & Schwartz, M. (2003) J. Neurotrauma 20, 559–569. [DOI] [PubMed] [Google Scholar]
- 44.Ziemssen, T., Kumpfel, T., Klinkert, W. E., Neuhaus, O. & Hohlfeld, R. (2002) Brain 125, 2381–2391. [DOI] [PubMed] [Google Scholar]
- 45.Iritani, S., Niizato, K., Nawa, H., Ikeda, K. & Emson, P. C. (2003) Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 801–807. [DOI] [PubMed] [Google Scholar]
- 46.Han, B. H. & Holtzman, D. M. (2000) J. Neurosci. 20, 5775–5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Markowitsch, H. J., Kessler, J., Van Der Ven, C., Weber-Luxenburger, G., Albers, M. & Heiss, W. D. (1998) Neuropsychologia 36, 77–82. [DOI] [PubMed] [Google Scholar]
- 48.Deutsch, S. I., Rosse, R. B., Schwartz, B. L. & Mastropaolo, J. (2001) Clin. Neuropharmacol. 24, 43–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.