Abstract
Recognition of pathogen-associated molecular patterns (PAMPs) by surface-localized pattern-recognition receptors (PRRs) activates plant innate immunity, mainly through activation of numerous protein kinases. Appropriate induction of immune responses must be tightly regulated, as many of the kinases involved have an intrinsic high activity and are also regulated by other external and endogenous stimuli. Previous evidences suggest that PAMP-triggered immunity (PTI) is under constant negative regulation by protein phosphatases but the underlying molecular mechanisms remain unknown. Here, we show that protein Ser/Thr phosphatase type 2A (PP2A) controls the activation of PRR complexes by modulating the phosphostatus of the co-receptor and positive regulator BAK1. A potential PP2A holoenzyme composed of the subunits A1, C4, and B’η/ζ inhibits immune responses triggered by several PAMPs and anti-bacterial immunity. PP2A constitutively associates with BAK1 in planta. Impairment in this PP2A-based regulation leads to increased steady-state BAK1 phosphorylation, which can poise enhanced immune responses. This work identifies PP2A as an important negative regulator of plant innate immunity that controls BAK1 activation in surface-localized immune receptor complexes.
Keywords: innate immunity, negative regulation, phosphatase, receptor kinase
Introduction
Recognition of pathogen-associated molecular patterns (PAMPs) by surface-localized pattern-recognition receptors (PRRs) is central to the establishment of innate immunity (Ronald & Beutler, 2010). However, the appropriate timing and intensity of innate immune responses must be tightly controlled. The negative regulation of PRR-triggered immunity (PTI) starts to be well understood in mammals (Kondo et al, 2012; Sasai & Yamamoto, 2013). In contrast, hardly anything is known in plants where over-activation of immune receptors can have a dramatic impact on growth.
Plant PRRs are surface-localized receptor kinases (RKs) or receptor-like proteins (RLPs) (Monaghan & Zipfel, 2012; Schwessinger & Ronald, 2012). These PRRs require dynamic association with regulatory kinases within plasma membrane-localized immune receptor complexes to initiate signaling (Monaghan & Zipfel, 2012). Notably, the regulatory leucine-rich repeat (LRR)-RK BAK1 (also named SERK3) is a key immune component that acts as a co-receptor required for the function of several LRR-containing PRRs (Monaghan & Zipfel, 2012; Santiago et al, 2013; Sun et al, 2013a,b; Liebrand et al, 2014).
The best-studied PRR-BAK1 complexes involve the Arabidopsis LRR-RKs FLS2 and EFR, which are the receptors for bacterial flagellin (or the derived immunogenic peptide flg22) and for elongation factor Tu (EF-Tu) (or the derived immunogenic peptide elf18), respectively (Monaghan & Zipfel, 2012; Schwessinger & Ronald, 2012). FLS2 and EFR form a ligand-induced complex with BAK1 leading to rapid phosphorylation of both proteins (Chinchilla et al, 2007; Heese et al, 2007; Schulze et al, 2010; Roux et al, 2011; Schwessinger et al, 2011; Sun et al, 2013b). In turn, downstream cytoplasmic kinases such as BIK1 (and its closest paralog PBL1) or BSK1 are phosphorylated and dissociate from the PRR-BAK1 complex (Lu et al, 2010; Zhang et al, 2010; Shi et al, 2013; Xu et al, 2013; Lin et al, 2014). These dynamic interactions and phosphorylation events lead to the activation of immune responses, including production of reactive oxygen species (ROS) by the NADPH oxidase RBOHD, activation of mitogen-activated protein kinase (MAPK) cascade, transcriptional reprogramming, and immunity to pathogens (Kadota et al, 2014; Li et al, 2014a; Macho & Zipfel, 2014).
However, mechanisms controlling the activation of PRR-BAK1 complexes prior to or upon ligand perception are still poorly understood. Recently, the identification of the LRR-RK BIR2 has highlighted a mechanism that limits the formation of the FLS2-BAK1 complex in absence of elicitation (Halter et al, 2014). In addition, the E3-ubiquitin ligases PUB12 and PUB13 were shown to regulate the degradation of the ligand-bound FLS2 after BAK1 activation, most likely to enable the replenishment of ligand-free receptor at the plasma membrane (Robatzek et al, 2006; Lu et al, 2011; Smith et al, 2014).
Phosphorylation is a reversible post-translational modification. Protein phosphatases have been implicated in the negative regulation of immune signaling at diverse steps. Several members of the Ser/Thr protein phosphatase type 2C (PP2C) family are involved in stress signaling and biotic responses (Schweighofer et al, 2004). For example, in Arabidopsis, the PP2C AP2C1 is a negative regulator of the MAPKs MPK4 and MPK6 and modulates the levels of the hormones jasmonic acid and ethylene during immunity (Schweighofer et al, 2007). In addition, MPK3 and MPK6 are also dephosphorylated by the Arabidopsis dual specificity phosphatases (DSPs) MKP1 and MKP2 during PTI or oxidative stress in Arabidopsis (Lee & Ellis, 2007; Anderson et al, 2011). Plant Ser/Thr protein phosphatase type 2A (PP2A) holoenzymes are equally involved in development and responses to external stimuli or hormones. Similarly to their metazoan counterparts, plant PP2A are trimeric holoenzymes composed of a conserved catalytic subunit (‘C’), associated via a scaffold or hook subunit (‘A’) to one of many regulatory subunits (B, B’, or B’’) that determine localization and substrate specificity (Uhrig et al, 2013). As such, different combinations of these subunits can potentially generate a wealth of different holoenzymes regulating distinct specific processes (Virshup & Shenolikar, 2009). Several plant PP2A substrates have been identified such as the auxin receptor PIN1, the blue light receptor Phot2, the ethylene biosynthetic enzyme ACS6 or the transcription factor BZR1, which regulates brassinosteroid (BR) responses (Michniewicz et al, 2007; Tseng & Briggs, 2010; Skottke et al, 2011; Tang et al, 2011). Interestingly, the PP2A-B’γ subunit may act as a negative regulator of immune responses under low light condition or day length-dependent oxidative stress (Trotta et al, 2011; Li et al, 2014b), and silencing of the PP2A-C subunits in Nicotiana benthamiana leads to enhanced immune responses (He et al, 2004). However, PP2A substrates involved in immunity are currently unknown.
Given the major role played by protein kinases in early PTI signaling, it is likely that protein phosphatases negatively regulate early immune components. Yet, so far, only a few examples of protein phosphatases acting at the level of plant PRR complexes are known. The PP2C KAPP interacts with the FLS2 cytoplasmic domain in yeast two-hybrid assays, and KAPP over-expression leads to flg22-insensitivity (Gomez-Gomez et al, 2001). However, no data are available on the effect of KAPP on FLS2 activity, and the specificity of KAPP is questionable since it was also reported to interact with many RKs (Ding et al, 2007). In rice, the PP2C XB15 interacts with and dephosphorylates the PRR XA21 leading to the negative regulation of XA21-dependent immunity (Park et al, 2008). RKs acting as PRR often belong to the non-RD (where RD refer to conserved Arg and Asp residues in the kinase subdomain VIb) kinase family and exhibit weak kinase activities (Dardick et al, 2012). In contrast, PRR-associated kinases, such as BAK1 and BIK1, generally have stronger kinase activity and can be involved in diverse plant signaling pathways (Wang et al, 2008; Lu et al, 2010; Zhang et al, 2010; Cheng et al, 2011; Schwessinger et al, 2011; Yan et al, 2012; Lin et al, 2013). Thus, a tight control of these kinases is required to avoid their activation in the absence of the appropriate stimulus, as well as to ensure optimal outputs upon ligand-induced activation. Yet, no protein phosphatase negatively regulating PRR complexes in Arabidopsis or other plant species has been identified, despite previous evidences suggesting that PTI is under constant negative regulation by various protein phosphatases (Felix et al, 1994; MacKintosh et al, 1994; Chandra & Low, 1995; Suzuki & Shinshi, 1995). In this study, we directly investigated the possible involvement of PP2A in the negative regulation of early PTI signaling. We used pharmacological, reverse-genetic and biochemical approaches to reveal that a specific PP2A holoenzyme potentially composed of the subunits A1, C4, and B’η/ζ negatively regulates the steady-state activity of the co-receptor BAK1 during immunity. Our results illustrate a novel function for a plant PP2A by controlling the activity of a common regulatory receptor kinase.
Results
Protein phosphatase 2A inhibits immune signaling
Previous evidences suggest that PTI is under constant negative regulation by protein phosphatases, including PP2A (Felix et al, 1994; Chandra & Low, 1995; Suzuki & Shinshi, 1995). However, the exact identity of the phosphatase(s) and the mechanisms involved remain elusive. We first tested whether PP2A is involved in regulating PTI in Arabidopsis. We used the well-established specific PP2A inhibitor cantharidin (Li & Casida, 1992; Bajsa et al, 2011) to investigate the effect of PP2A activity on PTI. PAMP-induced resistance restricts by more than tenfold the growth of the phytopathogenic bacterium Pseudomonas syringae pv. tomato (Pto) DC3000 (Zipfel et al, 2004). Strikingly, chemical inhibition of PP2A by cantharidin induced a comparable level of resistance to Pto DC3000 in mature wild-type (WT) Col-0 plants to that observed upon pre-treatment with flg22 (Fig1A). Importantly, cantharidin treatment did not trigger any visible symptoms by itself under the same conditions used for the pre-treatment experiments (Supplementary Fig S1A). This clearly suggests that PP2A negatively regulates immunity in Arabidopsis.
Figure 1. Treatment with the PP2A inhibitor cantharidin activates PAMP-triggered immunity.
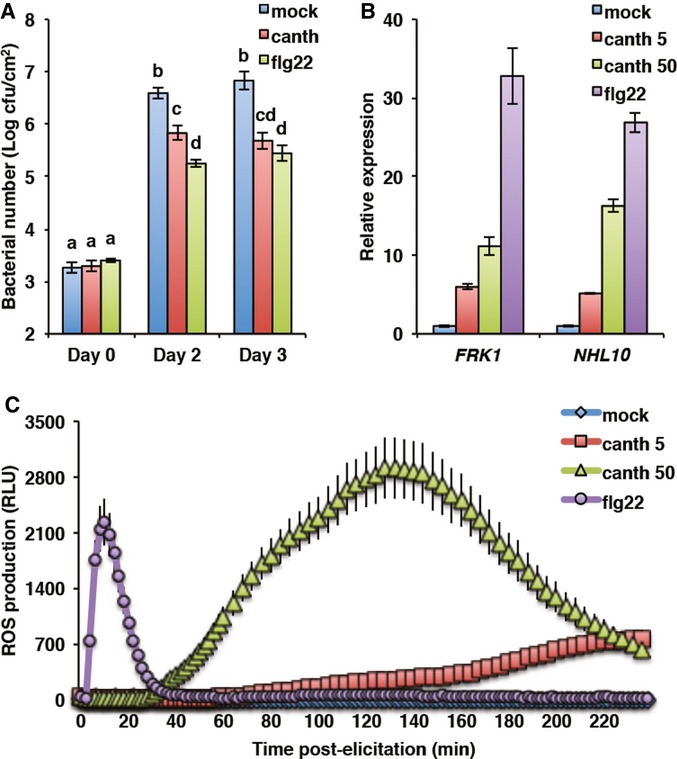
- PP2A chemical inhibition protects Arabidopsis from bacterial infection. Wild-type Col-0 plants were pre-treated 24 h with mock, 1 μM flg22, or 50 μM cantharidin. Bacterial number was determined at the indicated time following Pseudomonas syringae pv. tomato (Pto) DC3000 inoculation. Values presented are average of four biological repeats ± SE. Values labeled with different letters are statistically different as established by a one-way ANOVA (P < 0.05). cfu, colony-forming units.
- PP2A inhibits flg22-induced gene expression. Accumulation of marker gene transcripts FRK1 (At2g19190) and NHL10 (At2g35980) was assessed by qRT-PCR in Col-0 seedlings 1 h after treatment with mock, 100 nM flg22, 5 μM, or 50 μM cantharidin. Values are average of three biological repeats ± SE presented as fold induction compared with mock-treated samples.
- PP2A chemical inhibition triggers oxidative burst. ROS production was measured as relative luminescence units (RLU) in Col-0 in response to mock, 100 nM flg22, 5 μM, and 50 μM cantharidin. Values presented are average of three biological repeats ± SE.
To identify at which point of the PTI signaling network is PP2A acting, we tested the effect of cantharidin treatment on early PTI events. Cantharidin by itself induced the accumulation of PTI marker transcripts such as FRK1 and NHL10 in axenic Col-0 seedlings (Fig1B). Also, cantharidin triggered a dose-dependent ROS production in mature Col-0 leaves, which had similar characteristics but was slower than that observed upon flg22 treatment (Fig1C). This may be explained by different diffusion rate across the cell wall and/or dynamics of action between flg22 and cantharidin. Together, these data suggest that PP2A acts early in PTI signaling at the level of the PRR complex, or that PP2A-dependent processes control several downstream signaling components, such as the NADPH oxidase RBOHD or the cytoplasmic kinases involved in the responses measured.
PP2A associates with BAK1 in planta
To determine if PP2A acts directly at the level of the PRR complex, we tested if PP2A chemical inhibition is sufficient to induce PRR complex activation. Remarkably, cantharidin treatment alone triggered the phosphorylation of the receptor complex-associated cytoplasmic kinase BIK1, as measured by the mobility shift of BIK1 in a transgenic BIK1pro:BIK1-HA line (Fig2A). This observation does not necessary imply that PP2A targets BIK1. Indeed, BIK1 is phosphorylated by activated FLS2 and BAK1 upon flg22 perception (Lu et al, 2010; Zhang et al, 2010; Shi et al, 2013; Lin et al, 2014). Notably, we found that cantharidin-triggered ROS production was strongly reduced in the null bak1-4 and bik1 pbl1 mutants, while it was unaffected in fls2 and completely absent in rbohD (Fig2B). These data demonstrate that PP2A acts upstream or at the level of BAK1 and/or BIK1.
Figure 2. PP2A associates with BAK1 in planta.
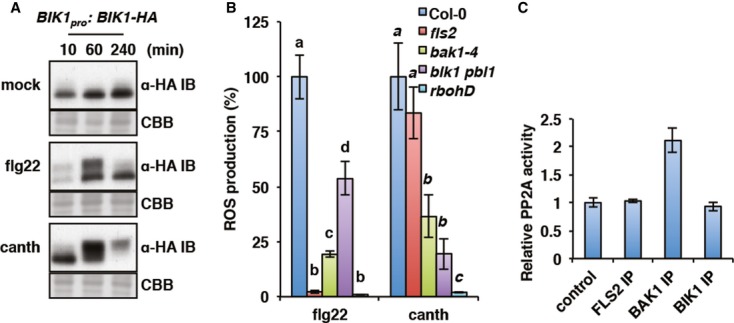
- PP2A chemical inhibition triggers BIK1 activation. BIK1 phosphorylation status was detected by anti-HA immunoblot in BIK1pro:BIK1-HA seedlings treated with mock, 100 nM flg22, or 50 μM cantharidin for the indicated time. Coomassie Brilliant Blue staining of the membrane (CBB) is shown to assess equal protein loading.
- Cantharidin-triggered oxidative burst is impaired in mutants of PRR complex components. ROS production in response to 100 nM flg22 or 50 μM cantharidin was measured in Col-0, fls2, bak1-4, bik1 pbl1, and rbohD. Values are average of three biological repeats ± SE presented as percentage of Col-0 response. Values labeled with different letters (regular and italic for flg22 and cantharidin treatment respectively) are statistically different as established by a one-way ANOVA (P < 0.05).
- PP2A activity is constitutively associated with BAK1. PP2A activity in un-elicited BIK1pro:BIK1-HA seedlings was measured by colorimetry on protein extracts enriched with anti-FLS2, anti-BAK1, or anti-HA antibodies. PP2A activity relative to background detected in non-enriched protein extracts in absence of antibodies (control) is presented as average of three biological repeats ± SE.
Source data are available online for this figure.
To identify which kinase from the PRR complex is associated with PP2A, we measured the PP2A activity present in FLS2, BAK1, and BIK1 immunoprecipitates from un-elicited seedlings. After subtraction of the background activity observed in non-enriched extract, a significant PP2A activity could be detected in complex with BAK1, but not with FLS2 or BIK1 (Fig2C). Moreover, the BAK1-associated PP2A activity was abolished by cantharidin treatment in vitro (Supplementary Fig S1B). These results indicate that PP2A associates constitutively with BAK1 in planta. The observations that cantharidin treatment is sufficient to induce BIK1 phosphorylation (Fig2A) and that PP2A does not associate with BIK1 (Fig2C) are consistent with BAK1 acting upstream of BIK1 (Lu et al, 2010; Zhang et al, 2010; Shi et al, 2013; Xu et al, 2013; Lin et al, 2014).
Specific PP2A subunits negatively regulate PAMP-triggered immunity
The key to understand the regulation of distinct cellular processes by PP2A is the identification of specific subunits composing PP2A holoenzymes (Shi, 2009; Virshup & Shenolikar, 2009). Of particular interest is the identification of the ‘B’ subunits that provide localization and substrate specificity. Thus, we conducted a reverse-genetic approach to identify PP2A subunits responsible for the negative regulation of early PTI signaling. The Arabidopsis genome encodes five isoforms of the catalytic ‘C’ subunit, three isoforms of the hook ‘A’ subunit, and up to 18 isoforms of the ‘B’ regulatory subunit, allowing the potential formation of multiple highly specific trimeric enzymes (Farkas et al, 2007). Expression of the ‘C’ and ‘A’ genes is globally ubiquitous and constant, whereas expression of several ‘B’ genes, mostly in the B’ subgroup, is altered by PAMP perception and/or biotic stresses (Supplementary Fig S2).
Despite the high homology between the catalytic isoforms and expected functional redundancy, single pp2a-c mutants yet display informative phenotype (Ballesteros et al, 2013). We obtained and characterized insertional mutant lines for the 5 ‘C’ and 3 ‘A’ genes, as well as for the 3 ‘B’ genes whose transcript accumulation is induced by biotic stress (Supplementary Figs S2 and S3). We postulate that given the negative role of PP2A in PTI, loss-of-function mutants in relevant subunits should be hyper-responsive to PAMPs. Using ROS production in response to increasing flg22 dose as a screening assay, we identified several pp2a mutants that show higher ROS production than Col-0 (Fig3A).
Figure 3. The PP2A subunits A1, B’η, B’ζ, and C4 regulate PTI signaling.
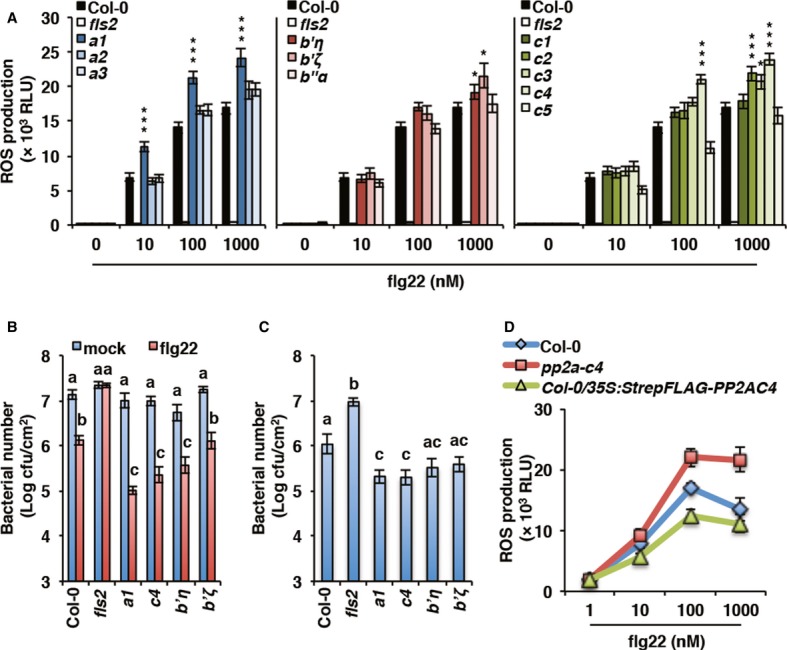
- Specific PP2A subunits regulate flg22-triggered oxidative burst. ROS production in response to increasing flg22 concentration was measured as relative luminescence units (RLU) in pp2a mutant lines compared to wild-type Col-0. Values presented are average of three biological repeats ± SE. Values labeled with asterisk are statistically different from Col-0 as established by one-way ANOVA (*P < 0.01 and ***P < 0.001).
- Specific PP2A subunits control flg22-mediated restriction of bacterial growth. Induced resistance to Pto DC3000 was determined 2 days post-infection in pp2a mutant lines compared to Col-0 following 24 h mock or 100 nM flg22 treatment. Values presented are average of four biological repeats ± SE. Values labeled with different letters are statistically different as established by a one-way ANOVA (P < 0.01). cfu, colony-forming units.
- Specific PP2A subunits control Arabidopsis susceptibility to bacterial infection. Susceptibility to Pto DC3000 was determined 2 days after spray-inoculation in pp2a mutant lines compared to Col-0. Values are presented as average of four biological repeats ± SE. Values labeled with different letters are statistically different as established by a one-way ANOVA (P < 0.01). cfu, colony-forming units.
- Over-expression of PP2A-C4 inhibits PAMP-triggered ROS burst. ROS production in response to increasing flg22 concentration was measured as relative luminescence units (RLU) in 5-week-old pp2a-c4 mutant and 35Spro:StrepFlag-PP2AC4 lines compared to Col-0. Values presented are average of three biological repeats ± SE.
We further characterized flg22 responsiveness in the pp2a-a1, pp2a-c4, pp2a-b’η, and pp2a-b’ζ mutants by testing flg22-induced protection against bacterial infection. In this assay, pp2a-a1, pp2a-c4, and pp2a-b’η allowed significantly less growth of Pto DC3000 than Col-0 after pre-treatment with a low dose of flg22 (Fig3B), demonstrating flg22 hypersensitivity in these lines. Consistent with an increased PAMP sensitivity, pp2a-a1, pp2a-c4, and to a lesser extent pp2a-b’η and pp2a-b’ζ were more resistant to spray-infection by Pto DC3000 (Fig3C). Additionally, we observed a reduced flg22-triggered ROS production in plants over-expressing PP2A-C4 (Fig3D). Moreover, complementation of the rcn1-1 (an A1 subunit mutant) by RCN1 expression suppressed the exaggerated elf18 response (Supplementary Fig S4). We used elf18 for this assay as rcn1-1 is the Ws ecotype background, which is a natural fls2 mutant (Zipfel et al, 2004). Finally, it is noteworthy that the flg22 hypersensitivity observed in pp2a-a1, pp2a-c4, pp2a-b’η, and pp2a-b’ζ mutant lines did not seem associated with constitutive immune responses, as suggested by the normal development of the plants when grown in soil and the absence of immune transcripts over-accumulation in un-elicited seedlings compared to WT (Supplementary Fig S5).
As BAK1 is also involved in BR signaling (Zhu et al, 2013; Liebrand et al, 2014), we tested whether the PP2A subunits identified in our study could also play a role in BR-triggered responses. Interestingly, the pp2a-a1 and pp2a-c4 mutants appeared slightly hypersensitive to exogenous BR treatment as measured by expression of the BR marker genes CPD and SAUR-AC1 (Supplementary Fig S6).
Specific PP2A subunits are part of a constitutive BAK1 complex
To test if the subunits identified by reverse-genetics are indeed part of the BAK1-associated PP2A holoenzyme, we assessed the impact of the corresponding mutations on BAK1-associated PP2A activity. We found that this activity in the pp2a-a1, pp2a-c4, and pp2a-b’η mutants was similar to that in the null bak1-4 mutant (Fig4A), indicating that the A1, C4, and B’η subunits constitute the core of the PP2A holoenzyme associated with BAK1 in planta. The reduced BAK1-associated PP2A activity (Fig4A) together with the increased flg22-induced ROS production and resistance to Pto DC3000 (Fig3A and C) observed in the pp2a-b’ζ mutant suggest that this subunit may also be part of the holoenzyme.
Figure 4. The PP2A subunits A1, B’η, B’ζ, and C4 are part of a constitutive BAK1 complex.
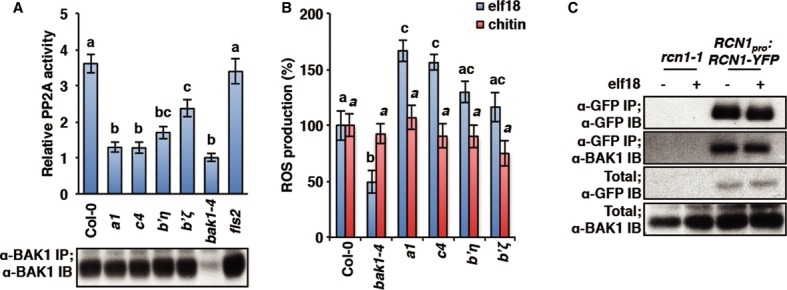
- BAK1-associated PP2A activity is impaired in pp2a mutants. PP2A activity (top) was detected in Col-0 and pp2a mutant protein extracts enriched with anti-BAK1 antibodies. PP2A activity is presented relative to the activity detected in bak1-4 protein extract as average of three biological repeats ± SE. Values labeled with different letters are statistically different as established by one-way ANOVA (P < 0.01). Equal amount of immunoprecipitated BAK1 (bottom) was assessed by immunoblot in the same samples.
- Specific PP2A subunits regulate BAK1-dependent PTI. ROS production in response to 100 nM elf18 or 1 mg/ml chitin was measured in pp2a mutant lines. Values presented are average of three biological repeats ± SE. Values labeled with different letters (regular and italic for elf18 and chitin treatment respectively) are statistically different as established by a one-way ANOVA (P < 0.01).
- PP2A-A1 (RCN1) subunit constitutively interacts with BAK1. PP2A/BAK1 interaction was detected by co-immunoprecipitation in rcn1-1 or rcn1-1/RCN1pro:RCN1-YFP protein extracts enriched on GFP-Trap beads. Seedlings were treated with water (−) or 100 nM elf18 (+) for 5 min.
Source data are available online for this figure.
Importantly, in accordance with a specific association of PP2A with BAK1 (Fig2C), we found that the pp2a-a1, pp2a-c4, and to a lesser extent pp2a-b’η and pp2a-b’ζ mutants were also hypersensitive to elf18, but not to chitin (Fig4B) whose responsiveness is BAK1-independent (Shan et al, 2008; Ranf et al, 2011).
In Arabidopsis seedlings, PP2A subunit A1 (RCN1) participates in most of PP2A activity (Deruere et al, 1999). We therefore tested the interaction between BAK1 and RCN1 by co-immunoprecipitation before and after elicitation with elf18. Consistent with Figs2C and 4A, BAK1 was detected in the RCN1 immunoprecipitate (using the rcn1-1/RCN1pro:RCN1-YFP transgenic line) independently of elf18 treatment (Fig4C). Interestingly, we found that, while PP2A does not dissociate from BAK1 upon elicitation (Fig4C and Supplementary Fig S7), BAK1-associated PP2A activity rapidly decreases by ˜50% upon flg22 treatment (Supplementary Fig S7B). Altogether, these data suggest that PP2A is constitutively associated with BAK1 and that the holoenzyme activity is attenuated upon ligand binding to the receptor complex.
PP2A negatively controls BAK1 phosphorylation status
Lastly, we investigated the impact of the PP2A association on BAK1 accumulation, its ligand-induced complex formation with FLS2 and its activation. The pp2a-a1, pp2a-c4, pp2a-b’η, and pp2a-b’ζ mutants accumulated similar amount of BAK1 protein as WT (Figs4A and 5A). Furthermore, co-immunoprecipitation experiments showed that flg22-induced complex formation with FLS2 is not affected in any of the pp2a mutants tested (Fig5A).
Figure 5. PP2A controls BAK1 activation in planta.
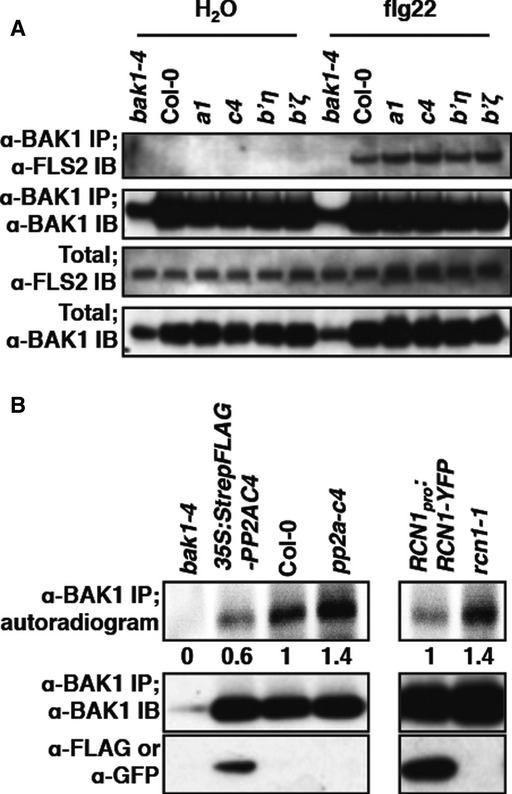
- pp2a mutants do not exhibit constitutive ligand-independent FLS2/BAK1 heteromerization. Ligand-dependent FLS2/BAK1 heteromerization was detected by co-immunoprecipitation and immunoblotting on pp2a mutant protein extracts enriched with anti-BAK1 antibodies, following 5 min of treatment with water or 100 nM flg22.
- BAK1 is hyper-activated in pp2a mutants. BAK1 kinase activity (top) was detected by incorporation of 32P on protein extracts enriched with anti-BAK1 antibodies. BAK1 activity is shown as relative band intensity below the autoradiogram. Equal amount of immunoprecipitated BAK1 (middle) and presence of tagged proteins (bottom) was assessed by immunoblot in the same samples.
Source data are available online for this figure.
We next analyzed BAK1 steady-state phosphorylation status, as BAK1 kinase activity is mainly controlled by its phosphorylation status (Oh et al, 2010). Interestingly, BAK1 kinase activity was increased by ˜40% in the pp2a-c4 mutant line and conversely reduced by ˜60% in the PP2A-C4 over-expressing line (Fig5B). A similar enhanced BAK1 activity was also observed in the rcn1-1 line compared to the rcn1-1/RCN1pro:RCN1-YFP complemented line (Fig5B). These results unveil a regulatory role of the C4 catalytic and A1 hook subunits in regulating BAK1 basal phosphorylation status.
Discussion
PAMP perception by LRR-containing PRRs leads to the rapid recruitment of the co-receptor BAK1 that acts as key mediator of immune signaling. Our findings suggest that PP2A negatively regulates the basal activity of BAK1 in the absence of stimulus, ultimately determining the intensity of the eventual PTI responses upon PAMP perception (Fig6). In the absence of elicitation, a PP2A potentially composed of the subunits A1, B’η/ζ, and C4 is associated with BAK1 and maintains low basal kinase activity. Upon ligand perception, PP2A most likely remains associated with BAK1 but its activity is rapidly attenuated, which ultimately allows increase in BAK1 kinase activity and consequent immune receptor activation. The primed immune responses observed in pp2a mutants could be due to increased phosphorylation upon elicitation of PRRs and/or PRR substrates, such as BIK1 and BSK1 (Lu et al, 2010; Zhang et al, 2010; Shi et al, 2013). Also, the enhanced BAK1 basal kinase activity in pp2a mutants may accelerate the release of BIR2 from BAK1, as this dissociation is BAK1 kinase activity-dependent (Halter et al, 2014), allowing a faster formation of an active BAK1-PRR immune complex upon low elicitation.
Figure 6. Model depicting the negative regulation of BAK1 activation by PP2A.
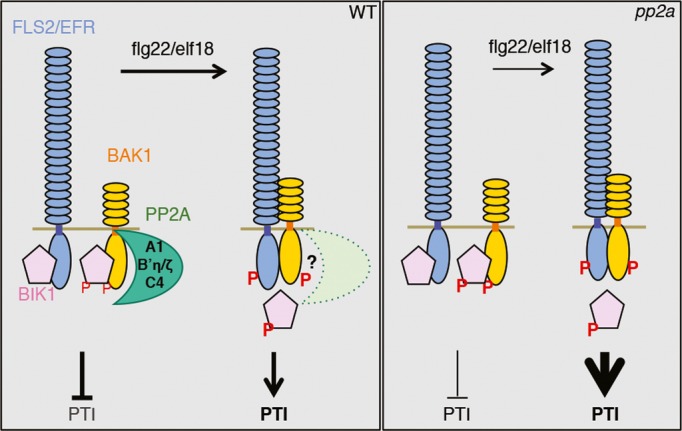
Interestingly, mutants in A1, C4 (which belongs to the subfamily II of catalytic subunits), or B’η/ζ subunits did not show any signs of constitutive immune responses and rather exhibit increased responsiveness to flg22 and elf18 (Fig3 and Supplementary Fig S5). This is consistent with the notion that the ‘primed’ BAK1 in these mutants still needs to form a ligand-induced complex with FLS2/EFR to activate downstream immune signaling. However, this is in contrast with the constitutive immune responses observed upon continuous cantharidin treatment (Fig1), or when knocking-down the subfamily I of catalytic subunits in Nicotiana benthamiana or PP2A-B’γ in Arabidopsis (He et al, 2004; Trotta et al, 2011), which suggests that other specific PP2A holoenzymes involving distinct subunits control additional steps of immune signaling. The composition of these heterocomplexes and their cellular target(s) remain to be determined.
The LRR-RKs BAK1 and the paralogous SERK proteins have emerged recently as key regulator of multiple pathways triggered by LRR-containing RKs and RLPs (Liebrand et al, 2014). Notably, BAK1 seems to exist in pre-formed complexes with ligand-binding receptors, which would explain the extremely rapid complex stabilization upon ligand binding (Schulze et al, 2010; Bucherl et al, 2013). Consistently, BAK1 was recently shown to act as a co-receptor for the LRR-RKs FLS2 and BRI1 (which is the BR receptor) forming direct interactions with both the ligand-bound receptors and the ligands (Santiago et al, 2013; Sun et al, 2013a,b). BAK1 is a constitutively highly active RD kinase capable of both auto- and trans-phosphorylation (Wang et al, 2008; Cheng et al, 2011; Schwessinger et al, 2011; Yan et al, 2012) and can spontaneously fold into an active kinase even in the absence of cellular context (Aan den Toorn et al, 2012). Thus, mechanisms that keep BAK1 activity under control must exist, although they are still poorly defined.
BRI1, another strong RD kinase, is negatively regulated by a combination of intramolecular inhibition, phosphorylation, and binding of inhibitory proteins such as BKI1 and PP2A (Wang et al, 2005, 2008; Wang & Chory, 2006; Jaillais et al, 2011; Oh et al, 2011, 2012; Wu et al, 2011). Recently, the C-terminal tail of BAK1 has been shown to regulate its activity both negatively and positively, and complex formation with BRI1 was proposed to relieve the inhibitory action of BAK1 C-terminal region (Oh et al, 2014).
Our work reveals PP2A as the first known inhibitory protein for BAK1. We show that PP2A constitutively associates with BAK1, but not with FLS2 or BIK1 in planta (Fig2). The fact that the BAK1-associated PP2A activity is abrogated in insertional mutants of the subunits A1, C4, and B’η (Fig4) suggests that these proteins constitute the core of the PP2A holoenzyme that associates with BAK1. This is further substantiated by the observation that BAK1 basal phosphorylation status is increased in the pp2a-c4 and rcn1-1 mutants, while it is reduced in a transgenic line over-expressing the C4 subunit (Fig5). Interestingly, the pp2a-a1, pp2a-c4, pp2a-b’η, and pp2a-b’ζ mutants displayed a similar amount of BAK1 protein as WT (Figs4A and 5A), which is in contrast to what has been observed previously with BRI1 whose degradation positively correlates with PP2A (Wu et al, 2011). Notably, the PP2A holoenzyme regulating BRI1 (whose exact composition is still unknown) does not affect BAK1 levels (Wu et al, 2011) further illustrating the specific roles played by distinct heteromeric PP2A enzymes.
The mechanisms by which PP2A negatively affects BAK1 phosphorylation status are, however, still unclear. An obvious possibility is that PP2A dephosphorylates important residues on BAK1. This hypotheses will be tested in future work, but the identification of in vivo BAK1 phosphosites playing roles in immunity is currently technically challenging due to the inhibitory impact of C-terminal immunological tags on BAK1 (Ntoukakis et al, 2011) and the poor protein coverage obtained by mass spectrometry after enrichment using native BAK1 antibodies (data not shown).
Interestingly, we observed that BAK1-associated PP2A activity is reduced by ˜50% within 2 min after flg22 treatment (Supplementary Fig S7B). We postulate that this inhibition is required to enable full immune signaling strength upon PAMP perception. PP2A activity can be regulated by post-translational modifications, such as phosphorylation, methylation or ubiquitination, ultimately affecting PP2A complex formation, stability or subcellular localization (Janssens et al, 2008; Virshup & Shenolikar, 2009). For example, BR perception leads to increased expression of the leucine carboxymethyltransferase SBI1 that methylates PP2A-C subunits (Wu et al, 2011). This methylation shifts the pool of PP2A toward the plasma membrane potentially leading to dephosphorylation and degradation of BR-activated BRI1 (Wu et al, 2011). Whether BRI1 is indeed a PP2A substrate remains to be determined.
We could not find any clear evidence for dissociation of the BAK1-RCN1 complex or for degradation of the A1 or C subunits upon PAMP treatment (Fig4C, Supplementary Fig S7 and data not shown). It will be interesting in the future to decipher the exact mechanisms underlying the inhibition of PP2A activity in response to PAMP perception.
Of note, PP2A has been previously implicated in both the negative and positive regulation of BR signaling at two distinct levels (Di Rubbo et al, 2011). PP2A can dephosphorylate the BR-activated LRR-RK BRI1 leading to its degradation (Wu et al, 2011). In addition, a PP2A enzyme potentially comprising the subunits A1, B’α, and B’β positively regulates BR-triggered responses by dephosphorylating the key transcriptional regulator BZR1, which enables its release from cytoplasmic 14-3-3 proteins and its transfer to the nucleus (Tang et al, 2011).
Interestingly, we found that the pp2a-a1 and pp2a-c4 mutants are slightly hypersensitive to exogenous BR treatment as measured by expression of BR marker genes (Supplementary Fig S6). However, we cannot completely exclude the possibility that the effect of the pp2a-a1 mutation may also be due to its previously described impact on the degradation of the ligand-activated BR receptor BRI1 (Wu et al, 2011). Importantly, our data suggest enhanced BR responsiveness in pp2a mutants, ruling out that the enhanced PTI responses in these lines are a direct consequence of the antagonism between BR and PTI signaling (Albrecht et al, 2012; Belkhadir et al, 2012; Lozano-Duran et al, 2013; Fan et al, 2014; Malinovsky et al, 2014).
In summary, our work reveals an important regulatory mechanism that fine-tunes PRR complex activation during innate immunity and illustrates a novel function of PP2A in the regulation of receptor kinase-based pathways. Given the central role of BAK1 in multiple receptor kinase complexes involved in immunity and other cellular processes, our findings have broad implications to understand and engineer plant adaptation to environmental stresses. Moreover, this work further illustrates how distinct PP2A holoenzymes have evolved to regulate multiple cellular processes.
Materials and Methods
Plant materials and growth conditions
The fls2, bak1-4, and bik1 pbl1 mutants have been described previously (Zipfel et al, 2004; Chinchilla et al, 2007; Zhang et al, 2010). The pp2a mutant lines used in this study are described in Supplementary Fig S3. Primers used to genotype the pp2a-b mutants are listed in Supplementary Table S1.
Arabidopsis plants used for ROS production measurement and infection assays were grown in soil at 21°C with a 10-h photoperiod. For Arabidopsis sterile seedlings, seeds were surface-sterilized and germinated on plates containing Murashige-Skoog medium (including vitamins; Duchefa) and 1% sucrose supplemented with 0.8% agar for the first 5 days at 22°C and with a 16-h photoperiod. Seedlings were then pricked out in liquid Murashige-Skoog medium supplemented with 1% sucrose.
Chemicals and elicitors
Elicitor peptides flg22 and elf18 were ordered from Peptron. Phosphatase inhibitor cantharidin was obtained from Enzo Life Sciences.
Measurement of ROS generation
Oxidative burst measurement was performed as described previously (Albrecht et al, 2012). ROS was elicited with cantharidin, flg22, or elf18, and mock elicitation was included in all experiments as negative control. Twelve leaf disks from 5-week-old plants were used for each condition. Luminescence was measured over time with a high-resolution photon counting system (HRPCS218; Photek).
RNA isolation and quantitative RT-PCR
Total RNA was prepared from 2-week-old seedlings grown in liquid medium. Total RNA was extracted using TRI reagent (Invitrogen) according to the manufacturer's instructions. RNA samples were treated with Turbo DNA-free DNase (Ambion) and quantified with a NanoDrop spectrophotometer (Thermo Scientific). First-strand cDNA was synthesized from 5 μg of RNA by using SuperScript RNA H-Reverse Transcriptase (Invitrogen) and an oligo(dT) primer, according to the manufacturer's instructions. cDNA were amplified in triplicate by quantitative PCR by using SYBR Green JumpStart Taq ReadyMix (Sigma) and the PTC-200 Peltier Thermal Cycler (MJ Research). The relative expression values were determined by using U-box gene (At5g15400) as reference and the comparative Ct method (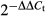 ). Primers used for quantitative PCR are listed in Supplementary Table S2.
). Primers used for quantitative PCR are listed in Supplementary Table S2.
Induced resistance and susceptibility to bacteria
Induced resistance assays were realized as described previously (Albrecht et al, 2012). Briefly, water, flg22, or cantharidin were infiltrated with a needleless syringe into leaves of 5-week-old Arabidopsis plants. After 24 h, the same leaves were syringe-infiltrated with 105 cfu/ml of Pto DC3000. Bacterial growth was determined 2 days after inoculation by plating serial dilutions of leaf extracts on L agar medium supplemented with appropriate antibiotics. To test susceptibility to Pto DC3000, 5-week-old plants were sprayed with a suspension of Pto DC3000 108 cfu/ml supplemented with 0.04% Silwett L-77 (Lehle seeds). Bacterial growth was determined 2 and 3 days after inoculation.
Protein extraction and immunoprecipitation
Protein extraction and immunoprecipitation using Arabidopsis seedlings were performed as described below (Kinase assay) and previously (Schwessinger et al, 2011).
Kinase assay
Two-week-old seedlings were treated with 1 μM flg22 or 1 μM elf18 and ground in liquid nitrogen. Proteins were extracted with 0.5 volume/weight of buffer [50 mM Tris–HCl, pH 7.5; 150 mM NaCl; 10% glycerol; 1 mM EDTA; 5 mM DTT; 1% (vol/vol) protease inhibitor cocktail (Sigma); 1% (vol/vol) Nonidet P-40, 2.5 mM Na3VO4, 50 nM calyculin A, 1 mM PMSF, 10 mM NaF, 5 mM Na2MoO4]. Samples were centrifuged 20 min at 4°C at 20,000 g. Supernatants were filtered and adjusted to 2–3 mg/ml protein; extracts were incubated with gentle agitation for 2 h at 4°C in the presence of 20 μl TrueBlot anti-rabbit Ig IP beads (eBioscience) and 15 μl anti-BAK1 antibodies. Beads were washed twice with washing buffer 1 (20 mM Tris–HCl, pH 7.5, 5 mM EDTA, 100 mM NaCl, 1% Nonidet P-40) and once with washing buffer 2 (20 mM Tris–HCl, pH 7.5, 5 mM EDTA, 1 M NaCl, 1% Nonidet P-40). Anti-BAK1 immunoprecipitates were washed once with kinase buffer (20 mM Tris–HCl, pH 7.5, 15 mM MgCl2, 5 mM EDTA, 1 mM DTT). Immunoprecipitates were finally incubated 30 min at 30°C and under vigorous shaking with 30 μl of kinase buffer supplemented with radioactive [32P]γ-ATP (183 kBq; Perkin-Elmer). The reactions were stopped by addition of 10 μl of NuPAGE 4× LDS sample buffer (Invitrogen) in presence of 1× reducing agent and denatured for 10 min at 70°C. Proteins were separated by SDS/PAGE 10% and analyzed by Western blot by using rabbit polyclonal anti-BAK1 antibodies. The membranes were subjected to autoradiography by using a FLA5000 PhosphorImager (Fuji).
Phosphatase assay
PP2A phosphatase activity present in immunoprecipitates was measured using a non-radioactive molybdate dye-based phosphatase assay kit (Promega) according to the manufacturer's instructions. The synthetic phosphopeptide, RRA[pT]VA, was used as the substrate. The reaction mixture (50 μl) contained PP2A buffer, 100 μM phosphopeptide substrate, and 3 mg/ml protein extract immunoprecipitated with anti-FLS2, anti-BAK1, or anti-HA antibodies. The reactions were incubated at 37°C for 1 h and stopped by adding 50 μl molybdate dye-additive. A standard curve for absorbance at 600 nm was prepared using 0, 2, 4, 10, 20, and 40 pmol inorganic phosphate solution. The phosphate released by the samples was then determined by extrapolating the absorbance at 600 nm against this standard curve.
Statistical analysis
All experiments were conducted in triplicate. Statistical significances based on one-way ANOVA analyses were determined with Prism 5.01 software (GraphPad).
Acknowledgments
We thank the John Innes Centre Horticultural Service for excellent technical assistance, Drs B. Schwessinger, Y. Kadota, J. Monaghan, and R. Lozano-Durán for critically reading the manuscript, all members of the Zipfel laboratory for fruitful discussions and helpful comments, and Drs J.-M. Zhou and A. DeLong for sharing biological materials. This research was funded by The Gatsby Charitable Foundation (CZ), the UK Biotechnology and Biological Sciences Research Council grant BB/G024944/1 (‘ERA-PG Pathonet’) (CZ), the European Research Council (CZ), and by the Spanish Ministry of Science and Innovation grant BIO2008-03052 (JJSS). APM was supported by a postdoctoral fellowship from the Federation of European Biochemical Societies. MS is supported by the Ramón y Cajal Researcher Programme of the Spanish Ministry of Economy and Competitivity.
Author contributions
CS and CZ designed research; CS, APM, and VN performed research; MS and JJSS contributed new materials; CS, APM, and VN analyzed data; CS and CZ wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information for this article is available online: http://emboj.embopress.org
References
- Aan den Toorn M, Huijbers MM, de Vries SC, van Mierlo CP. The Arabidopsis thaliana SERK1 kinase domain spontaneously refolds to an active state in vitro. PLoS ONE. 2012;7:e50907. doi: 10.1371/journal.pone.0050907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht C, Boutrot F, Segonzac C, Schwessinger B, Gimenez-Ibanez S, Chinchilla D, Rathjen JP, de Vries SC, Zipfel C. Brassinosteroids inhibit pathogen-associated molecular pattern-triggered immune signaling independent of the receptor kinase BAK1. Proc Natl Acad Sci U S A. 2012;109:303–308. doi: 10.1073/pnas.1109921108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JC, Bartels S, Gonzalez Besteiro MA, Shahollari B, Ulm R, Peck SC. Arabidopsis MAP Kinase Phosphatase 1 (AtMKP1) negatively regulates MPK6-mediated PAMP responses and resistance against bacteria. Plant J. 2011;67:258–268. doi: 10.1111/j.1365-313X.2011.04588.x. [DOI] [PubMed] [Google Scholar]
- Bajsa J, Pan Z, Duke SO. Transcriptional responses to cantharidin, a protein phosphatase inhibitor, in Arabidopsis thaliana reveal the involvement of multiple signal transduction pathways. Physiol Plant. 2011;143:188–205. doi: 10.1111/j.1399-3054.2011.01494.x. [DOI] [PubMed] [Google Scholar]
- Ballesteros I, Dominguez T, Sauer M, Paredes P, Duprat A, Rojo E, Sanmartin M, Sanchez-Serrano JJ. Specialized functions of the PP2A subfamily II catalytic subunits PP2A-C3 and PP2A-C4 in the distribution of auxin fluxes and development in Arabidopsis. Plant J. 2013;73:862–872. doi: 10.1111/tpj.12078. [DOI] [PubMed] [Google Scholar]
- Belkhadir Y, Jaillais Y, Epple P, Balsemao-Pires E, Dangl JL, Chory J. Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proc Natl Acad Sci U S A. 2012;109:297–302. doi: 10.1073/pnas.1112840108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucherl CA, van Esse GW, Kruis A, Luchtenberg J, Westphal AH, Aker J, van Hoek A, Albrecht C, Borst JW, de Vries SC. Visualization of BRI1 and BAK1(SERK3) membrane receptor heterooligomers during brassinosteroid signaling. Plant Physiol. 2013;162:1911–1925. doi: 10.1104/pp.113.220152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Low PS. Role of phosphorylation in elicitation of the oxidative burst in cultured soybean cells. Proc Natl Acad Sci U S A. 1995;92:4120–4123. doi: 10.1073/pnas.92.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Munkvold KR, Gao H, Mathieu J, Schwizer S, Wang S, Yan YB, Wang J, Martin GB, Chai J. Structural analysis of Pseudomonas syringae AvrPtoB bound to host BAK1 reveals two similar kinase-interacting domains in a type III Effector. Cell Host Microbe. 2011;10:616–626. doi: 10.1016/j.chom.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nurnberger T, Jones JD, Felix G, Boller T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- Dardick C, Schwessinger B, Ronald P. Non-arginine-aspartate (non-RD) kinases are associated with innate immune receptors that recognize conserved microbial signatures. Curr Opin Plant Biol. 2012;15:358–366. doi: 10.1016/j.pbi.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Deruere J, Jackson K, Garbers C, Soll D, Delong A. The RCN1-encoded A subunit of protein phosphatase 2A increases phosphatase activity in vivo. Plant J. 1999;20:389–399. doi: 10.1046/j.1365-313x.1999.00607.x. [DOI] [PubMed] [Google Scholar]
- Di Rubbo S, Irani NG, Russinova E. PP2A phosphatases: the “on-off” regulatory switches of brassinosteroid signaling. Sci Signal. 2011;4 doi: 10.1126/scisignal.2002046. pe25. [DOI] [PubMed] [Google Scholar]
- Ding Z, Wang H, Liang X, Morris ER, Gallazzi F, Pandit S, Skolnick J, Walker JC, Van Doren SR. Phosphoprotein and phosphopeptide interactions with the FHA domain from Arabidopsis kinase-associated protein phosphatase. Biochemistry. 2007;46:2684–2696. doi: 10.1021/bi061763n. [DOI] [PubMed] [Google Scholar]
- Fan M, Bai MY, Kim JG, Wang T, Oh E, Chen L, Park CH, Son SH, Kim SK, Mudgett MB, Wang ZY. The bHLH transcription factor HBI1 mediates the trade-off between growth and pathogen-associated molecular pattern-triggered immunity in Arabidopsis. Plant Cell. 2014;26:828–841. doi: 10.1105/tpc.113.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas I, Dombradi V, Miskei M, Szabados L, Koncz C. Arabidopsis PPP family of serine/threonine phosphatases. Trends Plant Sci. 2007;12:169–176. doi: 10.1016/j.tplants.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Felix G, Regenass M, Spanu P, Boller T. The protein phosphatase inhibitor calyculin A mimics elicitor action in plant cells and induces rapid hyperphosphorylation of specific proteins as revealed by pulse labeling with [33P]phosphate. Proc Natl Acad Sci U S A. 1994;91:952–956. doi: 10.1073/pnas.91.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gomez L, Bauer Z, Boller T. Both the extracellular leucine-rich repeat domain and the kinase activity of FSL2 are required for flagellin binding and signaling in Arabidopsis. Plant Cell. 2001;13:1155–1163. [PMC free article] [PubMed] [Google Scholar]
- Halter T, Imkampe J, Mazzotta S, Wierzba M, Postel S, Bucherl C, Kiefer C, Stahl M, Chinchilla D, Wang X, Nurnberger T, Zipfel C, Clouse S, Borst JW, Boeren S, de Vries SC, Tax F, Kemmerling B. The leucine-rich repeat receptor kinase BIR2 is a negative regulator of BAK1 in plant immunity. Curr Biol. 2014;24:134–143. doi: 10.1016/j.cub.2013.11.047. [DOI] [PubMed] [Google Scholar]
- He X, Anderson JC, del Pozo O, Gu YQ, Tang X, Martin GB. Silencing of subfamily I of protein phosphatase 2A catalytic subunits results in activation of plant defense responses and localized cell death. Plant J. 2004;38:563–577. doi: 10.1111/j.1365-313X.2004.02073.x. [DOI] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci U S A. 2007;104:12217–12222. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y, Hothorn M, Belkhadir Y, Dabi T, Nimchuk ZL, Meyerowitz EM, Chory J. Tyrosine phosphorylation controls brassinosteroid receptor activation by triggering membrane release of its kinase inhibitor. Genes Dev. 2011;25:232–237. doi: 10.1101/gad.2001911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens V, Longin S, Goris J. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail) Trends Biochem Sci. 2008;33:113–121. doi: 10.1016/j.tibs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, Ntoukakis V, Jones JD, Shirasu K, Menke F, Jones A, Zipfel C. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol Cell. 2014;54:43–55. doi: 10.1016/j.molcel.2014.02.021. [DOI] [PubMed] [Google Scholar]
- Kondo T, Kawai T, Akira S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012;33:449–458. doi: 10.1016/j.it.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Lee JS, Ellis BE. Arabidopsis MAPK phosphatase 2 (MKP2) positively regulates oxidative stress tolerance and inactivates the MPK3 and MPK6 MAPKs. J Biol Chem. 2007;282:25020–25029. doi: 10.1074/jbc.M701888200. [DOI] [PubMed] [Google Scholar]
- Li YM, Casida JE. Cantharidin-binding protein: identification as protein phosphatase 2A. Proc Natl Acad Sci U S A. 1992;89:11867–11870. doi: 10.1073/pnas.89.24.11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li M, Yu L, Zhou Z, Liang X, Liu Z, Cai G, Gao L, Zhang X, Wang Y, Chen S, Zhou JM. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe. 2014a;15:329–338. doi: 10.1016/j.chom.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Li S, Mhamdi A, Trotta A, Kangasjarvi S, Noctor G. The protein phosphatase subunit PP2A-B'gamma is required to suppress day length-dependent pathogenesis responses triggered by intracellular oxidative stress. New Phytol. 2014b;202:145–160. doi: 10.1111/nph.12622. [DOI] [PubMed] [Google Scholar]
- Liebrand TW, van den Burg HA, Joosten MH. Two for all: receptor-associated kinases SOBIR1 and BAK1. Trends Plant Sci. 2014;19:123–132. doi: 10.1016/j.tplants.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Lin W, Lu D, Gao X, Jiang S, Ma X, Wang Z, Mengiste T, He P, Shan L. Inverse modulation of plant immune and brassinosteroid signaling pathways by the receptor-like cytoplasmic kinase BIK1. Proc Natl Acad Sci U S A. 2013;110:12114–12119. doi: 10.1073/pnas.1302154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Li B, Lu D, Chen S, Zhu N, He P, Shan L. Tyrosine phosphorylation of protein kinase complex BAK1/BIK1 mediates Arabidopsis innate immunity. Proc Natl Acad Sci U S A. 2014;111:3632–3637. doi: 10.1073/pnas.1318817111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Duran R, Macho AP, Boutrot F, Segonzac C, Somssich IE, Zipfel C. The transcriptional regulator BZR1 mediates trade-off between plant innate immunity and growth. eLife. 2013;2:e00983. doi: 10.7554/eLife.00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Wu S, Gao X, Zhang Y, Shan L, He P. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc Natl Acad Sci U S A. 2010;107:496–501. doi: 10.1073/pnas.0909705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Lin W, Gao X, Wu S, Cheng C, Avila J, Heese A, Devarenne TP, He P, Shan L. Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science. 2011;332:1439–1442. doi: 10.1126/science.1204903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho AP, Zipfel C. Plant PRRs and the activation of innate immune signaling. Mol Cell. 2014;54:263–272. doi: 10.1016/j.molcel.2014.03.028. [DOI] [PubMed] [Google Scholar]
- MacKintosh C, Lyon GD, MacKintosh RW. Protein phosphatase inhibitors activate anti-fungal defence responses of soybean cotyledons and cell cultures. Plant J. 1994;5:137–147. [Google Scholar]
- Malinovsky FG, Batoux M, Schwessinger B, Youn JH, Stransfeld L, Win J, Kim SK, Zipfel C. Antagonistic regulation of growth and immunity by the Arabidopsis Basic helix-loop-helix transcription factor homolog of brassinosteroid enhanced expression2 interacting with increased leaf inclination1 binding bHLH1. Plant Physiol. 2014;164:1443–1455. doi: 10.1104/pp.113.234625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, Heisler MG, Ohno C, Zhang J, Huang F, Schwab R, Weigel D, Meyerowitz EM, Luschnig C, Offringa R, Friml J. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell. 2007;130:1044–1056. doi: 10.1016/j.cell.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Monaghan J, Zipfel C. Plant pattern recognition receptor complexes at the plasma membrane. Curr Opin Plant Biol. 2012;15:349–357. doi: 10.1016/j.pbi.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Ntoukakis V, Schwessinger B, Segonzac C, Zipfel C. Cautionary notes on the use of C-terminal BAK1 fusion proteins for functional studies. Plant Cell. 2011;23:3871–3878. doi: 10.1105/tpc.111.090779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MH, Wang X, Wu X, Zhao Y, Clouse SD, Huber SC. Autophosphorylation of Tyr-610 in the receptor kinase BAK1 plays a role in brassinosteroid signaling and basal defense gene expression. Proc Natl Acad Sci U S A. 2010;107:17827–17832. doi: 10.1073/pnas.0915064107. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Oh MH, Sun J, Oh DH, Zielinski RE, Clouse SD, Huber SC. Enhancing Arabidopsis leaf growth by engineering the BRASSINOSTEROID INSENSITIVE1 receptor kinase. Plant Physiol. 2011;157:120–131. doi: 10.1104/pp.111.182741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MH, Wang X, Clouse SD, Huber SC. Deactivation of the Arabidopsis brassinosteroid insensitive 1 (BRI1) receptor kinase by autophosphorylation within the glycine-rich loop. Proc Natl Acad Sci U S A. 2012;109:327–332. doi: 10.1073/pnas.1108321109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MH, Wang X, Kim SY, Wu X, Clouse SD, Huber SC. The Carboxy-terminus of BAK1 regulates kinase activity and is required for normal growth of Arabidopsis. Front Plant Sci. 2014;5:16. doi: 10.3389/fpls.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Park CJ, Peng Y, Chen X, Dardick C, Ruan D, Bart R, Canlas PE, Ronald PC. Rice XB15, a protein phosphatase 2C, negatively regulates cell death and XA21-mediated innate immunity. PLoS Biol. 2008;6:e231. doi: 10.1371/journal.pbio.0060231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranf S, Eschen-Lippold L, Pecher P, Lee J, Scheel D. Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage-associated molecular patterns. Plant J. 2011;68:100–113. doi: 10.1111/j.1365-313X.2011.04671.x. [DOI] [PubMed] [Google Scholar]
- Robatzek S, Chinchilla D, Boller T. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 2006;20:537–542. doi: 10.1101/gad.366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald PC, Beutler B. Plant and animal sensors of conserved microbial signatures. Science. 2010;330:1061–1064. doi: 10.1126/science.1189468. [DOI] [PubMed] [Google Scholar]
- Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tor M, de Vries S, Zipfel C. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell. 2011;23:2440–2455. doi: 10.1105/tpc.111.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J, Henzler C, Hothorn M. Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co-receptor kinases. Science. 2013;341:889–892. doi: 10.1126/science.1242468. [DOI] [PubMed] [Google Scholar]
- Sasai M, Yamamoto M. Pathogen recognition receptors: ligands and signaling pathways by Toll-like receptors. Int Rev Immunol. 2013;32:116–133. doi: 10.3109/08830185.2013.774391. [DOI] [PubMed] [Google Scholar]
- Schulze B, Mentzel T, Jehle AK, Mueller K, Beeler S, Boller T, Felix G, Chinchilla D. Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J Biol Chem. 2010;285:9444–9451. doi: 10.1074/jbc.M109.096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer A, Hirt H, Meskiene I. Plant PP2C phosphatases: emerging functions in stress signaling. Trends Plant Sci. 2004;9:236–243. doi: 10.1016/j.tplants.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Schweighofer A, Kazanaviciute V, Scheikl E, Teige M, Doczi R, Hirt H, Schwanninger M, Kant M, Schuurink R, Mauch F, Buchala A, Cardinale F, Meskiene I. The PP2C-type phosphatase AP2C1, which negatively regulates MPK4 and MPK6, modulates innate immunity, jasmonic acid, and ethylene levels in Arabidopsis. Plant Cell. 2007;19:2213–2224. doi: 10.1105/tpc.106.049585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B, Roux M, Kadota Y, Ntoukakis V, Sklenar J, Jones A, Zipfel C. Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet. 2011;7:e1002046. doi: 10.1371/journal.pgen.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B, Ronald PC. Plant innate immunity: perception of conserved microbial signatures. Annu Rev Plant Biol. 2012;63:451–482. doi: 10.1146/annurev-arplant-042811-105518. [DOI] [PubMed] [Google Scholar]
- Shan L, He P, Li J, Heese A, Peck SC, Nurnberger T, Martin GB, Sheen J. Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe. 2008;4:17–27. doi: 10.1016/j.chom.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Shi H, Shen Q, Qi Y, Yan H, Nie H, Chen Y, Zhao T, Katagiri F, Tang D. BR-Signaling KinaSE1 physically associates with Flagellin Sensing2 and regulates plant innate immunity in Arabidopsis. Plant Cell. 2013;25:1143–1157. doi: 10.1105/tpc.112.107904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skottke KR, Yoon GM, Kieber JJ, DeLong A. Protein phosphatase 2A controls ethylene biosynthesis by differentially regulating the turnover of ACC synthase isoforms. PLoS Genet. 2011;7:e1001370. doi: 10.1371/journal.pgen.1001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JM, Salamango DJ, Leslie ME, Collins CA, Heese A. Sensitivity to Flg22 is modulated by ligand-induced degradation and de novo synthesis of the endogenous flagellin-receptor FLAGELLIN-SENSING2. Plant Physiol. 2014;164:440–454. doi: 10.1104/pp.113.229179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Han Z, Tang J, Hu Z, Chai C, Zhou B, Chai J. Structure reveals that BAK1 as a co-receptor recognizes the BRI1-bound brassinolide. Cell Res. 2013a;23:1326–1329. doi: 10.1038/cr.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Li L, Macho AP, Han Z, Hu Z, Zipfel C, Zhou JM, Chai J. Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science. 2013b;342:624–628. doi: 10.1126/science.1243825. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Shinshi H. Transient activation and tyrosine phosphorylation of a protein kinase in tobacco cells treated with a fungal elicitor. Plant Cell. 1995;7:639–647. doi: 10.1105/tpc.7.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Yuan M, Wang R, Yang Y, Wang C, Oses-Prieto JA, Kim TW, Zhou HW, Deng Z, Gampala SS, Gendron JM, Jonassen EM, Lillo C, DeLong A, Burlingame AL, Sun Y, Wang ZY. PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat Cell Biol. 2011;13:124–131. doi: 10.1038/ncb2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta A, Wrzaczek M, Scharte J, Tikkanen M, Konert G, Rahikainen M, Holmstrom M, Hiltunen HM, Rips S, Sipari N, Mulo P, Weis E, von Schaewen A, Aro EM, Kangasjarvi S. Regulatory subunit B'gamma of protein phosphatase 2A prevents unnecessary defense reactions under low light in Arabidopsis. Plant Physiol. 2011;156:1464–1480. doi: 10.1104/pp.111.178442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng TS, Briggs WR. The Arabidopsis rcn1-1 mutation impairs dephosphorylation of Phot2, resulting in enhanced blue light responses. Plant Cell. 2010;22:392–402. doi: 10.1105/tpc.109.066423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrig RG, Labandera AM, Moorhead GB. Arabidopsis PPP family of serine/threonine protein phosphatases: many targets but few engines. Trends Plant Sci. 2013;18:505–513. doi: 10.1016/j.tplants.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Virshup DM, Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Mol Cell. 2009;33:537–545. doi: 10.1016/j.molcel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Wang X, Li X, Meisenhelder J, Hunter T, Yoshida S, Asami T, Chory J. Autoregulation and homodimerization are involved in the activation of the plant steroid receptor BRI1. Dev Cell. 2005;8:855–865. doi: 10.1016/j.devcel.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Wang X, Chory J. Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling, from the plasma membrane. Science. 2006;313:1118–1122. doi: 10.1126/science.1127593. [DOI] [PubMed] [Google Scholar]
- Wang X, Kota U, He K, Blackburn K, Li J, Goshe MB, Huber SC, Clouse SD. Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev Cell. 2008;15:220–235. doi: 10.1016/j.devcel.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Wu G, Wang X, Li X, Kamiya Y, Otegui MS, Chory J. Methylation of a phosphatase specifies dephosphorylation and degradation of activated brassinosteroid receptors. Sci Signal. 2011;4 doi: 10.1126/scisignal.2001258. ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wei X, Yan L, Liu D, Ma Y, Guo Y, Peng C, Zhou H, Yang C, Lou Z, Shui W. Identification and functional analysis of phosphorylation residues of the Arabidopsis botrytis-induced kinase1. Protein Cell. 2013;4:771–781. doi: 10.1007/s13238-013-3053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Ma Y, Liu D, Wei X, Sun Y, Chen X, Zhao H, Zhou J, Wang Z, Shui W, Lou Z. Structural basis for the impact of phosphorylation on the activation of plant receptor-like kinase BAK1. Cell Res. 2012;22:1304–1308. doi: 10.1038/cr.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li W, Xiang T, Liu Z, Laluk K, Ding X, Zou Y, Gao M, Zhang X, Chen S, Mengiste T, Zhang Y, Zhou JM. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe. 2010;7:290–301. doi: 10.1016/j.chom.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Zhu JY, Sae-Seaw J, Wang ZY. Brassinosteroid signalling. Development. 2013;140:1615–1620. doi: 10.1242/dev.060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, Boller T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764–767. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


