Abstract
The hippocampus is required for encoding spatial information. Little is known however, about how different attributes of learning are related to different types of synaptic plasticity. Here, we investigated the association between long-term depression (LTD) and long-term potentiation, both cellular models for learning, and novelty exploration. We found that exploration of a new environment containing unfamiliar objects and/or familiar objects in new locations facilitated LTD, whereas exploration of the new environment itself, in the absence of objects, impaired LTD. Furthermore, we found this phenomenon to be modulated by 5-hydroxytryptamine 4 receptor activation. In contrast, long-term potentiation was facilitated by exploration of an empty novel environment, but simultaneous object exploration caused depotentiation. We also found that no further LTD could be induced. These findings support a decisive role for LTD in the acquisition of object–place configuration and consolidate its candidacy as a learning mechanism.
The hippocampus is a key structure for formation of spatial memory (1). It has been proposed, however, that it may also contribute to certain forms of nonspatial memory (2, 3). While the debate continues, several investigations indicate that the hippocampus is involved in pairing configuration of stimuli, i.e., objects with location (4, 5). Furthermore, learning the sequential order of events requires an intact hippocampus (6); this corresponds to its proposed role in episodic memory in humans (7). In terms of recognition memory, a dissociation between hippocampus and the respective cortical area occurs (8); the perirhinal cortex encodes object recognition (9) and the piriform cortex encodes odor recognition (10). Although it may not contribute to the recognition of the distinct features characterizing stimuli, the hippocampus may act as a novelty detector, conducting mismatch predictions by comparing stored information with new incoming cues (11). The mechanism for encoding this type of information is as yet unidentified.
Memory is achieved by experience-dependent changes in synaptic strength. These changes can take the form of persistent enhancements (long-term potentiation, or LTP), or long-term depressions (LTD), of synaptic transmission. It has been proposed that LTD, working together with LTP, underlies storage of memory (12). Whereas a certain number of studies have investigated the correlation between LTP and memory (13–15), very few investigations have addressed what possible role LTD may have in memory formation. Little is known about whether LTD plays an independent role, thus underlying certain distinctive types of information storage, or whether it merely enhances the signal-to-noise ratio or alternatively functions as the means to erase stored engrams (16). An association has been demonstrated between LTD and the acquisition of novel information (17). Whereas low-frequency stimulation during exploration of a novel holeboard, containing novel objects, resulted in LTD, LTD was not inducible either without holeboard exploration or by exploration of a familiar holeboard. Thus, a clear relationship between LTD and novelty acquisition is evident. Novelty acquisition is by nature multimodal and consists of many different types of information. Mapping of allocentric space, features of objects, and location of objects each may rely on different types of networks as well as differences in synaptic plasticity. The information perceived might also have differential salience, which contributes to the strength of the memory trace (18).
This study set out to investigate the hypothesis that only a part of novelty acquisition is correlated to the facilitation of LTD. We found that exploration of a novel space had an inhibitory effect on LTD induction, which was overcome if novel object exploration occurred simultaneously. This was regulated by 5-hydroxytryptamine 4 (5-HT4) receptor activation. We also found a distinct correlation between LTD and the location of the objects, suggesting that LTD may encode the novel acquisition of object location rather than the novel objects themselves. On the other hand, we found that LTP was facilitated by spatial exploration but was inhibited when new objects were present. This depotentiation of LTP occluded further LTD induction. This indicates that, irrespective of stimulation protocol, the same cellular mechanism is activated by novel object exploration. Furthermore, it suggests that the CA1 region is more involved in novel object–place associations than in mapping space.
Methods
Electrophysiology. Male Wistar rats (Harlan Winkelmann, Borchen, Germany) (7–8 weeks old at time of surgery) had electrodes and a guide cannula implanted under anesthesia (Pentobarbital, 52 mg/kg), as described (19). After surgery, the animals were housed in single cages and were allowed 7–10 days of recovery before the experiments began.
Recordings were obtained in CA1 stratum radiatum by stimulation of Schaffer collaterals.
To determine the stimulus intensity that evoked field excitatory postsynaptic potentials (EPSPs), which were 40% of the maximum, every experiment was commenced with the recording of an input/output curve. Test EPSPs were evoked at a frequency of 0.025 Hz. Each time point was the average of five consecutive stimulations. The first six data points recorded served as baseline, and all data were expressed as mean percentage ± SEM of average baseline value. To ensure that there was no drift in the response to stimulation, control experiments were conducted where basal synaptic transmission was evoked by test pulses and followed for the same duration as plasticity experiments. Low-frequency stimulation (LFS) to elicit synaptic depression consisted of 900 pulses at 1 Hz. During LFS, the stimulus strength was raised to 70% of maximum. In this study, we defined LTD as a depression that endured for >4 h. Two high-frequency tetanus (HFT) protocols were used. To induce short-term potentiation, 1 train of 100 pulses at 100 Hz was given (weak HFT). To induce LTP (>4 h), 4 trains of 30 pulses at 100 Hz, with an intertrain interval of 5 min, were given. Statistical evaluations were performed by using ANOVA (repeated measures), and t tests were used to assess differences among individual time points. The level of significance was set to P < 0.05.
Drugs. The 5-HT4 receptor agonist RS67333 (Tocris Cookson, St. Louis) was dissolved in water to a concentration of 2 μg/μl. A volume of 5 μl was injected over a period of 5 min. In both the electrophysiological experiments and the behavioral experiment, injections were administered 30 min before holeboard exposure.
Novelty Exploration. To enable acclimatization, animals were always placed in the room where experiments were performed on the day before experimentation. The recording chamber measured 40 × 40 × 40 cm. It was constructed of gray Perspex, except for the removable front wall, which was made of clear Perspex. The boxes were open at the top. The implanted electrodes were connected by a flexible cable and swivel connector to the stimulation and recording equipment; thus, the animal could move around freely in the recording chamber. Each animal was assigned one recording chamber where all experiments were conducted. Plasticity experiments in the absence of a holeboard were always carried out a minimum of 8 days before holeboard experiments. In subsequent experiments, a holeboard (39.8 × 39.8 cm, washable blue plastic) was inserted into the floor of the recording chamber. This was done just before application of LFS/HFT and after baseline recordings. The holeboard was removed immediately after LFS/HFT (or after 15 min, in experiments where weak HFT was applied). Each corner of the holeboard corner contained a hole, 5.5 cm in diameter and 5 cm deep. An object was usually placed in each hole. The objects differed from each other in appearance and size and easily fitted within the holes. Each animal was always presented with the same four objects, except in the series where no objects were present (empty holeboard; see Results).
Behavioral Experiment. Sixteen animals were used. The holeboard used consisted of a gray Perspex box (80 × 80 × 80 cm). Each holeboard corner contained a hole (5.5 cm diameter and 4.5 cm deep) that contained objects. Two trials of 15 min duration were conducted at a 24-h interval. On the day before the first trial, the animals were placed in the experiment room and given an intracerebroventricle injection of 5 μl of saline to acclimatize them to the injection procedure. Thirty minutes before the first trial, eight rats received 5 μl of RS67333 (2 μg/μl); the remaining rats were given saline. These injections were administered blindly. The experimenter recorded the occurrence of rearings and head dips in the holes. No injections were given before reexposure to the holeboard.
Results
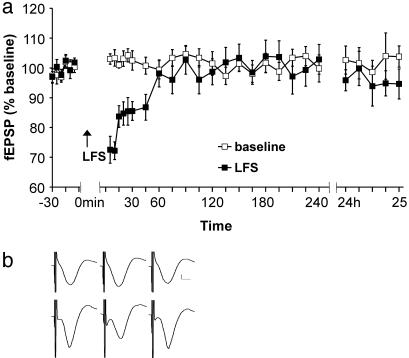
LFS Induces Short-Term Depression in the CA1 Region of Freely Moving Rats. The degree of LTD elicited by LFS is known to depend on rat strain (20). The rat strain used in this study, Harlan Winkelmann Wistar, consistently showed a depression of synaptic transmission after LFS at 1 Hz, which endured for ≈45 min (Fig. 1; n = 16, P < 0.001).
Fig. 1.
LFS does not induce LTD. (a) Application of LFS (arrow) induced short-term depression compared with test-pulse stimulated controls. Line breaks indicate changes in time scale. (b) Analog traces of averaged fEPSPs taken at t =–5 min, t = 5 min, and t = 24 h after LFS. (Upper) Baseline controls. (Lower) An LFS experiment. Vertical scale bar corresponds to 0.5 mV, and horizontal bar corresponds to 5 ms.
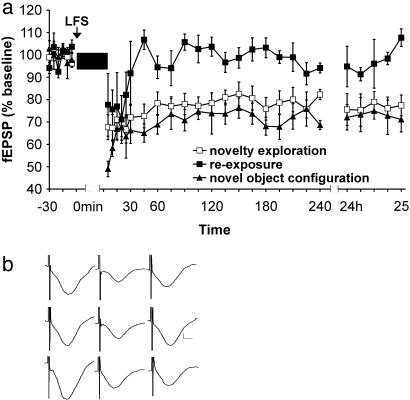
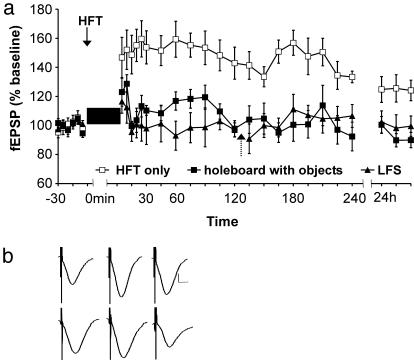
Exploration of a Holeboard Containing Objects During LFS Induces Robust LTD. At least 8 days after the control LFS experiments, the effect of novelty exposure on LFS-elicited responses was examined. Previous experiments showed that Hooded Lister rats express LTD when LFS is given simultaneously with novelty exposure (17, 20). We repeated this protocol using Harlan Winkelmann Wistar rats. The animals were allowed to explore a holeboard with four different objects during LFS (Fig. 2, n = 10). The insertion of the holeboard caused enhanced explorative behavior, and in only one case were signs of stress, i.e., freezing behavior, observed. This rat was excluded from data analysis. The exploration of the object-containing holeboard converted short-term depression into an LTD that lasted for at least 25 h [ANOVA: F (1,30) = 151.69, P < 0.0001 compared to LFS application in controls]. Individual time-point differences were assessed by t test analysis of LFS given alone and LFS given concurrently with holeboard exposure. This revealed a significantly enhanced depression in the holeboard group from t = 15 min (P < 0.05).
Fig. 2.
Exposure to a novel holeboard with objects facilitates LTD. (a) LFS given simultaneously with holeboard exposure (indicated by black box) facilitated LTD upon first-time exposure (□) or if the objects were repositioned (▴). No facilitation occurred upon reexposure to the holeboard containing the same object configuration as in the first exposure (▪). (b) Analog traces representing novelty exploration (Top), reexposure (Middle), and novel object configuration (Bottom). They illustrate (from right to left) levels before LFS, after LFS, and 24 h after LFS. Vertical scale bar corresponds to 0.5 mV, and horizontal bar corresponds to 5 ms.
To investigate whether the facilitatory effect of novelty exploration on LTD really was due to novelty acquisition and not to a nonmnemonic effect such as enhanced locomotor activity (which could elicit brain temperature-related changes in the magnitude of potentials) (21), we subsequently reexposed the animals to the same holeboard with the same constellation of objects after LTD had returned to pre-LFS levels (Fig. 2, reexposure; n = 5). The exposure to a now-familiar holeboard during LFS application no longer elicited LTD. A transient depression occurred, however, which declined faster than after LFS application in control experiments (t = 45 min; P < 0.05, t test; n = 5). From t = 60 min, there was a significant difference that occurred between first-time exposure and reexposure to the holeboard (P < 0.0019, t test). ANOVA revealed a highly significant habituation effect to the holeboard [F (1,30) = 101.56; P < 0.0001; reexposure compared to first-time exposure].
The hypothesis underlying this study is that LTD correlates with a specific part of novelty acquisition, such as detection of object location within space, as opposed to exploration of allocentric space or object recognition. To test whether this is true, we subjected the rats to a further experiment. The same holeboard was inserted into the recording chamber, but the objects were now randomly positioned in a constellation of holes that differed from the previous constellation. Thus, on this occasion, the only novel variable was the different location of the objects; familiarization with the holeboard had already occurred (Fig. 2, novel object configuration). With this configuration, intriguingly, LTD was once again induced [ANOVA: F (1,30) = 141.70, P < 0.0001, n = 5]. The field EPSP (fEPSP) slope values 5 min after LFS were 49.0% ± 3.5%, significantly lower than values seen after the first exposure to the holeboard (P < 0.05, t test). Thus, induction of LTD elicited by exposure to a novel spatial constellation of familiar objects was stronger than that elicited by a novel constellation of novel objects. No other differences in the profile of LTD were noted, however, and maintenance of LTD was not changed. These findings strongly indicate that LTD is associated with the spatial mapping of objects and not spatial exploration per se.
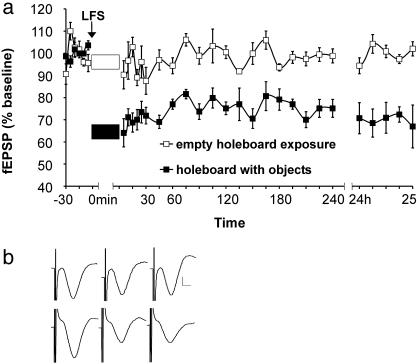
Spatial Exploration Is Not Sufficient to Trigger LTD. The next question we addressed was whether exploration of the objects was the sole contributor to the LTD observed. To explore this, we assigned the remainder of our animals to novel exploration of the holeboard in the absence of objects. Thus, we could examine the effect that novel acquisition of an unfamiliar environment (i.e., holeboard) has on LTD, without any masking effects from exploration of the object and their spatial configuration. When LFS was applied during the presence of the empty holeboard, it resulted in an impaired level of depression (Fig. 3a, n = 6); there was significantly less depression when the empty holeboard were present during LFS as observed in the control-LFS experiment [ANOVA: F (1,30) = 4.91, P = 0.0263]. Thus, exploration of the empty holeboard inhibited the induction of short-term depression (first three recordings after LFS, P < 0.01). In this case, not only was the LFS insufficient for induction of LTD, exploration of the novel holeboard also counteracted the effect of LFS. In the next experiment, the same group of animals was exposed to the now familiar holeboard, but this time in the presence of objects (Fig. 3, holeboard with objects, n = 6). Under these conditions, LFS facilitated the induction of LTD [ANOVA: F (1,30) = 307.91, P < 0.001, object present compared to empty holeboard, n = 6 in both experiments]. The LTD elicited did not significantly differ from that observed when the rats were allowed to explore an unfamiliar holeboard with novel objects for the first time. This implies that habituation to the holeboard alone did not affect LTD induction and further supports the likelihood that LTD is associated with exploration of novel items.
Fig. 3.
Exploration of empty holeboard inhibits LTD. (a) Concurrent with LFS, the animals were either exposed to an empty holeboard (white box) or a holeboard containing objects (black box). (b) Analog traces (Upper) represent levels before, after, and 24 h after LFS from a rat exposed to the empty holeboard. (Lower) Traces recorded from an experiment with an object-containing holeboard. Vertical scale bar corresponds to 0.5 mV, and horizontal bar corresponds to 5 ms.
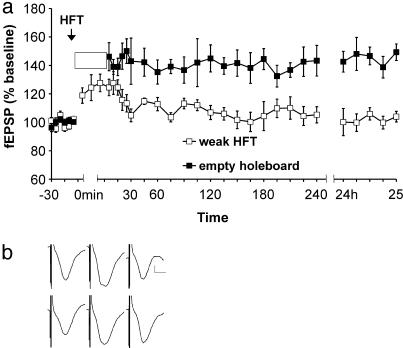
Spatial Exploration but Not Object Exploration Facilitates LTP. We were intrigued by the apparently inhibitory effect spatial exploration had on LTD induction. To further examine this effect, we repeated the above experiment with a new set of animals, but instead of LFS we now gave a weak HFT (100 pulses at 100 Hz), which under normal conditions produced short-term potentiation (Fig. 4a). HFT was applied immediately after the rats were introduced to the holeboard (i.e., t = 0 min); the animals were allowed to explore the holeboard for 15 min to make it comparable with the corresponding LFS experiment. Exposure to the holeboard significantly facilitated LTP (n = 9) compared to controls (n = 9) [ANOVA: F (1,30) = 230.37, P < 0.0001]. Twenty-four hours later, no potentiation was evident in animals that had not been exposed to the holeboard. However, fEPSP values were still potentiated in holeboard-exposed animals (P < 0.01, t test). Thus, the inhibiting effect of spatial exploration on LTD, which we observed, may be correlated to a shift toward a higher susceptibility for LTP.
Fig. 4.
Exploration of empty holeboard facilitates LTP. (a) Weak HFT (100 pulses at 100 Hz) given at the time point indicated by the arrow induces short-term potentiation. Weak HFT given at the beginning of 15 min of empty holeboard exploration (white box) facilitates short-term potentiation into LTP. (b) Analog traces (Upper) represent potentials recorded before HFT, 20 min after HFT, and 24 h after HFT, in an HFT experiment. (Lower) fEPSPs observed before HFT, 5 min after holeboard removal (i.e., 20 min after HFT), and 24 h after HFT. Vertical scale bar corresponds to 0.5 mV, and horizontal bar corresponds to 5 ms.
If exploration of a new environment and exploration of cues within this environment relies on opposite changes in synaptic strength, it could be hypothesized that the threshold for induction of LTP is raised during exploration of novel objects. We investigated this possibility by giving a strong HFT (4 trains of 30 pulses at 100 Hz, at 5-min intertrain intervals) during exploration of the holeboard (Fig. 5a). The tetanus induced LTP in the rats under control conditions. When the holeboard was present, it resulted in a depotentiation of LTP. Interestingly, this did not happen immediately. In the first 10 min after the removal of the holeboard, there was no significant difference between control HFT responses and HFT responses under holeboard exploration. The fEPSPs were initially potentiated to 146.7% ± 13.8% of baseline value without holeboard (n = 9) and 123.0% ± 9.7% with holeboard exploration (5 min after HFT, n = 6). When the rats had been exposed to the holeboard, a rapid depotentiation occurred 15 min after HFT (P < 0.001, t test). This depotentiation was long-lasting, and 24 h after HFT the value was 100.5% ± 5.7%. ANOVA revealed a significant effect of hole-board exploration on the HFT-induced LTP [F (1,28) = 155.16, P < 0.0001].
Fig. 5.
LTP is depotentiated by object exploration. (a) LTP was induced by strong HFT (4 trains of 30 pulses at 100 Hz with 5-min intertrain intervals, downward arrow) in animals in a familiar recording chamber. Exploration of a novel object-containing holeboard depotentiated LTP (▪). LFS applied 120 min after HFT (indicated by dashed upward arrow) did not elicit any synaptic depression (▴). (b) Analog traces (Upper) represent fEPSPs from an experiment without holeboard exploration before HFT, after HFT, and 24 h after HFT. (Lower) Analogs obtained at similar time points when novel exploration had occurred. Vertical scale bar corresponds to 0.5 mV, and horizontal bar corresponds to 5 ms.
If the observed depotentiation shares mechanistic properties with object-induced LTD, it could be expected that LFS applied after depotentiation would not cause further synaptic depression. We applied LFS2 h after depotentiation had been induced by holeboard exploration in HFT animals. This time point for application of LFS was chosen because there was a stabilization of the depotentiation at that time (n = 5). No significant LTD was induced by LFS under these conditions [ANOVA: F (1,28) = 0.94, P > 0,05], suggesting that common mechanisms are shared by LTD and depotentiation induced by holeboard exposure.
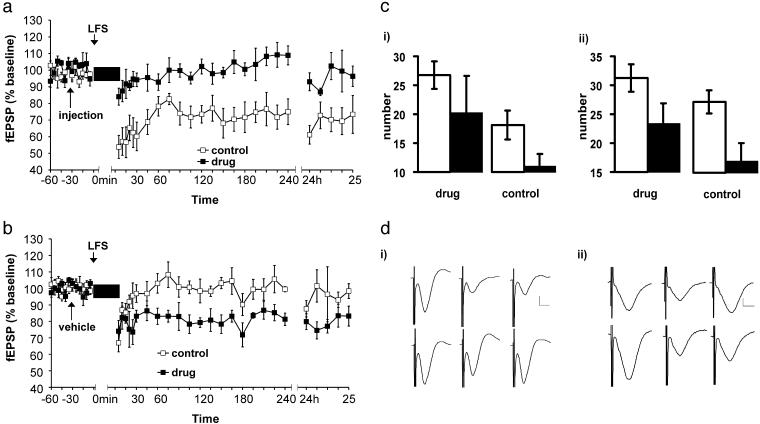
LTD Induced by Object Exploration Is Modulated by 5-HT4 Receptors. Activation of 5-HT4 receptors has an inhibitory effect on electrically induced LTD (unpublished data). Furthermore, a role for the 5-HT4 receptor has been described for multiple cognitive functions in the brain (22–25). Thus, activation of 5-HT4 receptors may also impair learning of object–place associations. A group of rats (n = 5) received an intracerebral injection of 5 μl of RS67333 (2 μg/μl), a selective 5-HT4 agonist, 30 min before they were introduced to the object-containing holeboard. A control group (n = 5) received the same amount of saline. The activation of 5-HT4 receptors completely blocked the exploration-induced expression of LTD (Fig. 6a) [ANOVA: F (1,24) = 169.8; P < 0.0001].
Fig. 6.
RS67333 inhibits exploration-induced LTD and habituation to novel holeboard. (a) LFS applied under novel exploration induced LTD when animals were injected with saline but not when injected with 10 μg of RS67333. (b) Reexposure to the holeboard. Both groups were injected with saline, as indicated by arrow. The animals that were injected with RS67333 before first exposure now expressed LTD after LFS. The vehicle group did not express LTD. (c) Habituation test in large holeboard. (i) Number of rears in animals injected with 10 μg of RS67333 (drug) or with saline (control). White bars comprise data from first exposure, and black bars are from reexposure 24 h later. (ii) Number of dips recorded in the same experiment as above. (d) Analog traces obtained before LFS, after LFS, and 24 h after LFS. (i) Experiment shown in a. (Upper) Vehicle experiment. (Lower) Injection of RS67333. (ii) Traces comprise the corresponding analogs from the experiment shown in b. Vertical scale bar corresponds to 0.5 mV, and horizontal bar corresponds to 5 ms.
The animals were then tested for LTD during reexposure to the holeboard (at least 7 days after first-time exposure). Before reexposure to the holeboard, all animals were injected with saline to mimic the conditions of the previous experiment. Whereas the animals that had received only vehicle injections (before first time and reexposure) showed no facilitation of LTD similar to effects described in Fig. 2, the group that had received RS67333 before first-time exposure showed significant LTD [Fig. 6b; ANOVA: F (1,24) = 72.61; P < 0.0001; drug group vs. control group]. In accordance with our postulate that LTD encodes novel information, the inhibition of LTD by RS67333 may have impaired the encoding of the object–place associations. Thus, when we reexposed the RS67333-treated animals to the holeboard with the same object constellation as previously, this effectively comprised a novel experience, and thus LTD facilitation occurred.
To obtain a measurement of habituation and further confirmation of this possibility, we made a similar behavioral experiment in an open-field holeboard (80 × 80 × 80 cm). The number of rears and head dippings into the holes is an expression of exploratory activity. If habituation occurs, it can be expected that reexposure should lead to significantly less rears and head-dippings. Two groups of eight rats were injected blindly with either 10 μg of RS67333 or saline 30 min before the first trial. Two trials, each lasting 15 min, occurred 24 h apart. The control group did significantly less head dipping and rearing when reexposed to holeboard compared to their performance in the first trial (t test, P < 0.05, both parameters, within group). This means that the control animals were able to remember the environment 24 h after their first encounter with the box. The drug group did not show any significant habituation effect to the holeboard, however (Fig. 6b). Thus, whereas habituation normally occurs after 15 min of exploration of the open field box, activation of 5-HT4 receptors inhibited this phenomenon.
Discussion
The results of this study provide strong evidence that LTD may encode the novel acquisition of object location, whereas LTP correlates with exploration of new space.
It has been reported that the induction of LTP is facilitated by preexposure to a novel environment (26). This phenomenon was independent of object presence. The current findings suggest that spatial exploration of new surroundings causes potentiated synaptic transmission, thereby inhibiting synaptic depression. A temporal relationship between the expression of LTP and novel spatial exploration seems to exist, however. Whereas holeboard exploration given after HFT depotententiates (27, 28), HFT given after (26) or during exploration facilitates LTP induction. This corresponds to observations in the dentate gyrus (29), where a narrow time window for facilitation of LTP has been described. Taken together with our data, these findings suggest that under specific conditions, information about novel space facilitates LTP expression.
Acquisition of information about novel objects, on the other hand, facilitates LTD expression. Thus, when animals are reexposed to a familiar holeboard containing novel objects, a robust LTD is observed. We also found that both LTD induction in and habituation to the holeboard were inhibited by 5-HT4 agonist application. This strengthens the likelihood that the facilitated induction of LTD is caused by the novelty of information and not by exploration itself. Therefore, whereas spatial exploration shifts the plasticity threshold toward LTP, acquisition of object information favors induction of LTD. LTD seems thus to be associated with the presence of new stimuli in a contextual frame. These biphasic changes in synaptic strength could be the means to separate the acquisition of different types of information. It has been reported that HFT-induced LTP is depotentiated by novelty exposure (17, 27, 28). We saw depotentiation in this study when novel object-containing holeboard exposure occurred after HFT. Depotentiation took ≈10 min to develop, suggesting that a temporal dissociation occurs between novel spatial exploration encoded by LTP and the acquisition of novel object–space information encoded by LTD.
The series of experiments using altered object constellations revealed two important facts. First, when novel objects are given during first-time exposure to the holeboard, LTD is induced. Bearing in mind that novel exposure to an empty holeboard does not facilitate LTD, this implies that the objects provide a stronger impetus for LTD formation than spatial novelty itself. This could be because they are more salient or because they create a context for acquiring information about the otherwise homogenous holeboard environment. The second interesting finding is that after two exposures to the holeboard with the same objects always located in the same spatial constellation, LTD is expressed merely by changing the configuration of the objects. As mentioned before, the exact role the hippocampus plays in memory of nonspatial elements is still a subject of debate. However, if the novelty of objects is the factor that triggers LTD, it would not be expected that the rearrangement of familiar objects would counteract the impairing effect habituation has on LTD induction. Our data support that it is not object recognition, but rather the association of objects within a particular spatial context, which triggers LTD. For LTD induction, spatial cues are the decisive factor.
Several neurotransmitter systems such as acetylcholine (30) or noradrenalin (31) may contribute to the effects of novelty exploration on synaptic plasticity. In this study, we chose to concentrate on the 5-HT4 receptor, because a role for this receptor has been described for multiple cognitive functions in the brain (22–25, 32). We found that both LTD induction in and habituation to the holeboard were inhibited by 5-HT4 agonist application. When animals were injected with RS67333 before their first encounter with the object-containing holeboard, LTD was inhibited. However, reexposure during LFS to the same holeboard a week later enabled LTD expression to occur. This effect was accompanied by diminished habituation. Although control animals did show habituation (expressed as less rearing and head dips) when reexposed to the holeboard after 24 h, no significant habituation was seen in the drug group. Although no injections were given before the second trial, the drug group still demonstrated a higher amount of rearing and head dips; thus, the effect could not be attributed to a possible higher explorative drive caused by 5-HT4 receptor application. This supports the interpretation that the facilitated induction of LTD is caused by the novelty of information and not by exploration.
Previous reports have shown that 5-HT4 receptors can influence learning and memory (22–25). In contrast to our study, most of these reports support a beneficial effect of 5-HT4 receptor activation; for instance, acquisition of spatial information in a water maze was improved by RS67333 when there was a distinct time interval between the trials (32). Besides being mildly aversive, the water maze is a purely spatial test, and the results of that study may correlate with our observations that spatial exploration without object exploration inhibited induction of LTD in a manner similar to RS67333. Another study showed that RS67333 improved acquisition but impaired memory consolidation (33). Thus, it may be that 5-HT4 receptor activation is beneficial for certain types of information acquisition that depend on LTP and detrimental for other types, such as LTD. Thus, if the encoding of memory traces depend on the biphasic changes in synaptic strength and RS67333 blocks LTD, LTP may be more easily induced.
We conclude that LTD may encode the novel acquisition of object–place configuration. Object location is an intrinsic feature of spatial information processing. Our data strongly support a role for LTD in this very specific aspect of spatial learning within the hippocampus and reinforce its candidacy as a learning mechanism.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft Grants SFB 515/B8 and SFB 509/B3 (to D.M.-V.).
Abbreviations: LTD, long-term depression; LTP, long-term potentiation; EPSP, excitatory postsynaptic potential; fEPSP, field EPSP; LFS, low-frequency stimulation; HFT, high-frequency tetanus; 5-HT4, 5-hydroxytryptamine 4.
References
- 1.O'Keefe, J. A. & Nadel, L. (1978) The Hippocampus as a Cognitive Map (Clarendon, Oxford).
- 2.Eichenbaum, H. (1996) Curr. Opin. Neurobiol. 6, 187–195. [DOI] [PubMed] [Google Scholar]
- 3.Hampson, R. E., Sineral, J. D. & Deadwyler, S. A. (1999) Nature 402, 610–614. [DOI] [PubMed] [Google Scholar]
- 4.Gaffan, D. & Parker, A. (1996) J. Neurosci. 16, 5864–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert, P. E. & Kesner, R. P. (2002) Behav. Neurosci. 116, 63–71. [DOI] [PubMed] [Google Scholar]
- 6.Fortin, N. J., Agster, K. L. & Eichenbaum, H. B. (2002) Nat. Neurosci. 5, 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Squire, L. R. & Zola-Morgan, S. (1996) Proc. Natl. Acad. Sci. USA 93, 13515–13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, M. W. & Aggleton, J. P. (2001) Nat. Rev. Neurosci. 2, 51–61. [DOI] [PubMed] [Google Scholar]
- 9.Wan, H., Aggleton, J. P. & Brown, M. W. (1999) J. Neurosci. 19, 1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson, D. A. & Stevenson, R. J. (2003) Trends Neurosci. 26, 243–247. [DOI] [PubMed] [Google Scholar]
- 11.Lisman, J. E. & Otmakhova, N. A. (2001) Hippocampus 11, 551–568. [DOI] [PubMed] [Google Scholar]
- 12.Bear, M. (1996) Proc. Natl. Acad. Sci. USA 93, 13453–13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsien, J. Z., Huerta, P. T. & Tonegawa, S. (1996) Cell 87, 1327–1338. [DOI] [PubMed] [Google Scholar]
- 14.Moser, E. I., Krobert, K. A., Moser, M. B. & Morris, R. G. M. (1998) Science 281, 2038–2042. [DOI] [PubMed] [Google Scholar]
- 15.Villarreal, D. M., Do, V., Haddad, E. & Derrick, B. E. (2002) Nat. Neurosci. 5, 48–52. [DOI] [PubMed] [Google Scholar]
- 16.Tsumoto, T. (1993) Neurosci. Res. 16, 263–270. [DOI] [PubMed] [Google Scholar]
- 17.Manahan-Vaughan, D. & Braunewell, K.-H. (1999) Proc. Natl. Acad. Sci. USA 96, 8739–8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris, R. G. M. & Frey, U. (1997) Philos. Trans. R. Soc. London B 352, 1489–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manahan-Vaughan, D. (1997) J. Neurosci. 17, 3303–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manahan-Vaughan, D. (2000) Cereb. Cortex 10, 483–487. [DOI] [PubMed] [Google Scholar]
- 21.Moser, E., Mathiesen, I. & Andersen, P. (1993) Science 259, 1324–1326. [DOI] [PubMed] [Google Scholar]
- 22.Lamirault, L. & Simon, H. (2001) Neuropharmacology 41, 844–853. [DOI] [PubMed] [Google Scholar]
- 23.Bockaert, J., Ansanay, H., Letty, S., Marchetti-Gauthier, E., Roman, F., Ronduin, G., Fagni, L., Soumireu-Mourat, B. & Dumuis, A. (1998) C. R. Acad. Sci. 321, 217–221. [DOI] [PubMed] [Google Scholar]
- 24.Letty, S., Child, R., Dumuis, A., Pantaloni, A., Bockaert, J. & Ronduin, G. (1997) Neuropharmacology 36, 681–687. [DOI] [PubMed] [Google Scholar]
- 25.Marchetti, E., Dumuis, A., Bockaert, J., Soumireu-Mourat, B. & Roman, F. S. (2000) Neuropharmacology 39, 2017–2027. [DOI] [PubMed] [Google Scholar]
- 26.Li, S., Cullen, W. K., Anwyl, R. & Rowan, M. J. (2003) Nat. Neurosci. 6, 526–531. [DOI] [PubMed] [Google Scholar]
- 27.Xu, L., Anwyl, R. & Rowan, M. J. (1998) Nature 394, 891–894. [DOI] [PubMed] [Google Scholar]
- 28.Abraham, W. C., Logan, B., Greenwood, J. M. & Dragunow, M. (2002) J. Neurosci. 22, 9626–9634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Straube, T., Kortz, V. & Frey, J. U. (2003) Neurosci. Lett. 344, 5–8. [DOI] [PubMed] [Google Scholar]
- 30.Bear, M. F. (1999) Proc. Natl. Acad. Sci. USA 96, 9457–9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sara, S. J., Dyon-Laurent, C. & Herve, A. (1995) Brain Res. Cognit. Brain Res. 2, 181–187. [DOI] [PubMed] [Google Scholar]
- 32.Lelong, V., Dauphin, F. & Boulouard, M. (2001) Neuropharmacology 41, 517–522. [DOI] [PubMed] [Google Scholar]
- 33.Meneses, A. & Hong, E. (1997) Pharmacol. Biochem. Behav. 56, 347–351. [DOI] [PubMed] [Google Scholar]