Abstract
Arginine vasopressin (AVP) and its nonmammalian homolog arginine vasotocin influence social behaviors ranging from affiliation to resident–intruder aggression. Although numerous sites of action have been established for these behavioral effects, the involvement of specific AVP cell groups in the brain is poorly understood, and socially elicited Fos responses have not been quantified for many of the AVP cell groups found in rodents. Surprisingly, this includes the AVP population in the posterior part of the medial bed nucleus of the stria terminalis (BSTMP), which has been extensively implicated, albeit indirectly, in various aspects of affiliation and other social behaviors. We examined the Fos responses of eight hypothalamic and three extra-hypothalamic AVP-immunoreactive (-ir) cell groups to copulation, nonaggressive male–male interaction, and aggressive male–male interaction in both dominant and subordinate C57BL/6J mice. The BSTMP cells exhibited a response profile that was unlike all other cell groups: from a control baseline of ~5% of AVP-ir neurons colocalizing with Fos, colocalization increased significantly to ~12% following nonaggressive male–male interaction, and to ~70% following copulation. Aggressive interactions did not increase colocalization beyond the level observed in nonaggressive male mice. These results suggest that BSTMP neurons in mice may increase AVP-Fos colocalization selectively in response to affiliation-related stimuli, similar to findings in finches. In contrast, virtually all other cell groups were responsive to negative aspects of interaction, either through elevated AVP-Fos colocalization in subordinate animals, positive correlations of AVP-Fos colocalization with bites received, and/or negative correlations of AVP-Fos colocalization with dominance. These findings greatly expand what is known of the contributions of specific brain AVP cell groups to social behavior.
Keywords: Extended amygdala, Hypothalamus, Neuromodulation, Social behavior
Introduction
The neuropeptide arginine vasopressin (AVP) and its avian homolog arginine vasotocin (AVT) influence a variety of social behaviors, including pair bonding in voles (Winslow et al., 1993; Insel and Hulihan, 1995; Lim et al., 2004); social recognition in male mice and rats (Engelmann et al., 1994; Everts and Koolhaas, 1997; Bielsky et al., 2004, 2005; Choleris et al., 2009); maternal behaviors in rats (Bosch and Neumann, 2008; Nephew and Bridges, 2008); and social communication in fishes, birds and rodents (Albers et al., 1986; Maney et al., 1997; Goodson, 1998a; Goodson and Bass, 2000). Modulation of aggression by AVT/AVP is complex and can vary across contexts and phenotypes (Goodson et al., 2009a; Kabelik et al., 2009; also see Beiderbeck et al., 2007), and although numerous neural loci are likely involved, resident–intruder aggression is potently facilitated by activation of V1a receptors in the anterior hypothalamus (AH), as shown in male Syrian hamsters (Mesocricetus auratus; Ferris et al., 1997) and prairie voles (Microtus ochrogaster; Gobrogge et al., 2009).
Sites of action have been established for many of AVP’s behavioral effects, but surprisingly little is known about the social stimulus properties that elicit responses from discrete AVP cell populations in the brain. For instance, AVP strongly promotes mating-induced behaviors in monogamous voles (Young and Wang, 2004; Lim and Young, 2006), but the source(s) of mating-induced AVP release has not been identified. Similarly, little or no functional data are available for most of the smaller populations of AVP cells in the extended amygdala, preoptic area (POA), and hypothalamus. These data are critical to understanding AVP-mediated effects as it is difficult to attribute site-specific effects to a particular AVP cell group, given that those effects may occur at sites distal from terminal distributions, and volumetric peptide release from dendrites may effectively bathe large amounts of the brain in AVP (Landgraf and Neumann, 2004; Ludwig and Leng, 2006). Adding to this complexity is that projections from multiple cell groups often appear to overlap. Indeed, functionally opposed AVT cell groups exhibit overlapping projection fields in songbirds, suggesting that the behavioral properties of a given cell group depend upon distributed patterns of neuromodulation across the brain, not simply site-specific actions (Goodson and Kabelik, 2009).
All of these observations highlight a strong need for behaviorally relevant data on the AVP cells themselves, and on the kinds of environmental stimuli to which AVP cell groups respond. Thus, a major goal of the present experiments was to address that need. A second goal was to test the hypothesis that AVP cell groups in mice exhibit opposing response profiles, as recently shown in songbirds for the AVT populations in the medial bed nucleus of the stria terminalis (BSTM) and paraventricular nucleus of the hypothalamus (PVN), which exhibit increased Fos activity to positive and negative social stimuli, respectively (Goodson and Wang, 2006; Goodson and Kabelik, 2009).
Finally, we sought to clarify the relationship of AVP neurons to male–male aggression. Although AVP acts within the AH to promote resident–intruder aggression in Syrian hamsters and prairie voles (Ferris et al., 1997; Gobrogge et al., 2009), findings in rats and songbirds suggest that AVT/AVP relates to aggression in complex ways that reflect social context, anxiety phenotype, and constitutive aggressiveness (Beiderbeck et al., 2007; Veenema and Neumann, 2007; Goodson et al., 2009a; Kabelik et al., 2009). In order to address these experimental goals, we quantified the immunocolocalization of Fos and AVP in male C57BL/6J mice following (1) copulation, (2) nonaggressive male–male interaction, and (3) aggressive male–male interactions. In the male–male aggressive interactions, both dominant and subordinate mice were examined, and data were collected for 11 AVP-immunoreactive (-ir) cell groups, representing virtually all definable groups in the mouse brain.
Methods
Animals and housing
Adult C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were individually housed and maintained on either a 12:12 light/dark cycle (resident–intruder tests) or a 14:10 cycle (copulation tests). Food and water were available ad libitum except during testing. All subjects were adults (>12 weeks old) at time of testing. All experiments were performed in accordance with the guidelines of the National Institute of Health Guide for the Care and Use of Laboratory Animals (1996) and were approved by the Institutional Animal Care and Use Committee at Indiana University.
Ovariectomies and estrus induction
At >10 weeks of age, female partners for copulation tests were bilaterally ovariectomized. Animals were deeply anesthetized for this procedure with an intraperitoneal (i.p.) injection of a cocktail comprised of ketamine (20 mg/ml; Henry Schein, Melville, NY), xylazine (4 mg/ml; Henry Schein), and 0.9% saline, or sodium pentobarbital (50 mg/ml; Henry Schein). Animals were returned to their home cages and dosed orally with meloxicam (Metacam, 1 mg/kg; Henry Schein) for 3 days following surgeries. Behavioral receptivity was later induced with subcutaneous injections of estradiol benzoate (10 μg/0.05 ml peanut oil; Sigma, St. Louis, MO) and progesterone (500 μg/0.1 ml peanut oil; Sigma) at 48 and 6 h, respectively, prior to testing. Steroid solutions were first dissolved in acetone and allowed ample time for evaporation prior to the addition of peanut oil.
Receptivity screening
To determine whether females were sexually receptive and to ensure that subject males were sexually motivated, animals were screened for copulatory behavior ~1 week prior to testing. Within the first hour after lights-off, females were placed in the male’s cage and males were allowed two bouts of intromission (i.e., periods that consist of a mount and subsequent thrusting), or until 30 min had elapsed. Only males that successfully intromitted twice within 30 min were included in the study.
Copulation testing
Males were habituated to the test room for 1 h after lights-off for two days prior to testing. On the day of testing and perfusion, animals were habituated to the test room 3 h prior to the start of testing. At lights-off, a receptive stimulus female was placed in the subject male’s cage. The number and latency to mounts, intromissions, and ejaculation were quantified by direct observation under red light. Tests were terminated at ejaculation, after which the male was placed in a dark, quiet area until perfusion. Control males received the same treatment, with handling at 0 and 45 min, except that no female was introduced.
Resident–intruder testing
To reduce the level of nonspecific Fos activation, resident–intruder pairs were acclimated to cage-swapping in the test room once per day for 7 days prior to testing. These acclimations occurred in the first hr after lights-off. Animals were placed in each other’s cage for 15 min, after which they were returned to their home cage. Control males were placed in a clean cage or handled and left in their own cage for 15 min.
On the day of testing and perfusion, subjects were moved to the test room for habituation 3 h prior to the start time. At lights-off, an intruder male was placed into a resident’s cage for 15 min. Interactions were observed under red light to determine dominant-subordinate status and were also recorded with a digital video camcorder (Canon ZR40) for quantification of latency to aggression, the number of bites, the number of aggressive bouts, and duration of aggressive bouts. In these tests, bites were always given by the dominant male, though residency did not determine social status (i.e., dominant males were not necessarily residents). After the 15 min test, the intruder was returned to his cage. Animals that chemoinvestigated but did not engage in aggressive interactions were designated as “nonaggressive” and were also euthanized for Fos analyses. Both resident and intruder males were handled at the start and finish of testing. All animals were kept in a dark, quiet area until perfusion.
Perfusion
Subjects in the resident–intruder experiment were perfused 75 min following the start of testing. The timing of perfusions in the copulation experiment varied from 75 to 90 min after the start of testing because subjects exhibited different latencies to copulation (i.e., subjects that ejaculated in 30 min or less were perfused at 75 min and those that exhibited longer latencies were perfused 45 min after ejaculation, with a maximum of 90 min allowed after the start of testing). Handled controls were perfused at 75 min. Animals were deeply anesthetized with an i.p. injection of a ketamine cocktail comprised of ketamine (20 mg/ml), xylazine (4 mg/ml), and 0.9% saline, or sodium pentobarbital and perfused transcardially with 0.1 M phosphate buffered saline (PBS) for exsanguination followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). Extracted brains were post-fixed overnight then transferred to a 30% sucrose solution for 2 days.
Immunocytochemistry
Brain tissue was cut into three series of 40 μm free-floating coronal sections using a cryostat and stored at −80 °C in cryoprotectant prior to immunocytochemical labeling. Immunocytochemistry was performed as follows: tissue was rinsed 6×10 min in 0.1 M PBS; followed by 20 min in 10 mM sodium citrate (pH 9.5, ceramic well plates placed into a shallow water bath heated to 70 °C); 2×10 min in PBS; and 1 h in PBS +5.0% bovine serum albumin (BSA)+0.3% triton-X. Tissue was then incubated with guinea pig anti-AVP (1:1000; Bachem, Torrance, CA) and rabbit anti-Fos (1:1000; Santa Cruz Biotech, Santa Cruz, CA), diluted in PBS+2.5% BSA+0.3% triton-X+0.05% sodium azide and incubated 40–44 h at 4 °C. This was followed by 2×30 min rinses in PBS; 1 h in biotinylated goat anti-guinea pig secondary (8 μl/ml; Jackson ImmunoResearch, West Grove, PA); 3×10 min in PBS; 2 h in goat anti-rabbit secondary conjugated to Alexa Fluor 594 (5 μl/ml) +streptavidin conjugated to Alexa Fluor 488 (3 μl/ml) in PBS+2.5% BSA+0.3% triton-X. Alexa Fluors were purchased from Molecular Probes/Invitrogen (Eugene, OR), with custom cross-adsorption against guinea pig in the goat anti-rabbit 594. Sections were extensively washed in PBS, mounted on slides, and coverslipped with ProLong Gold containing DAPI nuclear stain (Molecular Probes/Invitrogen).
Quantification of immunoreactivity
Colocalization of AVP and Fos was examined in the posterior BSTM (BSTMP; two levels), ventral BSTM (BSTMV), periventricular preoptic area (POA; two levels) and eight cell groups of the hypothalamus–anterior commissural nucleus (AC), anterior hypothalamus (AH), nucleus circularis (NC), supraoptic nucleus (SON), retrochiasmatic SON (rSON), paraventricular nucleus (PVN; two levels), suprachiasmatic nucleus (SCN), and tuberal region of the lateral hypothalamus (tuberal LH). The position of these cell groups in mice (shown in Figs. 1 and 2) has been described most fully by Castel and Morris (1988), although subdivisions of the BSTM were not described in that study (see The BSTM contains two AVP-ir cell groups) and the “accessory” cell groups corresponding to our AH, NC, and tuberal LH were not separately named (but are shown embedded within the hypothalamo-hypophyseal tract). The position of the AH and NC populations is comparable to that described for other rodents (e.g., Gobrogge et al., 2007), whereas the tuberal LH cell group has not been given a name by other authors and appears to be dramatically larger in mice than in other species. We reliably located the tuberal LH cells at the caudal pole of the rSON (Fig. 2D). Scattered AVP-ir neurons were also observed in the medial nucleus of the amygdala, but not in all subjects and not in sufficient numbers for analysis.
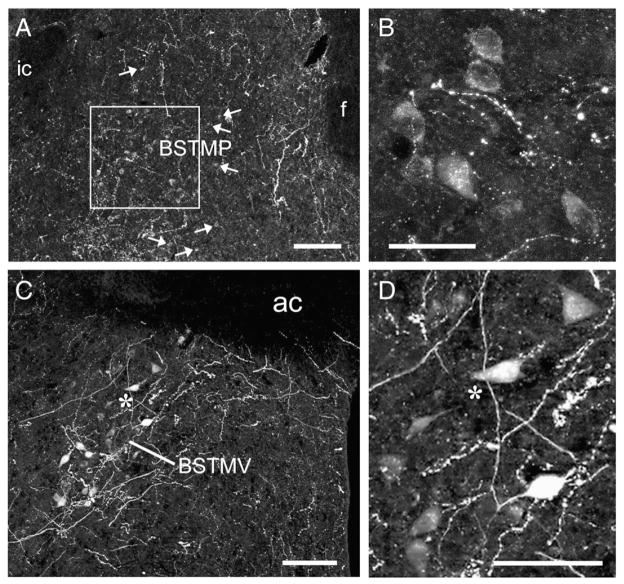
Fig. 1.
Photomicrographs showing the position and morphology of AVP-ir neurons in the posterior part of the medial bed nucleus of the stria terminalis (BSTMP; A, B) and ventral BSTM (BSTMV; C, D). Medial is to the right in all photos. The box in panel A shows an area expressing a high density of AVP-ir neurons. Panel B shows AVP-ir neurons from the same animal at a higher magnification, and the asterisk in panel C corresponds to the position of the asterisk in panel D. The AVP-ir neurons in the BSTMP are small, round, and weakly immunoreactive (also see Figs. 3A–C), whereas those in the BSTMV are larger, variably shaped, and strongly immunoreactive. All photos were taken at a rostrocaudal level comparable to plate 32 of the mouse brain atlas. Scale bars=100 μm in panels A and C; 25 μm in panel B; and 50 μm in panel D. Abbreviations: ac, anterior commissure; f, fornix; ic, internal capsule.
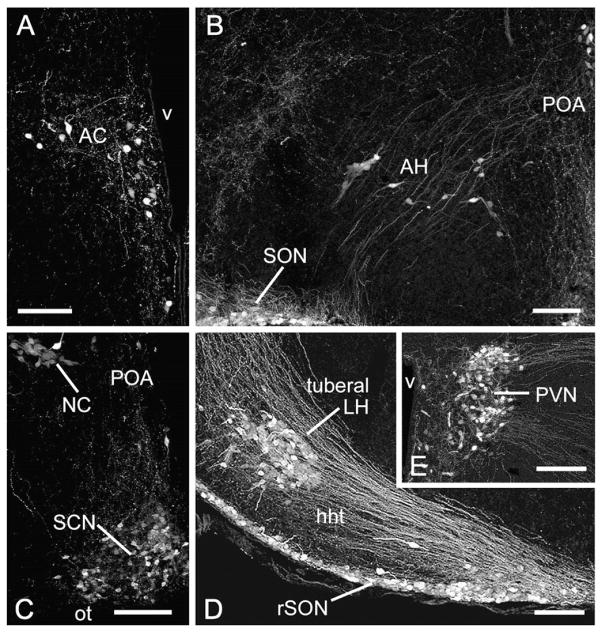
Fig. 2.
Photomicrographs showing the position and morphology of AVP-ir neurons in the preoptic area (POA) and hypothalamus: (A) anterior commissural nucleus (AC; caudoventral to the anterior commissure, and thus caudoventral to the photo in Fig. 1C); (B) POA, anterior hypothalamic area (AH) and supraoptic nucleus (SON); (C) nucleus circularis (NC) and suprachiasmatic nucleus (SCN); (D) tuberal region of the lateral hypothalamus (tuberal LH) and retrochiasmatic SON (rSON); and (E) caudal paraventricular nucleus (PVN). Medial is to the right in all photos except panel E, where medial is to the left. Rostrocaudal levels of these photos correspond, respectively, to plates 33, 36, 37, 39 and 38 of the mouse brain atlas. All scale bars=100 μm. Other abbreviations: hht, hypothalamo-hypophyseal tract; ot, optic tract; v, 3rd ventricle.
Photomicrographs of each brain area were shot at 10× using a Zeiss Axioimager microscope (Carl Zeiss Inc., Göttingen, Germany) outfitted with a Z-drive and a Zeiss Apotome optical dissector (which at 10x yields focal plane resolution of near-confocal quality while controlling for duplicate labeling of cells), a Zeiss Axiocam HRm camera and a high intensity X-Cite fluorescence illuminator (EXFO Photonic Solutions Inc., Mississauga, Canada). Images were captured in Zeiss AxioVision 4.6.3.0 and exported into Photoshop CS3 (Adobe Systems Inc., San Jose, CA). Data acquisition parameters were standardized for all subjects and cell counts were conducted by a blind observer from superimposed monochrome images of AVP, Fos, and DAPI. These methods have been employed in previous studies (e.g., Goodson and Wang, 2006; Goodson et al., 2009b). Representative double-labeling is shown in Fig. 3.
Fig. 3.
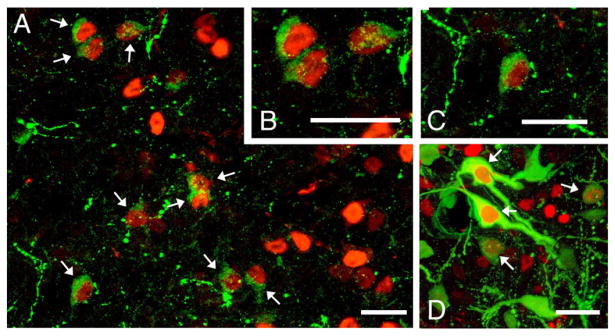
Representative colocalization of AVP (Alexa Fluor 488; green) and Fos (Alexa Fluor 594; red) in the posterior part of the medial bed nucleus of the stria terminalis (BSTMP; A–C) and paraventricular nucleus (D) following copulation. Scale bars=25 μm.
Statistical analysis
Mean group differences were analyzed with StatView 5.0 (SAS Institute, Inc., Cary, NC) using unpaired t-tests for the copulation experiment and one-way analysis of variance (ANOVA) for the resident–intruder experiment. Post hoc pair-wise comparisons were conducted using Fisher’s PLSD. Linear regression was used to examine correlations between behavioral measures and AVP-Fos colocalization. Differences were considered significant when p<0.05.
Due to sectioning damage to the ventral surface of the brain, SCN data are not available for two of the 32 subjects in the resident–intruder experiment (one nonaggressive and one subordinate), and data for the rSON and adjacent tuberal LH are not available for a single nonaggressive subject.
Results
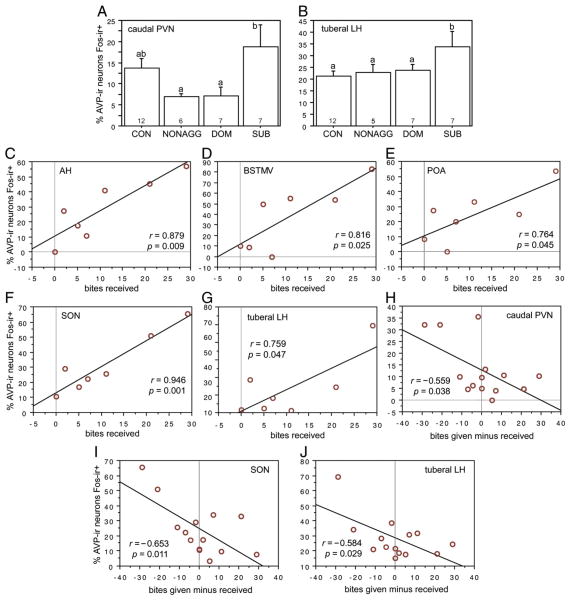
Distinctly different patterns of AVP-Fos colocalization were observed following sexual and aggressive interactions. In general, the BSTMP cell group appeared to respond to positive aspects of social interaction (copulation and social investigation). Copulation was accompanied by a large increase in colocalization within the BSTMP (Fig. 4A), with no notable differences between rostral and caudal levels of this nucleus (Table 1). Of the remaining cell groups, only the anterior SON exhibited a significant increase in AVP-Fos colocalization following copulation, although this response was quite modest as compared with the BSTMP (Table 1). Latency to mount, latency to intromit, and latency to ejaculate were negatively correlated with AVP-Fos colocalization in the BSTMP, and no other AVP-ir cell group exhibited significant correlations with sexual behaviors, with the exception of the BSTMV cell group (Table 2; also note trends in the opposing direction for the AH). The BSTMP cell group exhibited responses to male–male interactions only at the caudal level (Table 3 and Fig. 4B), but notably, the amount of colocalization in fighters was no higher than in nonaggressive males that simply chemoinvestigated, suggesting that the increase in colocalization following male–male encounters is an effect of social investigation, not aggressive interactions per se.
Fig. 4.
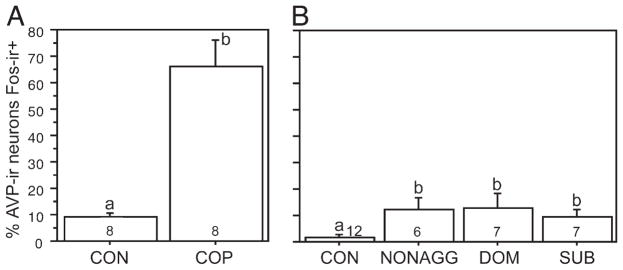
AVP-ir neurons in the posterior part of the medial bed nucleus of the stria terminalis (BSTMP) exhibit robust Fos responses to copulation (A) and comparatively modest responses to nonaggressive chemoinvestigation (B). Aggressive interactions produce no increase in colocalization above the levels observed in nonaggressive males that chemoinvestigated (note also that AVP-Fos colocalization in the BSTMP correlates with measures of sexual behavior, but not aggression; see Tables 2 and 4). The data shown in both panels are for the caudal portion of the cell group only; similar patterns were found rostrally but were only significant in the copulation study (Table 1). Data are shown as means± SEM and n’s per group are indicated in the bars. Different letters above the error bars denote significant group differences (Fisher PLSD p<0.05 following significant ANOVA).
Table 1.
Fos responses of AVP-ir neurons to copulation in male mice.
| Cell group | % AVP-ir cells Fos-ir+(mean±SEM)
|
t(1,14) | P | |
|---|---|---|---|---|
| Control | Copulation | |||
| AC | 13.5±4.2 | 19.6±4.1 | −1.045 | 0.31 |
| AH | 40.6±4.0 | 44.5±5.7 | −0.570 | 0.58 |
| BSTMP, rostral | 4.0±1.3 | 64.3±5.1 | −11.488 | <0.0001 |
| BSTMP, caudal | 9.2±1.5 | 66.2±10.1 | −5.575 | <0.0001 |
| BSTMP, total | 6.3±0.8 | 67.2±6.0 | −10.112 | <0.0001 |
| BSTMV | 28.4±5.8 | 44.2±7.1 | −1.712 | 0.10 |
| NC | 35.6±6.1 | 26.1±4.2 | 1.276 | 0.22 |
| POA | 12.7±7.3 | 12.4±7.3 | 0.373 | 0.92 |
| PVN, rostral | 33.9±3.4 | 28.6±6.1 | 0.750 | 0.46 |
| PVN, caudal | 27.1±2.7 | 26.4±3.4 | 0.160 | 0.88 |
| PVN, total | 29.7±2.6 | 27.1±3.8 | 0.562 | 0.58 |
| rSON | 23.9±2.6 | 21.3±2.4 | 0.749 | 0.46 |
| SCN | 20.8±9.2 | 22.4±9.0 | −1.557 | 0.74 |
| SON | 13.7±5.1 | 19.1±1.5 | −2.328 | 0.03 |
| Tuberal LH | 22.3±1.8 | 26.9±4.1 | −1.012 | 0.33 |
Table 2.
Correlations between AVP-Fos colocalization and measures of sexual performance in male mice.
| Cell group | Mounts
|
Latency to mount
|
Latency to intromit
|
Latency to ejaculate
|
||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| AC | 0.293 | 0.48 | −0.184 | 0.66 | 0.000 | 0.97 | 0.044 | 0.90 |
| AH | 0.672 | 0.06 | 0.512 | .19 | 0.536 | 0.09 | 0.661 | 0.07 |
| BSTMP, rostral | −0.507 | 0.20 | −0.270 | 0.51 | −0.375 | 0.35 | −0.479 | 0.23 |
| BSTMP, caudal | −0.571 | 0.13 | −0.800 | 0.01 | −0.832 | 0.01 | −0.731 | 0.03 |
| BSTMP, total | −0.643 | 0.08 | −0.720 | 0.04 | −0.775 | 0.02 | −0.781 | 0.03 |
| BSTMV | −0.616 | 0.10 | −0.612 | 0.10 | −0.782 | 0.02 | −0.691 | 0.05 |
| NC | −0.374 | 0.36 | −0.228 | 0.58 | −0.431 | 0.28 | −0.427 | 0.29 |
| POA | −0.089 | 0.83 | 0.110 | 0.80 | 0.105 | 0.80 | −0.071 | 0.86 |
| PVN, rostral | −0.322 | 0.43 | 0.582 | 0.13 | 0.164 | 0.69 | −0.110 | 0.79 |
| PVN, caudal | −0.032 | 0.92 | 0.386 | 0.34 | 0.315 | 0.44 | 0.257 | 0.53 |
| PVN, total | −0.261 | 0.53 | 0.603 | 0.11 | 0.266 | 0.52 | 0.032 | 0.93 |
| rSON | 0.045 | 0.90 | 0.377 | 0.35 | 0.237 | 0.57 | 0.202 | 0.63 |
| SCN | −0.367 | 0.37 | −0.495 | 0.21 | −0.610 | 0.11 | −0.499 | 0.21 |
| SON | 0.032 | 0.95 | −0.032 | 0.94 | 0.044 | 0.92 | 0.100 | 0.80 |
| Tuberal LH | −0.265 | 0.52 | 0.244 | 0.55 | −0.055 | 0.89 | −0.145 | 0.73 |
Table 3.
Fos responses of AVP-ir neurons to nonaggressive chemoinvestigation and fighting in male mice.
| Cell group | % AVP-ir cells Fos-ir+(mean±SEM)
|
F(3, 28)* | p | |||
|---|---|---|---|---|---|---|
| Control | Nonaggressive | Dominant | Subordinate | |||
| AC | 36.9±7.0 | 26.5±5.5 | 25.2±6.6 | 38.0±10.7 | 0.334 | 0.80 |
| AH | 31. 7±4.5 | 39.0±9.2 | 34.5±9.1 | 28.5±7.7 | 0.312 | 0.82 |
| BSTMP, rostral | 8.5±4.1 | 26.9±18.7 | 5.2±2.1 | 4.6±2.6 | 1.394 | 0.27 |
| BSTMP, caudal | 1.6±1.2 | 12.3±4.6 | 11.2±4.9 | 9.6±2.7 | 3.025 | 0.04 |
| BSTMP, total | 6.7±3.2 | 20.9±9.8 | 7.6±2.2 | 6.9±1.8 | 1.399 | 0.26 |
| BSTMV | 35.1±10.2 | 11.4±7.3 | 28.6±5.5 | 37.4±11.8 | 1.100 | 0.37 |
| NC | 24.3±6.6 | 26.7±7.5 | 22.4±5.7 | 33.2±6.3 | 0.867 | 0.46 |
| POA | 16.9±4.4 | 23.7±5.7 | 14.0±5.3 | 24.0±6.6 | 0.775 | 0.52 |
| PVN, rostral | 17.9±4.6 | 7.0±1.9 | 10.4±2.8 | 12.9±3.9 | 1.539 | 0.22 |
| PVN, caudal | 13.2±2.2 | 7.5±0.8 | 6.9±1.8 | 18.7±5.3 | 3.097 | 0.04 |
| PVN, total | 15.9±3.4 | 7.0±1.2 | 8.8±2.3 | 15.0±4.1 | 1.940 | 0.14 |
| rSON | 24.3±5.1 | 34.8±2.6 | 18.0±2.5 | 23.2±6.0 | 1.518 | 0.23 |
| SCN | 28.7±2.3 | 42.2±4.1 | 33.0±5.5 | 35.1±2.4 | 2.338 | 0.10 |
| SON | 22.0±6.4 | 30.5±3.0 | 17.5±5.4 | 31.7±7.4 | 1.546 | 0.22 |
| Tuberal LH | 18.7±2.6 | 23.0±3.4 | 22.4±2.5 | 33.8±6.5 | 2.950 | 0.05 |
Due to limited tissue damage, data are omitted for the SCN of two subjects (one nonaggressive and one subordinate), and the rSON and tuberal LH of a single nonaggressive subject.
Most cell groups other than the BSTMP showed a sensitivity to negative aspects of male–male interactions. Thus, subordinate males showed significantly higher levels of AVP-Fos colocalization than nonaggressive and dominant males within the caudal PVN and tuberal LH (Figs. 5A and B, respectively). The number of bites received positively correlated with colocalization in the AH, BSTMV, POA, anterior SON, and tuberal LH (Figs. 5C–G, respectively), and dominance (i.e., bites given minus bites received) was negatively correlated with colocalization in caudal PVN, anterior SON, and tuberal LH (Figs. 5H–J and Table 4, respectively). Other results for male–male interactions are shown in Tables 3 and 4.
Fig. 5.
AVP-ir neurons of the AH, BSTMV, POA, caudal PVN, SON, and tuberal LH exhibit response profiles that suggest a sensitivity to stressful or negative aspects of social interaction. AVP-Fos colocalization in these areas is greater in subordinate animals relative to dominant animals (A, B), positively correlated with bites received (C–G), or negatively correlated with dominance index (bites given minus bites received; H–J). Data in panels A and B are shown as means±SEM and n’s per group are indicated in the bars. Different letters above the error bars denote significant group differences (Fisher PLSD p<0.05 following significant ANOVA). Abbreviations as in Figs. 1 and 2. Additional data are provided in Tables 3 and 4.
Table 4.
Correlations between AVP-Fos colocalization and measures of agonistic behavior in male mice.
| Cell group | Bites given
|
Bites received
|
Dominance index (bites given minus received)
|
|||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| AC | −0.414 | 0.35 | 0.731 | 0.06 | −0.408 | 0.15 |
| AH | 0.295 | 0.52 | 0.879 | <0.01 | −0.147 | 0.62 |
| BSTMP, rostral | −0.055 | 0.91 | 0.596 | 0.15 | −0.300 | 0.30 |
| BSTMP, caudal | 0.548 | 0.20 | 0.318 | 0.48 | 0.214 | 0.46 |
| BSTMP, total | 0.045 | 0.91 | 0.514 | 0.23 | −0.161 | 0.58 |
| BSTMV | −0.295 | 0.57 | 0.816 | 0.03 | −0.438 | 0.12 |
| NC | 0.628 | 0.13 | 0.669 | 0.10 | −0.344 | 0.23 |
| POA | 0.100 | 0.95 | 0.764 | 0.05 | −0.499 | 0.07 |
| PVN, rostral | 0.474 | 0.28 | 0.612 | 0.14 | −0.272 | 0.35 |
| PVN, caudal | 0.192 | 0.68 | 0.511 | 0.24 | −0.559 | 0.04 |
| PVN, total | 0.436 | 0.32 | 0.579 | 0.17 | −0.436 | 0.12 |
| rSON | 0.630 | 0.12 | 0.300 | 0.51 | −0.263 | 0.38 |
| SCN | 0.114 | 0.80 | 0.028 | 0.96 | −0.027 | 0.93 |
| SON | 0.077 | 0.86 | 0.946 | <0.01 | −0.653 | 0.01 |
| Tuberal LH | 0.237 | 0.61 | 0.759 | <0.05 | −0.584 | 0.03 |
Discussion
Much of our knowledge about the behavioral functions of brain AVP has been obtained from microinjection studies, and although this approach has served well for determining behavioral effects and sites of action, the involvement of specific AVP cell groups remains unclear. The AVP cells of the PVN and SON have been most extensively studied, but largely in relation to the pituitary and peripheral physiology (Engelmann et al., 2004; Caldwell et al., 2008a), and we know far less about the contributions of those cell groups to the central modulation of behavior. Thus, the present experiments were designed to identify the social parameters that activate particular AVP cell groups, and to test specific hypotheses about several cell groups that are derived from studies of songbirds and other rodent species that exhibit behavioral profiles that are somewhat different from Mus musculus. We obtained strong support for the hypothesis that AVP cells of the BSTMP are primarily responsive to affiliation-related stimuli, and provide evidence that most other AVP cell groups respond to negative social stimulation (e.g., subjugation), which may reflect a general responsiveness to environmental stressors.
AVP cells of the BSTMP process affiliation-related stimuli
Studies in the 1980s demonstrated that male rats possess far more AVP-ir cells in the BSTM than do females (Van Leeuwen et al., 1985; also see Miller et al., 1989b), and this dimorphism generates a male-biased innervation of the lateral septum (LS; De Vries et al., 1981; De Vries and Buijs, 1983). Cell number and fiber density were also shown to be sensitive to hormones (De Vries et al., 1984, 1985, 1986; Miller et al., 1989a; De Vries and Al Shamma, 1990; Miller et al., 1992). Although fish lack a homologous AVT circuit (Greenwood et al., 2008), the basic features of this BSTM-LS circuit have been documented across every tetrapod class (Goodson and Bass, 2001; De Vries and Panzica, 2006), as have the behavioral effects of AVT/AVP manipulations, including site-specific manipulations within putative targets of the BSTM AVT/AVP neurons (Goodson and Bass, 2001; De Vries and Panzica, 2006; Lim and Young, 2006; Caldwell et al., 2008a; Goodson, 2008).
Despite this wealth of information, however, we cannot assert with confidence that AVP neurons of the BSTM mediate any of the known functions of AVP. This is rooted in the facts that (1) no behavioral studies have directly manipulated these neurons by knocking down local AVP production, and (2) the possible sources of AVP in postsynaptic targets are multiple and hard to identify. Receptors are often distributed a long way from known terminal zones, and volumetric release from the dendrites of magnocellular neurons may reach almost any neural target (Landgraf and Neumann, 2004; Ludwig and Leng, 2006; De Vries, 2008). In fact, although the BSTM appears to contribute most or all of the direct AVP innervation to the LS (De Vries and Buijs, 1983; De Vries and Panzica, 2006), neurophysiological studies demonstrate that ventral LS neurons are also modulated by AVP derived from the PVN (Disturnal et al., 1986), and septal V1a receptors potently mediate behavioral functions in animals that lack a BSTM-LS projection system (see second section below). Push-pull and microdialysis studies further confirm that AVP is present in brain areas that lack direct innervation (Landgraf and Neumann, 2004), and thus the known behavioral effects of AVP cannot be attributed to specific cell groups based on anatomy alone. Furthermore, at least a subset of avian and mammalian species exhibit intensely stained AVT-ir or AVP-ir fibers in the LS that are likely of hypothalamic origin (Rosen et al., 2007; Goodson and Kabelik, 2009), again suggesting caution when attributing pharmacological effects (e.g., behavioral effects of receptor antisense, antagonism or over-expression) to a specific AVT/AVP cell group.
Immediate early gene (IEG) studies are therefore a valuable tool for asking questions about specific cell groups, but to our knowledge, these data have not been reported for AVP neurons in the mammalian BSTM. Our only direct knowledge regarding the stimulus–response properties of this cell group in mammals is the fact that cohabitation with a female increases AVP mRNA in the BSTM of male prairie voles (Wang et al., 1994; but see Goodson and Wang, 2006). We now show that BSTMP AVP neurons in mice exhibit a robust Fos response to copulation and a relatively modest response to nonaggressive interaction, with no greater response to fighting than observed in nonaggressive males. Combined, these results suggest that activity of the BSTMP cell group is increased by a variety of stimuli that are related to affiliation, such as social investigation and sexual interactions.
This interpretation is consistent with findings in songbirds, which demonstrate that AVT cells of the BSTM exhibit Fos responses to affiliation-related social stimuli, but not to those that elicit aversion or aggression (Goodson and Wang, 2006; Goodson et al., 2009b). For instance, exposure to a same-sex conspecific increases AVT-Fos colocalization in the gregarious zebra finch, but decreases colocalization in the territorial (i.e., relatively asocial) violet-eared waxbill. These findings are also consistent with studies in rodents that have established roles for endogenous AVP in V1a-mediated behaviors such as social recognition, pair bonding, parental behavior, alloparental behavior, and nonsexual investigation (Young and Wang, 2004; Lim and Young, 2006; Carter et al., 2008; Veenema and Neumann, 2008; Choleris et al., 2009). At least some of AVP’s effects are also mediated by the V1b receptor subtype, which appears to have a central distribution restricted to the CA2 field of the hippocampus (Caldwell et al., 2008b).
Although studies of AVP-Fos colocalization are informative, they reveal only part of the picture about behavioral functions, even if we assume that Fos response equates to action potentials and peptide release, which might not always be the case (Herdegen and Leah, 1998). Other IEGs might reveal a different activity profile, and further, the stimuli to which a cell group responds may not necessarily reflect its influence on behavior. For instance, even though the present studies demonstrate a response of BSTMP AVP cells to copulation and chemo-investigation, peptide released by those cells during copulation could nonetheless influence mating-induced aggression (e.g., Gobrogge et al., 2009), or even broader emotional states such as anxiety (e.g., Landgraf et al., 1995; Bielsky et al., 2004). Similarly, there is no reason to assume that BSTMP AVP cells influence copulatory behavior just because they are activated in association with copulation. Rather, what the present results tell us is that specific cell groups have the potential to convey certain kinds of information to other cells in the brain; how those other areas use the information must be demonstrated via other means.
The BSTM contains two AVP-ir cell groups
To our knowledge, previous authors have not explicitly addressed the presence of multiple AVP-ir cell groups in the BSTM that would correspond to the BSTMP and BSTMV in mice. The classically steroid-sensitive, sexually dimorphic neurons of the BSTM in rats are small, round, and weakly immunoreactive (De Vries and Miller, 1998; De Vries and Panzica, 2006; De Vries and Södersten, 2009). This phenotype is comparable to AVP-ir cells of the BSTMP in mice, which lie adjacent to the stria terminalis. On the other hand, BSTMV cells are large, label robustly, and given their position just rostral and dorsolateral to the PVN, at the caudal pole of the anterior commissure (see Fig. 1), they could readily be mistaken for hypothalamic neurons of the PVN or AC. Interestingly, the response profile of BSTMV cells shows similarities to both the BSTMP population, with AVP-Fos colocalization being negatively correlated with intromission and ejaculation latencies, and several hypothalamic cell groups, with colocalization being positively correlated with the number of bites received.
Do AVP cells of the BSTM promote aggression?
Soon after male-biased sex differences were described in the AVP circuitry of the BSTM and LS (De Vries et al., 1981; Van Leeuwen et al., 1985), Ferris and colleagues demonstrated that infusions of AVP into the LS promote agonistic flank marking in male Syrian hamsters (Ferris et al., 1990; Irvin et al., 1990). The combined observations suggested the hypothesis that the male-biased circuitry of the BSTM-LS supports male-biased aggressive behavior, although numerous observations do not support this idea (for excellent discussions of this topic, see Rosen et al., 2006; De Vries, 2008). However, Syrian hamsters typically express no AVP-ir cells in the BSTM and no AVP-ir innervation of the LS (Dubois-Dauphin et al., 1990; Bolborea et al., 2010) – thus we cannot assume that behavioral effects of septal AVP with respect to male–male aggression are attributable to signaling via the BSTM-LS circuit.
Regardless of the source of peptide, the behavioral effects of septal AVP are complex and not consistent with the hypothesis that septal AVP promotes aggression. In songbirds, intraseptal infusions of AVT inhibit resident–intruder aggression (Goodson, 1998a,b), but facilitate aggression in the context of mate competition (Goodson and Adkins-Regan, 1999) and promote the use of spontaneous agonistic song (Goodson, 1998a). Intraseptal infusions of AVP (i.e., exogenous AVP) also facilitate aggression in male rats that have been cohabitated with a female (Koolhaas et al., 1990), but during aggressive encounters, endogenous release of AVP into the LS varies in complex ways that relate to the male’s anxiety phenotype and aggressiveness (Beiderbeck et al., 2007). Indeed, septal AVP/AVT potently regulates anxiety (Landgraf et al., 1995), and thus many effects on social behavior may relate to the context-dependent modulation of anxiety (Goodson, 2008).
Hypothalamic AVP cell groups are integrators of dominance, subordinance and stress
The present experiments demonstrate that most of the AVP cell groups in the brain are responsive to aggressive interactions in ways that suggest a sensitivity to stress or negative aspects of the social interaction (i.e., subjugation). We found that AVP-Fos colocalization was (1) significantly greater within the caudal PVN and tuberal LH of subordinate males relative to dominant males, (2) positively correlated with the number of bites received in the AH, BSTMV, POA, anterior SON, and tuberal LH, with a comparable near-significant effect in the AC (p<0.06), and (3) negatively correlated with an index of dominance in caudal PVN, anterior SON, and tuberal LH. No significant effects were obtained for the nucleus circularis, although the data weakly trended in the same direction.
These results are consistent with previous findings showing that subordinance is associated with widespread activation of the POA and hypothalamus, and that Fos activation of virtually all hypothalamic loci is significantly greater in subordinate animals as compared with dominant animals (Kollack-Walker et al., 1997; Motta et al., 2009; also see Ebner et al., 2005). In Syrian hamsters, only the SON exhibits greater activation in dominant males relative to subordinates (Kollack-Walker et al., 1997), and an extensive analysis of hypothalamic activation in mice found that no loci expressed Fos at higher levels in dominant males relative to subordinates, although dominant males did show activation of many areas above handled controls (Motta et al., 2009). Our results are likewise consistent with findings that suggest virtually all AVP cell groups of the hypothalamus, including the accessory cell groups, are responsive to metabolic challenge (Briski and Brandt, 2000).
Despite these consistencies, our findings are less readily reconciled with data from male Syrian hamsters and prairie voles, in which resident–intruder aggression is facilitated by AVP signaling in the AH. Following aggressive interactions, dominant hamsters exhibit increased AVP-Fos colocalization within the medial SON and NC, both of which project AVP-ir axons into the AH (Delville et al., 2000). As predicted by those findings, offensive aggression in hamsters is blocked by infusions of a V1a antagonist into either the AH or ventrolateral hypothalamus, whereas aggression is facilitated by infusions of AVP (Ferris and Potegal, 1988; Potegal and Ferris, 1989; Delville et al., 1996; Ferris et al., 1997; Jackson et al., 2005). Similar pharmacological and activation results are obtained for the AH in tests of selective post-mating aggression in male prairie voles (Gobrogge et al., 2007, 2009), and voles exhibit endogenous AVP release within the AH that is positively correlated with aggression (Gobrogge et al., 2009).
These apparent differences may reflect a variety of things. First, we cannot discount the possibility that there are substantial species differences in the properties of hypothalamic AVP circuits, given that large species differences have been documented in other aspects of nonapeptide anatomy and behavioral function (Goodson, 2008). However, it may also be the case that functional properties of this circuitry are more complex than previous experiments would suggest. As discussed above, the relevant AVP neurons are responsive to a wide range of stressors, and developmental exposure to maternal separation or subjugation can alter the anatomy of hypothalamic AVP systems and profiles of aggressive behavior in adulthood (Delville et al., 1998; Veenema et al., 2006; Veenema et al., 2007; Lukas et al., 2010). Finally, AVP circuits exhibit plastic changes in relation to experience and physiological state (De Vries and Miller, 1998; Goodson and Bass, 2001; Veenema, 2009), factors which likely vary across studies. Thus, as a first step toward distinguishing between these various possibilities, it would be advantageous to examine the behavioral effects of pharmacological manipulations in animals with divergent developmental backgrounds, and in a diversity of behavioral and physiological contexts. Such studies should provide a more accurate view of how AVP systems integrate myriad variables to dynamically coordinate behavior and physiology.
Acknowledgments
We thank Marcy A. Kingsbury for providing feedback on the manuscript and Sara E. Schrock for extensive contributions to data analysis. Related work under support from the National Institutes of Health (RO1 MH062656 to J.L.G.) has been key to the development of ideas in this paper. We thank Indiana University and the Department of Biology at Indiana University for funding this project.
References
- Albers HE, Pollock J, Simmons WH, Ferris CF. A V1-like receptor mediates vasopressin-induced flank marking behavior in hamster hypothalamus. J Neurosci. 1986;6:2085–2089. doi: 10.1523/JNEUROSCI.06-07-02085.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiderbeck DI, Neumann ID, Veenema AH. Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. Eur J Neurosci. 2007;26:3597–3605. doi: 10.1111/j.1460-9568.2007.05974.x. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bolborea M, Ansel L, Weinert D, Steinlechner S, Pevet P, Klosen P. The bed nucleus of the stria terminalis in the Syrian hamster (Mesocricetus auratus): absence of vasopressin expression in standard and wild-derived hamsters and galanin regulation by seasonal changes in circulating sex steroids. Neuroscience. 2010;165:819–830. doi: 10.1016/j.neuroscience.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc Natl Acad Sci U S A. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briski KP, Brandt JA. Oxytocin and vasopressin neurones in principal and accessory hypothalamic magnocellular structures express fos-immunoreactivity in response to acute glucose deprivation. J Neuroendocrinol. 2000;12:409–414. doi: 10.1046/j.1365-2826.2000.00469.x. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS., III Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008a;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Wersinger SR, Young WSI. The role of the vasopressin 1b receptor in aggression and other social behaviours. Prog Brain Res. 2008b;170:65–72. doi: 10.1016/S0079-6123(08)00406-8. [DOI] [PubMed] [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- Castel M, Morris JF. The neurophysin-containing innervation of the forebrain of the mouse. Neuroscience. 1988;24:937–966. doi: 10.1016/0306-4522(88)90078-4. [DOI] [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Phan A, Kavaliers M. Neuroendocrinology of social information processing in rats and mice. Front Neuroendocrinol. 2009;30:442–459. doi: 10.1016/j.yfrne.2009.05.003. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Sex differences in vasopressin and oxytocin innervation of the brain. Prog Brain Res. 2008;170:17–27. doi: 10.1016/S0079-6123(08)00402-0. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Al Shamma HA. Sex differences in hormonal responses of vasopressin pathways in the rat brain. J Neurobiol. 1990;21:686–693. doi: 10.1002/neu.480210503. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Sluiter AA. Gonadal hormone actions on the morphology of the vasopressinergic innervation of the adult rat brain. Brain Res. 1984;298:141–145. doi: 10.1016/0006-8993(84)91157-0. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Swaab DF. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain—presence of a sex difference in the lateral septum. Brain Res. 1981;218:67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, van Leeuwen FW, Caffe AR, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985;233:236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Duetz W, Buijs RM, Van Heerikhuize J, Vreeburg JTM. Effects of androgens and estrogens on the vasopressin and oxytocin innervation of the adult rat brain. Brain Res. 1986;399:296–302. doi: 10.1016/0006-8993(86)91519-2. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Miller MA. Anatomy and function of extrahypothalamic vasopressin systems in the brain. Prog Brain Res. 1998;119:3–20. doi: 10.1016/s0079-6123(08)61558-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Södersten P. Sex differences in the brain: the relation between structure and function. Horm Behav. 2009;55:589–596. doi: 10.1016/j.yhbeh.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Delville Y, Mansour KM, Ferris CF. Serotonin blocks vasopressin-facilitated offensive aggression: interactions within the ventrolateral hypothalamus of golden hamsters. Physiol Behav. 1996;59:813–816. doi: 10.1016/0031-9384(95)02166-3. [DOI] [PubMed] [Google Scholar]
- Delville Y, Melloni RH, Jr, Ferris CF. Behavioral and neurobiological consequences of social subjugation during puberty in golden hamsters. J Neurosci. 1998;18:2667–2672. doi: 10.1523/JNEUROSCI.18-07-02667.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disturnal JE, Veale WL, Pittman QL. The ventral septal area: electrophysiological evidence for putative arginine vasopressin projections onto thermo-responsive neurons. Neuroscience. 1986;19:795–802. doi: 10.1016/0306-4522(86)90299-x. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Pevet P, Tribollet E, Dreifuss JJ. Vasopressin in the brain of the golden hamster: the distribution of vasopressin binding sites and of immunoreactivity to the vasopressin-related glycopeptide. J Comp Neurol. 1990;300:535–548. doi: 10.1002/cne.903000408. [DOI] [PubMed] [Google Scholar]
- Ebner K, Wotjak CT, Landgraf R, Engelmann M. Neuroendocrine and behavioral response to social confrontation: residents versus intruders, active versus passive coping styles. Horm Behav. 2005;47:14–21. doi: 10.1016/j.yhbeh.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic–pituitary–adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ludwig M, Landgraf R. Simultaneous monitoring of intrace-rebral release and behavior: endogenous vasopressin improves social recognition. J Neuroendocrinol. 1994;6:391–395. doi: 10.1111/j.1365-2826.1994.tb00598.x. [DOI] [PubMed] [Google Scholar]
- Everts HGJ, Koolhaas JM. Lateral septal vasopressin in rats: role in social and object recognition? Brain Res. 1997;760:1–7. doi: 10.1016/s0006-8993(97)00269-2. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Gold L, De Vries GJ, Potegal M. Evidence for a functional and anatomical relationship between the lateral septum and the hypothalamus in the control of flank marking behavior in golden hamsters. J Comp Neurol. 1990;293:476–485. doi: 10.1002/cne.902930310. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Potegal M. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol Behav. 1988;44:235–240. doi: 10.1016/0031-9384(88)90144-8. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502:1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, Wang Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci U S A. 2009;106:19144–19149. doi: 10.1073/pnas.0908620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm Behav. 1998a;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Vasotocin and vasoactive intestinal polypeptide modulate aggression in a territorial songbird, the violet-eared waxbill (Estrildidae: Uraeginthus granatina) Gen Comp Endocrinol. 1998b;111:233–244. doi: 10.1006/gcen.1998.7112. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Nonapeptides and the evolutionary patterning of sociality. Prog Brain Res. 2008;170:3–15. doi: 10.1016/S0079-6123(08)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) J Neuroendocrinol. 1999;11:19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature. 2000;403:769–772. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D. Dynamic limbic networks and social diversity in vertebrates: from neural context to neuromodulatory patterning. Front Neuroendocrinol. 2009;30:429–441. doi: 10.1016/j.yfrne.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D, Schrock SE. Dynamic neuromodulation of aggression by vasotocin: influence of social context and social phenotype in territorial songbirds. Biol Lett. 2009a;5:554–556. doi: 10.1098/rsbl.2009.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Rinaldi J, Kelly AM. Vasotocin neurons in the bed nucleus of the stria terminalis preferentially process social information and exhibit properties that dichotomize courting and non-courting phenotypes. Horm Behav. 2009b;55:197–202. doi: 10.1016/j.yhbeh.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc Natl Acad Sci U S A. 2006;103:17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood AK, Wark AR, Fernald RD, Hofmann HA. Expression of arginine vasotocin in distinct preoptic regions is associated with dominant and subordinate behaviour in an African cichlid fish. Proc R Soc B: Biol Sci. 2008;275:2393–2402. doi: 10.1098/rspb.2008.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Irvin RW, Szot P, Dorsa DM, Potegal M, Ferris CF. Vasopressin in the septal area of the golden hamster controls scent marking and grooming. Physiol Behav. 1990;48:693–700. doi: 10.1016/0031-9384(90)90213-n. [DOI] [PubMed] [Google Scholar]
- Jackson D, Burns R, Trksak G, Simeone B, Deleon KR, Connor DF, Harrison RJ, Melloni RH., Jr Anterior hypothalamic vasopressin modulates the aggression-stimulating effects of adolescent cocaine exposure in Syrian hamsters. Neuroscience. 2005;133:635–646. doi: 10.1016/j.neuroscience.2005.02.047. [DOI] [PubMed] [Google Scholar]
- Kabelik D, Klatt JD, Goodson JL. Endogenous vasotocin exerts context-dependent behavioral effects in a semi-naturalistic colony environment. Horm Behav. 2009;56:101–107. doi: 10.1016/j.yhbeh.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–8855. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Moor E, Hiemstra Y, Bohus B. The testosterone-dependent vasopressinergic neurons in the medial amygdala and lateral septum: involvement in social behavior of male rats. In: Jaard S, Jamison R, editors. Vasopressin. INSERM/Libbey; Paris: 1990. pp. 213–220. [Google Scholar]
- Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci. 1995;15:4250–4258. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Lukas M, Bredewold R, Neumann ID, Veenema AH. Maternal separation interferes with developmental changes in brain vasopressin and oxytocin receptor binding in male rats. Neuropharmacology. 2010;58:78–87. doi: 10.1016/j.neuropharm.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Wingfield JC. Intraventricular infusion of arginine vasotocin induces singing in a female songbird. J Neuroendocrinol. 1997;9:487–491. doi: 10.1046/j.1365-2826.1997.00635.x. [DOI] [PubMed] [Google Scholar]
- Miller MA, De Vries GJ, al-Shamma HA, Dorsa DM. Decline of vasopressin immunoreactivity and mRNA levels in the bed nucleus of the stria terminalis following castration. J Neurosci. 1992;12:2881–2887. doi: 10.1523/JNEUROSCI.12-08-02881.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Urban JH, Dorsa DM. Steroid dependency of vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization. Endocrinology. 1989a;125:2335–2340. doi: 10.1210/endo-125-5-2335. [DOI] [PubMed] [Google Scholar]
- Miller MA, Vician L, Clifton DK, Dorsa DM. Sex differences in vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization. Peptides. 1989b;10:615–619. doi: 10.1016/0196-9781(89)90152-6. [DOI] [PubMed] [Google Scholar]
- Motta SC, Goto M, Gouveia FV, Baldo MVC, Canteras NS, Swanson LW. Dissecting the brain’s fear system reveals the hypothalamus is critical for responding in subordinate conspecific intruders. Proc Natl Acad Sci U S A. 2009;106:4870–4875. doi: 10.1073/pnas.0900939106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Arginine vasopressin V1a receptor antagonist impairs maternal memory in rats. Physiol Behav. 2008;95:182–186. doi: 10.1016/j.physbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potegal M, Ferris CF. Intraspecific aggression in male hamsters is inhibited by intrahypothalamic vasopressin-receptor antagonist. Aggress Behav. 1989;15:311–320. [Google Scholar]
- Rosen GJ, De Vries GJ, Goldman SL, Goldman BD, Forger NG. Distribution of vasopressin in the brain of the eusocial naked mole-rat. J Comp Neurol. 2007;500:1093–1105. doi: 10.1002/cne.21215. [DOI] [PubMed] [Google Scholar]
- Rosen GJ, De Vries GJ, Villalba C, Weldele ML, Place NJ, Coscia EM, Glickman SE, Forger NG. Distribution of vasopressin in the forebrain of spotted hyenas. J Comp Neurol. 2006;498:80–92. doi: 10.1002/cne.21032. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen FW, Caffe AR, De Vries GJ. Vasopressin cells in the bed nucleus of the stria terminalis of the rat: sex differences and the influence of androgens. Brain Res. 1985;325:391–394. doi: 10.1016/0006-8993(85)90348-8. [DOI] [PubMed] [Google Scholar]
- Veenema AH. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: what can we learn from animal models? Front Neuroendocrinol. 2009;30:497–518. doi: 10.1016/j.yfrne.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Blume A, Niederle D, Buwalda B, Neumann ID. Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur J Neurosci. 2006;24:1711–1720. doi: 10.1111/j.1460-9568.2006.05045.x. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, Neumann ID. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology. 2007;32:437–450. doi: 10.1016/j.psyneuen.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Neurobiological mechanisms of aggression and stress coping: a comparative study in mouse and rat selection lines. Brain Behav Evol. 2007;70:274–285. doi: 10.1159/000105491. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res. 2008;170:261–276. doi: 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- Wang Z, Smith W, Major DE, De Vries GJ. Sex and species differences in the effects of cohabitation on vasopressin messenger RNA expression in the bed nucleus of the stria terminalis in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Brain Res. 1994;650:212–218. doi: 10.1016/0006-8993(94)91784-1. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]