Abstract
20-Hydroxyvitamin D3 [20(OH)D3], the major product of CYP11A1 action on vitamin D3, is biologically active and like 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] can inhibit proliferation and promote differentiation of a range of cells, and has anti-inflammatory properties. However, unlike 1,25(OH)2D3, it does not cause toxic hypercalcemia at high doses and is therefore a good candidate for therapeutic use to treat hyperproliferative and autoimmune disorders. In this study we analyzed the ability of mouse liver microsomes to metabolize 20(OH)D3. The two major products were identified from authentic standards as 20,24-dihydroxyvitamin D3 [20,24(OH)2D3] and 20,25-dihydroxyvitamin D3 [20,25(OH)2D3]. The reactions for synthesis of these two products from 20(OH)D3 displayed similar Km values suggesting that they were catalyzed by the same cytochrome P450. Some minor metabolites were produced by reactions with higher Km values for 20(OH)D3. Some metabolites gave mass spectra suggesting that they were the result of hydroxylation followed by dehydrogenation. One product had an increase in the wavelength for maximum absorbance from 263 nm seen for 20(OH)D3, to 290 nm, suggesting a new double bond was interacting with the vitamin D-triene chromophore. The two major products, 20,24(OH)2D3 and 20,25(OH)2D3 have both previously been shown to have higher potency for inhibition of colony formation by melanoma cells than 20(OH)D3, thus it appears that metabolism of 20(OH)D3 by mouse liver microsomes can generate products with enhanced activity.
Keywords: 20-Hydroxyvitamin D3, Microsomes, Hydroxylation, Cytochrome P450, Liver
1. Introduction
Purified CYP11A1 acts on vitamin D3 to produce 20-hydroxyvitamin D3 [20(OH)D3] as the major metabolite, some of which is metabolized by the same enzyme to 20,23-dihydroxyvitamin D3 [20,23(OH)2D3], 20,22-dihydroxyvitamin D3 [20,22(OH)2D3], 17,20-dihydroxyvitamin D3 [17,20(OH)2D3] and 17,20,23-trihydroxyvitamin D3 (1–6). Nuclear magnetic resonance (NMR) of 20(OH)D3 produced by CYP11A1 indicates that it is the S-epimer, 20S(OH)D3 (4), which was further confirmed by chemical synthesis (7). More recently we have shown that 20(OH)D3 can be produced by human placentas, human epidermal keratinocytes, bovine and rodent adrenals (8), as well as by Caco-2 colon cells (8), all of which express CYP11A1 (9,10). A monohydroxyvitamin D3 metabolite corresponding in retention time to 20(OH)D3 has also been detected in human plasma (8). While a physiological role for endogenously produced 20(OH)D3 remains to be established, it displays biological activity on cultured cells of different lineage acting as a partial agonist on the vitamin D receptor (1,3,11–15). Thus, it inhibits proliferation and stimulates the differentiation of many cell types including epidermal keratinocytes and melanocytes, dermal fibroblasts and melanoma, leukemia and breast cancer cells with comparable potency to 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3]. It also displays anti-inflammatory effects, inhibiting the activity of NF-κB (1,12,14) and inhibiting production of pro-inflammatory cytokines, while promoting the production of the anti-inflammatory cytokine IL-10 (1). Most recently, we have demonstrated that 20(OH)D3 attenuates bleomycin-induced scleroderma in vivo (15). Moreover, unlike 1,25(OH)2D3, it lacks calcemic activity in rodents with no toxicity at doses as high as 30 μg/kg (11,13). Also in contrast to 1,25(OH)2D3, it is a poor inducer of the inactivating enzyme, CYP24A1 (13,16). These properties of 20(OH)D3 suggest that it may be a useful therapeutic agent for the treatment of proliferative and autoimmune disorders (1).
In vitro studies with the mitochondrial cytochromes P450 that are involved in vitamin D3 metabolism reveal that CYP27A1, CYP27B1 and CYP24A1 can act on 20(OH)D3. CYP27B1 produces 1α,20-dihydroxyvitamin D3 (16,17), and the addition of the 1α-hydroxyl group confers some calcemic activity to the derivative (13). We detected production of 1α-hydroxy derivatives of 20(OH)D3 and 20,23(OH)2D3 in vivo in human placenta and epidermal keratinocytes (8). Both CYP27A1 and CYP24A1 convert 20(OH)D3 to 20,25-dihydroxyvitamin D3 [20,25(OH)2D3] with this metabolite displaying greater inhibition of melanoma cell proliferation than the parent 20(OH)D3, or 1,25(OH)2D3 (18,19).
A number of microsomal cytochromes P450 are involved in vitamin D metabolism. CYP2R1 is generally regarded as the major 25-hydroxylase in humans (20,21) although there may be some contribution from mitochondrial CYP27A1 (19,22). The knockout of both Cyp2r1 and Cyp27a1 in mice only caused a 50% decrease in serum 25-hydroxyvitamin D3 [25(OH)D3] levels indicating that in rodents there is another vitamin D 25-hydroxylase (23). This could be Cyp2j3, which displays high 25-hydroxylase activity whereas its human counterpart, CYP2J2, had only low 25-hydroxylase activity (21,24). CYP3A4 in humans acts to catalyze 25-hydroxylation of vitamin D2 and the synthetic prodrugs 1α-hydroxyvitamin D3 [1α(OH)D3] and 1α-hydroxyvitamin D2 [1α(OH)D2]. It also catalyzes 24-hydroxylation of 1α(OH)D3, 1α(OH)D2 and vitamin D3 (21,25,26) but shows little ability to 25-hydroxylate vitamin D3 (21). Recently, Wang et al. (27) showed that 4β,25-dihydroxyvitamin D3, a form of vitamin D detectable in the bloodstream, was produced from 25(OH)D3 by CYP3A4 in human liver. In contrast to the human, mouse liver microsomes do not express CYP3A4, instead there are six different family 3A isoforms expressed: Cyp3a11, Cyp3a13, Cyp3a16, Cyp3a25, Cyp3a41 and Cyp3a44. These are believed to carry out the equivalent function of CYP3A4 (28).
Currently mice are being used to examine the in vivo therapeutic potential of 20(OH)D3 (1,15). It is therefore important to know how this active vitamin D3 metabolite is metabolized by the liver, and thus the aim of the current study was to characterize the metabolism of 20(OH)D3 by mouse liver microsomes.
2. Materials and Methods
2.1. Generation of secosteroid standards
20(OH)D3 was produced by the enzymatic action of recombinant bovine CYP11A1 on vitamin D3, and purified by TLC and reverse-phase HPLC (16). 22-Hydroxyvitamin D3 [22(OH)D3], 17,20(OH)2D3, 20,22(OH)2D3 and 20,23(OH)2D3 were also produced from vitamin D3 using bovine CYP11A1 (16). 20,24-dihydroxyvitamin D3 [20,24(OH)2D3] and 20,25(OH)2D3 were produced by the action of rat Cyp24a1 on 20(OH)D3 (18) while 20,26-dihydroxyvitamin D3 [20,26(OH)2D3] was made from 20(OH)D3 using human CYP27A1 (19). The structures of these metabolites have previously been determined by NMR (5,16,18).
2.2. Preparation of mouse liver microsomes
Ten-week-old female mice (C57BL/6J; Animal Resource Centre, Murdoch University) were euthanized and livers placed in 0.25 M sucrose on ice. The tissue was homogenized in 9 volumes of 0.25 M sucrose in a Potter-Elvehjem homogenizer by three low-speed passes of the teflon pestle. The homogenate was centrifuged at 600 × g for 10 min at 4°C. The supernatant was removed and centrifuged at 6,000 × g for 15 min at 4°C to sediment the mitochondrial fraction. The resulting supernatant was centrifuged at 11,000 × g for 15 min at 4°C to sediment any remaining mitochondria and the supernatant was then centrifuged at 107,000 × g for 1 h at 4°C. The microsomal pellet was resuspended in 0.25 M sucrose by hand homogenizing using a Potter-Elvehjem homogenizer and centrifuged again at 107,000 × g for 1 h at 4°C to wash the microsomes. The microsomal pellet was resuspended in 0.25 M sucrose by hand homogenizing and stored at −80°C.
2.3. Metabolism of 20(OH)D3 by the mouse liver microsomal fraction
Mouse liver microsomes at a final concentration of 1.5 mg/mL were incubated with 20(OH)D3 (see Figure Legends for concentrations) in 0.45% 2-hydroxypropyl-β-cyclodextrin (cyclodextrin) in buffer comprising 0.25 M sucrose, 50 mM HEPES (pH 7.4), 20 mM KCl, 5 mM MgSO4 and 0.2 mM EDTA. The cyclodextrin served to hold the 20(OH)D3 substrate in solution and is often used for activity assays with low solubility substrates where it forms a hydrophobic cage around them, with a hydrophilic exterior (29,30). It could solubilize 200 μM 20(OH)D3, the maximum concentration used in this study (3,8,31). The incubation also contained a cofactor and regeneration system comprising 2 mM glucose-6-phosphate, 2 U/mL glucose-6-phosphate dehydrogenase and 500 μM NADPH. The typical incubation volume was 0.5 mL. Samples were preincubated for 8 min in a 37°C water bath shaking at 50 cycles per min and then reactions started by the addition of NADPH. After incubation at 37 C (see Results for times), reactions were stopped with 2.5 volumes ice-cold dichloromethane. Samples were vortexed and centrifuged at 670 × g for 10 min and the lower organic phase was retained and the upper aqueous phase was extracted another three times with 2.5 volumes dichloromethane. The extracts were then dried under nitrogen at 30°C and dissolved in the solvent required for HPLC analysis.
2.4. Reverse-phase HPLC analysis of 20(OH)D3 metabolites
The analysis of 20(OH)D3 metabolites was carried out using a Perkin-Elmer HPLC system (Biocompatible Binary Pump 250) with a UV monitor set at 265 nm, equipped with a C18 column (Grace Alltima, 25 cm × 4.6 mm, particle size 5 μm). For routine analysis, metabolites were separated using a gradient of 45 – 100% acetonitrile in water for 30 min, followed by 100% acetonitrile for 35 min, at a flow rate of 0.5 mL/min (designated HPLC Program A in Figure Legends). As this system did not separate all metabolites or authentic standards, some 20(OH)D3 metabolites were subsequently separated using a gradient of 64 – 100% methanol in water for 20 min, followed by 100% methanol for 30 min, at a flow rate of 0.5 mL/min (designated HPLC Program B in the Figure Legends).
2.5. Mass spectrometry of 20(OH)D3 metabolites
Reverse-phase HPLC was initially used to purify and collect 20(OH)D3 metabolites using an acetonitrile in water gradient (HPLC Program A), then further purified and collected using a methanol in water gradient (HPLC Program B). The purified 20(OH)D3 metabolites (5 nmol) were then subjected to analysis by mass spectrometry. The system used involved liquid chromatography-mass spectrometry (LC/MS) combining 2-dimensional (2D) separation with two pentafluorophenyl (PFP) columns, to provide relatively pure material to the mass spectrometer, as described in detail previously (32). The system comprised an Agilent 1290 UPLC binary pump coupled to an Agilent 6460 triple quadrupole mass spectrometer with a Jetstream source. Electrospray ionization was used in the positive mode. Each sample was reconstituted in 500 μL of 70% methanol + 0.1% formic acid, with 20 μL being injected onto the LC/MS. Samples were subjected to an MS2 scan from 300 – 500 m/z. These assays were performed as a service by UWA Centre for Metabolomics.
2.6. Marker enzyme assays
Cytochrome c oxidase was used as a marker enzyme for mitochondria (33). The colorimetric assay measured the oxidation of ferrocytochrome c to ferricytochrome c by cytochrome c oxidase, which causes a decrease in absorbance at 550 nm (33). The assays were carried out on both mitochondrial and microsomal fractions at 30°C and the oxidation of cytochrome c was measured over 3 min, with rates being linear over this time span.
Testosterone hydroxylation was used as a microsomal marker (34,35). Rat liver mitochondrial and microsomal fractions were incubated with testosterone at a final concentration of 100 μM added from an acetonitrile stock (2% acetonitrile final concentration), in a buffer comprising 0.25 M sucrose, 50 mM HEPES (pH 7.4), 20 mM KCl, 5 mM MgSO4 and 0.2 mM EDTA. The appropriate cofactors were added; 5 mM isocitrate and 500 μM NADPH for mitochondria; 2 mM glucose-6-phosphate, 2 U/mL glucose-6-phosphatase and 500 μM NADPH for microsomes. Samples were preincubated for 8 min at 37°C and reactions were started with either NADPH or isocitrate/NADPH, then stopped with 2.5 volumes ice-cold dichloromethane and extracted as before. Testosterone metabolites were separated from testosterone by HPLC on a C18 column (Grace Alltima, 25 cm × 4.6 mm, particle size 5 μm) using a gradient of 30 - 75% acetonitrile in water for 25 min, followed by a gradient of 75 - 100% acetonitrile in water for 10 min, and then 100% acetonitrile for 10 min, all at a flow rate of 0.5 mL/min. Testosterone and its metabolites were detected with a UV monitor set at 240 nm (34). The rates of testosterone metabolism by the microsomal and mitochondrial fractions were calculated from the percentage conversion of testosterone to products and the initial testosterone concentration.
2.7. Other procedures
The Ponceau S protein assay (36) was used to determine the protein concentration of isolated mouse liver microsomal and mitochondrial fractions. The concentrations of vitamin D metabolites with a similar UV spectrum to vitamin D3, were determined using a Shimadzu UV-2450 spectrophotometer using an extinction coefficient at 263 nm of 18,000 M−1cm−1 (37). Hydroxy derivatives of vitamin D3 have been shown to have the same extinction coefficient as vitamin D3 (37).
3. Results
3.1. Purity of microsomal fraction
Since liver mitochondria contain at least one P450, CYP27A1, that can metabolize 20(OH)D3 (19), we tested the level of contamination of the microsomal fraction with mitochondria using cytochrome c oxidase as a marker. The mitochondrial fraction exhibited a cytochrome oxidase activity of 6.75 ± 0.6 nmol/min/mg protein (data are mean ± SD for triplicate determinations), while the microsomal fraction exhibited an activity of 0.37 ± 0.05 nmol/min/mg protein. This indicates only minor contamination of the microsomal fraction with mitochondria. Testosterone metabolism was used as a microsomal marker and its activity was 8.2 times higher in the microsomal fraction than for the mitochondrial fraction.
3.2. Metabolism of 20(OH)D3 by mouse liver microsomes
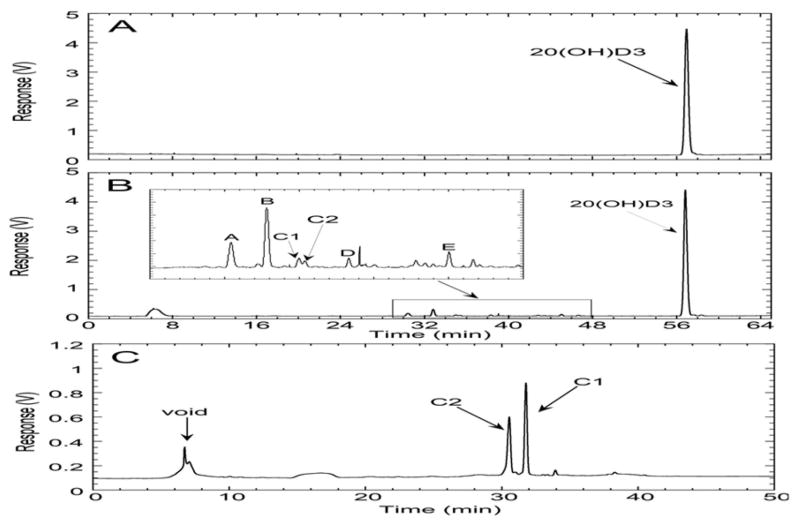
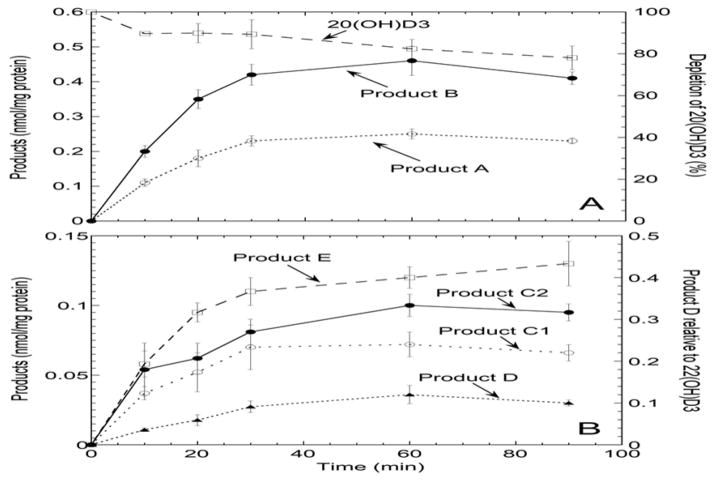
Incubation of the mouse liver microsomal fraction with 20(OH)D3 produced six metabolites (A to E, Fig. 1B), not present in the control (Fig. 1A), each of which represented greater than 0.5% of the total extracted secosteroids. Two products are labelled as C1 and C2 in the HPLC chromatogram (Fig. 1B) as they were not baseline separated using an acetonitrile-water gradient (and originally designated as product C). To determine the proportions of C1 and C2, the combined peak from the acetonitrile-water gradient was collected and separated into C1 and C2 using a methanol-water gradient, where they elute in reverse order (Fig 1C). The time course for appearance of products A–E showed that products A and B were the two major products at all time points (Fig. 2). The rates of production of all metabolites were similar and declined with time, presumably due to enzyme inactivation, as only 22% of the substrate was consumed by 90 min (Fig. 2A). No evidence for secondary metabolite formation was seen as none of the products showed a lag (requiring synthesis of a precursor) in their time course. Note that product D had a different absorption spectrum to all the other products that was not typical of vitamin D3 (see below) and thus it could not be assumed that it has the same extinction coefficient as the internal standard [22(OH)D3] used for quantitation. Hence its production is expressed as a relative response of the UV detector compared to that of the internal standard (Fig. 2B, right axis).
Figure 1.
HPLC analysis of 20(OH)D3 metabolism by mouse liver microsomes. 20(OH)D3 (50 μM) in 0.45% cyclodextrin was incubated with microsomes (1.5 mg/mL) for 30 min, products extracted and analyzed by HPLC as described in Experimental Procedures. (A), control incubation without the addition of NADPH, analyzed using HPLC Program A; (B), test incubation with the addition of NADPH, analyzed using HPLC Program A; (C), metabolites C1 and C2 collected from the acetonitrile gradient (HPLC Program A) and analyzed with a methanol gradient (HPLC Program B), showing the two peaks clearly separated.
Figure 2.
Time course for metabolism of 20(OH)D3 by mouse liver microsomes. 20(OH)D3 (50 μM) in 0.45% cyclodextrin was incubated with the microsomal fraction (1.5 mg/mL) for the times indicated, the reaction stopped with dichloromethane and 22(OH)D3 (1 nmol) added as internal standard for quantitation. Products were extracted and analyzed by HPLC using an acetonitrile gradient (HPLC Program A, see Experimental Procedures). 20(OH)D3 metabolites produced from the reaction are labeled as in Fig. 1 with products C1 and C2 being separated with a methanol gradient (HPLC Program B) as in Fig. 1. (A), depletion of substrate and formation of major products. (B), production of minor metabolites. Product D is plotted as the response of the UV detector relative to that of the internal standard since its UV spectrum was different to the internal standard and other products. Data are mean ± SD of triplicate determinations.
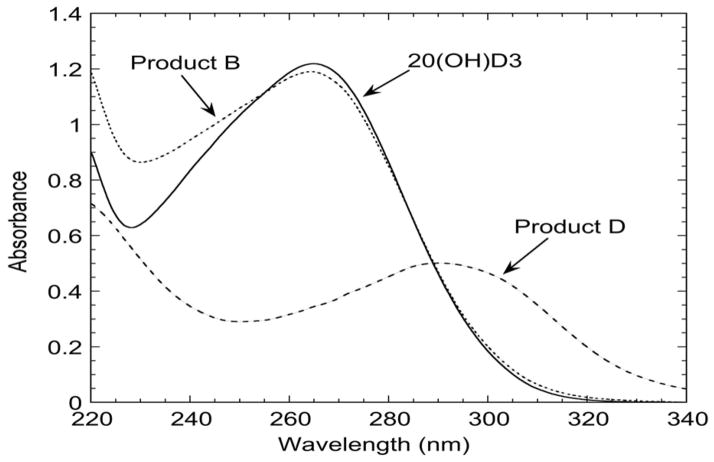
The electrospray mass spectra for products A, B and C2 showed the parent ion at m/z = 439.3 (416.3 + Na+), representing a dihydroxyvitamin D3 (m/z = 416.3) (Table 1). The Na+ adduct likely comes from the incubation buffer or the borosilicate glass tubes used for sample preparation (38). Products A and B also showed major ions at m/z = 381.3 (416.3 + H+ – 2H2O), 399.3 (416.3 + H+ – H2O) and for product A, 455.2 (416.3 + K+), affirming that both products are dihydroxyvitamin D3 (Table 1). Product C2 also displayed the ions at m/z 399.3 and 455.2. These 3 metabolites displayed the typical vitamin D UV absorption spectrum with a peak at 263 nm, as illustrated for product B in Fig. 3. The mass spectra for products C1, D and E showed the parent ion at m/z = 415.2 (414.2 + H+) and another ion at m/z = 437.2 (414.2 + Na+) (Table 1), suggesting that a hydroxyl group had been added (m/z = 416.3) followed by an alcohol to ketone conversion or the insertion of a double bond (reducing m/z by 2), resulting in a dehydro-dihydroxyvitamin D3. Other major ions seen for product D included m/z = 379.2 (414.2 + H+ – 2H2O) and 397.2 (414.2 + H+ – H2O), while the other major ion seen for products C1 and E was at m/z = 453.1 (414.1 + K+). The UV spectra for products C1 and E were similar to the parent 20(OH)D3 with a peak at 263 nm. In contrast, product D had its peak at 290 nm instead of the typical 263 nm of vitamin D (Fig. 3). This could be due to the new double bond (C=C or C=O) interacting with the vitamin D chromophore.
Table 1.
Analysis of 20(OH)D3 metabolites produced by mouse liver microsomes by mass spectrometry. Mass spectra were recorded as described in the Experimental section. Parent ions are highlighted in bold.
| Product | [M + Na]+ | [M + H]+ | [M + H - H2O]+ | [M + H - 2H2O]+ | [M + K]+ | Classification |
|---|---|---|---|---|---|---|
| A | 439.3 | - | 399.3 | 381.3 | 455.2 | Dihydroxyvitamin D3 |
| B | 439.3 | - | 399.3 | 381.3 | - | Dihydroxyvitamin D3 |
| C1 | 437.2 | 415.2 | - | - | 453.1 | Dehydro-dihydroxyvitamin D3 |
| C2 | 439.3 | - | 399.3 | - | 455.2 | Dihydroxyvitamin D3 |
| D | 437.3 | 415.2 | 397.2 | 379.2 | - | Dehydro-dihydroxyvitamin D3 |
| E | 437.1 | 415.2 | - | - | 453.1 | Dehydro-dihydroxyvitamin D3 |
Figure 3.
UV spectra of 20(OH)D3 and products B and D. Samples were dissolved in ethanol for recording their absorbance spectra.
Spiking of the reaction extract with authentic standards (see Section 2.1) and subsequent HPLC analysis using two different solvent systems enabled identification of product A as 20,25(OH)2D3 (Fig. 4, panels A, B, D and E). Product B had an identical retention time to both 20,24(OH)2D3 (Fig. 4C) and 20,26(OH)2D3 (not shown) as these two secosteroids can not be separated by reverse-phase HPLC using an acetonitrile gradient. These two secosteroids do separate with a methanol gradient enabling identification of product B as 20,24(OH)2D3 (Fig. 4, panels F–H). We also compared retention times of products C1, C2, D and E to a number of other dihydroxyvitamin D3 standards containing a 20-hydroxyl group, namely, 20,23(OH)2D3, 20,22(OH)2D3 and 17,20(OH)2D3, but none matched the observed products, excluding these dihydroxyvitamin D3 compounds as microsomal metabolites.
Figure 4.
Identification of product A as 20,25(OH)2D3 and B as 20,24(OH)2D3 by HPLC analysis. (A–C), an extract of the metabolites produced by the microsomal fraction was spiked with authentic 20,24(OH)2D3 or 20,25(OH)2D3 and analyzed using an acetonitrile gradient (HPLC Program A). (D–H), products A and B were collected from the acetonitrile gradient and further analyzed using a methanol gradient (HPLC Program B). (A), products without spiking; (B), products after spiking with 20,25(OH)2D3; (C), products after spiking with 20,24(OH)2D3; (D) product A collected from the acetonitrile gradient; (E), product A spiked with 20,25(OH)2D3; (F) product B collected from the acetonitrile gradient; (G), product B spiked with 20,26(OH)2D3; (H), product B spiked with 20,24(OH)2D3.
To test if any of products C1, D or E resulted from dehydrogenation of either 20,24(OH)2D3 (product B) or 20,25(OH)2D3 (product A), microsomes were incubated with 20,24(OH)2D3 or 20,25(OH)2D3 (25 μM) dissolved in 0.45% cyclodextrin, for 1 h at 37°C. HPLC analysis (as for Fig. 1) revealed that no products were formed (not shown) indicating that none of the dehydro-dihydroxyvitamin D3 products arise from 20,24(OH)2D3 or 20,25(OH)2D3. This experiment also confirms that neither 20,24(OH)2D3 nor 20,25(OH)2D3 is further metabolized by the microsomal fraction.
3.3. Kinetics of 20(OH)D3 metabolism by mouse liver microsomes
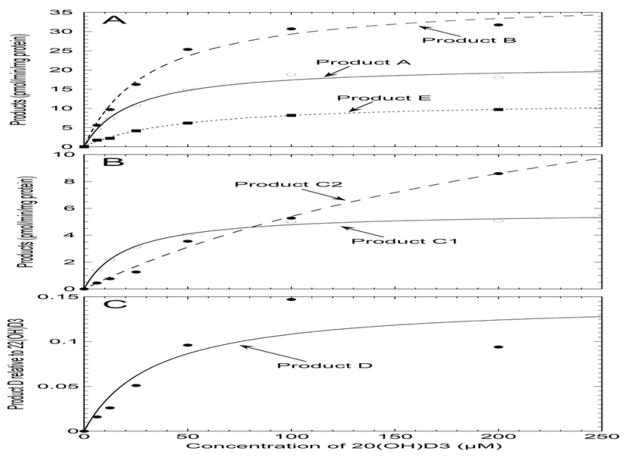
To determine the Km for 20(OH)D3 conversion to each product, the concentration of substrate was varied and the amount of each product measured under initial rate conditions. Products were analyzed by HPLC using an acetonitrile-water gradient. As for the time course (Fig. 2), the proportions of products C1 and C2 were determined by collecting the combined peak from the acetonitrile gradient and separating it into C1 and C2 using a methanol-water gradient. Data for all metabolites generally gave good fits to the Michaelis-Menten equation, as illustrated in Fig. 5, with saturation occurring at high 20(OH)D3 concentrations. For product D, the data points at 100 μM and 200 μM 20(OH)D3 were not close to the fitted curve resulting in a large standard error in the Km. The hyperbola did split the two points equally and the low activity at 200 μM 20(OH)D3 is unlikely to be due to substrate inhibition which was not observed for the other products, nor to insolubility of 20(OH)D3 at this concentration as cyclodextrin was present as a solubilizing agent (see Section 2.3). The large standard error is possibly associated with there being more than one catalytic step required for formation of product D.
Figure 5.
The effect of 20(OH)D3 concentration on production of products A–E by mouse liver microsomes. 20(OH)D3 at concentrations varying from 6.25 to 200 μM, dissolved in cyclodextrin at a final concentration of 0.45%, were incubated with the mouse liver microsomal fraction. Incubations were carried out for 30 min at 37 C, the reaction stopped with dichloromethane, 22(OH)D3 (1.0 nmol) added as internal standard, then products extracted and analyzed using HPLC Program A (see Experimental Procedures). Data were fitted to the Michaelis-Menten equation.
The kinetic parameters for all products are listed in Table 2. Product A, already identified as 20,25(OH)2D3, had a comparable Km value to that of product B, identified as 20,24(OH)2D3. Products C1 and D also had Km values similar to those for 20,24(OH)2D3 and 20,25(OH)2D3 while the Km for product E was higher. The Km for product C2 formation was 9 – 12 fold higher than values for formation of 20,24(OH)2D3 and 20,25(OH)2D3. Of the products with the same absorbance spectrum as 20(OH)D3, product B [20,24(OH)2D3] was produced with the greatest catalytic efficiency (Vmax/Km) while product E was produced with the lowest. The true Vmax for product D is unknown due to its different absorbance spectrum to the other products; its rate of production is therefore recorded as the HPLC peak area relative to that of the internal standard (Fig. 5C).
Table 2.
Kinetics of the metabolism of 20(OH)D3 by mouse liver microsomes. The Michaelis-Menten equation was fitted to the experimental data as in Fig. 5 and gave good fits with correlation coefficients ranging from 0.91 to 0.99. Data for Km and Vmax are presented as mean ± standard error of the curve fit.
| Product | Km (μM) | Vmax (pmol/min/mg protein) | Vmax/Km |
|---|---|---|---|
| A [20,25(OH)2D3] | 22.9 ± 3.5 | 21.4 ± 1.0 | 0.93 |
| B [20,24(OH)2D3] | 32.3 ± 4.9 | 38.8 ± 2.0 | 1.20 |
| C1 | 20.3 ± 1.6 | 5.8 ± 0.1 | 0.28 |
| C2 | 287.9 ± 75.1 | 20.9 ± 3.7 | 0.073 |
| D | 34.1 ± 23.4 | N/A | N/A |
| E | 47.6 ± 3.8 | 12.0 ± 0.4 | 0.25 |
N/A = Not Applicable
4. Discussion
We have found that mouse liver microsomes can metabolize 20(OH)D3 to a number of metabolites with the two major ones being identified from mass spectrometry and authentic standards as 20,24(OH)2D3 and 20,25(OH)2D3. These major metabolites did not undergo further transformation by the microsomal fraction. However, we have reported previously that both of these metabolites can be converted to their 1α-hydroxy derivatives by the mitochondrial enzyme, CYP27B1, of which highest activity is expressed in kidney (17). Both 20,24(OH)2D3 and 20,25(OH)2D3, as well as 1,24,25-trihydroxyvitamin D3, have previously been shown to display higher biological activity than 20(OH)D3 towards melanoma cells (18).
20(OH)D3 exhibits Km values of 23 – 32 μM for formation of 20,24(OH)2D3 and 20,25(OH)2D3 by the mouse liver microsomal fraction. The similarity of the Km values for formation of these two products suggests that it reflects binding to a single site on a P450 with a specific orientation that permits hydroxylation at the two neighboring carbons on the vitamin D side chain. Km values for production of C1 (20 μM) and D (34 μM) are also comparable to those for formation of 20,24(OH)2D3 and 20,25(OH)2D3 and might also be catalyzed by the same P450. In contrast, the Km values for formation of products E (47 μM) and C2 (291 μM) are higher than for the other products, suggesting that one or more different P450s are involved. The Km for the formation of 20,24(OH)2D3, the major product among those with a similar absorbance spectrum as 20(OH)D3, is 7.6-fold higher than for 24R-hydroxylation of 1,25(OH)2D3 by mouse liver microsomes which is believed to be catalyzed by CYP3A isoforms (28). However, the kcat for the conversion of 20(OH)D3 to 20,24(OH)2D3 is 16.9-fold higher than for this conversion of 1,25(OH)2D3 to 1,24R,25-trihydroxyvitamin D3 by microsomes from control animals, so the catalytic efficiency is 2-fold higher for 20(OH)D3. Human liver microsomes can metabolize 25(OH)D3 to 4β,25-dihydroxyvitamin D3 with a Km of 19 μM, similar to that for formation of 20,24(OH)2D3 and 20,25(OH)2D3 by mouse liver microsomes. The 4β-hydroxylation of 25(OH)D3 is mediated by CYP3A4 in a reaction suggested to contribute to the reduction of 25(OH)D3 levels with chronic drug treatment (27).
The mass spectral data indicate that products C1, D and E are the result of two separate reactions, hydroxylation and dehydrogenation. Dehydrogenation reactions involving alcohol to ketone or aldehyde conversions are well known reactions catalyzed by P450s and for example, both reactions are observed in the C24 oxidation pathway of vitamin D catabolism catalyzed by CYP24A1 (39). Although uncommon, some P450s have been reported to catalyze dehydrogenations involving conversion of a C-C single bond to a double bond (40). Thus, while it would seem more likely that a separate dehydrogenase enzyme catalyzes the dehydrogenation if it involves C-C bond oxidation, it is possible that a single P450 species can catalyze both the hydroxylation and subsequent dehydrogenation required to form products C1, D and E from 20(OH)D3. In the case of product D, the increase in the wavelength for maximum absorbance from 263 to 290 nm suggests that the new double bond interacts with the triene chromophore of vitamin D3 involving the A ring and broken B ring of the secosteroid. Such an increase in the absorbance maximum is seen when the C-C double bond of vitamin D between C10 and C19 is replaced by a ketone group at C10 (41).
This study, as well as our previous reports (18,19), indicate that there are several P450s in mammals that can hydroxylate 20(OH)D3 at C25. While the identity of the mouse liver microsomal enzyme(s) responsible for this remain to be established, candidates include Cyp2r1, Cyp2j3, and Cyp3a family members (see Introduction). Purified mitochondrial Cyp24a1 can hydroxylate 20(OH)D3 at C24 and C25 producing 20,24(OH)2D3 and 20,25(OH)2D3, as for microsomes (19). It should be noted that the microsomes we used had minimal mitochondrial contamination and Cyp24a1 is predominantly a kidney enzyme and is not expressed in liver (39), excluding this enzyme as the source of 20,24(OH)2D3 and 20,25(OH)2D3 production by mouse liver microsomes. Human CYP27A1 found in liver mitochondria can also hydroxylate 20(OH)D3 at C25 producing 20,25(OH)2D3, but produces comparable amounts of 20,26(OH)2D3 (19) which is not seen as a product of mouse liver microsomes, indicating no involvement of Cyp27a1.
20(OH)D3 is produced by the action of CYP11A1 on vitamin D3 and we have previously presented data showing that this reaction occurs in vivo (8). 20(OH)D3 inhibits proliferation of normal and malignant cells, promotes cell differentiation and has anti-inflammatory properties (reviewed in (1)). The above properties renders it a good candidate as an anti-cancer and/or anti-inflammatory drug, especially because it does neither induce hypercalcemia nor any identifiable cytotoxicity at pharmacological doses, unlike 1,25(OH)2D3, the biologically active form of vitamin D (1,11,13). Furthermore, most recently we demonstrated anti-fibrosing activity of 20(OH)D3 in animal models of scleroderma (15) and have found marked inhibition of collagen-induced arthritis in mice (Postlethwaite, Slominski et al., in preparation). The clinical utility of 20(OH)D3 requires its oral application. 20(OH)D3 after absorption in the gastrointestinal tract will further pass through the liver with its subsequent metabolism and likely production of 20,24(OH)2D3 and 20,25(OH)2D3, as described in this paper. The significance of these studies is further strengthened by our recent molecular modeling studies with the vitamin D receptor that predict that both 20,24(OH)2D3 and 20,25(OH)2D3 bind more tightly to the receptor than the parent 20(OH)D3, with their 1α-hydroxy derivatives predicted to bind even tighter (1). This is consistent with our previous report that these products cause significantly greater inhibition of colony formation by SKMEL-188 melanoma cells than either the parent 20(OH)D3, or 1,25(OH)2D3 (19).
In conclusion, we demonstrate that mouse liver microsomes hydroxylate 20(OH)D3 producing 20,24(OH)2D3 and 20,25(OH)2D3 as major metabolites. The action of liver microsomal enzymes on 20(OH)D3 suggests that its passage through the liver can activate this vitamin D analog, producing metabolites with predicted (1) and demonstrated greater anti-cancer potency (19).
Highlights.
Mouse liver microsomes metabolize 20-hydroxyvitamin D3.
Major products identified were 20,24-dihydroxyvitamin D3 and 20,25-dihydroxyvitamin D3.
A novel 20-hydroxyvitamin D3 metabolite with an altered chromophore was detected.
Formation of major products displayed Km values lower than for the other products.
Acknowledgments
This work was supported by funding from the University of Western Australia. Writing of this paper was supported in part by a grant 2R06AR052190 from NIH/NIAMS.
Footnotes
Conflict of Interest: The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J Steroid Biochem Mol Biol. 2013 doi: 10.1016/j.jsbmb.2013.10.012. http://dx.doi.org/10.1016/j.jsbmb.2013.1010.1012. [DOI] [PMC free article] [PubMed]
- 2.Strushkevich N, MacKenzie F, Cherkesova T, Grabovec I, Usanov S, Park HW. Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system. P Natl Acad Sci USA. 2011;108:10139–10143. doi: 10.1073/pnas.1019441108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuckey RC, Li W, Shehabi HZ, Janjetovic Z, Nguyen MN, Kim TK, Chen J, Howell DE, Benson HAE, Sweatman T, Baldisseri DM, Slominski A. Production of 22-hydroxy metabolites of vitamin D3 by cytochrome P450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab Dispos. 2011;39:1577–1588. doi: 10.1124/dmd.111.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, Tuckey RC. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272:4080–4090. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuckey RC, Li W, Zjawiony JK, Zmijewski MA, Nguyen MN, Sweatman T, Miller D, Slominski A. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J. 2008;275:2585–2596. doi: 10.1111/j.1742-4658.2008.06406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc Natl Acad Sci U S A. 2003;100:14754–14759. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W, Chen J, Janjetovic Z, Kim TK, Sweatman T, Lu Y, Zjawiony J, Tuckey RC, Miller D, Slominski A. Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity. Steroids. 2010;75:926–935. doi: 10.1016/j.steroids.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EKY, Nguyen MN, Benson HAE, Korik E, Janjetovic Z, Chen J, Yates CR, Postlethwaite A, Li W, Tuckey RC. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26:3901–3915. doi: 10.1096/fj.12-208975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar CC, Tuckey RC. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sidler D, Renzulli P, Schnoz C, Berger B, Schneider-Jakob S, Fluck C, Inderbitzin D, Corazza N, Candinas D, Brunner T. Colon cancer cells produce immunoregulatory glucocorticoids. Oncogene. 2011;30:2411–2419. doi: 10.1038/onc.2010.629. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Slominski A, Tuckey RC, Janjetovic Z, Kulkarni A, Chen J, Postlethwaite AE, Miller D, Li W. 20-Hydroxyvitamin D3 inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012;32:739–746. [PMC free article] [PubMed] [Google Scholar]
- 12.Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, Slominski AT. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-κB activity by increasing IκBα levels in human keratinocytes. PLoS ONE. 2009;4:e5988. doi: 10.1371/journal.pone.0005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, Sweatman T, Li W, Zjawiony J, Miller D, Chen TC, Lozanski G, Holick MF. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS ONE. 2010;5:e9907. doi: 10.1371/journal.pone.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janjetovic Z, Brozyna AA, Tuckey RC, Kim TK, Nguyen MN, Jozwicki W, Pfeffer SR, Pfeffer LM, Slominski AT. High basal NF-kappaB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br J Cancer. 2011;105:1874–1884. doi: 10.1038/bjc.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slominski A, Janjetovic Z, Tuckey RC, Nguyen MN, Bhattacharya KG, Wang J, Li W, Jiao Y, Gu W, Brown M, Postlethwaite AE. 20S-Hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J Clin Endocr Metab. 2013;98:E298–E303. doi: 10.1210/jc.2012-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slominski AT, Kim TK, Janjetovic Z, Tuckey RC, Bieniek R, Yue J, Li W, Chen J, Nguyen MN, Tang EK, Miller D, Chen TC, Holick M. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol. 2011;300:C526–541. doi: 10.1152/ajpcell.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang EK, Chen J, Janjetovic Z, Tieu EW, Slominski AT, Li W, Tuckey RC. Hydroxylation of CYP11A1-derived products of vitamin D3 metabolism by human and mouse CYP27B1. Drug Metab Dispos. 2013;41:1112–1124. doi: 10.1124/dmd.113.050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tieu EW, Tang EKY, Chen J, Li W, Nguyen MN, Janjetovic Z, Slominski A, Tuckey RC. Rat CYP24A1 acts on 20-hydroxyvitamin D3 producing hydroxylated products with increased biological activity. Biochem Pharmacol. 2012;84:1696–1704. doi: 10.1016/j.bcp.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tieu EW, Li W, Chen J, Baldisseri DM, Slominski AT, Tuckey RC. Metabolism of cholesterol, vitamin D3 and 20-hydroxyvitamin D3 incorporated into phospholipid vesicles by human CYP27A1. J Steroid Biochem. 2012;129:163–171. doi: 10.1016/j.jsbmb.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. P Natl Acad Sci USA. 2004;101:7711–7715. doi: 10.1073/pnas.0402490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu J, DeLuca HF. Vitamin D 25-hydroxylase – Four decades of searching, are we there yet? Arch Biochem Biophys. 2012;523:30–36. doi: 10.1016/j.abb.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Sawada N, Sakaki T, Ohta M, Inouye K. Metabolism of Vitamin D3 by Human CYP27A1. Biochem Bioph Res Co. 2000;273:977–984. doi: 10.1006/bbrc.2000.3050. [DOI] [PubMed] [Google Scholar]
- 23.Zhu JG, Ochalek JT, Kaufmann M, Jones G, DeLuca HF. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. P Natl Acad Sci USA. 2013;110:15650–15655. doi: 10.1073/pnas.1315006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamasaki T, Izumi S, Ide H, Ohyama Y. Identification of a novel rat microsomal vitamin D3 25-hydroxylase. J Biol Chem. 2004;279:22848–22856. doi: 10.1074/jbc.M311346200. [DOI] [PubMed] [Google Scholar]
- 25.Gupta RP, Hollis BW, Patel SB, Patrick KS, Bell NH. CYP3A4 is a human microsomal vitamin D 25-hydroxylase. J Bone Miner Res. 2004;19:680–688. doi: 10.1359/JBMR.0301257. [DOI] [PubMed] [Google Scholar]
- 26.Gupta RP, He YA, Patrick KS, Halpert JR, Bell NH. CYP3A4 as a vitamin D-24- and 25-hydroxylase: analysis of structure function by site-directed mutagenesis. J Clin Endocr Metab. 2005;90:1210–1219. doi: 10.1210/jc.2004-0966. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Lin YS, Zheng XE, Senn T, Hashizume T, Scian M, Dickmann LJ, Nelson SD, Baillie TA, Hebert MF, Blough D, Davis CL, Thummel KE. An inducible cytochrome P450 3A4-dependent vitamin D catabolic pathway. Mol Pharmacol. 2012;81:498–509. doi: 10.1124/mol.111.076356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deb S, Pandey M, Adomat H, Guns ES. Cytochrome P450 3A-mediated microsomal biotransformation of 1alpha,25-dihydroxyvitamin D3 in mouse and human liver: drug-related induction and inhibition of catabolism. Drug Metab Dispos. 2012;40:907–918. doi: 10.1124/dmd.111.041681. [DOI] [PubMed] [Google Scholar]
- 29.De Caprio J, Yun J, Javitt NB. Bile acid and sterol solubilization in 2-hydroxypropyl-beta-cyclodextrin. J Lipid Res. 1992;33:441–443. [PubMed] [Google Scholar]
- 30.Wallimann P, Marti T, Furer A, Diederich F. Steroids in Molecular Recognition. Chem Rev. 1997;97:1567–1608. doi: 10.1021/cr960373b. [DOI] [PubMed] [Google Scholar]
- 31.Slominski AT, Kim TK, Shehabi HZ, Tang EK, Benson HA, Semak I, Lin Z, Yates CR, Wang J, Li W, Tuckey RC. In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol Cell Endocrinol. 2014;383:181–192. doi: 10.1016/j.mce.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke MW, Tuckey RC, Gorman S, Holt B, Hart PH. Optimized 25-hydroxyvitamin D analysis using liquid–liquid extraction with 2D separation with LC/MS/MS detection, provides superior precision compared to conventional assays. Metabolomics. 2013;9:1031–1040. [Google Scholar]
- 33.Storrie B, Amadden E. [16] Isolation of subcellular organelles. In: Murray PD, editor. Method Enzymol. Academic Press; 1990. pp. 203–225. [DOI] [PubMed] [Google Scholar]
- 34.Yamazaki H, Shimada T. Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes. Arch Biochem Biophys. 1997;346:161–169. doi: 10.1006/abbi.1997.0302. [DOI] [PubMed] [Google Scholar]
- 35.Pearce R, Greenway D, Parkinson A. Species differences and interindividual variation in liver microsomal cytochrome P450 2A enzymes: Effects on coumarin, dicumarol, and testosterone oxidation. Arch Biochem Biophys. 1992;298:211–225. doi: 10.1016/0003-9861(92)90115-d. [DOI] [PubMed] [Google Scholar]
- 36.Pesce MA, Strande CS. A new micromethod for determination of protein in cerebrospinal fluid and urine. Clin Chem. 1973;19:1265–1267. [PubMed] [Google Scholar]
- 37.Hiwatashi A, Nishii Y, Ichikawa Y. Purification of cytochrome P-450D1 alpha (25-hydroxyvitamin D3-1 alpha-hydroxylase) of bovine kidney mitochondria. Biochem Biophys Res Commun. 1982;105:320–327. doi: 10.1016/s0006-291x(82)80047-8. [DOI] [PubMed] [Google Scholar]
- 38.Thurman EM, Ferrer I, Barceló D. Choosing between Atmospheric Pressure Chemical Ionization and Electrospray Ionization Interfaces for the HPLC/MS Analysis of Pesticides. Anal Chem. 2001;73:5441–5449. doi: 10.1021/ac010506f. [DOI] [PubMed] [Google Scholar]
- 39.Schuster I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim Biophys Acta. 2011;1814:186–199. doi: 10.1016/j.bbapap.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 40.Meunier B, de Visser SP, Shaik S. Mechanism of oxidation reactions catalyzed by cytochrome p450 enzymes. Chem Rev. 2004;104:3947–3980. doi: 10.1021/cr020443g. [DOI] [PubMed] [Google Scholar]
- 41.Napoli JL, Sommerfeld JL, Pramanik BC, Gardner R, Sherry AD, Partridge JJ, Uskokovic MR, Horst RL. 19-Nor-10-ketovitamin D derivatives: unique metabolites of vitamin D3, vitamin D2, and 25-hydroxyvitamin D3. Biochemistry. 1983;22:3636–3640. doi: 10.1021/bi00284a015. [DOI] [PubMed] [Google Scholar]