Abstract
Background
Long-term exposure to microgravity during space flight may lead to cardiac remodeling and rhythm disturbances. In mice, hindlimb unloading (HU) mimics the effects of microgravity and stimulates physiological adaptations, including cardiovascular deconditioning. Recent studies have demonstrated an important role played by changes in intracellular Ca handling in the pathogenesis of heart failure and arrhythmia. In this study, we tested the hypothesis that cardiac remodeling following HU in mice involves abnormal intracellular Ca regulation through the cardiac ryanodine receptor (RyR2).
Methods and Results
Mice were subjected to HU by tail suspension for 28 to 56 days in order to induce cardiac remodeling (n=15). Control mice (n=19) were treated equally, with the exception of tail suspension. Echocardiography revealed cardiac enlargement and depressed contractility starting at 28 days post-HU versus control. Moreover, mice were more susceptible to pacing-induced ventricular arrhythmias after HU. Ventricular myocytes isolated from HU mice exhibited an increased frequency of spontaneous sarcoplasmic reticulum (SR) Ca release events and enhanced SR Ca leak via RyR2. Western blotting revealed increased RyR2 phosphorylation at S2814, and increased CaMKII auto-phosphorylation at T287, suggesting that CaMKII activation of RyR2 might underlie enhanced SR Ca release in HU mice.
Conclusion
These data suggest that abnormal intracellular Ca handling, likely due to increased CaMKII phosphorylation of RyR2, plays a role in cardiac remodeling following simulated microgravity in mice.
Keywords: Arrhythmia, calcium, Ca/calmodulin-dependent kinase II, microgravity, ryanodine receptor
INTRODUCTION
Exposure to microgravity during space flight causes various changes in the human cardiovascular system, including a cephalic fluid shift,[1] changes in cardiac systolic volume,[2, 3] and, over time, a loss of left ventricle mass.[4, 5] It has been suggested that microgravity leads to a reduction in cardiac output and stroke volume due to cardiac remodeling, triggered by a reduction in circulating blood volume.[2, 6] In addition, documented observations in crew members over several years suggest that long-term exposure to microgravity can alter the electrical properties of the heart, increasing the propensity towards cardiac arrhythmias.[7–10] One factor thought to contribute to enhanced arrhythmogenic risk is the cardiac sympathovagal imbalance observed in response to microgravity. However, at present, it remains unclear whether microgravity is a direct cause of arrhythmias and cardiac dysfunction, or whether it is caused indirectly by previously asymptomatic cardiovascular disease.
It is well established that abnormal intracellular Ca handling can contribute to both contractile dysfunction in heart failure (HF) and arrhythmogenesis.[11–14] Diastolic release of Ca from the sarcoplasmic reticulum (SR) via the type-2 ryanodine receptor (RyR2) can activate the Na/Ca-exchanger, causing delayed afterdepolarizations (DADs). DADs can trigger premature action potentials that can initiate arrhythmias through local reentry. In addition, the leak of Ca from the SR (via RyR2) can interfere with SR reloading, associated with weakened cardiac contractility.[15] One major reason for enhanced RyR2 activity, or “leaky” RyR2 channels, in heart failure is increased phosphorylation by Ca/calmodulin-dependent protein kinase II (CaMKII).[16–18] This enzyme becomes activated in diseased hearts, whereas inhibition of CaMKII can improve cardiac contractility and prevent cardiac arrhythmias.[19] Specifically, CaMKII phosphorylates residue S2814 on RyR2 in normal and diseased hearts, while genetic inhibition of S2814 phosphorylation in mice subjected to pressure-overload ameliorates cardiac failure and ventricular arrhythmias.[13, 20] At this time, however, it is unknown whether this signaling pathway also contributes to the pathogenesis of HF and arrhythmias associated with microgravity-induced cardiac remodeling.
Hindlimb unloading (HU) - the classical ground-based model of microgravity - is used to study the effects of weightlessness in rodents,[21] and is widely accepted due to its similarity with the 6 degree head-down tilt bed-rest model for human volunteers.[21, 22] In this model, the hindlimbs of mice are elevated by suspending the tail to produce a 30-degree head-down tilt. The tilt and unloading of the hindquarters leads to a cephalad fluid shift, hindlimb muscle atrophy, osteoporosis, and other physiological consequences, similar to what is observed in humans during space flight.[23, 24] Here, we demonstrate that HU in mice leads to progressive cardiac deconditioning associated with structural remodeling, contractile dysfunction, and an increased propensity towards ventricular arrhythmias. Ventricular myocytes isolated from mice after HU exhibited more spontaneous SR Ca release events with enhanced SR Ca leak, suggestive of RyR2 dysfunction. Western blotting revealed increased activation of CaMKII, associated with enhanced CaMKII-mediated phosphorylation of RyR2 at S2814, consistent with increased SR Ca leak. Thus, CaMKII-mediated activation of RyR2 resulting in diastolic Ca leakage from the SR might be a novel mechanism involved in microgravity-related cardiac deconditioning.
MATERIAL AND METHODS
Surgical procedures
All animal studies were performed according to protocols approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine, conforming to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). Wire loops made of sterile 2-0 surgical steel suture (Ethicon Inc., USA) were implanted [4] and hindlimb unloading (HU) was performed as described.[21] Sham mice were treated equally except for elevation of the hind limbs. Mice were monitored twice daily for behavior, urination, defecation, and for food and water intake. Body weights were monitored on a weekly basis to ensure that mice were not experiencing excessive weight loss due to malnutrition or dehydration.
Body composition analysis
Mice were placed in an induction chamber filled with 2% isoflurane (in 100% oxygen) to initiate anesthesia, and transferred onto a disposable PIXImus measuring tray for proper alignment during the experiment (Inside Outside Sales Llc, Fitchburg, WI). Anesthesia was maintained using a loose-fitting nose cone for administration of Isoflurane (2%). Dual x-ray absorptiometry (DXA) was performed, after which mice were returned to a heated cage for recovery. After mice were sacrificed, the soleus and gastrocnemius muscles were individually dissected and weighed.
Transthoracic echocardiography
Echocardiography was performed using the VisualSonics VeVo 770 Imaging System (VisualSonics, Toronto, Canada) equipped with high-frequency 30 MHz probe, as described.[13] Cardiac function was assessed prospectively at 28 and 56 days after HU. All measurements were made while mice maintained heart rates within 450 ± 50 beats per minute. In addition, body temperatures were maintained within a narrow range (37.0 ± 0.5 °C) to avoid confounding effects of hypothermia.
Programmed electrical stimulation
In vivo electrophysiology studies were performed in mice as previously described.[25] Briefly, atrial and ventricular intracardiac electrograms (ECGs) were recorded using an 1.1F octapolar catheter (EPR-800, Millar Instruments, Houston, Texas) inserted via the right jugular vein. Surface and intracardiac electrophysiology parameters were assessed simultaneously at baseline using a computer-based data acquisition system (EMKA Technologies, Falls Church, VA). ECG waveform results included a clearly defined P wave, denoting atrial depolarization, and a QRS wave which signified ventricular depolarization. PR, RR, and QTc (corrected QT interval) were evaluated at baseline. Next, right ventricular pacing was performed using 2-ms current pulses delivered by an external stimulator (STG-3008, Multi Channel Systems, Reutlingen, Germany). Standard pacing protocols were used to determine basic electrophysiologic parameters such as effective refractory periods. Inducibility of ventricular tachycardia (VT) was determined by using single extra stimuli protocols. Premature ventricular complexes (PVCs) were defined as spontaneous abnormal ventricular contractions that occurred prior to programmed stimulation protocols. These ventricular contractions were differentiated from aberrated atrial premature beats and catheter ectopy based on intracardiac electrograms and surface morphology. Non-sustained VT was defined as an episode of 4–9 beats of VT, whereas sustained VT was defined as 10 or more consecutive beats of VT.[13, 26] All occurrences were tested twice for reproducibility.
Calcium imaging in ventricular myocytes
Single ventricular myocytes were isolated using a modified collagenase method as described.[13] Ventricular myocytes were loaded with 5 mmol/L Fluo-4 AM (Invitrogen, Carlsbad, CA) for 30 min at room temperature (RT), and perfused with 1.8 mM Ca normal Tyrode (NT) solution to wash out excessive dye. Intracellular Ca concentrations ([Ca]i) were measured using an illumination device (model Lambda DG-4, Sutter Instruments, Novato, CA), and an electro-multiplier intensified back-illuminated charge coupled device (CCD) camera (model Cascade 512B, Photometrics, Tucson, AZ). SR Ca leak was measured as described in detail by Shannon et al.[27] Myocytes were paced at 40 V, 3 Hz for 20 seconds, followed by a pause, then quickly perfused with 5 μmol/L tetracaine in 0 Na/0 Ca NT, followed by application of caffeine (10 mM) in 0 Na/0 Ca NT to estimate steady-state SR Ca. The tetracaine-dependent shift of Ca from cytosol to SR (decrease in cytosolic [Ca]i and increase in SR Ca content) is proportional to SR Ca leak in the absence of tetracaine.[27] Spontaneous Ca release events (SCaREs) during the period between termination of pacing and addition of tetracaine (TTC) were visualized and quantified.
Western blot analysis
Mouse cardiac homogenates were prepared as described, subjected to electrophoresis on 6% (for RyR2) or 12% acrylamide gels (for CaMKII and PLN), and transferred onto polyvinyl difluoride (PVDF) membranes.[28] Membranes were probed with monoclonal anti-RyR2 (1:5,000; Thermo Fisher Scientific, Waltham, MA), polyclonal anti-pS2814-RyR2 (1:1,000),[17] polyclonal anti-pS2808-RyR2 (1:1,000),[28] monoclonal anti-pT286-CaMKII (1,1,000; Cayman Chemicals, Ann Arbor, MI), polyclonal anti-CaMKII (1:1,000; custom made for our lab), polyclonal anti-pT17-PLN (1:5,000; Badrilla, Leeds, UK), monoclonal anti-PLN (1:5,000; Badrilla, Leeds, UK), and monoclonal anti-GAPDH (1:10,000; Millipore, Temecula, CA) antibodies. Membranes were then incubated with secondary anti-mouse and anti-rabbit antibodies conjugated to Alexa-Fluor 680 (Invitrogen Molecular Probes, Carlsbad, CA) and IR800Dye (Rockland Immunochemicals, Gilbertsville, PA), respectively, and bands were quantified using infrared visualization (Odyssey System, Lincoln, NE) and densitometry (ImageJ Data Acquisition Software, National Institute of Health, Bethesda, MD). GAPDH was used as a loading control.
Statistical Analysis
All data are represented as average ± SEM. Statistical significance of differences between experimental groups was determined using Student’s paired or independent t-test, or Fischer’s exact test when appropriate. A value of P<0.05 was considered statistically significant.
RESULTS
Validation of the mouse hindlimb unloading (HU) model of simulated microgravity
To assess the functional effects of microgravity on cardiac physiology, we subjected C57Bl/6 mice to experimental hindlimb unloading (n=15). Wire rings were implanted between the 6th and 7th intervertebral space of the tail, allowing for suspension at a 30-degree angle (of the HU group) (Fig. 1A). Control mice (n=19) also receive the rings but were not suspended. The design of the suspension assembly enabled mice to move around the cage freely, with unrestricted access to food and water (Fig. 1B,C).
Figure 1. Mouse hindlimb unloading (HU) model of simulated microgravity.
A. Modified wire loop implant placed in the 6–7th intervertebral space of the mouse-tail, which reduced tail inflammation and the possibility of escape from the suspension apparatus during prolonged suspension. B. Photograph of assembled hindlimb suspension cage. Mice subjected to HU had unrestricted access to food and water. C. Zoom image of tail suspension assembly. Suspended mice were able to move freely without restriction. D. Time line summarizing experimental protocol.
To validate the model, the body weight of each animal was monitored on a weekly basis, prior to and following HU (or sham). The body-mass of HU mice prior to suspension (26.1 ± 1.5g) was not significantly different from sham (26.4 ± 1.5g; P=0.65). After 7 days of HU, mice displayed a minor loss in body weight (-5.4%). However, there were no statistically significant changes in body weight following 14, 21, or 28 days of HU, compared to day 7. Furthermore, no significant changes in body weight were evident between mice subjected to HU for 28 days (25.0 ± 1.0g) and sham mice (26.4 ± 0.8g; P=0.21), consistent with previous studies.
Dual-energy X-ray absorptiometry (DXA) was also performed to analyze body composition in these HU and sham mice, prior to and at day 14 and 28 following HU (Fig. 2A). Analysis of whole body images revealed no significant changes in the amounts of lean muscle tissue or bone mineral content (BMC) in mice following 14 or 28 days of HU compared to their baseline values or sham mice (data not shown). In contrast, analysis of the femur region revealed a progressive decline in lean muscle mass (Fig. 2B) and BMC (Fig. 2C), starting at 14 days and continuing up to 28 days after HU. Following completion of the experiments, hindlimb muscles were dissected to confirm the extent of muscle mass loss. The soleus muscle weight was reduced by 54% in HU mice (5.9±1.7 mg) compared with sham mice (12.8±3.6 mg; P<0.05). The gastrocnemius weight was also reduced, by 20%, in HU mice (117.5±16.2 mg) compared with sham mice (147.5±14.6 mg; P<0.05). Thus, simulated microgravity by means of HU caused changes in body composition including bone demineralization and muscle atrophy of the lower extremities.
Figure 2. Body composition analysis to validate HU model.
A. Representative image of dual X-ray absorptiometry used for body composition analysis. Analysis was performed of the femur area (box). B,C. Quantification of the femur region revealed significant decreases in the amounts of lean muscle mass (B) and femur bone mineral content (BMC) (C) in mice at 14 and 28 days of HU. Numbers in bars indicate number of mice used per cohort. * P<0.05, *** P<0.001 vs. pre-HU; # P<0.05, ### P<0.001 vs. sham.
Development of cardiac dysfunction following hindlimb unloading
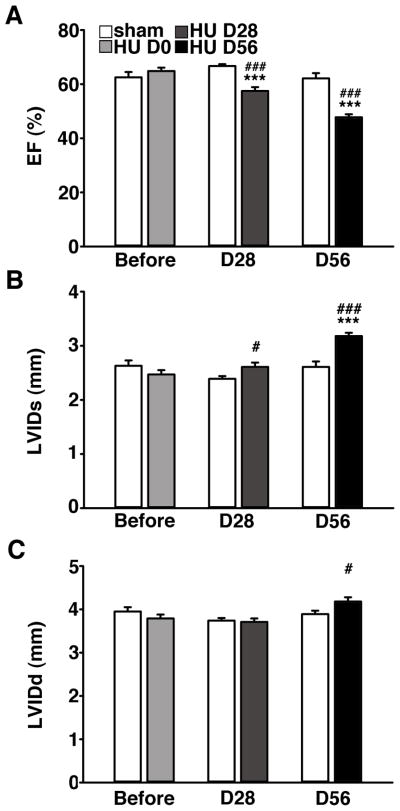
To evaluate the functional consequences of simulated microgravity on cardiac function, transthoracic echocardiography was performed prior to, 28, and 56 days after HU. At baseline (1 day before HU), left ventricular ejection fraction (LV-EF) and cardiac dimensions were similar in sham and HU mice (Fig. 3). However, after 28 days of HU, LV-EF was significantly decreased in HU mice (57.5 ± 1.4) compared to baseline (64.9 ± 1.3; P<0.001) and sham (66.7 ± 0.7; P<0.001) (Fig. 3A). After 56 days of HU in a separate group of mice, the LV-EF was decreased further (47.8 ± 1.1) compared with baseline (64.9 ± 1.3; P<0.001) or sham (62.1 ± 1.9; P<0.001). In contrast, there were no significant changes in sham mice over time (P=0.64).
Figure 3. Cardiac function declines following HU.
A. Echocardiography revealed progressive worsening of left ventricular (LV) ejection fraction (EF) in HU mice (n=15) compared to control mice (n=13). B–C. Progressive increase in LV internal diameter during both systole (LVIDs) and diastole (LVIDd) in HU mice. *** P<0.001 vs. pre-HU; # P<0.05, ### P<0.001 vs. sham.
The end-systolic LV internal diameter (LVIDs) increased in the HU mice starting at 28 days and further progressed up to 56 days after HU (Fig. 3B). Specifically, the LVIDs was significantly increased in HU mice at 56 days of HU (3.2 ± 0.1 mm) compared with HU mice at baseline (2.5 ± 0.1 mm; P<0.001), and compared to sham mice at 56 days of HU (2.6 ± 0.1 mm; P<0.001). Furthermore, the end-diastolic LV internal diameter (LVIDd) was also significantly enhanced after 56 days of HU (P<0.05; Fig. 3C). Together, these data show that HU leads to progressive cardiac remodeling associated with cardiac dilatation and mild systolic heart failure.
Increased susceptibility to ventricular arrhythmias following hindlimb unloading
To determine whether simulated microgravity alters the electrical properties of the heart, we first performed surface electrocardiograms (ECGs) on anesthetized mice using the MouseMonitor (Indus Instruments, Houston, TX) heating pad with embedded ECG leads to reduce noise (Fig. 4A,B). Analysis of ECG intervals revealed no significant differences in RR interval and PR intervals (Fig. 4C,D). There was a mild trend towards prolongation of the QTc interval (QT interval corrected for RR) in experimental mice (n=10) at 28 days after HU (40.9 ± 1.0 ms) compared to sham (n=14) controls (38.3 ± 1.3 ms; P=0.14) (Fig. 4E), a possible occurrence consistent with the possible presence of a potentially arrhythmogenic substrate.[8]
Figure 4. Unaltered ECG parameters in mice following HU.
A–B. Representative ECG tracings obtained from anesthetized mice at day 28 post-HU. C–E. ECG analysis revealed unaltered RR intervals (A), unaltered PR intervals (B), and a trend towards prolonged QTc (QT interval corrected to RR) intervals (C) in HU mice. Numbers in bars indicate number of mice used for all bars.
Because spontaneous arrhythmias were not observed during the brief ECG recording periods (<5 min), basal surface ECG recordings were evaluated for the presence of spontaneous premature ventricular contractions (PVCs) during a 30-min period. Whereas none of the sham controls exhibited any PVCs (0 of 14 mice), 20% (2 of 10) of the HU mice showed PVCs (P<0.05).
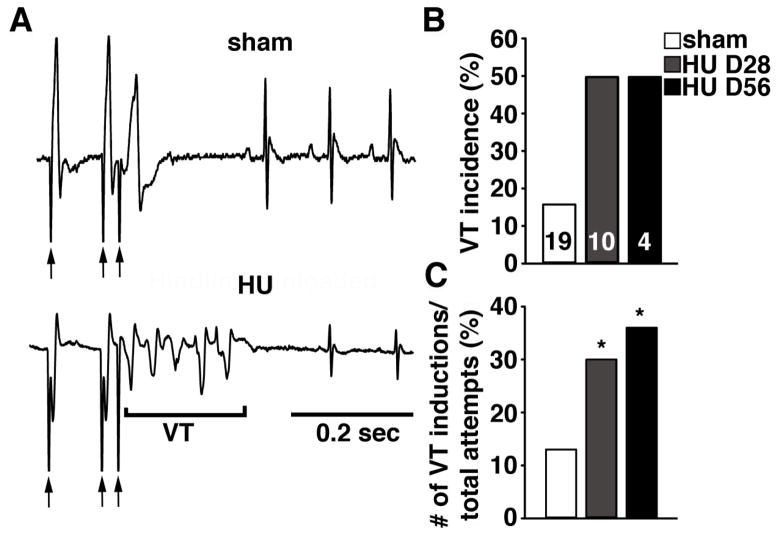
Next, we performed programmed electrical stimulation (PES) using an intracardiac pacing catheter. Ventricular pacing was performed at basic cycle lengths of both 90 and 70 ms (Fig. 5A, first 2 arrows below tracings depict 90 ms cycle length), followed by a premature beat (last arrow below tracings). PES provoked runs of non-sustained ventricular tachycardia (VT) in 50% of mice at 28 (n=10) or 56 days (n=4) of HU, respectively, compared to sham mice (16%; P=0.08) combined from both time points (n=19) (Fig. 5B). To gain more insight into the potential differences in arrhythmogenesis among the groups, we expressed the induction of VT as a percentage of total induction attempts (Fig. 5C). The percent of pacing protocols leading to VT induction was significantly greater in mice at 28 (30%) and 56 days (36%) after HU, compared to sham mice (13%; P<0.05) from both groups (Fig. 5C). Together, these data suggest that simulated microgravity increases the susceptibility of VT.
Figure 5. Increased arrhythmia susceptibility in HU mice.
A. Representative ECG tracings after intracardiac pacing stimuli (arrows). The sham mouse exhibited normal sinus rhythm following an extra-stimulus, whereas the HU mouse developed a non-sustained ventricular tachycardia (VT). B. Bar graph showing incidence of pacing-induced non-sustained VT in sham and HU mice at 28 or 56 days of HU. Numbers in bars indicate number of mice used for all bars. C. Percent of pacing attempts resulting in non-sustained VT in sham and HU mice. * P<0.05 versus sham.
Altered sarcoplasmic reticulum Ca handling following hindlimb unloading
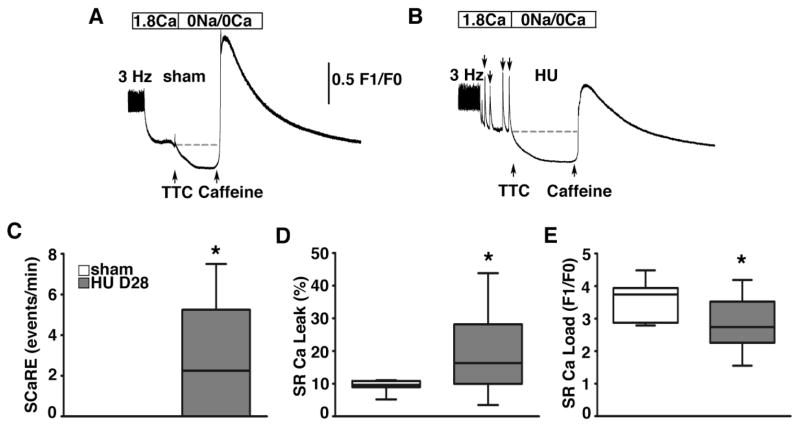
To determine the mechanisms underlying enhanced arrhythmogenesis and contractile dysfunction, we studied SR Ca handling in ventricular myocytes isolated from mice at 28 days of HU (n=3) or sham (n=2) (Fig. 6). After a steady-state pacing train at 3-Hz, spontaneous SR Ca release events (SCaREs) were observed in myocytes from HU (n=11 cells) but not sham (n=7 cells) mice (Fig. 6A, B). Quantification of these SCaREs revealed more frequent, significant events in HU mice (2.25 [interquartile range, 0.0 to 5.3 events/min]) compared with sham mice (0 events/min; P<0.05; Fig. 6C). Next, tetracaine (TTC) was applied to measure the diastolic Ca leak from the SR, as described.[27] Myocytes from HU mice exhibited enhanced SR Ca leak (16.3 [interquartile range, 10.0 to 28.2%]) compared with sham mice (9.7 [interquartile range, 8.9 to 10.8%]; P<0.05) (Fig. 6D). Finally, application of caffeine caused the release of Ca stored in the SR, resulting in a large Ca transient from which the SR Ca load was calculated. There was a trend toward reduced SR Ca content in myocytes from HU mice, consistent with enhanced SR Ca leak (P=0.09; Fig. 6E). These cellular studies show that simulated microgravity causes profound alterations in intracellular Ca handling in ventricular myocytes, consistent with depressed contractility and enhanced arrhythmogenesis.
Figure 6. Spontaneous Ca release events and enhanced SR Ca leak in ventricular myocytes from HU mice.
A–B. Representative tracing of intracellular [Ca] in ventricular myocytes from sham and HU mice following a 3-Hz pacing train. Myocytes from HU mice (11 myocytes from 3 mice) exhibited more spontaneous SR Ca release events compared to sham mice (7 myocytes from 2 mice) (arrows in B). Next, application of tetracaine (TTC) in 0 Na/0 Ca Tyrode solution caused Ca resequestration into the SR. Caffeine was applied to measure the SR Ca content. C. Bar graph showing incidence of spontaneous SR Ca release events (SCaREs). D. Quantification of SR Ca leak using the TTC protocol revealed enhanced leak normalized to SR Ca load, in HU mice. E. Quantification of SR Ca content using the caffeine protocol revealed reduced SR Ca content in HU mice. * P<0.05 versus sham.
Hindlimb unloading causes enhanced CaMKII activation and phosphorylation of Ca handling proteins RyR2 and PLN
To gain more insight into the signaling pathways possibly involved in the enhancement of SR Ca leak, we determined whether CaMKII was activated in the hearts of mice subjected to 28-days of HU. Western blotting of ventricular lysates revealed unaltered levels of total CaMKII levels, but increased levels of CaMKII auto-phosphorylation at T287 (Fig. 7A). Thus, the degree of CaMKII auto-phosphorylation was increased by about 40%, indicative of increased CaMKII activity in the heart (Fig. 7D). To assess the potential effects on key Ca handling proteins, the CaMKII phosphorylation levels of RyR2 (Fig. 7B) and PLN (Fig. 7C) were determined. Normalized to total protein levels, CaMKII phosphorylation of RyR2 at S2814 and PLN at T17 were both significantly elevated in hearts from HU mice compared with sham (Fig. 7D). In contrast, PKA phosphorylation of RyR2 at S2808 (Fig. 7B,D) and PLN at S16 (not shown) were unaltered in HU mice. Together, these data show that simulated microgravity activates CaMKII and promotes CaMKII-dependent phosphorylation of Ca-handling proteins RyR2 and PLN, which might be responsible for enhanced SR Ca leak.
Figure 7. Increased CaMKII activation of phosphorylation of RyR2 following HU.

A. Representative Western blots for CaMKII and CaMKII auto-phosphorylation at T287, B. RyR2 levels, and phosphorylation at S2814 and S2808, and C. PLN levels and phosphorylation at T17. GAPDH levels served as a loading control. D. Quantification of phosphorylation levels normalized to total protein levels revealed increased levels of CaMKII auto-phosphorylation, CaMKII-phosphorylation of RyR2, and CaMKII-phosphorylation of PLN following HU. Numbers in bars indicate number of mice used per group. * P<0.05, ** P<0.01, *** P<0.001 versus sham.
DISCUSSION
Cardiac arrhythmias in astronauts during space flight have been relatively benign, but arrhythmias do occur quite commonly.[7] A number of reports have been published with detailed information regarding in-flight arrhythmias.[7–9] With only limited medical assistance and supplies available on space crafts and the international space station, concerns exists about the potential detrimental consequences of cardiac rhythm disorders. Cardiac arrhythmias could jeopardize mission objectives and possibly the lives of crewmembers. The risks of fatal arrhythmias could be even more profound in case of potential future flights to Mars, which could last over one year. In addition, the recent expansion of commercial space flights exposes participants during short-term orbital flights to developing serious arrhythmias, in particular since these individuals are typically older and less healthy than career astronauts.[29] However, the mechanisms that contribute to arrhythmogenesis during long-duration spaceflight have not been elucidated.
Several factors might contribute to arrhythmogenesis during spaceflight. Once exposed to microgravity, the human cardiovascular system undergoes profound changes, including alterations in autonomic regulation that may adversely influence cardiac repolarization and precipitate rhythm disturbances,[8] In addition, long duration spaceflight has been shown to prolong cardiac conduction and repolarization.[8] In astronauts involved in the Skylab missions, several instances of arrhythmias including PVCs premature atrial contractions and re-entrant tachycardias have been recorded. These events occurred throughout the entire mission and in particular during effort tests, extravehicular activities, and lower body negative pressure sessions.[7] In addition, an isolated incident of a non-sustained 14-beat ventricular tachycardia was recorded in a crewmember, using in-flight Holter monitoring, aboard the Mir.[9] Although not part of a systematic scientific study, these specific cases suggest that exercise and/or sympathetic activation might promote or exacerbate cardiac arrhythmias during long-duration space flight.
A recent study by Moffitt et al.[30] suggests that exposure to microgravity increases the predisposition to cardiac arrhythmias during an acute stressor as a result of altered function of gap junctions, in particular connexin 43 (Cx43). In this study, electrocardiographic data were obtained over a short period of HU using an implantable telemetry device following administration of a sympathetic stressor, isoproterenol, in combination with animal restraint. Our findings suggest that activation of simulated microgravity in mice also leads to activation of CaMKII, an enzyme also activated by sympathetic activation.[31] Activated CaMKII was associated with enhanced phosphorylation of S2814 on RyR2 and T17 on PLN (Fig. 7). The absolute increase in CaMKII auto-phosphorylation levels and RyR2-S2814 phosphorylation were statistically significant but relatively modest (about 30%) in HU mice (Fig. 7). However, prior studies have demonstrated that such increases can functionally alter RyR2 open probability and may contribute to arrhythmogenic Ca release events.[13, 17] Moreover, recent studies have demonstrated that diastolic Ca leakage via CaMKII phosphorylated RyR2 is a major contributor to proarrhythmic events [13] and cardiac failure resulting from dilated cardiomyopathy (DCM).[20] Indeed, our findings revealed enhanced SR Ca leak in ventricular myocytes isolated from mice subjected to hindlimb unloading (Fig. 6). Diastolic SR Ca release can lead to delayed afterdepolarizations, PVCs, an enhanced susceptibility to arrhythmias, and heart failure. Interestingly, Jennings et al.[29] reported that treatment with a calcium channel blocker suppressed reoccurring PVCs in a spaceflight participant. However, it remains very difficult to obtain mechanistic insights from such observational studies in human subjects. Other ion channels can also contribute to triggered activity promoted by enhanced CaMKII activity, in particular activation of potassium and sodium channels. Our experimental animal studies suggest that abnormal intracellular Ca release due to CaMKII activation might contribute to arrhythmogenesis, and the eventual decline of cardiac performance due to long-term exposure to simulated microgravity.
Study limitations
One of the limitations of our study is that we did not perform long-term telemetry ECG recordings to assess whether mice subjected to HU exhibited an increased incidence of spontaneous PVCs and arrhythmias. Whereas our data revealed PVCs in some of the HU mice during the brief recording period, these studies were insufficiently powered to draw more definitive conclusions. On the other hand, our programmed electrical stimulation studies did establish that HU mice developed a substrate for arrhythmias. PES studies are routinely used to assess whether patients are at risk of developing cardiac arrhythmias, even when non-invasive Holter ECG monitoring remains negative. As such, the data obtained using PES studies in HU mice provide valuable insights into the mechanisms underlying arrhythmia development during microgravity, and for the first time implicated CaMKII in this process. Another limitation is that DXA measurements are not as exact of a measurement as the assessment of actual muscle and bone weight. However, the PIXImus body composition/densitometry apparatus has been optimized for mice, and allows for very accurate measurements of bone and tissue mass in small animals. Prior studies have demonstrated excellent correlations between bone and lean measurements to total extraction weights (r= 0.99).[32] In addition, our confirmation of weights of selected single muscles such as the soleus and gastrocnemius also correlated well with our DXA data. Some of the measurements were performed in subsets of mice, which could potentially affect the results. Finally, our studies were performed in rodents, and the mechanistic basis for arrhythmias might be different in humans. Therefore, further studies in humans subjected to space flight would be required to confirm our findings and further improve current knowledge in the field.
Perspectives and Significance
Taken together, our present work has demonstrated that hindlimb unloading (HU) represents a valid model for studying microgravity-related cardiovascular dysfunction in mice. Mice subjected to HU displayed decreases in bone mineral density and differential muscle atrophy in the femur regions. In addition, transthoracic echocardiography studies showed that prolonged exposure to simulated microgravity affects cardiac performance. Moreover, the number of NSVT episodes was significantly increased, consistent with an increased susceptibility to pacing-induced VT after 28 and 56 days of HU. The arrhythmias are likely caused by an increased susceptibility to calcium leak from the sarcoplasmic reticulum. Our study suggests that enhanced activation of CaMKII, which in turn promotes RyR2 phosphorylation at serine 2814. Future strategies to prevent arrhythmias in space flight participants might focus on normalizing intracellular calcium handling or prevention of excessive activation of CaMKII in the heart.
Highlights.
Long-term exposure to microgravity during space flight may promote cardiac arrhythmias.
Simulated microgravity in mice subjected to hindlimb unloading promotes arrhythmias.
Enhanced sarcoplasmic reticulum calcium leak promotes cellular arrhythmic events.
Increased ryanodine receptor phosphorylation promotes calcium leak.
Acknowledgments
The authors would like to thank Dr. Margaret E. Conner, Ph.D., of the Department of Molecular Virology and Microbiology at Baylor College of Medicine for her expertise and experimental support. We would also like to thank Dr. Corey Reynolds, Ph.D., Director of the Mouse Phenotyping Core at Baylor College of Medicine for the usage of equipment. X.H.T.W. is an American Heart Association Established Investigator (grant 13EIA14560061), and is supported by NIH grants HL089598, HL091947, and HL117641, a Muscular Dystrophy Association grant (186530), and the Juanita P. Quigley Endowed Chair in Cardiology (at Baylor College of Medicine). J.L.R. is a recipient of the NIH Training Grant Science Award T32-HL07676. P.M.G. is a recipient of the National Space Biomedical Research Institute (NSBRI) Postdoctoral Fellowship Award (NCC 9-58). J.P.S. acknowledges the support of the Friedkin Chair for Research in Sensory System Integration and Space Medicine (at Baylor College of Medicine).
GLOSSARY
- BMC
bone mineral content
- BW
body weight
- CaMKII
Ca/calmodulin-dependent protein kinase II
- DXA
dual X-ray absorptiometry
- ECG
electrocardiogram
- EF
ejection fraction
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HF
heart failure
- HU
hindlimb unloading
- HW
heart weight
- LVIDd
left ventricular internal diameter during diastole
- LVIDs
left ventricular internal diameter during systole
- NSVT
nonsustained ventricular tachycardia
- PKA
protein kinase A
- PLN
phospholamban
- RyR2
ryanodine receptor type 2
- SCaRE
spontaneous Ca release event
- SERCA2a
sarco/endoplasmic reticulum Ca-ATPase 2a
- SR
sarcoplasmic reticulum
- TL
tibia length
- VT
ventricular tachycardia
- WT
wildtype
Footnotes
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
DISCLOSURES
Dr. Wehrens is a founding partner of Elex Biotech, a company that develops drugs that modulate ryanodine-receptor channels.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thornton WE, Moore TP, Pool SL. Fluid shifts in weightlessness. Aviat Space Environ Med. 1987;58:A86–90. [PubMed] [Google Scholar]
- 2.Bungo MW, Goldwater DJ, Popp RL, Sandler H. Echocardiographic evaluation of space shuttle crewmembers. J Appl Physiol. 1987;62:278–83. doi: 10.1152/jappl.1987.62.1.278. [DOI] [PubMed] [Google Scholar]
- 3.Caiani EG, Sugeng L, Weinert L, Capderou A, Lang RM, Vaida P. Objective evaluation of changes in left ventricular and atrial volumes during parabolic flight using real-time three-dimensional echocardiography. J Appl Physiol. 2006;101:460–8. doi: 10.1152/japplphysiol.00014.2006. [DOI] [PubMed] [Google Scholar]
- 4.Summers RL, Martin DS, Meck JV, Coleman TG. Mechanism of spaceflight-induced changes in left ventricular mass. Am J Cardiol. 2005;95:1128–30. doi: 10.1016/j.amjcard.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Perhonen MA, Franco F, Lane LD, Buckey JC, Blomqvist CG, Zerwekh JE, et al. Cardiac atrophy after bed rest and spaceflight. J Appl Physiol. 2001;91:645–53. doi: 10.1152/jappl.2001.91.2.645. [DOI] [PubMed] [Google Scholar]
- 6.Charles JB, Lathers CM. Cardiovascular adaptation to spaceflight. J Clin Pharmacol. 1991;31:1010–23. doi: 10.1002/j.1552-4604.1991.tb03665.x. [DOI] [PubMed] [Google Scholar]
- 7.Leguay G, Seigneuric A. Cardiac arrhythmias in space. Role of vagotonia. Acta Astronaut. 1981;8:795–801. doi: 10.1016/0094-5765(81)90019-9. [DOI] [PubMed] [Google Scholar]
- 8.D’Aunno DS, Dougherty AH, DeBlock HF, Meck JV. Effect of short- and long-duration spaceflight on QTc intervals in healthy astronauts. Am J Cardiol. 2003;91:494–7. doi: 10.1016/s0002-9149(02)03259-9. [DOI] [PubMed] [Google Scholar]
- 9.Fritsch-Yelle JM, Leuenberger UA, D’Aunno DS, Rossum AC, Brown TE, Wood ML, et al. An episode of ventricular tachycardia during long-duration spaceflight. Am J Cardiol. 1998;81:1391–2. doi: 10.1016/s0002-9149(98)00179-9. [DOI] [PubMed] [Google Scholar]
- 10.Hawkin W, Zieglschmid JF. Clinical aspects in crew health. Biomedical results of Apollo. 1975:286–304. (NASA SP-386) [Google Scholar]
- 11.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003;93:592–4. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 12.Guo T, Zhang T, Mestril R, Bers DM. Ca2+/Calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res. 2006;99:398–406. doi: 10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- 13.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, et al. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–79. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ather S, Respress JL, Li N, Wehrens XH. Alterations in ryanodine receptors and related proteins in heart failure. Biochim Biophys Acta. 2013;1832:2425–31. doi: 10.1016/j.bbadis.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Luczak ED, Lee EJ, Hidalgo C, Yang J, Gao Z, et al. CaMKII effects on inotropic but not lusitropic force frequency responses require phospholamban. J Mol Cell Cardiol. 2012;53:429–36. doi: 10.1016/j.yjmcc.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y, Luczak ED, Lee EJ, Hidalgo C, Yang J, Gao Z, et al. CaMKII effects on inotropic but not lusitropic force frequency responses require phospholamban. J Mol Cell Cardiol. 2012;53:429–36. doi: 10.1016/j.yjmcc.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–51. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobrev D, Wehrens XH. Calmodulin kinase II, sarcoplasmic reticulum Ca2+ leak, and atrial fibrillation. Trends Cardiovasc Med. 2010;20:30–4. doi: 10.1016/j.tcm.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, et al. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–40. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Respress JL, van Oort RJ, Li N, Rolim N, Dixit SS, deAlmeida A, et al. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res. 2012;110:1474–83. doi: 10.1161/CIRCRESAHA.112.268094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol. 2002;92:1367–77. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- 22.Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation. 1997;96:517–25. doi: 10.1161/01.cir.96.2.517. [DOI] [PubMed] [Google Scholar]
- 23.Dehority W, Halloran BP, Bikle DD, Curren T, Kostenuik PJ, Wronski TJ, et al. Bone and hormonal changes induced by skeletal unloading in the mature male rat. Am J Physiol. 1999;276:E62–9. doi: 10.1152/ajpendo.1999.276.1.e62. [DOI] [PubMed] [Google Scholar]
- 24.Fluckey JD, Dupont-Versteegden EE, Montague DC, Knox M, Tesch P, Peterson CA, et al. A rat resistance exercise regimen attenuates losses of musculoskeletal mass during hindlimb suspension. Acta Physiol Scand. 2002;176:293–300. doi: 10.1046/j.1365-201X.2002.01040.x. [DOI] [PubMed] [Google Scholar]
- 25.Li N, Wehrens XH. Programmed electrical stimulation in mice. J Vis Exp. 2010:1730. doi: 10.3791/1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–6. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 27.Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res. 2002;91:594–600. doi: 10.1161/01.res.0000036914.12686.28. [DOI] [PubMed] [Google Scholar]
- 28.Sood S, Chelu MG, van Oort RJ, Skapura D, Santonastasi M, Dobrev D, et al. Intracellular calcium leak due to FKBP12.6 deficiency in mice facilitates the inducibility of atrial fibrillation. Heart Rhythm. 2008;5:1047–54. doi: 10.1016/j.hrthm.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jennings RT, Stepanek JP, Scott LR, Voronkov YI. Frequent premature ventricular contractions in an orbital spaceflight participant. Aviation, space, and environmental medicine. 2010;81:597–601. doi: 10.3357/asem.2742.2010. [DOI] [PubMed] [Google Scholar]
- 30.Moffitt JA, Henry MK, Welliver KC, Jepson AJ, Garnett ER. Hindlimb unloading results in increased predisposition to cardiac arrhythmias and alters left ventricular connexin 43 expression. Am J Physiol Regul Integr Comp Physiol. 2013;304:R362–73. doi: 10.1152/ajpregu.00391.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y, Gao Z, Chen B, Koval OM, Singh MV, Guan X, et al. Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proc Natl Acad Sci US A. 2009;106:5972–7. doi: 10.1073/pnas.0806422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagy TR, Clair AL. Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obes Res. 2000;8:392–8. doi: 10.1038/oby.2000.47. [DOI] [PubMed] [Google Scholar]