Abstract
Blood pressure regulation by 5-HT has proven to be a complex story to unravel. The work by Cuesta et al in this issue of Vascular Pharmacology adds another layer of complexity by providing sound in vivo data that 5-HT, through the 5-HT7 receptor, can inhibit the vasodepressor actions of the sensory nervous system and thereby promote blood pressure maintenance. This interaction of 5-HT with the sensory nervous system is inhibitory, whereas 5-HT is understood to be stimulatory in other systems. Moreover, activation of the 5-HT7 receptor has been linked to both reduction and elevation of blood pressure. These interactions are discussed in this mini-review, as are potential steps forward in understanding the interplay of 5-HT, the sensory nervous system and blood pressure.
Keywords: 5-HT, 5-HT7 receptor, blood pressure, sensory nervous system, CGRP
1. Introduction
5-hydroxytryptamine (5-HT, serotonin) was originally discovered in the blood, and thus its actions in the cardiovascular system has long been of interest. Decades of research have revealed that 5-HT alters the cardiovascular system in complicated ways. For example, the cardiovascular parameter of blood pressure is both decreased and increased by 5-HT, depending on the site of administration (reviewed in Watts et al, 2012). Work by Cuesta et al (2014) in this issue of Vascular Pharmacology raises a new mechanism by which 5-HT could influence blood pressure. Specifically, activation of 5-HT7 receptors inhibits the vasodepressor effect of the sensory nervous system.
2. 5-HT and the sensory nervous system
5-HT acts as an inhibitory hormone in the nervous system through presynaptic inhibition of neurotransmitter release (reviewed in Feuerstein, 2008; Restrepo et al, 2010). This includes both the sympathetic and parasympathetic branches of the autonomic nervous system and, of current interest, the sensory nervous system. The neurotransmitters of the sensory nervous system include calcitonin gene related peptide (CGRP) and substance P (SP). Cuesta et al isolated the sensory nervous system in the rat through pithing and administration of the nicotinic receptor ganglionic blocker; blood pressure was supported by administration of the α adrenergic agonist methoxamine. The spinal cord was electrically stimulated to activate the sensory nervous system with a resultant decrease in blood pressure. AS-19 is a tetralin derivative ((2S)-(+)-5-(1,3,5-Trimethylpyrazol-4-yl)-2-(dimethylamino)tetralin) that has been accepted as a potent agonist of 5-HT7 receptors (Sanin et al., 2004). AS-19 inhibited the EFS – induced vasodepression but did not directly inhibit vasodepression caused by CGRP itself, indicating that the effect of AS-19 was at the level of the stimulated cord and not at the level of the CGRP receptor. Importantly, pimozide blocked the ability of AS-19 to inhibit EFS-vasodepression. Pimozide was originally developed as a dopamine D2 receptor antagonist but has high affinity for the 5-HT7 receptor as well as the 5-HT1A, 5-HT2A, alpha 2 adrenergic and DA3 receptors (http://pdsp.med.unc.edu/pdsp.php with pimozide as test ligand; Boublike and Funder, 1984). As such, the conclusion that a 5-HT7 receptor is assuredly involved would be stronger with use of a more selective antagonist such as LY215840 or SB269970 (http://www.iuphar-db.org/DATABASE/ObjectDisplayForward?objectId=12).
The model of the Cuesta work is a pithed, instrumented and pharmacologically supported rat. If it took these maneuvers to uncover the inhibitory actions of 5-HT at the sensory nervous system, is this mechanism normally in play? Can 5-HT itself elicit the effects observed? Our greatest knowledge of 5-HT and the sensory nervous system is in the inhibition of CGRP/SP release through triptan and ergot-sensitive receptors in the trigeminovascular neurons and intracranial blood vessels in the treatment of migraine (Burstein et al., 2005; Silberstein, 2013). By contrast, 5-HT7 receptors have also been supported to promote CGRP release in experimental models of migraine (Wang et al, 2010).
The role of the sensory nervous system in blood pressure regulation is underappreciated. Work by DiPette and colleagues as well as other laboratories supports that the sensory nervous system may act as an important ‘brake’ or dampening system in models of hypertension. Removal of the sensory system early in development or blockade of the receptors of CGRP allow blood pressure to develop to a higher magnitude (Supowit et al, 1997). Use of genetically modified animals that lack CGRP also supports the idea that the sensory nervous system acts to dampen a hypertensive response (Smillie et al, 2014). This raises a long-standing question as to how 5-HT itself participates in blood pressure regulation via the sensory nervous system. Circulating levels of 5-HT are elevated in both genetically and experimentally derived models of rodent hypertension (Watts et al, 2012). Does this elevated endogenous 5-HT: 1) promote direct vasoconstriction; 2) act as a break like CGRP by relaxing vasculature (an adaptive response); and/or 3) promote hypertension by inhibiting the vasodepressor effects of the sensory nervous system? One could argue that all of the above could occur, making 5-HT a not so simple target with which to intervene in the treatment of hypertension.
3. A Paucity of tools to study the 5-HT7 receptor
Basic information about the 5-HT7 receptor is described in Figure 1. This receptor was first cloned in 1993 (Ruat et al, 1993) and identified as a heptahelical receptor with several splice variants of the C terminus (Gellyncyk et al, 2008; Jasper et al, 1997; Krobert et al, 2001). The 5-HT7 receptor has been predominantly linked with Gs and activation of adenylate cyclase. The discovery of this receptor helped people solve a mystery as to mechanisms of 5-HT-induced acute hypotension (Terron, 1997). The present study by Cuesta places 5-HT7 receptors in a different light, in that the findings of these authors suggests 5-HT7 receptor does not promote but indirectly inhibits hypotension. Thus, the involvement of 5-HT7 receptors in determining blood pressure becomes more complicated with the location of the 5-HT7 receptor largely governing the response.
Figure 1. Description of the 5-HT7 receptor.
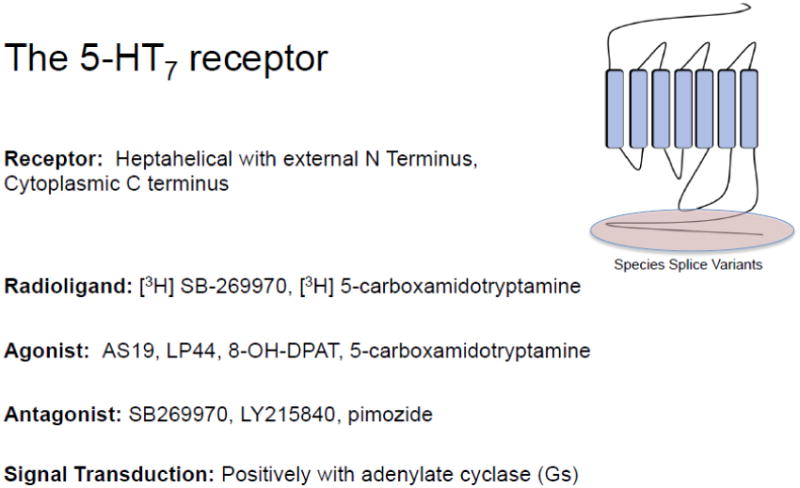
AS-19 is the 5-HT7 agonist that has been used to the greatest extent in vivo and was central to the work of Cuesta et al. LP-44 is another 5-HT7 receptor with a different pharmacophore. Outside of these two agonists, there is a lack of specific agonists with which to challenge the 5-HT7 receptor. The field has come to learn that compounds such as the 5-HT1A receptor agonist 8-OH-DPAT has significant affinity for the 5-HT7 receptor (Eriksson et al, 2008; Sprouse et al, 2004). Antagonists for the 5-HT7 receptor are more firmly established, having been validated in both in vitro and in vivo experiments; SB2699670 and LY215840 have proven useful drugs in this regard. Pimozide was used in the study of Cuesta and, while effective in blocking the 5-HT7 receptor, its significant affinity leads one to question whether antagonism was as specific as could be. This is a standing concern with amines which can act in promiscuous, non-selective manners at other amine receptors and especially in amine transporters (Daws, 2009).
4. Future Directions?
The study by Cuesta raises a number of interesting questions.
Does a vasodepressor inhibition of the sensory nervous system by 5-HT exist in the human? There are no solid studies that would suggest this is the case.
Is endothelin (ET-1) released by stimulation of the 5-HT7 receptor to reduce blood pressure? Blockade of AS-19 induced inhibition of EFS vasodepression by the ET receptor antagonist sulfisoxazole is an interesting finding that raises several questions. ET-1 and CGRP have long been regarded as physiological antagonists of one another (Labruijere et al, 2013), and ET-1-induced increases in blood pressure are prevented by the sensory nervous system (Wang and Wang, 2004). Sulfisoxazole is best known as an antibiotic, and better developed ET receptor antagonists (atrasentan, for example) exist, the use of which would add more confidence to this conclusion.
5. Conclusions
The study by Cuesta et al is an example of rigorously performed whole animal studies, a laborious effort that is necessary for us to appreciate physiological mechanisms. The discovery of the ability of 5-HT to inhibit sensory outflow is important because it sheds needed light on the complexity of the ability of 5-HT to change blood pressure. To ever consider 5-HT as a therapeutic endpoint means needing to have solid understanding of exactly how 5-HT works, and the present study adds to this understanding.
Acknowledgments
SW Watts was supported by NHLIB HL107495.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boublike JH, Funder JW. Interaction of dopamine receptor ligands with subtypes of the opiate receptor. Eur J Pharmacol. 1984;107:11–16. doi: 10.1016/0014-2999(84)90085-2. [DOI] [PubMed] [Google Scholar]
- Burstein R, Levy D, Jakubowski M. Effects of sensitization of trigeminovascular neurons to triptan therapy during migraine. Rev Neurol. 2005;161:658–660. doi: 10.1016/s0035-3787(05)85109-4. [DOI] [PubMed] [Google Scholar]
- Cuesta C, Garcia-Pedraza JA, Garcia M, Villalon CM, Moran A. Role of 5-HT7 receptors in the inhibition of the vasodepressor sensory CGRPergic outflow in pithed rats. Vascular Pharmacol. 2014 doi: 10.1016/j.vph.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Daws LC. Unfaithful neurotransmitter transporters: focus on serotonin update and implications for antidepressant efficacy. Pharmacol Ther. 2009;121:89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson TM, Golkar A, Ekstrom JC, Svenningsson P, Ogren SO. 5-HT7 receptor stimulation by 8-OH-DPAT counteracts the impairing effects of 5-HT(1A) receptor stimulation on contextual learning in mice. Eur J Pharmacol. 2008;596:107–110. doi: 10.1016/j.ejphar.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Feuerstein TJ. Presynpatic receptors for dopamine, histamine and serotonin. Handb Exp Pharmacol. 2008;184:289–338. doi: 10.1007/978-3-540-74805-2_10. [DOI] [PubMed] [Google Scholar]
- Gellynck E, Laenen K, Andressen KW, Lintermans B, De Martelaere K, Matthys A, Levy FO, Haegeman G, Vanhoenacker P, Van Craenenbroeck K. Cloning, genomic organization and functionality of 5-HT (7) receptor splice variants from mouse brain. Gene. 2008;426:23–31. doi: 10.1016/j.gene.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Jasper JR, Kosaka A, To ZP, Chang DJ, Eglen RM. (1997) Cloning, expression and pharmacology of a truncated splice variant of the human 5-HT7 receptor (h5-HT7b) Br J Pharmacol. 1997;122:126–132. doi: 10.1038/sj.bjp.0701336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krobert KA, Bach T, Syversveen T, Kvingedal AM, Levy FO. The cloned human 5-HT7 receptor splice variants: a comparative characterization of their pharmacology, function and distribution. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:620–632. doi: 10.1007/s002100000369. [DOI] [PubMed] [Google Scholar]
- Labruijere S, Compeer MG, van den Bogaerdt AJ, van den Brink AM, De Mey JG, Danswer AH, Batenburg WW. Long-lasting physiological antagonism of calcitonin gene-related peptide towards endothelin-1 in rat mesenteric arteries and human coronary artery. Eur J Pharmacol. 2013;720:303–309. doi: 10.1016/j.ejphar.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Restrepo B, Martin ML, San Roman L, Moran A. Peripheral 5-HT1A and 5-HT7 serotonergic receptors modulate parasympathetic neurotransmission in long-term rats. Exp Diabetes Res. 2010 doi: 10.1155/2010/686734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrange JM, Schwartz JC. Molecular cloning, characterization and localization of highaffinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci USA. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanin A, Brisander M, Rosqvist S, Mohell N, Malberg A, Johansson A. 5-Aryl substituted (S)-2-(dimethylamino)-tetralins novel serotonin 5HT7 receptor ligands. Proceedings of the 14th Camerino-Noord Symposium Ongoing Progress in the Receptor Chemistry. 2004:27. [Google Scholar]
- Silberstein SD. Emerging target based paradigms to prevent and treat migraine. Clin Pharmacol Ther. 2013;93:78–85. doi: 10.1038/clpt.2012.198. [DOI] [PubMed] [Google Scholar]
- Smillie SJ, King R, Kodji X, Outzen E, Pozsgai G, Fernandes E, Marshall N, de Winter P, Heads RJ, Dessapt-Baradez G, Gnudi L, Sams A, Shah AM, Siiw RC, Brain SD. An ongoing role of α-calcitonin gene-related peptide as part of a protective network against hypertension, vascular hypertrophy and oxidative stress. Hypertension. 2014;63:1056–1062. doi: 10.1161/HYPERTENSIONAHA.113.02517. [DOI] [PubMed] [Google Scholar]
- Sprouse J, Rennolds L, Li X, Braelton J, Schmidt A. 8-OH-DPAT as a 5-HT7 agonist: phase shifts of the circadian biological clock through increases in cAMP production. Neuropharmacol. 2004;46:52–62. doi: 10.1016/j.neuropharm.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Supowit SC, Zhao H, Hallman DM, DiPette DJ. Calcitonin gene-related peptide is a depressor of deoxycorticosterone-salt hypertension in the rat. Hypertension. 1997;29:945–950. doi: 10.1161/01.hyp.29.4.945. [DOI] [PubMed] [Google Scholar]
- Terron JA. Role of 5-ht7 receptors in the long-lasting hypotensive responses induced by 5-hydroxytryptamine in the rat. Br J Pharmacol. 1997;121:563–571. doi: 10.1038/sj.bjp.0701134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fang Y, Liang J, Yin Z, Miao J, Luo N. Selective inhibition of 5-HT7 receptor reduces CGRP release in an experimental model for migraine. Headache. 2010;50:579–587. doi: 10.1111/j.1526-4610.2010.01632.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang DH. Prevention of endothelin-1-induced increases in blood pressure: role of endogenous CGRP. Am J Physiol Heart Circ Physiol. 2004;287:H1868–H1874. doi: 10.1152/ajpheart.00241.2004. [DOI] [PubMed] [Google Scholar]
- Watts SW, Morrison SF, Davis RP, Barman SM. Serotonin and blood pressure regulation. Pharmacol Rev. 2012;64:359–388. doi: 10.1124/pr.111.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]


