Abstract
Objectives
Adding antimicrobial/anti-MMP quaternary ammonium methacrylates (QAMs) to comonomer blends should not weaken the mechanical properties of dental resins. This work evaluated the degree conversion and mechanical properties of BisGMA/TEGDMA/HEMA (60:30:10) containing 0–15 mass% QAMs A–E (A:2-acryloxyethyltrimethyl ammonium chloride; B:[3-(methacryloylamino)propyl]trimethylammonium chloride; C:[2-(methacryloxy)ethyl] trimethyl ammonium chloride; D:diallyldimethyl ammonium chloride; E:2-(methacryloyloxy) ethyltrimethyl ammonium methyl sulfate.
Methods
Unfilled resins with and without QAM were placed on ATR-FTIR and light-polymerized for 20 s in a thin film t 30°C. Unfilled resin beams were casted from square hollow glass tubings. Half of the beams were tested after 3 days of drying (control); the other half were tested wet after 3 days of water storage.
Results
Addition of QAMs in control resins significantly increased conversion 600s after light termination, with the exception of 5% MAPTAC (p<0.05). Increase of QAM content within a formulation significantly increased conversion. Control beams gave dry Young’s moduli of ~700 MPa. Addition of 5, 10 or 15 mass% QAMs produced significant reductions in dry Young’s moduli except for 5% B or C. 15 mass% A, B and C lowered the wet Young’s moduli of the resin beams by more than 30%. The ultimate tensile stress (UTS) of control dry resin was 89±11 MPa. Addition of 5–10 mass% QAMs had no adverse effect on the dry UTS. After water storage, the UTS of all resin blends fell significantly (p<0.05), especially when 15 wt% QAMs was added. Control dry beams gave fracture toughness (KIC) values of 0.88±0.1 MPa√m. Wet values were significantly higher at 1.02±0.06 (p<0.05). KIC of dry beams varied from 0.85±0.08 at 5% QAMs to 0.49±0.05 at 15% QAMs. Wet beams gave KIC values of 1.02±0.06 MPa√m that fell to 0.23±0.01 at 15% QAMs.
Significance
Addition of 10% QAMs increased the degree of conversion of unfilled resins, but lowered wet toughness and UTS; addition of 15% QAMs lowered the mechanical properties of wet resins below acceptable levels.
Keywords: percent-conversion, TPO, dry-testing, fracture toughness, quaternary ammonium methacrylates, ultimate tensile strength, Young’s modulus, wet-testing
1. Introduction
Quaternary ammonium methacrylates (QAMs) have been added to dental comonomer blends for their antibacterial [1–5] and anti-MMP activity [6,7]. Because most QAMs are monomethacrylates, it is important to determine if addition of QAMs to comonomer blends cause any weakening of their mechanical properties, and if so, at what concentration. A good example of a QAM used in a dental product is 12-methacryloyloxydodecylpyridinium bromide (MDPB). It is used in Clearfil SE Protect as an antibacterial primer [1–3,8].
It is well-known that water sorption into hydrophilic polymers plasticizes them and lowers their mechanical properties [9–13]. Unfilled resins tend to have low mechanical properties values because they have no reinforcing fillers. The lower flexural strength and flexural modulus of unfilled resins results in a lower fracture toughness [14], giving them values similar to compomers [14,15].
As one incorporates any monomethacrylate into comonomer blends at the expense of dimethacrylates, the degree of cross-linking of the polymer decreases. This results in lower flexural strength and flexural modulus. Presumably, this should also lower other mechanical properties of resins.
The purpose of this study was to determine if addition of 5, 10 or 15 wt% QAMs to a BisGMA/TEGDMA/HEMA blend would lower the degree of conversion or mechanical properties of beam specimens of unfilled resins. The first null test hypotheses was that 1) addition of 5, 10 or 15 wt% QAMs has no effect on the degree of conversion or dimethacrylate blends, 2) addition of 5, 10 or 15 wt% QAMs has no effect on the mechanical properties of dimethacrylate blends, 3) that addition of 5, 10 or 15% QAMs to dimethacrylate blends immersed in water does not produce water-sorption induced changes in the mechanical properties of QAM-containing dimethacrylates.
2. Materials and methods
2.1 Degree of conversion
Before testing the effect QAM-containing adhesives on the mechanical properties of unfilled resins, a careful investigation of the effect of incorporation of QAMs into dental adhesives is necessary to verify their role in polymerization. Indeed, it was clearly shown that the degree of conversion (DC) of dental adhesives is an important parameter since low mechanical properties associated with monomer elution are related with low percentage of monomer to polymer conversion within resin-based materials [16–18].
Previous investigations showed that DC depends on resin type, hydrophilicity, solvent content and photo-initiator blended within the monomers. It has been shown that even though camphoroquinone (CQ) is the most common photo-initiating system in dental resin restorative materials [19], it may impair the polymerization of hydrophilic monomers due to CQ’s intrinsic hydrophobic nature. On the other hand, the use of CQ-alternative photoinitiators, such as diphenyl(2,4,6-trimethylbenzoyl)-phosphine oxide), TPO, may improve the extent of polymerization (Ep) of hydrophilic adhesive formulations [20].
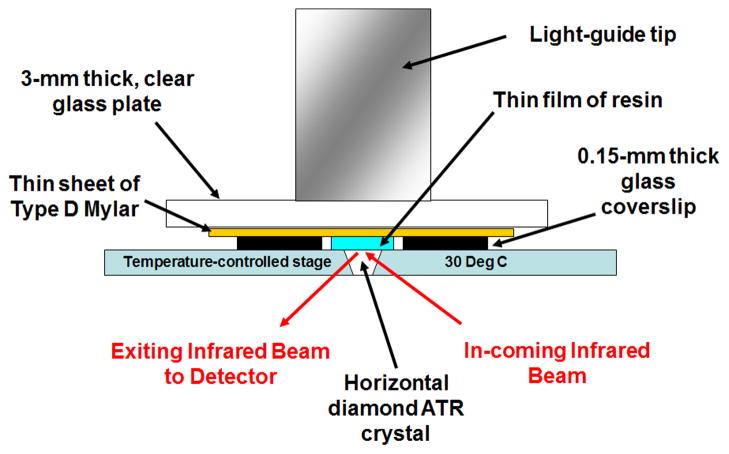
One drop of each resin mixture was placed between two, 0.15-mm thick, laterally placed microscope cover glass plates acting as shims, directly on the surface of a diamond crystal in a horizontal attenuated total reflectance heated unit (25°C) stage (Golden Gate Mk II, SPECAC Inc., Cranston, RI) using a 1 mL disposable syringe (Norm-Ject, Tuttlingen, Germany). The attachment was positioned in the optical compartment of a Fourier transform infrared spectrophotometer (FTS-40, Digilab/BioRad, Cambridge, MA). A 1.5 × 1.5 cm × 76 μm piece of polyester film (Mylar, Type D, Polymer Plastics Corporation, Reno, NY, USA) was immediately placed over the top of the deposited resin (covering the two glass shims laterally) to exclude oxygen (Fig. 1), and a 3-mm thick, clear glass plate was positioned over the top of the assembly and pressed down, forcing the fluid to spread and attain the final thickness of the two lateral glass shims. The flat end of the light guide of a quartz-tungsten-halogen light-curing unit (Optilux 401, Demetron/Kerr, Danbury, CT) was placed coincident with the underlying diamond element, and directly against the upper glass surface. The irradiance of the curing light was measuring through the same 3-mm thick glass plate, using a laboratory grade spectroradiometric system, consisting of a 6″ NIST-traceable integrating sphere (Labsphere, North Sutton, NH) and a spectrometer (USA2000, Ocean Optics, Dunedin, FL) to be 525 mW/cm2. The resins were exposed to blue light for 20 s and spectra were obtained continuously until 605 seconds from droplet application. The experimental setup simulated dispensing of an adhesive resin in a thin layer on a tooth prepared surface clinically, but without air-drying (Fig. 1).
Figure 1.
Schematic diagram of test device for obtaining infrared spectra.
In addition, this experimental set-up is similar to that used to measure polymerization shrinkage with the Watts Bonded Disc apparatus, allowing conversion data to ultimately by correlated with shrinkage values (not presented in the current work). Kinetic infrared (IR) spectra were obtained between 4000 and 700 cm−1 at 2 cm−1 resolution at one scan per second, with no averaging. Spectral acquisition was initiated immediately upon resin droplet deposition to obtain the IR spectra of each solution group in the uncured state and the curing light was activated 5 s after droplet deposition.
Preliminary FTIR spectra of one QAM monomer (MAPTAC) revealed an absorption band at 1653 cm (circled in Fig. 2, right) that interfered with the use of the conventional method of determining monomer conversion of methacrylate-based dental resins. That method uses the ratio of aliphatic C=C absorption (1636 cm−1) to that of the internal standard aromatic C=C band (1608 cm−1) in the cured and uncured state and relies on the absence of any other peak within the range of analysis (Fig. 1) [16,17]. MAPTAC’s spectrum, not allowing the conventional spectral analysis to be performed to determine monomer conversion. Comparison of spectral absorbance of representative resins in the unhydrogenated and hydrogenated states confirms the presence of an interfering peak at 1653 cm−1 in MAPTAC, even after total removal of the aliphatic C=C groups (Fig. 3). In Fig. 3, the left panel shows the absence of the C=C group at 1636 cm−1 in the MCMS resin following hydrogenation. The right panel shows the total loss of the 1636 cm−1 C=C group in MAPTAC following hydrogenation but the residual presence of an interfering absorption band at 1635 cm-1 that prevents conventional method of spectral analysis to be used to determine monomer conversion in this frequency range.
Figure 2.
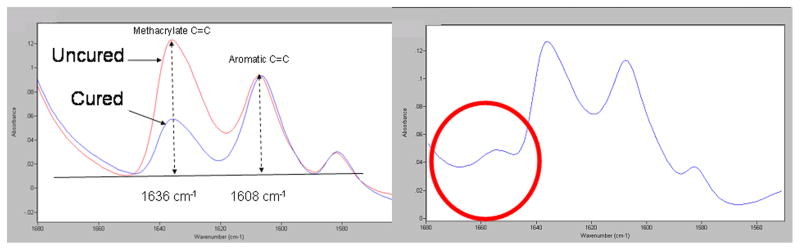
A: FTIR spectra using changes in relative heights of aliphatic vs. aromatic C=C peaks for monomer conversion analysis (left panel). B: Right panel shows presence of interfering peak at 1653 cm−1 (circle) in the spectrum of MAPTAC, one of the tested QAMs.
Figure 3.
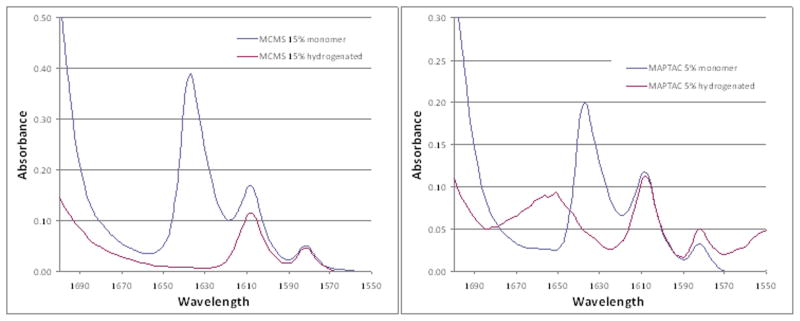
A: Left panel shows the absence of the C=C group at 1636 cm−1 in resin MCMS following hydrogenation. B: Right panel shows total loss of the 1636 cm−1 C=C group in MAPTAC following hydrogenation, but the presence of a residual presence of an interfering absorption band at 1653 cm−1, preventing the use of conventional methods of spectral analysis to determine monomer conversion in this frequency range.
Thus, the extent of polymerization (Ep) was determined by comparing changes in the loss of absorbance of a relatively isolated peak at 1318 cm−1, that represents the trans p(=CH)s in-plane=CH deformations [21]. The method used to determine conversion using 1318 cm−1 absorption peak was that of determining the absorption of the peak height with respect to a baseline drawn between the troughs in either side of the peak (Fig. 4).
Figure 4.
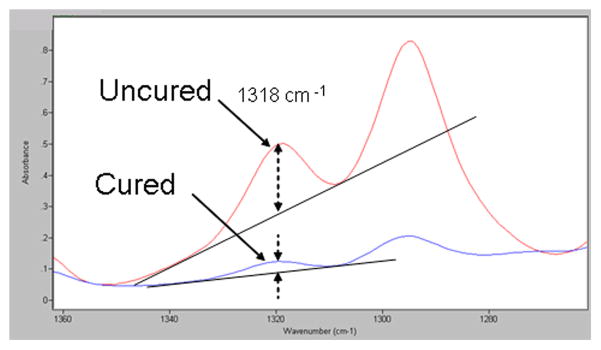
IR spectra showing the method for determining decreases in peak absorbance heights at 1318 cm−1 in the upper uncured versus the lower cured states.
IR spectra showing the method for determining the peak absorbance height at 1318 cm−1 in the uncured (upper) and cured (lower) states.
Verification that the 1318 cm−1 peak was indeed measuring C=C, absorbance was made by comparing the IR spectra of unhydrogenated and hydrogenated Scotchbond Multi-Purpose resin. Total absence of the 1318 cm−1 peak confirmed identity of the functional group associated with this peak (Fig. 5).
Figure 5.
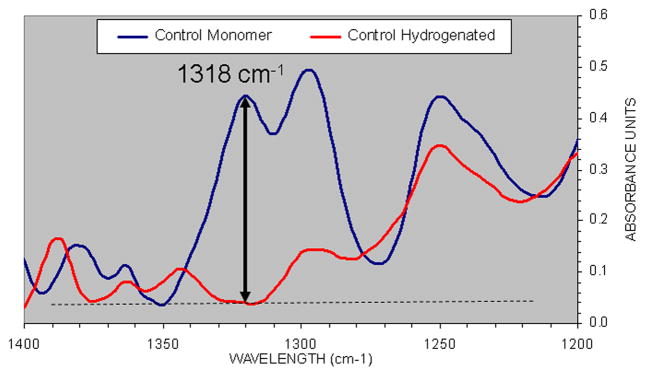
Comparison of the unhdrogenated BisGMA/TEGDMA-based Scotchbond Multi-Purpose adhesive (SBMP) (upper scan) showing the presence of a high absorption located at 1318 cm−1, and the total absence of this peak at that wavenumber following saturation of the C=C bond by hydrogenation (lower scan).
Comparison of unhydrogenated Bis-GMA/TEGDMA-based adhesive (SBMP) upper scan, showing the presence of a high absorption located in 1318 cm−1 and the total absence of this peak at that wavenumber, following hydrogenation of the C=C (lower line).
Prior to utilization of the 1318 cm−1 peak, conversion of a Bis-GMA/TEGDMA-based dental resin (SBMP) was compared using the conventional method, and that when using the 1318 cm−1 peak, to show that no significant differences were found (Fig. 6).
Figure 6.
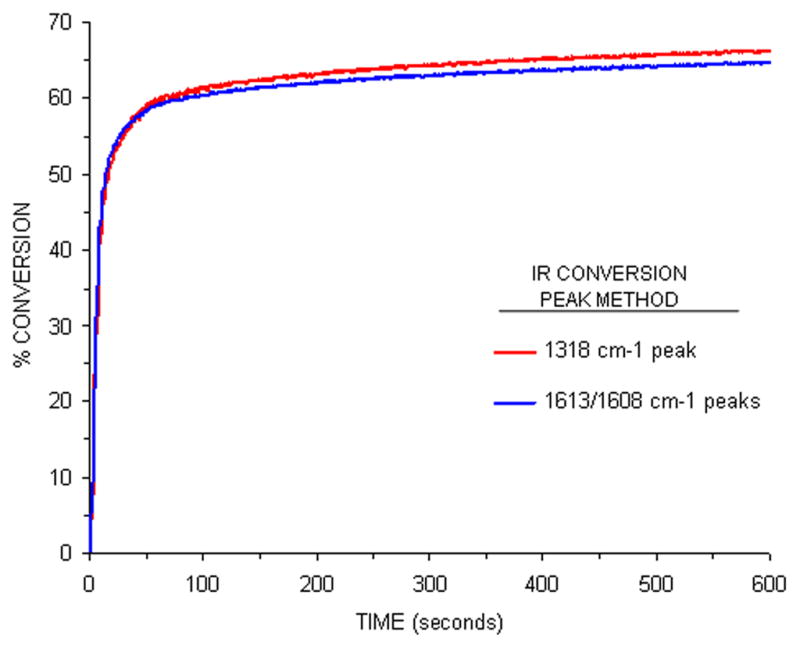
Comparison of real-time conversion profiles for SBMP adhesive using the conventional 1636 cm−1/1608 cm−1 ratio technique (lower line), and that using changes in the 1318 cm−1 absorption peak (upper line).
Monomer conversion values were determined for each second following light initiation, and conversion at specific times following light initiation were grouped: 20, 40, 60 and 600 seconds. Five repetitions for each test condition were made.
Comparison of real-time monomer conversion profiles for SBMP using the conventional 1636 cm−1/1608 cm−1 ratio technique (lower line) and that using change in the absorption peak located at 1318 cm−1 (upper line ).
The rate of cure was obtained by calculating the first derivative of the smoothed conversion curve with respect to time, using data-analysis software (Logger Pro 3.5, Vernier Software & Technology, Beaverton, OR). The maximum polymerization rate (expressed as DC%/s) was obtained during the first 20 s of exposure.
2.2 Statistical analysis
One-way ANOVAs were used to evaluate how resin formulation affected the maximum rate of conversion and extent of the DC 600 s following light initiation, as measured by FTIR. Differences between groups were calculated using Tukey’s post hoc test. Statistical significance was preset at α = 0.05.
2.3 Mechanical properties of resins
The control resin blend was 60 wt% BisGMA, 30% TEGDMA, 9% HEMA 1.0% [diphenyl](2,4,6-trimethylbenzoyl)-phosphine oxide (TPO). It was used without solvent. The QAMs were: ATA = 2-acryloxyethyltrimethylammonium chloride; METMAC = [3-(methacryloylamino)propyl]trimethylammonium chloride; DDAC = diallyldimethylammonium chloride; MCMS = methacryloylcholine methyl sulfate; MAPTAC = [2-(methyloyloxy)ethyl]trimethylammonium chloride. When 5, 10 or 15 wt% QAMs were added to the control blend, the BisGMA was reduced an equal amount.
The beams used for fracture toughness were made in a stainless steel mold with internal dimensions of 24 mm in length, 5 mm in height, 2.5 mm width, with a razor blade incorporated into the mold 12 mm from either end, projecting 2.5 mm into the empty mold space. The mold was placed on a glass plate covered with Mylar film. After the mold was filled with neat comonomers, the filled mold was covered by another Mylar film and then a glass slide. The mixture was exposed to a Demitron dental light (600 mW/cm3) for 40 s, overlapping the diameter of the light-guide down the length of the beam for 4 exposures. Then the glass plates were removed and the light-curing repeated on the other side. After disassembling the molds, the polymerized beams were carefully removed laterally to avoid damage to the preformed notch. Twenty beams were made of each resin (Table 1). Each group was randomly divided into “dry” and “wet” groups. Dry beams were stored dry for 3 days at 37°C prior to testing. All beams were sanded with dry 2000 grit SiC abrasive paper to remove any irregularities and to make them perfectly flat.
TABLE 1.
Composition of dimethacrylate/QAM comonomer blends, their maximum rates of cure, and their degrees of conversion at specific times following initiation of a 20-second light exposure.
| QAM | Composition of comonomer blends | Max Cure Rate (%/s) | DC 20 s | DC 40 s | DC 60 s | DC 600s |
|---|---|---|---|---|---|---|
| 5% ATA | 5% ATA/52.75% BisGMA/30% TEGDMA/10% HEMA/1.0% TPO/1.25% H2O | 18.2 ± 0.7B,C,D | 72.3 ± 0.4 | 76.3 ± 1.1 | 79.5 ± 0.0 | 84.4 ± 0.2E,F |
| 10% ATA | 10% ATA/46.5% BisGMA/30% TEGDMA/10% HEMA/0.0% TPO/2.5% H2O | 18.8 ± 0.8A,B,C | 75.4 ± 2.3 | 81.1 ± 0.9 | 82.4 ± 1.0 | 89.1 ± 0.1B,C,D |
| 15% ATA | 15% ATA/40.25% BisGMA/30% TEGDMA/10% HEMA/1.0% TPO/3.75% H2O | 19.9 ± 0.7A | 79.2 ± 1.4 | 82.4 ± 0.6 | 83.7 ± 0.5 | 90.4 ± 0.3A,B |
| 5% MAPTAC | 5% MAPTAC/49% BisGMA/30% TEGDMA/10% HEMA/10% TPO/5.0% H2O | 15.1 ± 1.0F | 65.9 ± 3.7 | 69.7 ± 5.1 | 71.9 ± 4.6 | 78.5 ± 3.9G,H |
| 5% DDAC | 5% DDAC/51.3% BisGMA/30% TEGDMA/10% HEMA/10% TPO/2.7% H2O | 18.2 ± 1.2B,C,D | 76.8 ± 1.9 | 81.4 ± 1.5 | 82.1 ± 2.0 | 90.2 ± 0.5A,B,C |
| 5% MCMS | 5% MCMS/52.75% BisGMA/30% TEGDMA/10% HEMA/10% TPO/1.25% H2O | 17.4 ± 0.5C,D,E | 69.9 ± 0.6 | 73.4 ± 0.6 | 74.6 ± 0.8 | 81.5 ± 1.5F,G,H |
| 10% MCMS | 10% MCMS/46.5% BisGMA/30% TEGDMA/10% HEMA/1.0% TPO/2.5% H2O | 17.2 ± 0.2D,E | 72.6 ± 2.7 | 76.7 ± 2.7 | 78.0 ± 2.6 | 85.8 ± 2.2D,E |
| 15% MCMS | 15% MCMS/40.25% BisGMA/30% TEGDMA/10% HEMA/1.0% TPO/3.75% H2O | 18.1 ± 0.7B,C,D | 79.6 ± 1.2 | 83.9 ± 0.9 | 85.3 ± 0.9 | 91.5 ± 1.2A |
| 5% METMAC | 5% METMAC/52.75% BisGMA/30% TEGDMA/10% HEMA/1.0% TPO/ 1.25% H2O | 17.0 ± 0.1D,E | 68.1 ± 1.9 | 71.8 ± 1.8 | 73.7 ± 0.9 | 78.9 ± 0.9G,H |
| 10% METMAC | 10% METMAC/46.5% BisGMA/30% TEGDMA/10% HEMA/1.0% TPO/ 2.5% H2O | 17.3 ± 0.7D,E | 70.8 ± 1.2 | 75.0 ± 0.5 | 76.4 ± 0.6 | 81.8 ± 0.5F,G |
| 15% METMAC | 15% METMAC/3=40.25% BisGMA/30% TEGDMA/10% HEMA/1.0% TPO/3.75% H2O | 18.9 ± 0.5A,B | 77.2 ± 0.5 | 80.6 ± 0.9 | 81.8 ± 0.8 | 87.4 ± 1.0C,D,E |
| Control | 60% BisGMA/30% TEGDMA/10% HEMA/1.0% TPO | 16.4 ± 0.7E | 65.6 ± 1.9 | 70.6 ± 0.9 | 72.1 ± 0.9 | 78.3 ± 02.2H |
| SBMP | 60% BisGMA/40% HEMA/1% DPIHP (Guo et al., 2007) | 6.5 ± 0.3G | 51.7 ± 0.4 | 56.9 ± 1.1 | 59.4 ± 0.6 | 66.2 ± 0.6I |
All compositions are mass %. Abbreviations: ATA = 2-acryloxyethyltrimethylammonium chloride; MAPTAC = [3-(methacryloyamino)propyl]trimethylammonium chloride; DDAC = diallyldimethylammonium chloride; MCMS = methacryloyl choline methyl sulfate; METMAC = [2-(methacryloyloxy)ethyl]triammonium chloride; SBMP = Scotchbond Multi-Purpose adhesive, 3M ESPE; BisGMA = 2,2-bis[4-(2-hydroxy-3-methacryloxypropoxy)phenyl]-propane; TEGDMA = triethyleneglycol dimethacrylate; HEMA = 2-hydroxyethylmethacrylate; TPO = [dipheny](2,4,6-trimethylbenzoyl)-phosphine oxide.
N=5 replications per specimen composition
Max cure rate and conversion values at 600 s identified by similar upper case letters are not significantly different.
“Wet” beams were placed in distilled water for 3 days at 37°C prior to testing. All beams were tested on the same rounded supports 22 mm apart. The dry beams were tested in air. The wet beams were tested under water at a compressive cross-head speed of 0.1 mm/min. The universal testing machine used a 22 N load cell to measure load. Displacement was measured from the cross-head movement. The exact notch depth (a) was measured with a microscope but was nominally 2.5 mm. KIC was calculated [22] as:
where KIC = fracture toughness in MPa√m, a = notch depth (mm), W = beam width (mm), B = beam height (mm), P maximum load at fracture (N).
Resin beams used for testing modulus of elasticity toughness or ultimate tensile strength were created by aspirating the neat comonomer blends into square profile (1 × 1 × 20 mm) borosilicate glass tubing [12,13] (Vitrocells™, Cat. 8100, Fiber Optic Center, New Bedford, MA, USA). The tensile properties were measured for all small specimens after either 24 h of dry storage in air at room temperature, or after 24 h storage in water at 25°C. Larger specimens used for measuring fracture toughness were incubated in water for 3 days. All specimens were attached to Bisco microtensile testers using viscous cyanoacrylate (Zapit brand, cyanoacrylate cement, Dental Ventures of America, Corona, CA, USA). The modulus of elasticity was measured as the slope of the linear portion of the stress-strain curve, between 5–15% strain, and expressed in MPa. The toughness of the specimens (MPa) was calculated as the area under the stress-strain curve. The ultimate tensile strength (UTS, MPa) of each specimen was calculated as the maximum force at tensile failure, divided by the cross-sectional area of the specimen.
2.4 Statistical analysis
Results were statistically analyzed for normal distributions and quality of variance using the Kolmogorow-Smirnoff (p=0.05) and Levene tests (p=0.05), respectively. As the normality and homoscedasticity assumptions were valid, the data were analyzed using a two-way ANOVA with resin type of one factor and state of hydration the other factor. Post-hoc comparisions were performed with the Tukey test. Statistical significance was preset at α = 0.05.
3. Results
Table 1 summarizes the rates and degrees of conversion (DC) of BisGMA/TEGDMA/HEMA/QAM blends and their controls. The lowest cure rate was seen to be that of the commercial SBMP product (6.5 ± 0.3% s−1), while the highest was noted for 15% ATA (19.9 ± 0.7 %/s). For those QAM formulations that were concentration-based (ATA, MCMS and METMAC), the maximum rates of cure appeared to increase the additional presence of the QAM. These observations are also in accordance with a recent study in which [9,22] adhesive systems containing hydrophilic photoinitiators dramatically increased their degree of conversion compared to CQ-based system when photopolymerized in presence of 7.8% water [23]. Interestingly, all of the QAM-containing compounds exhibited maximum rates of cure significantly greater than either the commercial product (SMAP 6.5 ± 0.3%/s) or the control mixture (16.4 ± 0.7 %/s), with the exception of 5% MAPTAC (15.1 ± 1.0 %/s), which was significantly lower than all other QAM-containing formulations. Among the remaining QAM formulations, maximum conversion rates ranged from a low of 17.0 ± 0.1%/s for 5% METMAC, to a high of 19.9 ± 0.7% for 15% ATA. There was a significant positive linear correlation between 20 s DC and the maximum rate of polymerization for all the tested resins: p-value of slope <0.001, R2 = 0.852 (not shown).
Table 2 summarizes the mechanical properties of the unfilled resins. The use of square cross-section resin beams (1 × 1 × 20 mm) was first used by Ye et al. [12]. Such beams have many advantages over hour-glassed shaped specimens. The glass tubing created smooth surfaces without the need for polishing, and without air bubbles. After light-curing for 30 s, the resin beams could be pushed out of the glass molds with the end of a paper-clip.
Table 2.
Composition of dimethacrylates/QAM comonomer blends and their fracture toughness, KIC and UTS values.
| Fracture Toughness (MPa√m) | Young’s Modulus (MPa) | Toughness (MPa) | Ultimate Stress (MPa) | |||||
|---|---|---|---|---|---|---|---|---|
| Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | |
| 5% ATA | 0.85±0.08A,ac | 1.0±0.11B,1 | 620±101A,ac | 526±55B,1 | 11.9±5.5A,b | 7.4±1.6B,1 | 98±12A,a | 58±2B,1 |
| 10% ATA | 0.76±0.06A,bc | 0.60±0.05C,3 | 607±106A,ac | 488±51B,1 | 8.8±3A,ab | 2.8±0.8B,2 | 83±8A,ab | 43±4B,2 |
| 15% ATA | 0.49±0.05e | PC | 472±73b | PC | 4.6±1.7a | PC | 53±12c | PC |
| 5% MAPTAC | 0.65±0.05A,d | 0.93±0.09B,1 | 728±73A,a | 432±102B1 | 7.7±1.6A,ab | 7.4±3.1A,1 | 87±10A,ab | 65±8B,1 |
| 5% DDAC | 0.76±0.07A,be | 1.01±0.09B,1 | 541±70A,bc | 545±84A,1 | 12.4±4A,b | 5.5±3.7B,1,2 | 95±12A,ab | 53±9B,1,4 |
| 5% MCMS | 0.85±0.06A,ab | 1.00±0.09B,1 | 726±147A,ac | 520±89B,1 | 9.9±3.9A,b | 5.8±1.7B,1,2 | 100±18A,a | 60±4B,1,4 |
| 10% MCMS | 0.80±0.08A,ab | 0.82±0.08A,1 | 629±97A,bc | 563±69A,1 | 10.1±2.6A,b | 3.8±1.8B,1 | 96±10A,ab | 49±6B,1,4 |
| 15% MCMS | 0.77±0.07A,bc | 0.26±0.02B,2 | 530±92A,bc | 304±43B,2 | 11.1±3.9A,b | 0.8±0.4B,3 | 86±9A,ab | 18±3B,3 |
| 5% METMAC | 0.81±0.05A,ab | 1.01±0.11B,2 | 561±100A,bc | 452±72B,1 | 9.8±2A,b | 4.9±0.9B,1,2 | 94±11A,ab | 56±4B,1,4 |
| 10% METMAC | 0.77±0.06A,bc | 0.79±0.08A,1 | 581±75A,bc | 441±66B,1 | 9.1±3.5A,ab | 3.9±2B,1 | 90±17A,ab | 45±8B,2,4 |
| 15% METMAC | 0.77±0.11A,bc | 0.23±0.01B,2 | 586±111A,bc | 236±23B,2 | 8.8±5.8A,ab | 0.6±0.3B,3 | 79±14A,b | 13±4B,3 |
| Control (c) | 0.87±0.09A,a | 1.02±0.06B,1 | 724±116A,a | 476±95B,1 | 7.6±1.9A,ab | 5.3±1.2B,1,2 | 89±12A,ab | 61±6B,1 |
Abbreviations: PC = phase changes; ATA = 2-acryloxyethyltrimethylammonium chloride; MAPTAC = [2-(methyloylamino)propyl]trimethylammonium chloride; DDAC = diallyldimethylammonium chloride; MCMS = methacryloylcholine methyl sulfate; METMAC = [3-(methacryloylamino)propyl]trimethylammonium chloride; control = 60 mass% BisGMA, 30 mass% TEGDMA, 9 mass% HEMA, 1.0 mass% [diphenyl] (2,4,6-trimethylbenzoyl)-phosphine oxide (TPO). Student t tests comparing significant differences between dry and wet values are indicated by uppercase letters. Different uppercase letters signify significant differences (p<0.05). One-way ANOVA looked for significant differences between test and control values. Differences among dry values are indicated by different lowercase letters. Differences between wet values are indicated by different uppercase letters (p<0.05).
The Young’s modulus of BisGMA/TEGDMA/HEMA controls gave a mean ± SD (N=10) of 724 ± 116 MPa dry and 476 ± 95 MPa wet (p<0.05) (Table 2). When 5, 10 or 15 wt% ATA was added to the blend at the expense of BisGMA, the Young’s modulus of dry resin beams fell to 620 ± 101, 607 ± 106 and 488 ± 51 MPa, respectively. The only significant difference was between the 15% ATA and the 5 or 10% ATA (p<0.05) (Table 2). The blends containing 5 or 10 wt% ATA were not significantly different from each other in the dry state. When ATA-containing specimens were immersed in water for 3 days, the Young’s moduli fell somewhat, but not significantly compared to their dry condition (p>0.05). When 15% ATA was blended with 40.25% BisGMA, 30% TEGDMA, 10% HEMA and 1% TPO, it underwent a phase change that interfered with polymerization, hence the mechanical properties of 15% ATA in BisGMA/TEGDMA/HEMA could not be measured (Table 2).
Addition of 5, 10 or 15% METMAC with BisGMA/TEGDMA/HEMA/TPO produced a slight, statistically insignificant reduction in Young’s modulus in dry beams of 561 ± 100, 581 ± 75 and 586 ± 111 MPa, respectively (Table 1). When these beams were immersed in water for 3 days, their stiffness values fell to values of 452 ± 72, 441 ± 66 and 236 ± 23 MPa, respectively. Only the 15% ATA value was significantly (p<0.05) lower than the 5 or 10% ATA values (Table 2).
Addition of 5 or 10 wt% MCMS to BisGMA/TEGDMA/HEMA blends had no effect on the Young’s modulus of the blends compared to controls (p>0.05). However, when 15 wt% MCMS was added to the blends, the stiffness values fell significantly (p<0.05) (Table 2).
Addition of 5 wt% DDAC to the control blend produced a slight, nonsignificant reduction in stiffness of dry or wet beams, compared to controls. In contrast, addition of 5 wt% MAPTAC to the control blend produced no change in the stiffness of dry beams, although the stiffness did fall significantly when the beams were immersed in water for 3 days. All attempts to increase the weight percent of DDAC or MAPTAC above 5 wt% caused phase changes and poor polymerization (Table 2).
When the toughness of the BisGMA/TEGDMA/HEMA-QAM blends was measured as the area under the stress-strain curve, it became clear that dry beams containing 5, 10 or 15 wt% QAMs were not different from control resins (Table 2), with the exception of 15% ATA. In that case, the toughness of dry beams was significantly lower (p<0.05) than that of controls or 5 or 10% ATA (Table 2).
When the resin beams containing ATA or MCMS were immersed in 37°C water for 3 days, their toughness did not fall significantly (p>0.05) regardless of the ATA concentration. In contrast, beams containing 5, 10 or 15 wt% METMAC that had absorbed water for 3 days showed significantly lower toughness (p<0.05). Beams containing 5 wt% DDAC or 5% MAPTAC showed similar toughness both dry and after water sorption (Table 2).
Addition of 5, 10 or 15 wt% ATA to BisGMA/TEGDMA/HEMA (with increases in QAM concentration substituted by equivalent decreases in BisGMA) caused little effects on the ultimate tensile strength (UTS) of dry beams (Table 2). The UTS values of dry specimens varied from 53.3 ± 12.1 MPa to 99.8 ± 17.8 MPa (Table 2). The UTS values of these control and experimental (i.e. QAM-containing) blends all fell significantly (p<0.05) when the beams were immersed in water for three days (Table 2).
Addition of 15 wt% METMAC or MCMS to the blends produced very low UTS values after they were soaked in water. Thus, these UTS values were similar qualitatively and quantitatively to the toughness values listed above (Table 2).
Table 1 summarizes the fracture toughness (FT) results. The control BisGMA/TEGDMA/HEMA dry resin gave a median value of 0.87 ± 0.03 MPa√m. When the same control resin was measured after 3 days of water sorption, the FT values increased significantly (p<0.05) to 1.03 ± 0.10. When 5 wt% ATA was added to the control blend, the dry FT median value was 0.85 ± 0.03 MPa√m, which was slightly but significantly lower than controls. When that blend was tested after water sorption, the FT values were not different from controls (i.e. they increased to the wet control values). When ATA was increased to 10 wt% in the blend, the dry FT values decreased significantly (p<0.05) to 0.78 ± 0.04 (median ± SEM). When 10% ATA-containing blends were allowed to absorb water for 3 days, the FT values fell to 0.60 ± 0.05 MPa√m. When 15 wt% ATA was added to the blend, the dry FT values fell to 0.50 ± 0.52 (median ± SEM) (p<0.05). When this blend was allowed to absorb water, the resin disintegrated (Table 2).
When 5 wt% METMAC was added to the blend, the dry FT value was 0.81 ± 0.05. This value was significantly lower than the dry control resin (p<0.05). After 3 days of water sorption, the wet FT value increased significantly to 1.01 ± 0.11 (p<0.05), a value that was not different from the wet control resin (Table 2). Ten wt% METMAC gave dry values of 0.77 ± 0.06 that were lower than controls, but not different from 5% METMAC (Fig. 2). After 3 days of water sorption, the wet FT values 0.79 ± 0.08 were not different from the dry FT values of 10 wt% METMAC (Fig. 2). When 15 wt% METMAC was added, the dry FT value, 0.77 ± 0.11 was not different from the other METMAC values, but after 3 days of water sorption, the wet FT value fell (p<0.05) to 0.23 ± 0.01, the lowest measured FT value (Table 2).
When 5 wt% MCMS was added to the blend, the dry FT value was 0.85 ± 0.06. After 3 days of water sorption, the wet FT values increased to 1.00 ± 0.09. When 10 wt% MCMS was added, the dry FT values was 0.80 ± 0.08. After 3 days of water sorption, the wet FT was 0.82 ± 0.08, a value not different from the dry FT value. Addition of 15 wt% MCMS lowered the dry FT value to 0.77 ± 0.07 (p<0.05). Three days of water sorption lowered the wet FT value (p<0.05) to 0.26 ± 0.02 (Table 2).
When 5 wt% DDAC was added, the dry FT value fell to 0.77 ± 0.06, a value that was lower than dry FT control values (Table 2). After 3 days of water sorption, the wet FT value increased to 1.01 ± 0.11 (p<0.05), a value not different from wet FT control values. When 10 or 15 wt% DDAC were added to the mixture, several phase changes occurred, so the mixtures could not be used (Table 2).
When 5 wt% MAPTAC was added, the dry FT value was only 0.65 ± 0.05 which was significantly lower than dry control values (Table 2). After 3 days of water sorption, the wet FT values increased significantly (p<0.05) to 0.91 ± 0.06 (Table 2). Attempts to add more MAPTAC to the mixtures resulted in phase changes that precluded further testing.
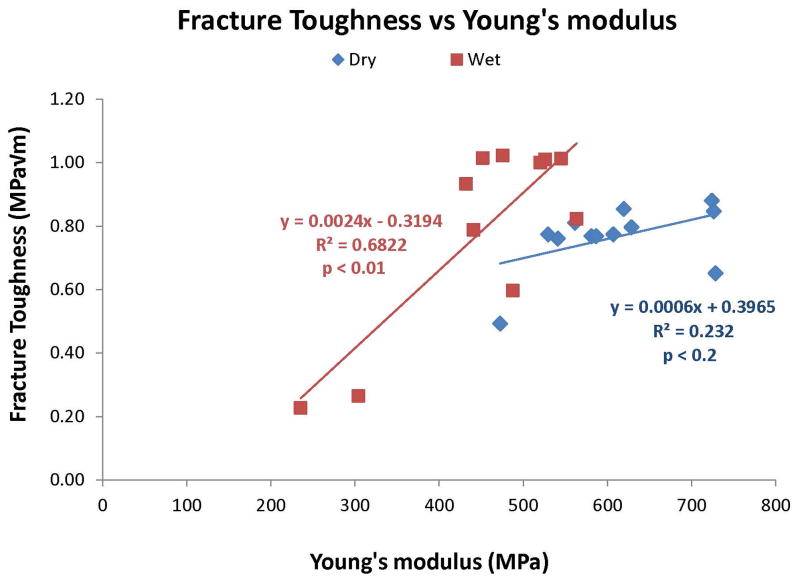
Figure 8 shows the relationship between fracture toughness and the modulus of elasticity of the control and test resins in the dry versus wet state. In the dry state, the fracture toughness values were largely similar (varying between 0.65–0.88 MPa√m, and the moduli of elasticity varied between 470–730 MPa. The slope of the line relating fracture toughness to modulus of elasticity was too low to be significant (R2 = 0.232, p<0.2). In the wet condition, both 15% MCMS and 15% METMAC absorbed so much water that their fracture toughness and stiffness values fell so much that the slope relating fracture toughness to modulus of elasticity was highly significant (R2 = 0.68, p<0.001).
Figure 8.
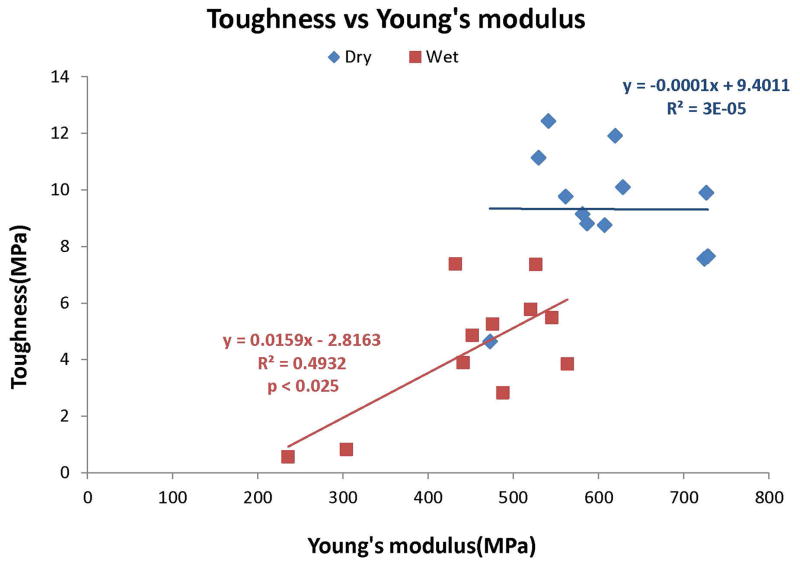
Relationship between toughness (area under the stress-strain curve) and Young’s modulus, in dry (◆) versus water-soaked (■) resin beams. Note that there was no significant relationship (i.e. slope of zero) between toughness and stiffness in dry specimens, but positive, significant (p<0.01) relationship (R2 = 0.493) between the two variables in water-saturated resin.
Figure 9 shows the relationship between toughness (measured as the area under the stress-strain curve) and moduli of elasticity of control and test resin blends measured dry or wet. Dry specimens showed no significant correlation (R2 = 0.00003, not significant). However, wet specimens showed a highly significant (p<0.001) relationship between toughness and stiffness (R2 = 0.70).
Figure 9.
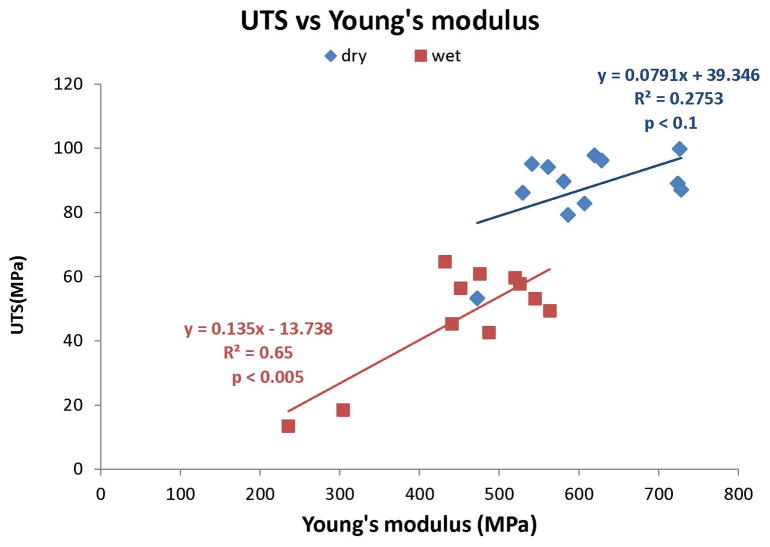
Relationship between ultimate tensile stress (UTS) and Young’s modulus in dry (◆) versus water-saturated (■) unfill resins. There was a positive, significant (p<0.025) (R2 = 0.525) relationship between fracture toughness and Young’s modulus in dry specimens. A similar positive significant (p<0.001) relationship (R2 = 0.65) was found between fracture toughness and Young’s modulus in water-saturated resin, although the UTS values were lower than were found in dry resin.
In Fig. 10, the relationships between the ultimate tensile stress and stiffness are shown in dry vs. moist specimens. Although there was no significant relationship with dry specimens, the wet specimens showed a highly significant correlation (R2 = 0.65, p<0.005).
Figure 10.
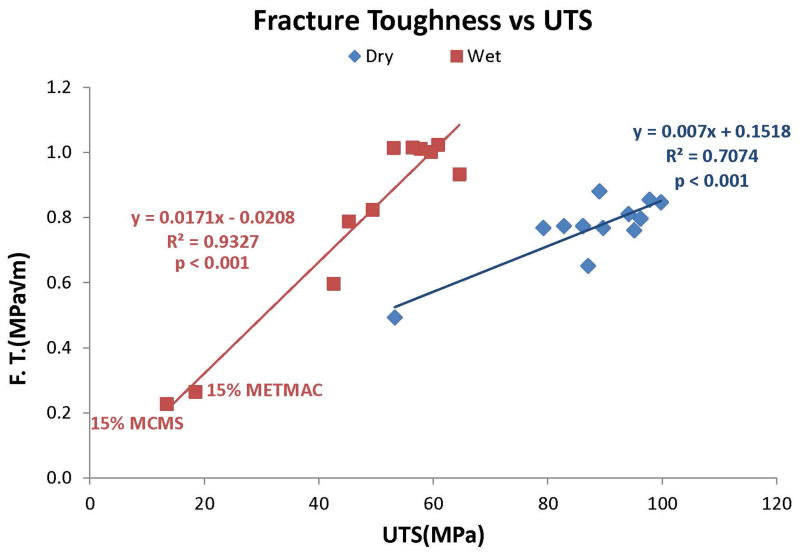
Relationship between fracture toughness and ultimate tensile stress (UTS) in dry (◆) versus wet (■) resins. With the exception of 15% MCMS and 15% METMAC added to control resins, water-saturated resins had higher KIC values than dry resins. Wet resins had a higher slope than dry resins with an R2 = 0.93, p<0.001).
Figure 11 shows the significant relationships between fracture toughness versus UTS of the dry and wet specimens. Both relationships were significant (R2 = 0.71, p<0.001 in dry specimens, versus R2 = 0.93, p<0.001 in wet specimens.
4. Discussion
The results of this study showed that increasing the QAM content of unfilled resins, increased the degree of conversion of the tested resin blends. Thus, the first tested null hypothesis was rejected. Increasing the QAM concentration of control resins decreased the mechanical properties of the unfilled resins, requiring rejection of the second null hypothesis. Since water storage of 5, 10 or 15 wt% QAM-containing dimethacrylate blends altered the mechanical properties of the resin, the results require rejection of the third null test hypothesis.
CQ and tertiary amines coinitiators (such as ethyl 4-dimethylaminobenzoate, EDMAB) are the most common photo-initiating systems in dental resin restorative materials [9]. However, they present some undesirable adverse effects such as yellow-color that can negatively affect the shade of the composite restoration [19], and a reduced reactivity of the amine co-initiatior that may be neutralized by the low pH of self-etch adhesives [24,25]. Because CQ has a very low water solubility, some investigators have avoided its use in water-containing resin blends [12]. This led others to investigate the application of alternative photo-initiators [26].
Acylphosphine oxides photo-initiating systems such as TPO, have been used in commercial dental products [27]. A previous study validated its efficacy by comparing experimental TPO-containing adhesive systems with their commercial CQ-containing counterparts, showing comparable DC values [26]. Similarly, TPO-containing methyl methacrylate monomer polymerized with a halogen light curing unit showed higher curing efficiency compared to the use of CQ, phenylpropanedione- (PPD), and bisacylphosphine oxide (Irgacure 819) as photoinitiators [28,29]. In a study by Leprince et al. [30], TPO had a higher reactivity and was a more efficient photoinitiator compared to CQ/DMAEMA in polymerizing Bis-GMA/TEGDMA resins used either unfilled or filled. TPO has been shown to be particularly helpful in improving the Ep of hydrophilic resin blends [20] because common hydrophobic initiators such as CQ are not very soluble in hydrophilic domains [23]. In a previous investigation, the addition of TPO significantly increased the DC of resin mixtures solvated with 10% ethanol plus 10% water, compared to CQ/EDMAB [Cadenaro et al., 2010]. Similarly, ATA, MCMS and METMAC, provided as aqueous solutions (with ATA and MCMS containing 40 wt% water and METMAC 50 wt% water respectively) or mixed with TEGDMA/HEMA, showed higher DC values when polymerized using TPO. These observations are also in accordance with a recent study in which adhesive systems containing hydrophilic photoinitiators dramatically increased their degree of conversion compared to CQ-based system when photopolymerized in presence of water [23].
Increasing the QAM concentrations of control unfilled resins lowered most of the mechanical properties of unfilled resins requiring acceptance of the second null hypothesis. Fracture toughness is the ability of a material to plastically deform and redistribute stresses as the material is loaded below the yield stress. Brittle materials with low fracture toughness undergo sudden catastrophic fractures as they are loaded. In a ductile material, plastic deformation, such as the ability to bend, occurs without fracture. The amount of energy required to fracture a material is that material’s fracture toughness. Fracture toughness is the relative value of a material’s ability to resist crack propogation [22,31–36]. A material fractures when the stress intensity reaches a critical value, KIC. The units of KIC are MPa m½. Unfilled resins have fracture toughness values of 0.8–1.0 MPa m½. Adding fillers to resins increases the fracture toughness to 2.0–2.2 MPa m½ [31].
In the current study, the control resins made up of neat 60 mass% BisGMA, 30 mass% TEGDMA, 9 mass% HEMA, 1% [diphenyl] (2,4,6-trimethylbenzoyl)-phosphine oxide (TPO), had a dry KIC of 0.87 ± 0.03 and a water-saturated KIC of 1.03 ± 0.10 MPa m½. This significant increase in the KIC of water saturated control or QAM-containing unfilled resin was the exception to the trend of addition of QAMs to unfilled resin causing decreases in mechanical properties. However, when 15% QAMs were added to control resin they all lowering KIC. That is, 5 or 10% QAMs in control resins increased KIC in wet resin specimens, while 15% QAMs lowered the wet KIC.
All of the five QAMs tested in this study were aliphatic monomethacrylate QAMs. That is they contained one acrylate (ATA) or methacrylate group at one end of the molecule, and a quaternary ammonium cation at the other end. Because they are monomethacrylates, they cannot crate cross-linking between growing polymers. The presence of monomethacrylate QAMs reduces the concentration of dimethacrylates in the test adhesives and lowered the modulus of elasticity of the control resin (Table 2). Addition of QAMs also lowered the toughness and UTS of the adhesive in proportion to their concentration. Diallyldimethyammonium chloride (DDAC) has two methacrylate groups, but they are on the same end of the QAM and would not contribute much to cross-linking. The solubility of DDAC was so low in the adhesive that only 5 wt% could be tested.
In all QAMs (ATA, MCMS, METMAC) where 5, 10 and 15 wt% concentrations could be tested, the dry fracture toughness values of QAM-containing BisGMA/TEGDMA adhesives were significantly (p<0.05) lower than the QAM-free control adhesive (Table 2) when 15 wt% QAM was added. However, when 5 wt% QAMs were added to dimethacrylates and tested under wet conditions, the fracture toughness values of controls and QAM-containing adhesives increased significantly (p<0.05) compared to their dry counterparts. High (15 wt%) concentrations of QAMs reduced wet fracture toughness values (Table 2).
The decrease in dry fracture toughness seen where QAMs were added to BisGMA/TEGDMA adhesives was not due to decreases in the degree of conversion. The DC of QAM-containing adhesives actually increased significantly (p<0.05) over controls as increasing concentrations of QAMs were added (Table 2). It is more likely that decreased in fracture toughness of QAM-containing adhesives may have been due to dilution of dimethacrylates by the monomethacrylate QAMs, leading to decreases in the cross-linked density of the resulting copolymers. The significant increase in fracture toughness seen after water immersion for three days may have been due to slight decreases in the elastic modulus of resins after water plasticization. This may have increased the ability of the water sorbed matrix to resist crack propogation. Increases in QAM concentrations to 15 wt% caused wet KIC values to fall below that of control (QAM-free) adhesives.
Most of the aliphatic, monomethacrylate QAMs that we tested had relatively low potencies. That is, in previously published work, it was found that 15–30% of these QAMs was necessary to inhibit the endogenous proteases of dentin [6], whereas 12-methacryloyloxydodecylpyridinium bromide (MDPB), a more potent QAM, was effective at 5 wt%. Clearly, the aliphatic QAMs tested in the current study lower the mechanical properties of dimethacrylate adhesives when used at anti-protease concentrations. Had fillers been added to these QAM-containing resins, the concentration of the QAMs would have fallen in proportion to the filler content that is typically 70–80 vol%. This would dilute the QAM concentrations below their effective concentrations.
In the current study, water sorption at 37°C was only allowed for 3 days. Our previous work shows that 3 days of water sorption produces 68% of the maximum water sorption that can occur in 60 days. [37–39]. Much longer water sorptions are required for resin composites [40,41]. When 5 mass% ATA or MCMS were added to the control resin, there was no change in the KIC values; wet or dry. However, when 5% MAPTAC or DDAC, or METMAC were added to the control resin, then lowered the KIC values significantly (p<0.05). These results require partial rejection of the first null hypothesis that addition of QAMs to control resins does not lower their mechanical properties. That is, the fracture toughness of the water-saturated control resin was significantly (p<0.05) higher than that of the dry resin. When 5 mass% QAMs were added to the control resin, all five test QAMs increased the KIC of the water-saturated dentin compared to their dry controls (Table 1). When 10% QAMs were added to the dimethacrylate control resin, there was no difference between dry vs. wet KIC except for 10% ATA which was significantly lower than the dry control. Addition of 15% QAM to the control resin produced significantly lower KIC values for water-saturated resins compared to dry controls when using MCMS, or METMAC. Using 15% ATA, there were no significant differences in KIC values for dry vs. wet resin. These results require rejection of the second null hypothesis that water sorption causes no change in the mechanical properties of QAM-containing dimethacrylates.
In general, addition of 15 wt% QAMs lowered the mechanical properties of the control resin below acceptable levels. We speculate that the mechanical properties fell because the 15 wt% QAMs (monomethacrylates), reduced the dimethacrylate BisGMA from 60 to 45 mass%. This would reduce the polymer cross-linking. Additionally, QAMs are hydrophilic and tend to increase water sorption [38]. Water sorption plasticizes the polymers, allowing more plastic deformation [9,10]. That is the presumed mechanism explaining why control resins had higher KIC values after water sorption than when tested dry. Low concentrations of QAMs (5 or 10%) produced even more water sorption. However, 15 wt% QAMs produced too much water sorption, allowing excessive plastic deformation of the polymers, lowering the modulus of elasticity so much that the yield stress of the resins fell allowing increased strain.
This does not mean that all quaternary ammonium methacrylates (QAMs) would lower the mechanical properties of dental polymers. Some potent QAMs such as 12-methacryloyloxydodecylpyridinium bromide (MDPB) are very antimicrobial at 5 mass %. Small linear QAMs like ATA and METMAC are not as antimicrobial or anti-MMP as QAMs with longer chain lengths such as MDPB or 3-(3,4-dimethyl-9-oxo-9H thioxanthen-2-yloxy)-2-hydroxytrimethyl ammonium chloride (OTX), a photoaccelerator that inhibits soluble and matrix-bound MMPs at 0.2 wt% [6]. Li et al. [42] reported that chain lengths shorter than 18 carbons reduced the antimicrobial activity of QAMs. Relatively short, linear QAMs as used in the current study, when incorporated into conventional dental adhesives do not provide the same cationic charge density as do polycationic polymers such as polyethylenimines (PEIs). The high cationic charge density of PEIs allows their use at low concentrations (ca. 1 mass %), that gives more antimicrobial activity than 10–15 mass % cationic monomers [43]. These polycationic PEIs can be made into nanoparticles that do not plasticize dental polymers or cause significant water sorption.
To create effective antimicrobial resin composites, the antimicrobial agent must be incorporated into the filler, since many posterior composites are composed of 75–80 mass % fillers. Song et al. [44] used 3-(trimethyloxylsilyl)-propyldimethyloctadecyl ammonium chloride to coat glass or silica filter particles. They reported 16 or 28 nm were more antimicrobial than 54 nm nanoparticles.
Figure 7.
Relationship between fracture toughness versus Young’s modulus in dry (◆) or wet (■) specimens. Note the insignificant relationship between these two variables in dry specimens (R2 = 0.232, p>0.02). In wet specimens, the relationship was positive and highly significant (R2 = 0.68, p<0.01). With the exception of 15% MCMS or 15% METMAC, water-saturated resins had higher KIC than dry resins.
Acknowledgments
The authors thank Professor Jack Ferracane for the loan of the single-edge notched-beam mold used in this study and ESSTEC for supplying the HEMA and dimethacrylates used in this work. We also thank Professor Jeff Standsbury for hydrogenating some of our QAM/dimethacrylate blends. This work was supported, in part, by USPHS NIH/NIDCR R01 DE015306 (P.I. DP). The authors are grateful to Mrs. Michelle Barnes for her secretarial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Imazato S, Kinomoto Y, Tarumi H, Torii M, Russell RR, McCabe JF. Incorporation of antibacterial monomer MDPB into dentin primer. J Dent Res. 1997;76:768–772. doi: 10.1177/00220345970760030901. [DOI] [PubMed] [Google Scholar]
- 2.Imazato S, Tay FR, Kaneshivo AV, Takahashi Y, Ebisu S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dent Mater. 2007;23:170–176. doi: 10.1016/j.dental.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Imazato S. Bio-active restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dent Mater J. 2009;28:11–19. doi: 10.4012/dmj.28.11. [DOI] [PubMed] [Google Scholar]
- 4.Li F, Chai ZG, Sun MN, Wang F, Ma S, Zhang L, Fang M, Chen JH. Anti-biofilm effect of dental adhesive with cationic monomer. J Dent Res. 2009;88:372–376. doi: 10.1177/0022034509334499. [DOI] [PubMed] [Google Scholar]
- 5.Li F, Weir MD, Xu HHK. Effects of quaternary ammonium chain length on antibacterial bonding agents. J Dent Res. 2013;92:932–938. doi: 10.1177/0022034513502053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, Breschi L, Nishitani Y, Tay FR, Pashley DH. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J Dent Res. 2011;90:535–540. doi: 10.1177/0022034510389472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tezvergil-Mutluay A, Mutluay MM, Gu L-S, Zhang K, Agee KA, Carvalho RM, Manso A, Carrilho M, Tay FR, Breschi L, Suh B-I, Pashley DH. The anti-MMP activity of benzalkonium chloride. J Dent. 2011;39:57–64. doi: 10.1016/j.jdent.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pashley DH, Tay FR, Imazato S. How to increase the durability of resin-dentin bonds. Comp Contin Edu Dent. 2011;32:60–64. [PubMed] [Google Scholar]
- 9.Paul SJ, Leach M, Rueggeberg FA, Pashley DH. Effect of water content on the physical properties of model dentin primer and bonding resin. J Dent. 1999;27:209–214. doi: 10.1016/s0300-5712(98)00042-6. [DOI] [PubMed] [Google Scholar]
- 10.Hosaka K, Tagami J, Nishitani Y, Yoshiyama M, Carrilho M, Tay FR, Agee KA, Pashley DH. Effect of wet versus dry testing on the mechanical properties of hydrophilic primer polymers. Eur J Oral Sci. 2007;115:1–7. doi: 10.1111/j.1600-0722.2007.00452.x. [DOI] [PubMed] [Google Scholar]
- 11.Hosaka K, Nakajima M, Takahashi M, Itoh S, Ikeda M, Tagami J, Pashley DH. Relationship between mechanical properties of one-step adhesives and water sorption. Dent Mater. 2010;26:360–367. doi: 10.1016/j.dental.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Ye Q, Park JG, Topp E, Wang Y, Misra A, Spencer P. In vitro performance of nanoheterogenous dentin adhesive. J Dent Res. 2008;87:829–833. doi: 10.1177/154405910808700911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo X, Wang Y, Spencer P, Xe Q, Yao X. Effect of water content and initiator composition on photopolymerization of a model BisGMA/HEMA resin. Dent Mater. 2008;24:824–831. doi: 10.1016/j.dental.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eick JD, Smith RE, Pinzino CS, Kotha SP, Kostoryz EL, Chappelow CC. Photopolymerization of developmental monomers for dental cationically initiated matrix resins. Dent Mater. 2005;21:384–380. doi: 10.1016/j.dental.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Faltermeier A, Rosentritt M, Müssig D. Acrylic removable appliances: comparative evaluation of different postpolymerization methods. Am J Orthod Dentofacial Orthop. 2007;131:301.e16–301e21. doi: 10.1016/j.ajodo.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Ferracane JL, Greener EH. The effect of resin formulations on the degree of conversion and mechanical properties of dental restorative resins. J Biomed Mater Res. 1986;20:121–131. doi: 10.1002/jbm.820200111. [DOI] [PubMed] [Google Scholar]
- 17.Ferracane JL, Greener EH. Elution of leachable components from composites. J Oral Rehab. 1994;21:41–452. doi: 10.1111/j.1365-2842.1994.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 18.Munksgaard EC, Peutzfeldt A, Asmussen E. Elution of TEGDMA and BisGMA from a resin and a resin composite cured with halogen or plasma light. Eur J Oral Sci. 2000;108:341–345. doi: 10.1034/j.1600-0722.2000.108004341.x. [DOI] [PubMed] [Google Scholar]
- 19.Jakubiak J, Allonas X, Fouassier JP, Sionkowska A, Andrzejewska E, Linden LÅ, Rabek JF. Camphorequinine-amines photoinitiating system for the initiation of free radical polymerization. Polymer. 2003;44:5219–5226. [Google Scholar]
- 20.Cadenaro M, Antoniolli F, Condan B, Agee K, Tay FR, DeStefano D, Dorigo E, Pashley DH, Breschi L. Influence of different initiators on the degree of conversion of experimental adhesive blends in relation to their hydrophilicity and solvent content. Dent Mater. 2010;26:288–294. doi: 10.1016/j.dental.2009.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nava D, Parada TR, González E, Boscán N, Cruz CDL. An infrared spectroscopic comparison of Cis and trans-CH=CH and vinyl CH-CH2 group frequencies of some hexens, heptences, and stereospecific and non-steriospecific polybutadiences. Spectrochim Acta Part A. 1996;52:1201–1210. [Google Scholar]
- 22.Ilie N, Hickel R, Valceanu AJ, Huth KC. Fracture toughness of dental restorative materials. Clin Oral Invest. 2011 doi: 10.1007/S00784-011-0525-Z. [DOI] [PubMed] [Google Scholar]
- 23.Ye G, Park J, Topp E, Spencer P. Effect of photoinitiators on the in vitro performance of a dentin adhesive exposed to simulated oral environment. Dent Mater. 2009;25:452–458. doi: 10.1016/j.dental.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheong C, King NM, Pashley DH, Ferrari M, Toledano M. Incompatibility of self-etch adhesives with chemical/dual-cured composites – two steps vs. one-step systems. Oper Dent. 2003;28:747–755. [PubMed] [Google Scholar]
- 25.Sanares AME, Itthagarun A, King NM, Tay FR, Pashley DH. Adverse surface interactions between one-bottle light-cured adhesives and chemical-cured composites. Dent Mater. 2001;17:542–556. doi: 10.1016/s0109-5641(01)00016-1. [DOI] [PubMed] [Google Scholar]
- 26.Ilie N, Hickel R. Can CQ be completely replaced by alternative initiators in dental resins? Dent Mater J. 2008;27:221–228. doi: 10.4012/dmj.27.221. [DOI] [PubMed] [Google Scholar]
- 27.Moszner N, Salz U, Zimmermann I. Chemical aspects of self-etching enamel-dentin adhesives: a systematic review. Dent Mater. 2005;21:895–910. doi: 10.1016/j.dental.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Neumann MG, Miranda WG, Jr, Schmitt CL, Rueggeberg FA, Correa IC. Molar extinction coefficients and the photon absorption efficiency of dental photoinitiators and light curing units. J Dent. 2005;33:525–532. doi: 10.1016/j.jdent.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Neumann MG, Schmitt CC, Ferreira GC, Correa IC. The initiating radical yields and the efficiency of polymerization for various dental photoinitiators excited by different light-curing lights. Dent Mater. 2006;22:576–584. doi: 10.1016/j.dental.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Leprince JG, Hadis M, Shortall AC, Ferracane JL, Devaux J, Leloup G, Palin WM. Photoinitiator type and applicability of exposure reciprocity law in filled and unfilled photoactive resins. Dent Mater. 2011;27:157–164. doi: 10.1016/j.dental.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Roberts JC, Powers JM, Craig RG. Fracture toughness of composite and unfilled restorative resins. J Dent Res. 1977;56:748–753. doi: 10.1177/00220345770560070801. [DOI] [PubMed] [Google Scholar]
- 32.Hill RG, Bates JF, Lewis TT, Rees N. Fracture toughness of acrylic denture base. Biomaterials. 1983;4:112–120. doi: 10.1016/0142-9612(83)90050-9. [DOI] [PubMed] [Google Scholar]
- 33.Annual book of ASTM standards ASTM E399–90. Standard test method for plane-strain fracture toughness of metallic materials 03(01:431–432.
- 34.Higgs WAJ, Lucksanasombool P, Higgs RJED, Swain MW. Comparison of the material properties of PMMA and glass-ionomer based cements for use in orthopaedic surgery. J Mater Sci: Mater in Med. 2001;12:453–460. doi: 10.1023/a:1011261322881. [DOI] [PubMed] [Google Scholar]
- 35.Balkenhol M, Köhler H, Orbach K, Wöstman B. Fracture toughness of cross-linked and non-cross-linked temporary crown and fixed partial denture materials. Dent Mater. 2009;25:917–928. doi: 10.1016/j.dental.2009.01.099. [DOI] [PubMed] [Google Scholar]
- 36.Puri C, Berzins DW, Dhuru VB, Raj PA, Rambhia SK, Dhir G, Dentino AR. Effect of phosphate group addition on the properties of denture base resins. J Prosthet Dent. 2008;100:302–308. doi: 10.1016/S0022-3913(08)60210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yiu CKY, King NM, Carrilho MRO, Sauro S, Rueggeberg FA, Prati C, Carvalho RM, Pashley DH, Tay FR. Effect of resin hydrophilicity and temperature on water sorption of dental adhesive resins. Biomater. 2005;26:6863–6872. doi: 10.1016/j.biomaterials.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 38.Malacarne J, Carvalho RM, De Goes MF, Svizero N, Pashley DH, Tay FR, Yiu CK, Carrilho MR. Water sorption/solubility of dental adhesive resins. Dent Mater. 2006;22:973–980. doi: 10.1016/j.dental.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 39.Donnelly A, Sword J, Nishitani Y, Yoshiyama M, Agee KA, Tay FR, Pashley DH. Water sorption and solubility of methacrylate-based root canal sealers. J Endod. 2007;33:990–994. doi: 10.1016/j.joen.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 40.Sideriduo I, Achilias DS, Spyroudi C, Karabela M. Water sorption characteristics of light-cured dental resins and composites based on Bis-EMA/PCDMA. Biomaterials. 2004;25:367–376. doi: 10.1016/s0142-9612(03)00529-5. [DOI] [PubMed] [Google Scholar]
- 41.Palin WM, Fleming GJP, Burke FJT, Marquis PM, Randall RC. The influence of short and medium-term water immersion on the hydrolytic stability of novel low-shrink dental composites. Dent Mater. 2005;21:852–863. doi: 10.1016/j.dental.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Li F, Weir MD, Xu HHK. Effects of quaternary ammonium chain length on antimicrobial bonding agents. J Dent Res. 2013;92:932–938. doi: 10.1177/0022034513502053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beyth N, Yudovin-Farber I, Blair R, Domb AJ, Weiss EI. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006;27:3995–4002. doi: 10.1016/j.biomaterials.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 2004;29:449–457. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 45.Song