Abstract
The goal of this study was to assess integrity of the cingulum bundle in patients diagnosed with first episode schizophrenia, chronic schizophrenia, and matched controls as well as to determine the relationship between diffusion measures of cingulum bundle integrity and severity of patients’ delusions of reference. Participants, who comprised 18 first episode patients, 20 chronic patients, and two groups of matched controls (20 subjects in each), underwent 3 Tesla MRI diffusion tensor imaging. Patients diagnosed with schizophrenia (chronic + first episode) showed decreased fractional anisotropy in the right cingulum bundle compared with controls. First episode patients exhibited higher trace bilaterally, compared with matched controls, and on the left compared with chronic patients. Axial diffusivity was increased in first episode patients, bilaterally, compared with matched controls and chronic patients. Radial diffusivity was also higher, bilaterally, in first episode patients compared with matched controls, and on the right compared with chronic patients. Trace diffusity and radial diffusivity in first episode patients were significantly correlated with increased severity of delusions of reference. Given that the abnormalities were present only in first episode patients and were not observed in chronic cases, it appears that they normalize over time. These abnormalities in first episode patients involved diffusivity measures in all directions (trace, radial and axial), suggesting a likely acute, partially reversible process in which there is an increase in brain water content, i.e., swelling, edema, or inflammation, that may reflect an early neuroinflammatory response in first episode patients.
Keywords: Schizophrenia, Diffusion Tensor Imaging, Delusions, Delusions, MRI
1. Introduction
Delusions of reference are a characteristic symptom of schizophrenia whereby patients misinterpret social and interpersonal stimuli as having exaggerated personal significance, such as believing a television newscaster is talking directly to or about them. It has been suggested that delusions of reference might be caused by insignificant events being given abnormal emotional valence (Fudge et al., 1998). The current study focuses on the cingulum bundle, a major white matter tract that connects the neocortex (e.g., cingulate gyrus) with the limbic system (e.g., hippocampal-amygdala complex). Given the anatomy of the cingulum bundle, and its role in regulating emotional and social cognition (Hadland et al., 2003; Brunet-Gouet and Decety, 2006), we examined the integrity of the cingulum bundle in patients diagnosed with schizophrenia and correlated these findings with delusions of reference. We hypothesized that while some degree of conduction delay is necessary for the formation of delusions of references, delays beyond a certain threshold might not give rise to psychotic symptoms. The cingulum bundle is an anatomical structure that is often portrayed as forming the bridge between emotion and cognition (Allman et al., 2001), and impairments of both of those processes are found in schizophrenia (Tamminga et al., 2000).
In studies with diffusion tensor imaging (DTI), a method that quantifies the tissue integrity of the physical connections between different regions of the brain by measuring the diffusion of water, patients with chronic schizophrenia have been shown to exhibit lower fractional anisotropy and increased mean diffusivity in the cingulum bundle, relative to matched healthy controls (see reviews in Kubicki et al., 2007; Shenton et al., 2010). This decrease in fractional anisotropy in patients with chronic schizophrenia has been correlated with intelligence (e.g., Nestor et al., 2008), passivity symptoms (e.g., Sim et al., 2009), prosaccade latency (Manoach et al., 2007), and auditory hallucinations (e.g., Hubl et al., 2004). In addition, mean diffusivity in the cingulum bundle in patients with chronic schizophrenia has also been correlated with performance time on the Stroop Test (Takei et al., 2009), suggesting that disruptions in white matter fiber connections may slow processing speed.
The cingulum bundle has been less well studied in patients with first episode or recent onset schizophrenia than in chronic patients. The studies that do exist have observed a mixture of decreased (Kumra et al., 2005; Hao et al., 2006; Tang et al., 2010; Voineskos et al., 2010; Lee et al., 2013), increased (Segal et al., 2010), both increased and decreased, (Hoptman et al., 2008), and no differences in fractional anisotropy (Peters et al., 2008), relative to matched healthy controls. For example, looking at clinical symptomatology, Tang et al. (Tang et al., 2010) showed that decreased fractional anisotropy was associated with increased positive symptoms as measured by the Positive and Negative Syndrome Scale (Kay et al., 1987). In first episode patients, Moriya et al. (Moriya et al., 2010) reported abnormally high mean diffusivity in the right anterior cingulate gyrus, and Lee et al. (Lee et al., 2013) reported widespread increased mean diffusivity, including in the cingulum.
While mean diffusivity (or trace = mean diffusivity × 3) and fractional anisotropy have been the standard measures for many years in DTI studies, and are believed to reflect overall white matter health, maturation and organization (Basser et al., 1994; Basser and Pierpaoli, 1996). Two additional measures, axial and radial diffusivity, have recently become available and are useful in furthering our understanding of the underlying physiology. Axial diffusivity, which is a measure of the degree of diffusion along the primary diffusion direction of the fiber, has been shown to reflect axon integrity, whereas the orthogonal measure, radial diffusivity, has been shown to reflect myelin integrity (Song et al., 2002; Song et al., 2003; Song et al., 2005). The present study compared the cingulum bundle in patients with schizophrenia at first episode with patients with chronic schizophrenia, and each of these patient groups’ matched controls, using fractional anisotropy, trace diffusity, axial diffusivity, and radial diffusivity. Given the possible role of the cingulum bundle in the formation of delusions of reference, we hypothesized an association between these four diffusion measures and the severity of patients’ delusions of reference.
2. Methods
2.1. Subjects
This study included 18 patients diagnosed with first episode schizophrenia, 20 patients diagnosed with chronic schizophrenia, and 20 controls for each patient group, for a total of 78 subjects (see Table 1). First episode patients were recruited as part of the larger Boston Center for Intervention Development and Applied Research (CIDAR) study, and they met criteria for a DSM-IV-TR (2000) diagnosis of schizophrenia, schizoaffective disorder, or schizophreniform disorder. Only patients diagnosed with schizophrenia were included in this study. They ranged in age from 15 to 31 years, and none had longer than 1 year of continuous antipsychotic treatment (see Table 1). Chronic patients were defined as meeting DSM-IV-TR criteria for a diagnosis for schizophrenia or schizoaffective disorder and ranged in age from 23 to 53 years, with a minimum of 5 years post-onset of illness (see Table 1). Patients were excluded if they had a history of electroconvulsive therapy within the past 5 years. Diagnoses were based on a diagnostic interview using the Structured Clinical Interview for the DSM-IV-TR (SCID), with the Research Version used for ages >18, and the Childhood Version for subjects 13–17 years of age (First et al., 2002b). The severity of delusions of reference was assessed in the first episode sample with the Scale for the Assessment of Positive Symptoms (item 14), which was administered on average 18.6 days from the magnetic resonance imaging (MRI) scan (Andreasen, 1984).
Table 1.
SD = Standard Deviation
| Group | A | Gender | Handedness | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | SD | Femal | Male | Left | Right | |||
| First Episode Schizophrenia Patients |
15–31 | 21.56 | 4.25 | 4 | 1 4 |
3 | 1 5 |
0 | |
| Controls Matched to First Episode |
17–32 | 23.47 | 3.86 | 5 | 1 5 |
0 | 1 7 |
3 | |
| Chronic Schizophrenia Patients |
23–53 | 41.90 | 9.29 | 1 | 1 9 |
0 | 2 0 |
0 | |
| Controls Matched to Chronic |
34–55 | 43.90 | 6.34 | 1 | 1 9 |
0 | 2 0 |
0 | |
Control subjects were screened for the presence of an Axis I disorder using the Structured Clinical Interview for DSM-IV, Non-patient Edition (First et al., 2002a), and were excluded if (1) they currently met the criteria for any psychosis, major depressive disorder, dysthymic disorder, bipolar disorder, obsessive-compulsive disorder, posttraumatic stress disorder, dissociative disorders, anorexia nervosa, bulimia nervosa, or developmental disorders; (2) they had a history of any psychosis, major depression (recurrent), bipolar disorder, obsessive-compulsive disorder, posttraumatic stress disorder, developmental disorders, or psychiatric hospitalization; (3) they had current or past use of antipsychotics for any psychiatric condition (other past psychotropic medication use acceptable, but patients must have been off medicine for at least 6 months before participating in the study, except for prn medications like sleeping medications or anxiolytic agents, like beta-blockers for performance anxiety, tremors, etc.); (4) they had any history of electro-convulsive therapy; (5) there was evidence of any prodromal symptoms, or schizotypal or other Cluster A personality disorders; or (6) they reported having a first degree relative with psychosis.
For all subjects exclusion criteria included sensory-motor handicaps, neurological disorders, medical illnesses that significantly affect neurological functioning, diagnosis of mental retardation, education of less than 9th grade (or less than 5th grade for subjects under 18), non-fluency in English (exposure to English by age 6), substance abuse in the past month as defined by the DSM-IV-TR, substance dependence (excluding nicotine) in the past 3 months as defined by the DSM-IV-TR, and current suicidality. For their safety during the MRI scanning, all subjects were screened for foreign metal in their body, pacemakers, pregnancy, claustrophobia, or any other circumstance that may pose a risk. All study participants (or legal guardians for those under 18) gave written informed consent before study participation. The study was approved by the local IRB committees at Harvard Medical School, Beth Israel Deaconess Medical Center, Massachusetts General Hospital, Brigham and Women’s Hospital and at the Veterans Affairs Boston Healthcare System, Brockton campus.
2.2. DTI scanning and processing
Subjects received diffusion imaging on a General Electric Echospeed system, using an echo planar imaging DTI sequence double echo option to reduce eddy-current related distortions (Alexander et al., 1997; Heid, 2000) at a resolution of 1.7 mm × 1.7 mm × 1.7 mm (51 diffusion directions at b=900, 8 baseline scans at b=0, 17000 ms repetition time, 78 ms echo time, 24 cm field of view, 144 × 144 matrix) at Brigham and Women’s Hospital, Boston.
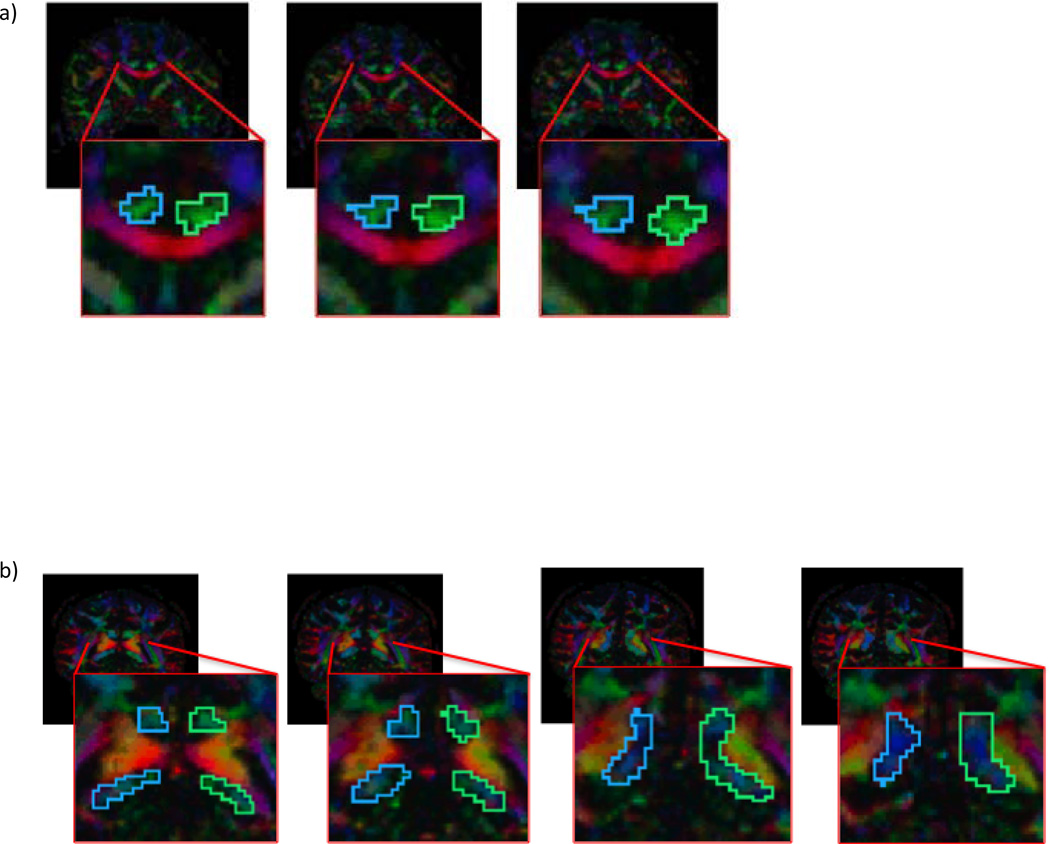
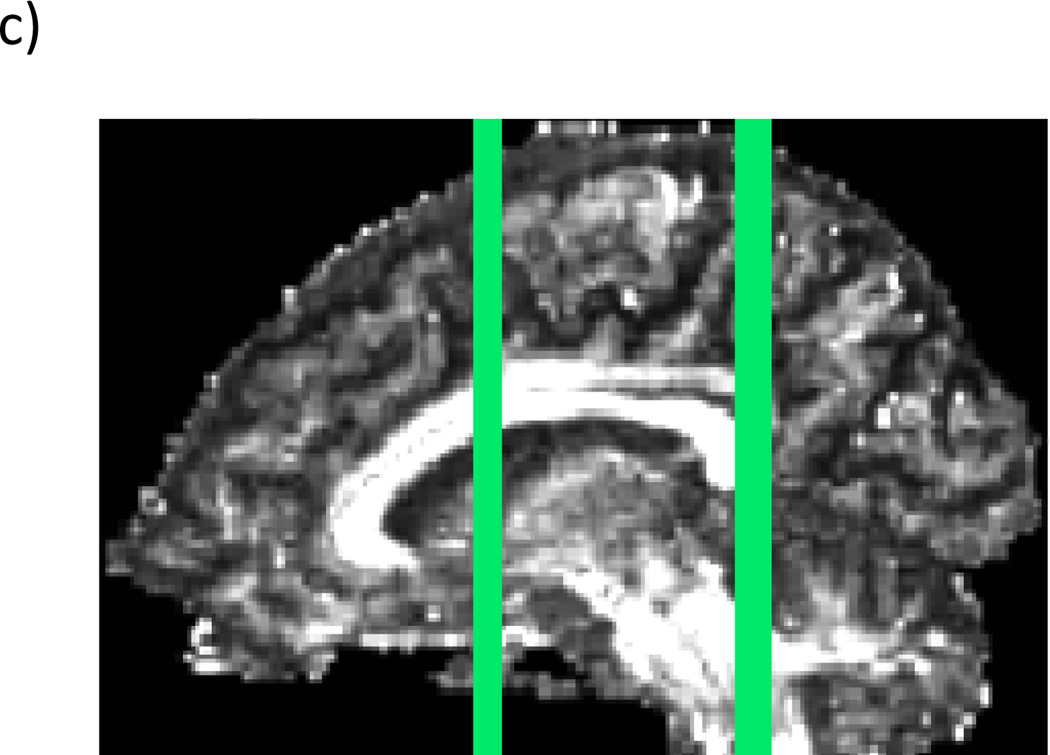
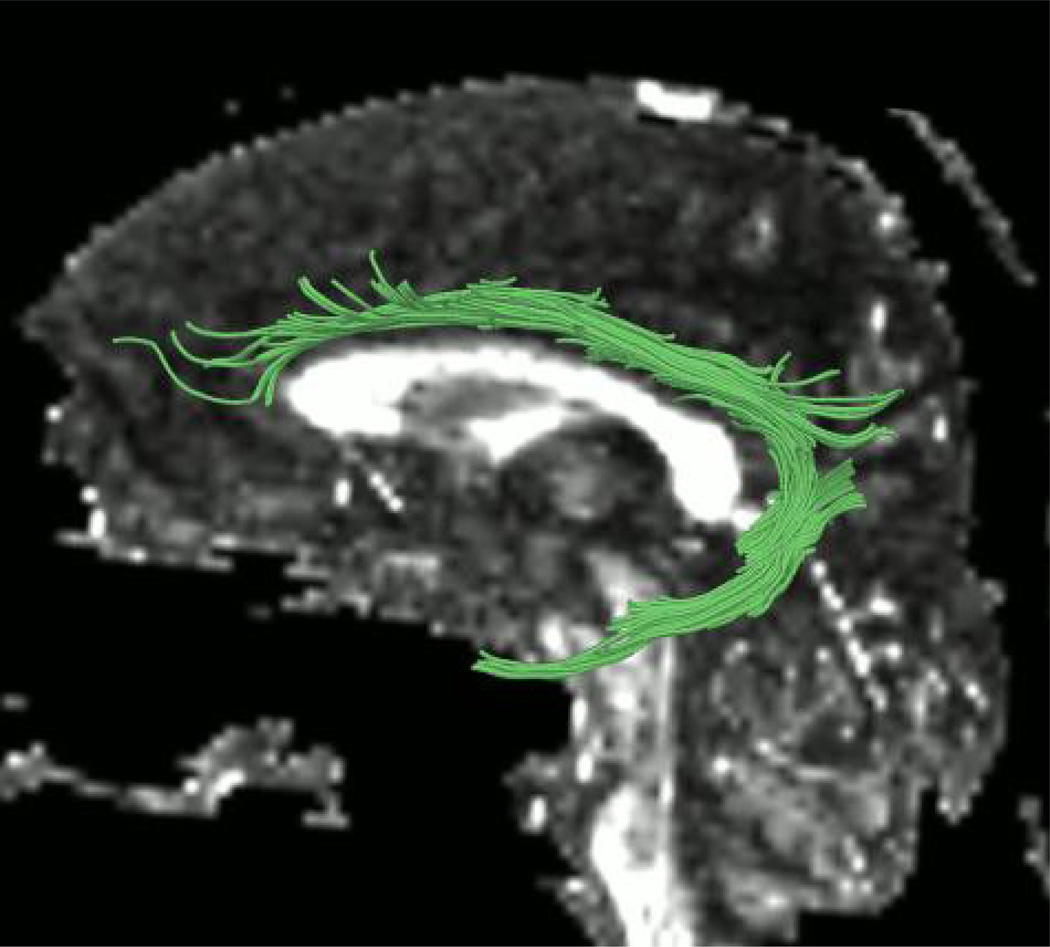
Manual regions of interest (ROIs) were drawn for every case. The cingulum was traced with two distinct ROIs. The first one was drawn on three sequential coronal slices where the anterior commissure is most visible, and the second one was drawn on two consecutive coronal slices that were anterior and posterior to the splenium of the corpus callosum (see Fig. 1). The placement of these regions was guided by two atlases (Mori et al., 2005; Catani and Thiebaut de Schotten, 2008). Streamline tractography for the cingulum was initiated (seeded) in both ROIs, and then filtered through a midline exclusion region (see Fig. 2). An eigenvector-tracking algorithm based on the fourth order Runge-Kutta method for tracking axonal fibers was used in this approach (Basser et al., 2000). A regularization scheme was used, where a small bias toward the previous tracking direction to the current tensor is added. The tractography stopping criteria were Westin’s measures (Westin at al., 2002) below 0.15 and rapid change of direction angle above 45 degrees. Westin’s linear measure was used to terminate tractography, in order to avoid the confound of using the same measure to construct fiber tracts and to quantify their integrity. After fiber tracking, mean fractional anisotropy, trace, axial diffusivity, and radial diffusivity were calculated for all voxels through which any fiber tracts passed. Trace and fractional anisotropy are independent of each other, with trace measuring the size of the diffusion tensor and fractional anisotropy measuring the shape of the diffusion tensor. Axial and radial diffusivity, which are substrates of trace, have been shown to be differentially sensitive to certain pathologies; that is, axial diffusivity has been shown to be sensitive to axonal pathology while radial diffusivity is sensitive to myelin pathology (Song et al., 2002; Song et al., 2003; Song et al., 2005; Hanson and Gottesman, 2005). For the ROIs, we used the streamline tractography method that is one of the modules in the 3D Slicer software (http://www.slicer.org) and has been described in detail in previous publications (Rosenberger et al., 2008; Whitford et al., 2011).
Figure 1.
Regions of interest o n color by orientation maps, a) anterior regions of interest, b) posterior regions of interest in a representative subject. c) Location of slices in a&b on a sagittal fractional anisotropy image in a representative subject.
Figure 2.
Left cingulum bundle resulting from region of interest based tractography.
2.3. Statistics
All statistical analyses were performed with SPSS Release 18 (IBM Corporation). For each diffusion measure (fractional anisotropy, trace, axial diffusivity, and radial diffusivity), a mixed design analysis of variance (ANOVA) was performed with hemisphere as the within-subject variable and age group (Young [first episode patients and their matched controls]; Older [chronic patients and their matched controls]) and diagnosis as the between-subject variables. When interactions were found in the ANOVA involving age group or diagnosis, after Bonferroni correction was applied, four sets of protected Student’s t-tests were performed to examine the age effect on both healthy control (older vs. young controls) and patient groups (first episode vs. chronic) separately and for each patient and their respective normal control group (first episode patients vs. their matched controls and chronic patients vs. their matched controls) for each hemisphere. To explore the effect of gender, all ANOVAs were repeated with only the male subjects, as the number of female subjects was insufficient to compare as a separate group. For the correlational analysis, Spearman’s rho was used to quantify the linear relationship between the diffusion measures and patients’ delusions of reference scores given the ordinal nature of the Scale for the Assessment of Positive Symptoms subscales. Spearman’s rho was also used to explore if there was a correlation between current medication dosage, in chlorpromazine equivalents, with any of our diffusion measures.
3. Results
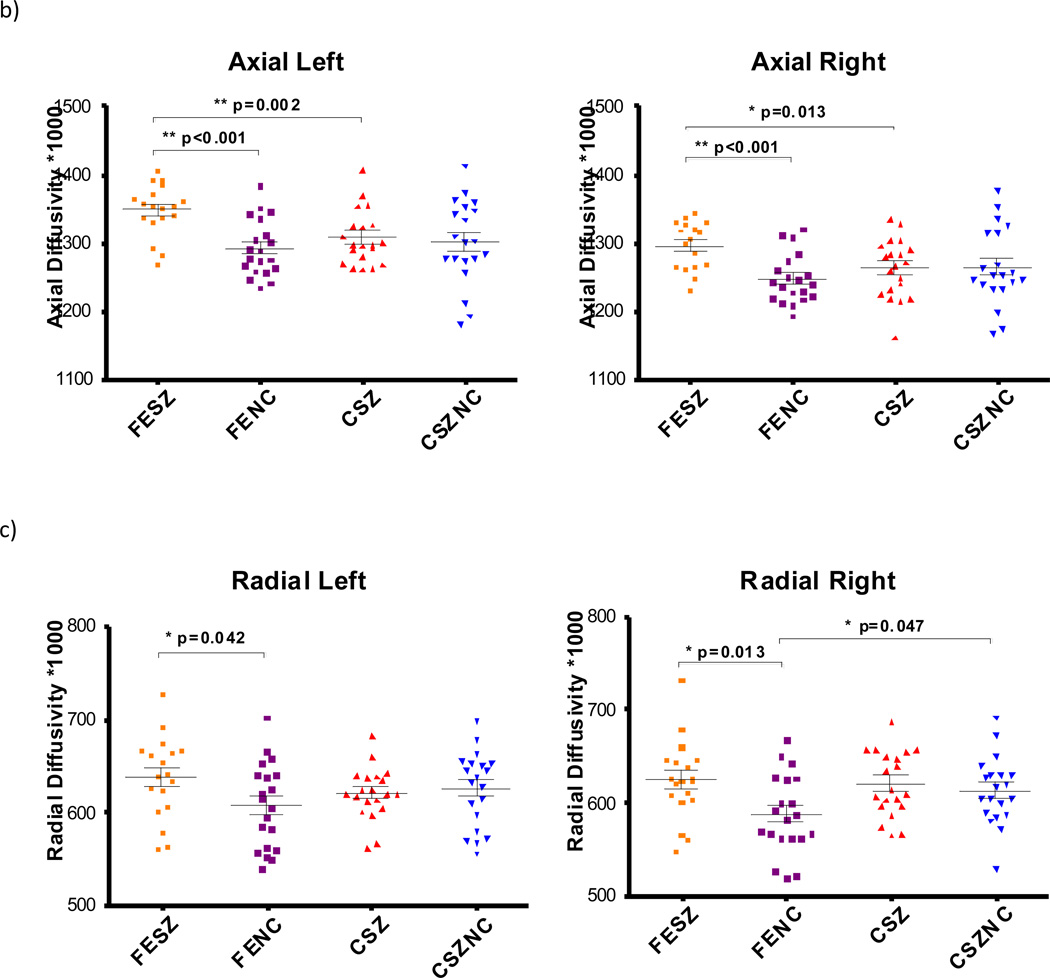
We measured fractional anisotropy, trace diffusivity, axial diffusivity, and radial diffusivity in all subjects using the statistical methods described above (see Table 2 for the ANOVA results and Fig. 3 for the t-test results). We confirmed that the data followed a normal distribution using the Kolmogorov-Smirnov test. Only significant results are discussed below. In this study. we observed increases in trace, axial, and radial diffusivity in patients with first episode schizophrenia, relative to both age-matched control and patients with chronic schizophrenia (Fig. 3). For fractional anisotropy, a main effect of hemisphere was observed, with the left cingulum showing higher fractional anisotropy values than the right. A significant effect for hemisphere by diagnosis was also observed, with all schizophrenia patients showing decreased fractional anisotropy in the right compared with the left, while all controls showed approximately the same fractional anisotropy in both hemispheres. Hemispheres differences were thus due to the patient samples. On follow-up t-tests, where first episode patients and chronic patients were considered separately, no significant differences in fractional anisotropy were found between groups, although there was a trend for increased fractional anisotropy in the first episode patients relative to chronic patients in the right hemisphere, which was absent in the left hemisphere.
Table 2.
Analyses of variance for dependent measures of fractional anisotropy, trace, axial diffusivity and radial diffusivity with all subjects. Degrees of freedom for all Analyses of variance = 1,74. Statistically significant findings are in bold.
| Measure | Comparison | F | p |
|---|---|---|---|
| Fractional Anisotropy | Hemisphere | 7.307 | 0.009 |
| Diagnosis | 0.605 | 0.377 | |
| Age | 3.292 | 0.074 | |
| Age * Diagnosis | 0.605 | 0.439 | |
| Hemisphere * Diagnosis | 6.021 | 0.016 | |
| Hemisphere * Age | 1.546 | 0.218 | |
| Hemisphere * Diagnosis * Age | 0.143 | 0.707 | |
| Trace | Hemisphere | 64.801 | <0.001 |
| Diagnosis | 6.964 | 0.010 | |
| Age | 0.000 | 0.991 | |
| Age * Diagnosis | 5.806 | 0.018 | |
| Hemisphere * Diagnosis | 0.318 | 0.575 | |
| Hemisphere * Age | 3.012 | 0.087 | |
| Hemisphere * Diagnosis * Age | 0.133 | 0.716 | |
| Axial Diffusivity | Hemisphere | 123.363 | <0.001 |
| Diagnosis | 8.524 | 0.005 | |
| Age | 1.560 | 0.216 | |
| Age * Diagnosis | 7.129 | 0.009 | |
| Hemisphere * Diagnosis | 0.976 | 0.326 | |
| Hemisphere * Age | 1.115 | 0.294 | |
| Hemisphere * Diagnosis * Age | 0.003 | 0.959 | |
| Radial Diffusivity | Hemisphere | 16.030 | <0.001 |
| Diagnosis | 4.253 | 0.043 | |
| Age | 0.493 | 0.485 | |
| Age * Diagnosis | 3.509 | 0.065 | |
| Hemisphere * Diagnosis | 2.393 | 0.126 | |
| Hemisphere * Age | 3.381 | 0.070 | |
| Hemisphere * Diagnosis * Age | 0.274 | 0.602 |
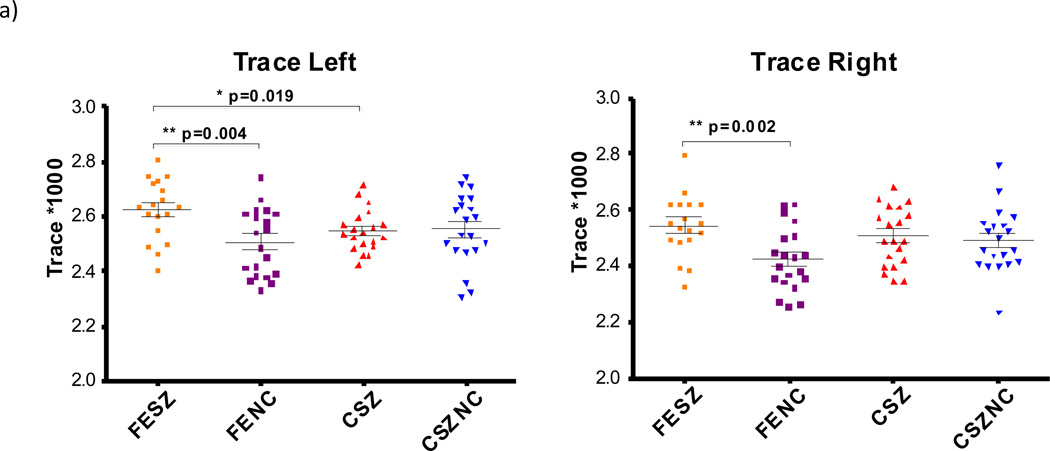
Figure 3.
Trace, Radial Diffusivity and Axial Diffusivity for all subject groups. Significant t-test results are marked with * p<0.05 and ** p<0.005. FESZ = first episode schizophrenia patients, FENC = controls matched to first episode patients, CSZ = chronic schizophrenia patients, CSZNC = controls matched to chronic patients.
For trace, a significant main effect of hemisphere was observed, with trace being higher in the left hemisphere than in the right in both patients and controls. There was a main effect of diagnosis, with patients exhibiting higher trace than controls. A diagnosis by age interaction was also observed where first episode patients showed higher trace than chronic patients. There was no such significant difference between the control groups. On the follow-up t-tests, first episode patients exhibited higher trace in comparison to their matched controls in both hemispheres and on the left in first episode patients compared with chronic patients (see Fig. 3a), suggesting that, overall, first episode patients had abnormally high levels of water diffusion in the cingulum.
For axial diffusivity there was a main effect of hemisphere with higher axial diffusivity in the left than on the right in all subjects. There was also a main effect of diagnosis, with patients exhibiting higher axial diffusivity than controls. A diagnosis by age effect was also observed, with axial diffusivity in first episode patients being higher than in chronic patients but with no differences being found between the two control groups. The additional follow-up t-tests indicated that axial diffusivity was increased in first episode patients bilaterally compared with their matched controls, and in first episode patients compared with chronic patients (see Fig. 3b).
For radial diffusivity, there was a main effect of hemisphere, with the left cingulum showing higher radial diffusivity than the right in all subjects. There was also a main effect of diagnosis, with patients exhibiting higher radial diffusivity. Follow-up t-tests revealed that radial diffusivity was higher in first episode patients compared with their matched controls, bilaterally (see Fig. 3c). Radial diffusivity was also higher in the right hemisphere in the group of older controls matched to the group of younger controls.
When females were excluded from the analyses, the ANOVA results described above remained the same, with the following three exceptions where statistical significance was lost: hemisphere by diagnosis for fractional anisotropy (F(1,63)=2.740, p=0.103), diagnosis for trace (F(1,63)=3.255, p=0.076), and diagnosis for radial diffusivity (F(1,63)=1.643, p=0.205). The effect sizes for all three of these effects did decrease slightly when removing females and leaving only males (fractional anisotropy, Hemisphere × Diagnosis: all subjects, r=0.274; males only, r=0.204; Trace Diagnosis: all subjects, r=0.293; males only, r=0.222; radial diffusivity, Diagnosis: all subjects, r=0.233; males only, r=0.159). See Table 2 for effects with all subjects included.
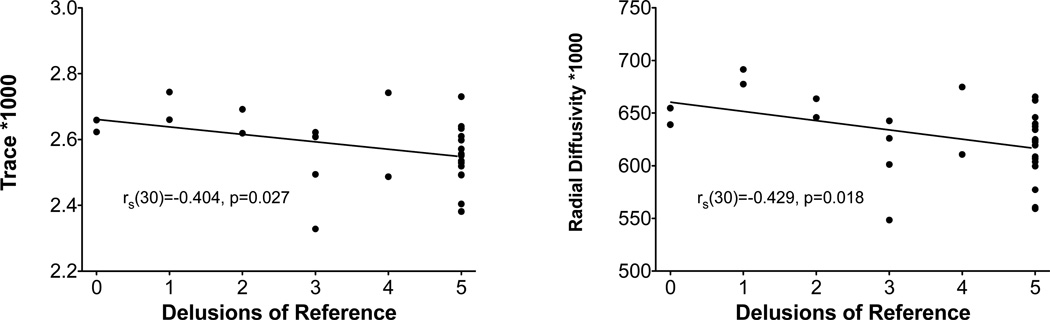
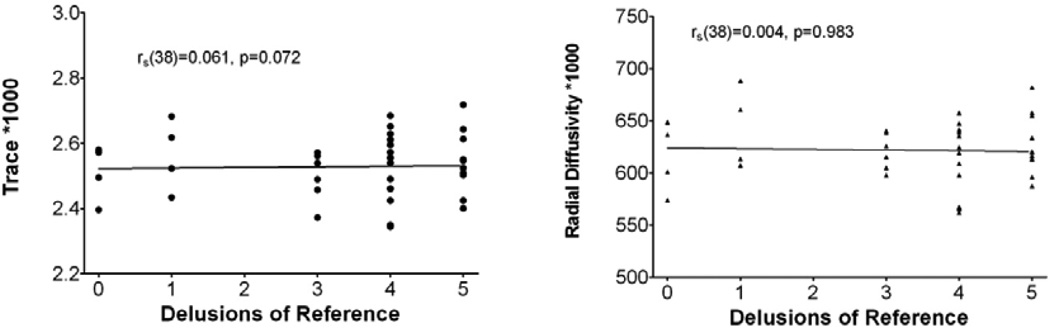
Trace and radial diffusivity were found to be correlated with delusions of reference in first episode patients (see Fig. 4; trace rs (30)=−0.404, p=0.027; radial rs (30)=−0.429, p=0.018), but not in chronic patients (trace rs (38)=0.060, p=0.720; radial rs(38)=0.004, p=0.983). No significant correlations were found with fractional anisotropy or axial diffusivity, and delusions of reference. No significant correlations were found with medication dosage and fractional anisotropy, trace, radial diffusivity or axial diffusivity in either the right or left cingulum in patients with schizophrenia (p>0.05 for all analyses). Further, no significant correlations were found between medication dosage and fractional anisotropy, trace, radial diffusivity or axial diffusivity in either the right or left cingulum in patients with schizophrenia (p>0.05 for all analyses). No significant associations were found between handedness and diffusion indices in the cingulum. The average chlorpromazine-equivalent dose of medication for the chronic patient group was 418.01 mg (SD=331.42 mg), and the average duration of medication was 20.25 years (SD=8.78 years).
Figure 4.
Correlation of trace and radial diffusivity with maximum delusions of reference score in first episode patients.
4. Discussion
Findings from this study show increases in trace, axial, and radial diffusivity in the cingulum bundle in patients with a first episode of schizophrenia compared with their age-matched healthy controls, but not in patients with chronic schizophrenia relative to their matched healthy controls. One explanation for these findings is that in first episode schizophrenia there are abnormalities in myelin development and this abnormal myelin cannot sustain the health of the underlying axons, resulting in partial degeneration of these axons (Song et al., 2002), which, in turn, results in abnormalities in both axon integrity and myelination. This process, however, would be characterized by an increase in radial diffusion as well as an increase in trace (Song et al., 2003; Song et al., 2005; Hanson and Gottesman, 2005; Pasternak et al., 2012), but would also most likely result in decreased axial diffusivity, not the increase that was observed in this study. As the increases were in trace, axial and radial diffusivity concurrently, and since they seemed to partially resolve over time, given that they were not observed in chronic cases, we suggest that the observed DTI abnormalities in the first episode participants may reflect an acute increase in water diffusion in the cingulum bundle due to an inflammatory process. This possibility, which has been proposed previously by Pasternak et al. in our group (Pasternak et al., 2012), is consistent with previous positron emission tomography studies that have found increases in activated microglia in patients with recent onset schizophrenia (Hanson and Gottesman, 2005; Muller and Schwarz, 2006; Potvin et al., 2008; van Berckel et al., 2008; Doorduin et al., 2009). The reversibility of diffusion measures has also been observed in the vasogenic edema phase of stroke as well as during the acute phase of multiple sclerosis, suggesting its sensitivity to edema/inflammation (Balashov et al., 2011). Pasternak et al. (Pasternak et al., 2012) suggest that the initial phase of inflammatory response that is observed in the early stages of schizophrenia resolves and is followed by myelin degeneration in later stages of the disease. Such dynamics could account for the findings in our study, where both chronic and first episode patients demonstrate white matter pathology (fractional anisotropy decrease), but only first episode subjects demonstrate trace increase. The first episode patients in this sample are being followed longitudinally, and follow-up studies are planned to investigate further whether there are progressive changes in these diffusion measures and to determine over what time course any changes occur.
As hypothesized, given the known role of the cingulum bundle in connecting areas involved in emotional and social cognition such as the anterior cingulate and prefrontal cortices, we found significant correlations between diffusion measures in the cingulum bundle and the severity of delusions of reference in first episode patients (Hadland et al., 2003; Brunet-Gouet and Decety, 2006). The first episode patients’ delusions of reference scores were negatively correlated with trace and radial diffusivity in the cingulum bundle, such that first episode patients with the most severe delusions had the least abnormal white matter in the cingulum bundle. While this finding may seem counterintuitive, it is consistent with several other studies showing that patients with increased levels of positive symptoms show correlations with diffusion measures that are relatively close to those seen in healthy control, while subjects with grossly abnormal diffusion measures show relatively low levels of positive symptoms. For example, Szeszko et al. (Szeszko et al., 2008) observed a positive correlation between overall positive symptomatology and fractional anisotropy in the inferior fronto-occipital fasciculus. Similarly, Shergill et al. (Shergill et al., 2007) reported a relationship between greater hallucination severity and elevated fractional anisotropy in the superior longitudinal fasciculus, the tapetum of the corpus callosum, and the cingulate bundle. Hubl et al. (Hubl et al., 2004) also reported elevated fractional anisotropy in patients with greater levels of auditory hallucinations in comparison to patients with lower levels of auditory hallucinations in the arcuate fasciculus and the anterior corpus callosum. The relationship between increased levels of fractional anisotropy and higher levels of symptoms was also demonstrated by Whitford et al. (Whitford et al., 2010), who observed that the severity of patients' reality distortion was positively correlated with fractional anisotropy in the anterior corpus callosum. In light of the established association between axonal transmission velocity and white matter integrity, severely subnormal fractional anisotropy levels may be associated with such effects as severe neural desynchronization that may, in fact, preclude the formation of stable, coherent, yet aberrant cognitive associations and thus the development of highly systematized hallucinations and delusions. This proposal, while speculative, is consistent with the results of the present study.
In this case, a small delay between the cingulum cortex, involved in error monitoring, and the limbic system, involved in emotional valence, may lead to inappropriate environmental events being given emotional salience and hence delusions of reference, but larger delays in conductance may lead to complete disassociation between the error signals and the environmental stimuli, resulting in negative symptomatology (Hubl et al., 2004; Shergill et al., 2007; Szeszko et al., 2008; Whitford et al., 2010). To the extent that neural conduction velocities can be estimated on the basis of diffusion indices such as trace and radial diffusivity (Whitford et al., 2011), these results suggest that while some degree of conduction delay might be necessary for the formation of delusions of reference; delays beyond a certain threshold may actually preclude the formation of highly systematized delusions of reference, possibly, as suggested previously, because they cannot be incorporated into a coherent phenomenological framework (Kremen et al., 1994; Whitford et al., 2010).
In this study we reported decreased fractional anisotropy in the right cingulum bundle in all patients with schizophrenia compared with the left cingulum bundle. Healthy controls showed no differences in fractional anisotropy in either hemisphere. The absence of anisotropic asymmetry differences in the cingulum bundle in healthy controls in our study differs from the findings in other studies. For example, Park et al. (Park et al., 2013) reported that normal controls showed leftward asymmetry of the paracingulate sulcus, while patient groups did not show this asymmetry. Additionally, Takao et al. (Takao et al., 2013) reported leftward asymmetry of fractional anisotropy in the cingulum in healthy controls. The inconsistent findings reported suggest that multiple confounds, such as sex, handedness, and the use of different methods, may account for such inconsistencies in the findings (Yucel et al., 2001; Fornito et al., 2008), and thus further investigation is needed.
There are several limitations worth noting in the present study. There were relatively few female participants. Future work will be needed to replicate the results and also to explore further the effect of gender. Cingulum bundle trace and radial diffusivity correlated significantly with the severity of delusions of reference in first episode patients. However, these correlations should be replicated in a larger sample. Additionally, all of the patients with chronic schizophrenia had a long-term history of receiving antipsychotic medications, which makes it impossible to determine which of the changes observed in this group are the result of disease and which are the result of long-term medication. This issue continues to be a challenge in the field of schizophrenia research, due to the difficulty of finding patients with chronic schizophrenia who are medication naive, and it is one of the reasons why research, including our own, has shifted to a focus on first episode patients who do not have a chronic history of antipsychotic medication usage.
The cross-sectional design is also a limitation, as it is not known whether the first episode patients in this sample will progress in such manner as to be comparable to the patients with chronic schizophrenia. We will need a prospective cohort design to follow up the patients with first episode schizophrenia until they reach a stage of chronicity, to determine whether there are any white matter changes. As mentioned previously, the first episode patients in this study are currently being followed longitudinally, and this question can then be more readily addressed. Finally, while we did not see statistically significant differences in fractional anisotropy between the chronic patients and their matched controls, such differences have been reported in previous studies (Kubicki et al., 2007).
Since we used much higher image resolution than in previous reports, we believe that by limiting partial volume effects we increased power and accuracy of DTI measures. It is still, however, possible that our sample might be too small to detect differences in the chronic patient group (see reviews in Kubicki et al., 2007; Shenton et al., 2010). In this case, though the inflammatory model is most consistent with our data, the hypothesis of demyelination and axonal degeneration should not be discarded as one of the explanations for our results.
In summary, this study observed increases in trace, axial, and radial diffusivity in patients with first episode schizophrenia, relative to both age-matched controls and patients with chronic schizophrenia. These findings suggest the presence of a pathophysiological abnormality in the cingulum bundle in first episode patients such as an inflammatory process that is not present in chronic patients. Additionally, a correlation was observed between trace and radial diffusivity in the cingulum bundle and the severity of delusions of reference in first episode patients. This suggests that the cingulum bundle may be involved in the etiology of delusions of reference, possibly due to its functional role in connecting the neocortex with the limbic system.
Future studies are needed to investigate further the role of the cingulum bundle in first episode patients by following them over time to chart changes in white matter pathology. Current studies are also underway to use more advanced tractography methods to dissect the cingulum bundle into its constituent sub-fascicles, which will enable a finer grained investigation into the relationship between white matter structure and function. A study of people at risk for developing schizophrenia is also needed and is underway. Investigating these at-risk individuals will be important to determine whether scalar measures of diffusion in the cingulum bundle might serve as imaging biomarkers that predict conversion to a first episode of psychosis, thereby making it possible to identify those most vulnerable to schizophrenia very early, in order to implement possible treatment interventions.
Figure 5.
Correlation of trace and radial diffusivity with maximum delusions of reference score in patients with chronic schizophrenia.
-
-
The goal of this study was to assess the associations between the change of diffusivity in the cingulum bundle and delusions of reference in patients with first episode schizophrenia and chronic schizophrenia using diffusion tensor imaging.
-
-
We observed increase in trace, axial, and radial diffusivity in patients with first episode schizophrenia.
-
-
Additionally a correlation was observed between trace and radial diffusivity in the cingulum bundle and the severity of delusions of reference in first episode patients.
Acknowledgements
The authors thank Catherine Peckinpaugh Vrtis, MFA, for her assistance in editing this manuscript.
Funding source
This work was also supported by NIH P50MH080272 (JF, JSS, MAN, PEP, DPT, TS, RIM, LJS, JMG, MK), R01MH050740 (JF, JSS, PEP, DPT, TS, MK), T32MH016259 (JSS, WRM), National Health and Medical Research Council of Australia 520627 (TJW*), NARSAD Brain and Behavior Research Fund 17537 (TJW, P.I.), VA Merit Awards (JF, PEP, DPT, TS, MK), and a VA Schizophrenia Center Grant (PEP, DPT, TS, MK, JF). This project was also supported by a Clinical Translational Science Award UL1RR025758 to Harvard University and Beth Israel Deaconess Medical Center from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander AL, Tsuruda JS, Parker DL. Elimination of eddy current artifacts in diffusion-weighted echo-planar images: the use of bipolar gradients. Magnetic Resonance in Medicine. 1997;38:1016–1021. doi: 10.1002/mrm.1910380623. [DOI] [PubMed] [Google Scholar]
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex: the evolution of an interface between emotion and cognition. Annals of the New York Academy of Sciences. 2001;935:107–117. [PubMed] [Google Scholar]
- American Psychiatric Association. DSM-IV. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: APA; 2000. text revision. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms. University of Iowa: Iowa City; 1984. [Google Scholar]
- Balashov KE, Aung LL, Dhib-Jalbut S, Keller IA. Acute multiple sclerosis lesion: conversion of restricted diffusion due to vasogenic edema. Journal of Neuroimaging. 2011;21:202–204. doi: 10.1111/j.1552-6569.2009.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysics Journal. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magnetic Resonance in Medicine. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Magnetic Resonance B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Brunet-Gouet E, Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Research: Neuroimaging. 2006;148:75–92. doi: 10.1016/j.pscychresns.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009;50:1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002a. [Google Scholar]
- First MB, Spritzer RL, Miriam G, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002b. [Google Scholar]
- Fornito A, Wood SJ, Whittle S, Fuller J, Adamson C, Saling MM, Velakoulis D, Pantelis C, Yücel M. Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: associations with cortical thickness, surface area, volume, and sulcal depth. Human Brain Mapping. 2008;29(2):222–236. doi: 10.1002/hbm.20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Powers JM, Haber SN, Caine ED. Considering the role of the amygdala in psychotic illness: a clinicopathological correlation. Journal of Neuropsychiatry and Clinical Neuroscience. 1998;10:383–394. doi: 10.1176/jnp.10.4.383. [DOI] [PubMed] [Google Scholar]
- Hadland KA, Rushworth MF, Gaffan D, Passingham RE. The effect of cingulate lesions on social behaviour and emotion. Neuropsychologia. 2003;41:919–931. doi: 10.1016/s0028-3932(02)00325-1. [DOI] [PubMed] [Google Scholar]
- Hanson DR, Gottesman II. Theories of schizophrenia: a genetic-inflammatory- vascular synthesis. BMC Medical Genetics. 2005;6:7. doi: 10.1186/1471-2350-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Liu Z, Jiang T, Gong G, Liu H, Tan L, Kuang F, Xu L, Yi Y, Zhang Z. White matter integrity of the whole brain is disrupted in first-episode schizophrenia. Neuro Report. 2006;17:23–26. doi: 10.1097/01.wnr.0000195664.15090.46. [DOI] [PubMed] [Google Scholar]
- Heid O. Eddy current-nulled diffusion weighting. Proceedings of the International Society of Magnetic Resonance in Medicine. 2000;8:799. [Google Scholar]
- Hoptman MJ, Nierenberg J, Bertisch HC, Catalano D, Ardekani BA, Branch CA, Delisi LE. A DTI study of white matter microstructure in individuals at high genetic risk for schizophrenia. Schizophrenia Research. 2008;106:115–124. doi: 10.1016/j.schres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, Maier SE, Schroth G, Lovblad K, Dierks T. Pathways that make voices: white matter changes in auditory hallucinations. Archives of General Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Seidman LJ, Goldstein JM, Faraone SV, Tsuang MT. Systematized delusions and neuropsychological function in paranoid and nonparanoid schizophrenia. Schizophrenia Research. 1994;12:223–236. doi: 10.1016/0920-9964(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. Psychiatry Research: Neuroimaging. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumra S, Ashtari M, Cervellione KL, Henderson I, Kester H, Roofeh D, Wu J, Clarke T, Thaden E, Kane JM, Rhinewine J, Lencz T, Diamond A, Ardekani BA, Szeszko PR. White matter abnormalities in early-onset schizophrenia: a voxel-based diffusion tensor imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:934–941. doi: 10.1097/01.chi.0000170553.15798.94. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kubicki M, Asami T, Seidman LJ, Goldstein JM, Mesholam-Gately RI, McCarley RW, Shenton ME. Extensive white matter abnormalities in patients with first-episode schizophrenia: a diffusion tensor imaging (DTI) study. Schizophrenia Research. 2013;143:231–238. doi: 10.1016/j.schres.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Ketwaroo GA, Polli FE, Thakkar KN, Barton JJ, Goff DC, Fischl B, Vangel M, Tuch DS. Reduced microstructural integrity of the white matter underlying anterior cingulate cortex is associated with increased saccadic latency in schizophrenia. Neuroimage. 2007;37:599–610. doi: 10.1016/j.neuroimage.2007.04.062. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM. MRI Atlas of Human White Matter. New York: Elsevier; 2005. [Google Scholar]
- Moriya J, Kakeda S, Abe O, Goto N, Yoshimura R, Hori H, Ohnari N, Sato T, Aoki S, Ohtomo K, Nakamura J, Korogi Y. Gray and white matter volumetric and diffusion tensor imaging (DTI) analyses in the early stage of first-episode schizophrenia. Schizophrenia Research. 2010;116:196–203. doi: 10.1016/j.schres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz M. Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotoxicity Research. 2006;10:131–148. doi: 10.1007/BF03033242. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Niznikiewicz M, Gurrera RJ, McCarley RW, Shenton ME. Neuropsychological disturbance in schizophrenia: a diffusion tensor imaging study. Neuropsychology. 2008;22:246–254. doi: 10.1037/0894-4105.22.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Hwang JY, Jung WH, Shin NY, Shim G, Jang JH, Kwon JS. Altered asymmetry of the anterior cingulate cortex in subjects at genetic high risk for psychosis. Schizophrenia Research. 2013;150:512–518. doi: 10.1016/j.schres.2013.08.027. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Westin CF, Bouix S, Seidman LJ, Goldstein JM, Woo TU, Petryshen TL, Mesholam-Gately RI, McCarley RW, Kikinis R, Shenton ME, Kubicki M. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. Journal of Neuroscience. 2012;32:17365–17372. doi: 10.1523/JNEUROSCI.2904-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, de Haan L, Dekker N, Blaas J, Becker HE, Dingemans PM, Akkerman EM, Majoie CB, van Amelsvoort T, den Heeten GJ, Linszen DH. White matter fiber tracking in first-episode schizophrenia, schizoaffective patients and subjects at ultra-high risk of psychosis. Neuropsychobiology. 2008;58:19–28. doi: 10.1159/000154476. [DOI] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biological Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Rosenberger G, Kubicki M, Nestor PG, Connor E, Bushell GB, Markant D, Niznikiewicz M, Westin CF, Kikinis R, A JS, McCarley RW, Shenton ME. Age-related deficits in fronto-temporal connections in schizophrenia: a diffusion tensor imaging study. Schizophrenia Research. 2008;102:181–188. doi: 10.1016/j.schres.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D, Haznedar MM, Hazlett EA, Entis JJ, Newmark RE, Torosjan Y, Schneiderman JS, Friedman J, Chu KW, Tang CY, Buchsbaum MS, Hof PR. Diffusion tensor anisotropy in the cingulate gyrus in schizophrenia. Neuroimage. 2010;50:357–365. doi: 10.1016/j.neuroimage.2009.12.071. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Whitford TJ, Kubicki M. Structural neuroimaging in schizophrenia: from methods to insights to treatments. Dialogues in Clinical Neuroscience. 2010;12:317–332. doi: 10.31887/DCNS.2010.12.3/mshenton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill SS, Kanaan RA, Chitnis XA, O'Daly O, Jones DK, Frangou S, Williams SC, Howard RJ, Barker GJ, Murray RM, McGuire P. A diffusion tensor imaging study of fasciculi in schizophrenia. American Journal of Psychiatry. 2007;164:467–473. doi: 10.1176/ajp.2007.164.3.467. [DOI] [PubMed] [Google Scholar]
- Sim K, Yang GL, Loh D, Poon LY, Sitoh YY, Verma S, Keefe R, Collinson S, Chong SA, Heckers S, Nowinski W, Pantelis C. White matter abnormalities and neurocognitive deficits associated with the passivity phenomenon in schizophrenia: a diffusion tensor imaging study. Psychiatry Research: Neuroimaging. 2009;172:121–127. doi: 10.1016/j.pscychresns.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson DG, Ashtari M, Vogel J, Betensky J, Sevy S, Ardekani BA, Lencz T, Malhotra AK, McCormack J, Miller R, Lim KO, Gunduz- Bruce H, Kane JM, Bilder RM. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33:976–984. doi: 10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- Takao H, Hayashi N, Ohtomo K. White matter microstructure asymmetry: effects of volume asymmetry on fractional anisotropy asymmetry. Neuroscience. 2013;231:1–12. doi: 10.1016/j.neuroscience.2012.11.038. [DOI] [PubMed] [Google Scholar]
- Takei K, Yamasue H, Abe O, Yamada H, Inoue H, Suga M, Muroi M, Sasaki H, Aoki S, Kasai K. Structural disruption of the dorsal cingulum bundle is associated with impaired Stroop performance in patients with schizophrenia. Schizophrenia Research. 2009;114:119–127. doi: 10.1016/j.schres.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Vogel M, Gao X, Lahti AC, Holcomb HH. The limbic cortex in schizophrenia: focus on the anterior cingulate. Brain Research Review. 2000;31:364–370. doi: 10.1016/s0165-0173(99)00053-3. [DOI] [PubMed] [Google Scholar]
- Tang J, Liao Y, Zhou B, Tan C, Liu T, Hao W, Hu D, Chen X. Abnormal anterior cingulum integrity in first episode, early-onset schizophrenia: a diffusion tensor imaging study. Brain Research. 2010;1343:199–205. doi: 10.1016/j.brainres.2010.04.083. [DOI] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD, Cahn W, Lammertsma AA, Kahn RS. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biological Psychiatry. 2008;64:820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Lobaugh NJ, Bouix S, Rajji TK, Miranda D, Kennedy JL, Mulsant BH, Pollock BG, Shenton ME. Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain. 2010;133:1494–1504. doi: 10.1093/brain/awq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin CF, Maier SE, Mamata H, Nabavi A, Jolesz FA, Kikinis R. Processing and visualization for diffusion tensor MRI. Medical Image Analysis. 2002;6:93–108. doi: 10.1016/s1361-8415(02)00053-1. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Ghorashi S, Schneiderman JS, Hawley KJ, McCarley RW, Shenton ME, Spencer KM. Predicting inter-hemispheric transfer time from the diffusion properties of the corpus callosum in healthy individuals and schizophrenia patients: a combined ERP and DTI study. Neuroimage. 2011;54(3):2318–2329. doi: 10.1016/j.neuroimage.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Schneiderman JS, O'Donnell LJ, King R, Alvarado JL, Khan U, Markant D, Nestor PG, Niznikiewicz M, McCarley RW, Westin CF, Shenton ME. Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biological Psychiatry. 2010;68:70–77. doi: 10.1016/j.biopsych.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Stuart GW, Maruff P, Velakoulis D, Crowe SF, Savage G, Pantelis C. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate paracingulate cortex in normal volunteers: an MRI morphometric study. Cerebral Cortex. 2001;11(1):17–25. doi: 10.1093/cercor/11.1.17. [DOI] [PubMed] [Google Scholar]