Abstract
This review covers the intrinsic organization and afferent and efferent connections of the midbrain dopaminergic complex, comprising the substantia nigra, ventral tegmental area and retrorubral field, which house, respectively, the A9, A10 and A8 groups of nigrostriatal, mesolimbic and mesocortical dopaminergic neurons. In addition, A10dc (dorsal, caudal) and A10rv (rostroventral) extensions into, respectively, the ventrolateral periaqueductal gray and supramammillary nucleus are discussed. Associated intrinsic and extrinsic connections of the midbrain dopaminergic complex that utilize gamma-aminobutyric acid (GABA), glutamate and neuropeptides and various co-expressed combinations of these compounds are considered in conjunction with the dopamine-containing systems. A framework is provided for understanding the organization of masssive afferent systems descending and ascending to the midbrain dopaminergic complex from the telencephalon and brainstem, respectively. Within the context of this framework, the basal ganglia direct and indirect output pathways are treated in some detail. Findings from rodent brain are briefly compared with those from primates, including human. Recent literature is emphasized, including traditional experimental neuroanatomical and modern gene transfer and optogenetic studies. An attempt was made to provide sufficient background and cite a representative sampling of earlier primary papers and reviews so that people new to the field may find this to be a relatively comprehensive treatment of the subject.
Keywords: substantia nigra, compacta, reticulata, ventral tegmental area, retrorubral field, periaqueductal gray
Introduction
The vertebrate mesencephalic ventral tegmentum houses a collection of dopamine (DA)-expressing neurons that influences neural systems subserving important adaptive functions such as arousal and locomotion (Kelly et al., 1975), control of intended movements (Mink, 1996), reinforcement (Yokel and Wise, 1975), learning (Schultz et al., 1997; Wise, 2004; Matsumoto and Hikosaka, 2009; Matsumoto and Takada, 2013) and state of mind, as in, e.g., wanting and willingness to exert effort (Berridge and Robinson, 1998; Salamone and Correa, 2012). Changes in patterns of DA neuron activity and metabolism caused by drugs of abuse, such as psychostimulants and opioids, contribute to drug addiction (Wise and Koob, 2014; Zahm, 2010). Degeneration of DA-expressing neurons underlies the deficits in movement initiation and posture experienced by Parkinson’s disease patients (Lang and Lozano, 1998a; b). Dysregulation of DA neurons underlies the positive symptoms of schizophrenia (Moore et al., 1999; Goto and Grace, 2007; Lau et al., 2013). In view of the broad spectrum of brain functions in which the DAergic (DA) ventral mesencephalic tegmentum has a role and its clinicopathologic significance, it is critically important to understand its interconnections, both intrinsic and with other parts of the brain. This review thus is presented as an update on recent progress mainly with regard to connectivity. A broader perspective on the structure and function of the midbrain ventral tegmentum can be acquired by reference to many outstanding prior reviews devoted to various aspects of the topic (e.g., Moore and Bloom, 1978; Lindvall and Björklund, 1983; Björklund and Lindvall, 1984; Oades and Halliday, 1987; Graybiel et al., 1990; van Domburg and ten Donkelaar, 1991; Langer et al., 1991; Gerfen, 1992; Parent et al., 1995; Fallon and Loughlin, 1995; Heimer et al., 1985; 1995; Haber and Fudge, 1997; Joel and Weiner, 2000; Haber, 2003; Bentivoglio and Morelli, 2005; Marinelli et al., 2006; Björklund and Dunnett, 2007; Fields et al., 2007; Ikemoto, 2007; Prensa et al., 2009; Haber and Knutson, 2010; Sesack and Grace, 2010; Schultz, 2010; Morikawa and Paladini, 2011; Hong, 2013; Roeper, 2013; Ikemoto and Bonci, 2014; Lammel et al., 2014).
Intrinsic organization and connections
A9, A10 and A8 groups of catecholamine-fluorescing cells occupying the ventral tegmental area (VTA), substantia nigra pars compacta (SNc), and retrobrubral field (RRF), respectively, were discovered by the great Swedish histochemists (Dahlström and Fuxe, 1964; Anden et al., 1964, 1965; 1966a; 1966b; Ungerstedt et al., 1971), who verified the DA nature of these cell groups (reviewed in Björklund and Lindvall, 1984; see also Björklund and Dunnett, 2007) and also showed that they give rise to a mesolimbic pathway to the limbic forebrain and orbitofrontal cortex and a nigrostriatal pathway to the striatum. We previously have referred to them collectively as the ‘ventral mesencephalic dopaminergic complex’ (Zahm et al., 2011b), which herein will be shortened to, ‘midbrain DA complex’. It should be clear that the A8–10 taxonomy refers explicitly to DA neurons, whereas the VTA, SNc, RRF are brainstem structures that also contain locally and distantly projecting neurons that utilize as transmitters, either co-expressed with DA or separately, gamma-aminobutyric acid (GABA), glutamate (GLU), various neuropeptides and possibly as yet unknown compounds.
A9, A10 and A8 comprise a single constellation of DA neurons that approximates the shape of an ellipsoid anchored in the VTA by A10 (Fig. 1). A9, more rostrally (Fig. 1A–E), and then A8 (Fig. 1D and E) extend lateralward from A10 beneath and above the medial lemniscus, through the SNc and RRF, respectively, to meet again in the ventrolateral tegmentum. A8 extends caudomedialward beyond the caudal limits of A9 and A10, ultimately to arch toward the periaqueductal gray (PAG). Rostroventral (rv), rostrodorsal, caudal (c) and dorsocaudal (dc) extensions of A10 into, respectively, supramammillary nucleus, medial habenula, dorsal midline of the caudal VTA and the rostral ventrolateral (vl) quadrant of the PAG (Fig. 1F) were designated later by Hökfelt et al. (1984b). A10rv and A10dc provide rich innervations of he lateral septum and extended amygdala, respectively, to be described in more detail below. Where they intermingle, neurons of A8 are morphologically indistinguishable from those in A9 or A10, as are A9 and A10 neurons at any imaginary boundary between the VTA and SNc (Fig. 1A–E). Nonetheless, A8, A9 and A10 are structurally and functionally differentiated and this is reflected in the relatively distinct, albeit broadly overlapping, topographies and functions of their ascending projections (Fallon et al., 1978; Fallon and Moore, 1978; Swanson, 1982; Lindvall and Björkland, 1983; Fallon and Loughlin, 1985; 1995; Deutch et al., 1988; Fallon, 1988).
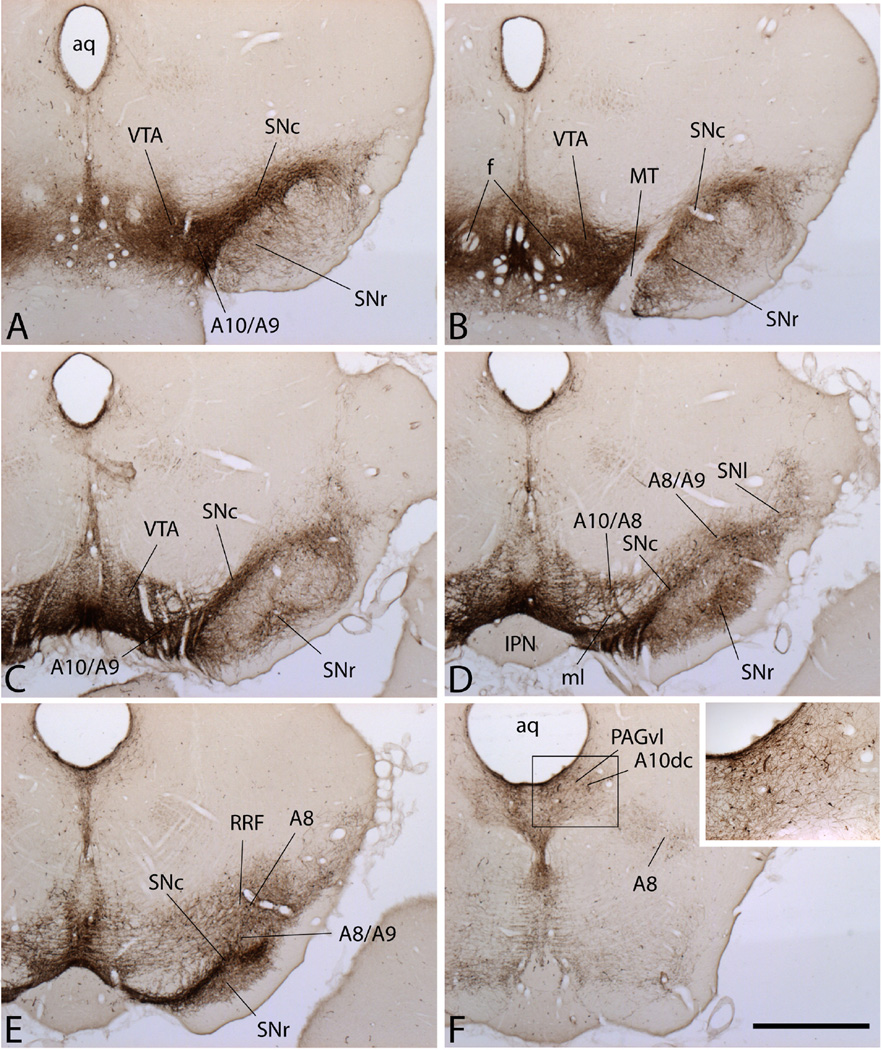
Figure 1.
Micrographs illustrating sections through the rat ventral mesencephalon in rostrocaudal sequence from A through F processed for tyrosine hydroxylase (TH) immunoreactivity (brown reaction product), which shows the continuities between various parts of the midbrain dopaminergic complex provided as names of structures, including the ventral tegmental area (VTA), substantia nigra compacta (SNc), retrorubral field (RRF) and ventrolateral quadrant of the periaqueductal gray (PAGvl), and as the catecholeamine-fluorescing dopaminergic (DAergic) cell groups (A10, A9, A8 and A10dc) that occupy the respective structures. Note that much TH immunoreactive material, including neurons, dendrites and axons is present in the substantia nigra reticulata (SNr), belying its common designation as a non-DAergic basal ganglia structure. A10/A9, A10/A8 and A8/A9 designate regions of transition between the respective cell groups. The inset in panel F shows the numerous TH-immunoreactive neurons in the PAGvl. Additional abbreviations: aq - cerebral aqueduct; dc - dorsal, caudal; f - fornix; IPN - interpeduncular nucleus; ml -medial lemniscus; MT - mammillothalamic tract; SNl - substantia nigra pars lateralis. Scale bar: A-F - 1 mm; inset F - 0.5 mm.
The VTA is a paramedian sector of the reticular formation at the ventral surface of the midbrain (Fig. 1A–E) that has within it a number of cell groups that have been categorized as nuclei (Taber, 1961; Berman, 1968; Phillipson, 1979a; Oades and Halliday, 1987; van Domburg and ten Donkelaar, 1991). These, which were reviewed in some detail by Ikemoto (2007), comprise a ventromedial, cell-dense nucleus paranigralis, a more lateral and dorsal nucleus parabrachialis pigmentosus and some midline structures, including a nucleus interfascicularis (Phillipson, 1979a) and a of couple linear nuclei - rostral and, depending on the account, either caudal (Phillipson, 1979a) or central (Swanson, 1982; Ikemoto, 2007), the latter of which extends in the midline from the main body of the VTA to the PAG and houses the A10c group of Hökfelt et al. (1984b). Neurons of the VTA are said to comprise a heterogeneous mixture of DA (~65%), GABAergic (GABA, ~30%) and GLUergic (GLU, ~5%) neurons (Nagai et al. 1983; Mugnaini and Oertel, 1985; Kosaka et al. 1987; Steffensen et al. 1998; Carr and Sesack, 2000a; Kawano et al., 2006; Margolis et al. 2006; 2012; Nair-Roberts et al. 2008; Steffensen et al., 2008; Dobi et al. 2010; Yamaguchi et al. 2011) of which none is distributed in a manner that correlates systematically with the aforementioned nuclear organization. Most VTA DA neurons occupy regions taken up by the paranigral and parabrachialis pigmentus nuclei, consistent with the greater proportional size of those VTA subnuclei (Dahlstorm and Fuxe, 1964; Swanson, 1982; Olson and Nestler, 2007), but significant numbers also are centered in the midline, particularly within the central linear nucleus and interfascicularis, where, despite its smaller size, the density of DA neurons is said to be greatest (Ikemoto, 2007). DA neurons are sparse in the rostral linear nucleus and a caudal part of the VTA which extends into the paramedian mesopontine tegmentum (Perrotti et al., 2005; Ikemoto, 2007; Olson and Nestler, 2007). In contrast, GABA neurons are scattered throughout the VTA, but are densest in a rostral, dorsolateral cluster identified by Olson and Nestler (2007) and the aforementioned DA neuron-sparse caudal region, where ovoid aggregates of GABA neurons flank the interpeduncular nucleus on both sides of the midline (see Fig. 2E–H in Jhou et al., 2009b). Perrotti et al. (2005) and others (Ikemoto, 2007; Kaufling et al., 2009; Bourdy and Barrot, 2012) have designated this region as the ‘tail’ of the ventral tegmental area (tVTA), but others regarded it as a structure distinct from the VTA (e.g., Jhou, 2005; Geisler et al., 2008) and it was recently given a name, rostromedial tegmental nucleus (RMTg, Jhou et al., 2009a; b; Lavezzi and Zahm, 2011), to formalize the distinction. Presently, both names are used in the literature. It should be noted, however, that the cell-dense structure identified as the ventral tegmental tail (their VTT) in Figure 8 of the review by Ikemoto (2007) does not represent what subsequently was identified as the RMTg (compare with Fig. 1 in Jhou et al., 2009b, and Fig. 2 in Lavezzi and Zahm, 2011; see also Jhou et al., 2009a; Kaufling et al., 2009; Barrot and Thome, 2011; Bourdy and Barrot, 2012).
Figure 2.
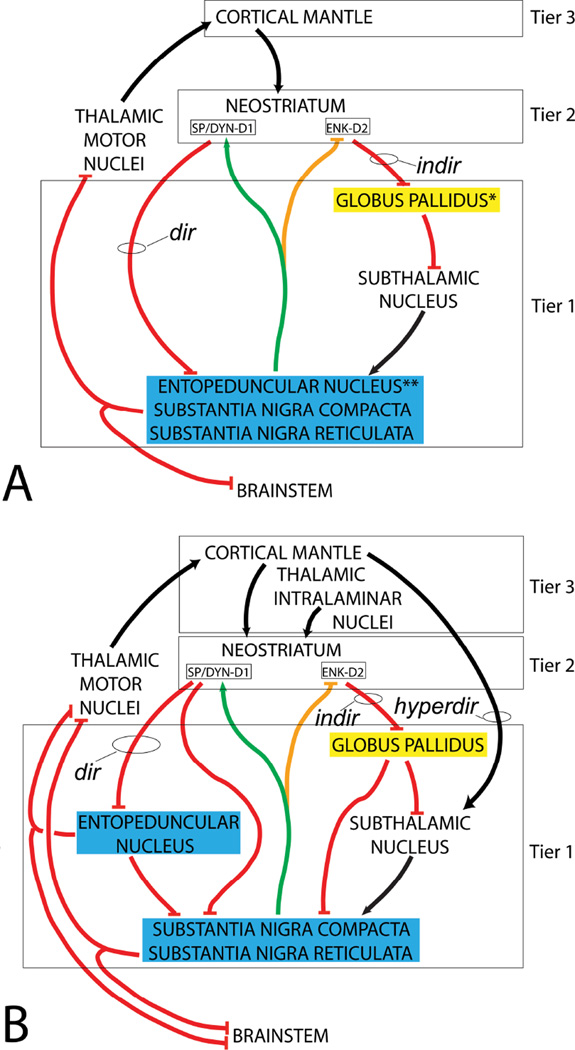
Diagram depicting the ‘balkanized’ connectivity of the basal ganglia as commonly shown (A) and in a more inclusive rendering (B). Spontaneously active structures are in Vcolored boxes and include output nuclei (blue - note that the substantia nigra compacta is not an output nucleus, but is embedded within the substantia nigra reticulata, which is) and intrinsic nuclei (yellow). Black and green (for dopaminergic) lines depict excitatory connections; inhibitory connections are shown as red and orange (for dopaminergic). Boxes just beneath NEOSTRIATUM indicate medium densely spiny neurons expressing substance P (SP), dynorphin (DYN) and dopamine (DA) D1 receptors that are the origin of the direct pathway (dir) and enkephalin (ENK) and D2 receptors that give rise to the indirect pathway (indir). Summation of the signs of the connecting links shows how the direct pathway may inhibit the output nuclei, thus disinhibiting the thalamocortical and brainstem targets, and how the indirect pathway may activate output structures, which suppresses the thalamocortical and brainstem targets. Note that DA acting at D1 receptors facilitates the activity of the direct pathway (green line) and, acting at D2 receptors, attenuates that of the indirect pathway (orange line). Panel B shows additional connectivity not typically included in discussions of the direct and indirect pathways. Conventions in B are as in A. Note, for example, that the entopeduncular nucleus (EPN) is split out from the substantia nigra reticulata (SNr) because it has no DA neurons. Serial inhibitory connections from SP/DYN neurons to the SNr, via EPN, would oppose the action of the direct pathway. More pathways not included in the original theory include the hyper direct pathway (hyperdir) from cortex to subthalamic nucleus and a pathway from the globus pallidus (GP) to the SNr. GP also projects to EPN (not drawn). Note also in B that the thalamic intralaminar nuclei are shown. These are important inputs to the direct and indirect pathways not typically regarded in the original format of the theory. Three tiers of innervation of DA neurons are indicated by black boxes and on the right. * Globus pallidus in the rat is homologous to the lateral (a.k.a. external) pallidal segment in primate. ** Entopeduncular nucleus in the rat is homologous to the medial (a.k.a. internal) pallidal segment in the primate.
The VTA also contains GLU neurons, said to be relatively few and most numerous rostromedially (Yamaguchi et al., 2007; Nair-Roberts et al., 2008). However, numerous recent multidisciplinary studies have provided evidence for abundant functionally significant release of GLU and/or GABA from the axons of DA neurons, irrespective of the relative strength of the corresponding neuroanatomical evidence for co-localization (Joyce and Rayport, 2000; Chuhma et al., 2004; 2009; Hnasko et al., 2010; 2012; Stuber et al., 2010; Alsiö et al., 2011; Fortin et al., 2012; Tritsch et al., 2012; Stamatakis et al., 2013). Moreover, in the case of GLU, in vivo co-expression is reported to peak early and decline as rats mature (Mendez et al., 2008). Consequently, the true extent of overlap of DA, GLU and GABA expression in the midbrain DA complex is presently uncertain. In contrast to GABA and GLU, which only recently have been shown persuasively to co-express with DA, co-expression of the neuropeptides cholecystokinin (CCK) and neurotensin (NT) in midbrain DA neurons has been recognized for decades (Hökfelt et al., 1980a; b; 1984a; Kalivas and Miller, 1984; Kalivas 1985; Seroogy et al., 1987, 1988, 1989; Studler et al., 1988; Crawley, 1991; Tan et al., 1999; Geisler et al., 2006).
The SNc, PAGvl, RRF and SN pars lateralis (SNl), which occupies the interval between the lateralmost border of the SNr and ventromedial border of the inferior colliculus (Fig. 1B–D), also comprise combinations of DA, GABA and GLU neurons (Moriizumi et al., 1992, Rogriguez and Gonzalez-Hernandez, 1999; Nair-Roberts et al., 2008; Geisler et al., 2007; Omelchenko and Sesack, 2010; Yamaguchi et al., 2013). The SNc and RRF are reported to contain approximately 29% and 58% GABA neurons, respectively, but apparently few GLU neurons (Nair-Roberts et al., 2008). However, Yamaguchi et al. (2013) have reported, based on a double-labeling protocol, that approximately 15% of SNc neurons expressed VGLUT2 and 85% expressed tyrosine hydroxylase (TH, the rate limiting enzyme in catecholamine synthesis, characteristically indicates DA phenotype in the midbrain DA complex). In the RRF, approximately 36% of total TH and VGLUT2 neurons expressed VGLUT2, with the numbers increasing in a rostralcaudal gradient, and 63% expressed TH. Interestingly, these data leave little room for significant co-expression of GLU and DA in the SNc and RRF. The PAG also contains GLU (Geisler et al., 2007) and GABA projection neurons (Omelchenko and Sesack, 2010).
The heterogeneous composition of the midbrain DA complex is also reflected in the distribution within subpopulations of DA neurons of different calcium buffering enzymes. Calbindin-D 28kD is expressed in the VTA, preferentially in neurons projecting to the accumbens (Acb) core (Tan et al., 1999; Barrot et al., 2000), RRF and a dorsal tier of the SNc (Gerfen et al., 1985). Another calcium binding protein, calretinin (Rogers, 1992; Isaacs and Jacobowitz, 1994), is expressed by about equivalent numbers of Acb core- and Acb shell-projecting DA neurons (Tan et al., 1999) and occupies mainly the ventral tier of the SNc (Haber et al., 1995; Krzywkowski et al., 1995).
Intrinsic connections
Following injections of the anterograde tracer Phaseolus vulgaris-leucoagglutinin (PHA-L) into various parts of the VTA, Ferreira et al. (2008) showed that [1] the rostral VTA projects weakly to the caudal, [2] the caudal VTA projects substantially to the rostral, [3] the caudal pole of the VTA heavily and in widespread fashion innervates the VTA, SNc and RRF, [4] the medial supramammillary nucleus innervates the ventro- and caudomedial VTA and [5] the ventrolateral VTA (within subnucleus paranigralis) projects to the interfascicular nucleus. They noted ([5] being the exception) that anterograde labeling most typically tends to spread from medial injections to more lateral VTA terminal fields, consistent with a tendency for medial to lateral flow of information in the midbrain DA complex (see below). Their remarkable injections into the caudal pole of the VTA produced robust labeling throughout the midbrain DA complex, especially in the SNc, where they saw TH immunoreactive neurons and dendrites richly entwined by PHA-L labeled axons and abundantly contacted by labeled axonal varicosities. The caudal injections of Ferreira et al. (2008) foreshadow papers that were to appear in a couple years announcing discovery of the RMTg (Jhou et al., 2009a;b; Kaufling et al., 2009).
In addition, the RRF projects to the VTA, SNc, and PAGvl (Deutch et al., 1988; Arts et al. 1996; Zahm et al., 2011b). The PAGvl has intra-PAG associational connections and GABA (Kirouac et al., 2004) inputs from the VTA (Vianna and Brandao, 2003; Zahm et al., 2011b), GLU outputs to the VTA (Geisler et al., 2007), GABA outputs to the VTA contacting DA and GABA neurons (Omelchenko and Sesack, 2010), and outputs to the RRF of undetermined phenotype (Zahm et al., 2011b).
Interconnectivity of VTA neurons is thought to originate mainly from non-DA VTA neurons because, following VTA tracer injections, few anterogradely labeled axon terminals or retrogradely labeled VTA neurons have TH immunoreactivity (Bayer and Pickel, 1990; Ferreira et al., 2008; Jhou et al., 2009b). Where axons labeled anterogradely following PHA-L injections into the VTA do exhibit synapses (Omelchenko and Sesack, 2009), the membrane appositions typically are symmetric (signifying inhibitory) and involve VTA neurons expressing TH. Many such synaptic profiles are co-labeled with glutamate decarboxylase (GAD, GABA synthesizing enzyme) immunoreactivity. Recent gene transfer and optogenetic studies indicate that VTA DA neurons are mainly inhibited following optogenetic stimulation of VTA GABA neurons (van Zessen et al., 2012; Cohen et al., 2012; Tan et al., 2012; Padgett et al., 2012). However, the extent in these studies to which the illumination may have involved the RMTg is problematic, so that the relative roles played by VTA GABA neurons versus RMTg inputs in regulating the activity of VTA and SNc neurons remains to be worked out (Hong et al., 2011). The few asymmetric (presumably excitatory) contacts observed by Omelchenko and Sesack (2009) fit with evidence provided by Dobi et al. (2010) that GLU neurons establish synaptic contacts with both TH-positive and TH-negative neurons in the VTA. Taken together, these results indicate that GABA and GLU VTA neurons (but few DA neurons) project to DA and non-DA VTA neurons and so represent a potentially strong and nuanced modulatory influence over DA released both locally and from the ascending DA projections. The actions of intrinsic circuitry may not be uniform throughout the midbrain DA complex, however, in that Ferreira et al. (2008) observed following PHA-L injections into the VTA that close contacts of anterogradely labeled axons with DA neurons were significantly fewer in the VTA as compared to the SNc and RRF. Insofar as Olmelchenko and Sesack (2009) have confirmed that such close contacts commonly are synaptic, intrinsic connectivity may subserve a net synaptically-mediated transfer of information from the VTA to the SNc.
Somatodendritic DA release and sensitivity to circulating hormones and peptides
DA neurons in both the SNc and VTA may also influence the local environment through somatodendritic DA release (Kalivas et al., 1989; Geffen et al. 1976; Rice et al. 1994; 1997; 2011). Whereas local DA release in the SNc appears to be exclusively somatodendritic, somatodendritic release of DA in the VTA is supplemented by DA released from the axons of DA neurons residing in the VTA and SNc, although, as noted above, the local DA innervation is much subordinate to local non-DA innervation (Bayer and Pickel 1990; Ferriera et al., 2008; Jhou et al., 2009b). Somatodendritic release of DA within the SNc and VTA likely activates D1 receptors residing on the terminals of VTA afferents, which might indirectly (via local GABA neurons) facilitate the activity of DA neurons (e.g., Bocklisch et al., 2013). In conditions of repeated exposure to psychostimulant drugs this effect may contribute to the initiation of behavioral sensitization to DA (Pierce et al., 1996; Bocklisch et al., 2013). Alternatively, local release of DA acts on DA D2 autoreceptors (Sesack et al., 1994; Yung et al., 1995), which act to inhibit both local somatodendritic DA release and release of DA from axon terminals in the mesencepalon and forebrain (Lacey et al., 1987; Kalivas and Duffy, 1991; Cragg and Greenfield, 1997). Indeed, intra-VTA infusions of DA D2 agonists are utilized by research neuroscientists as a means to suppress the activity of DA neurons (e.g., Liu et al., 2008).
Much evidence indicates that the sensitivity with which neurons in the midbrain DA complex respond to physiological stimuli is dependent on concentrations of circulating hormones (e.g., Deroche et al., 1995; Rougé-Pont et al., 1995) and peptides (e.g., Abizaid et al., 2006; Fulton et al., 2006; Hommel et al., 2006). These important effects may be mediated at least in part via afferent connections (e.g., Leinninger et al., 2009), but are commonly due to the direct actions of neuroactive compounds on DA neurons and local circuit, e.g., GABA, neurons. This is a large and important field of inquiry that, however, will not be considered further in this review of neuroanatomical connectivity.
Gap junctions
DA and GABA neurons in the VTA and SNc have gap junctions early (Vandecastelle et al., 2005) and later (Grace and Bunney, 1983; Stobbs et al., 2004; Allison et al., 2006) in development. The firing rate of DA neurons in the SNc is reported to be modulated by conduction of currents through gap junctions (Grace and Bunney, 1983; Vandecastelle et al., 2005). VTA GABA neurons are interconnected by connexin 36-expressing gap junctions, through which conduction is enhanced by corticotegmental input and dopamine (Allison et al., 2006). Gap junctional interconnection of VTA GABA neurons may affect addictive behaviors, such as, e.g., cocaine and alcohol consumption (Stobbs et al., 2004; Steffensen et al., 2011), by disseminating disinhibitory influences among coupled VTA dopamine neurons.
Afferent Connections
VTA
Inputs to the VTA arise in manifold structures in the cortex, basal forebrain, hypothalamus and brainstem (Nauta and Domesick, 1978; Phillipson, 1979b; Oades and Halliday, 1987; Wallace et al., 1989; 1992; Carr and Sesack, 1999; 2000b; Zahm et al., 2001; Fadel and Deutch, 2002; Philpot et al., 2005; Omelchenko and Sesack, 2005; 2006; 2007; 2009; 2010; Ikemoto, 2007; Balcita-Pedicino and Sesack, 2007; Balcita-Pedicino et al., 2011; Ferreira et al., 2008; Omelchenko et al., 2009; Watabe-Uchida et al., 2012) reported to comprise mainly a highly interconnected network (Geisler and Zahm, 2005; 2006; Geisler et al., 2007; Yetnikoff et al., 2013). Table 1, modified after Table 1 in Geisler and Zahm (2005), lists structures in which retrograde label was found following injections of tracer into the VTA, with those that provide robust input bolded. An initial impression conveyed by such a long list of such diverse structures is that the organization of VTA afferents must be rather diffuse. Certainly, the general pattern of retrograde labeling also contributes to this impression, inasmuch as the scatter of labeled neurons is conspicuous in its indifference to neuroanatomical boundaries. That is, mapped VTA-projecting neurons are as numerous between as within different brain structures (see, e.g., Figs. 3 and 8 in Geisler and Zahm, 2005, Figs. 3 and 4 in Zahm et al., 2011b, and 4 in Yetnikoff et al., 2013) and the structures themselves are often mutually coalescent, i.e., separated by ill-defined transition zones rather than discrete boundaries (see Discussion in Zahm et al., 2013).
Table 1.
Structures containing retrogradely labeled neurons following injections of FluoroGold into the VTA (adapted from Geisler and Zahm, 2005)
| Telencephalon: |
| dorsal peduncular, infralimbic, prelimbic*, cingulate and agranular insular cortex; claustrum, endopiriform nucleus, olfactory tubercle, core, shell and rostral pole of the accumbens, bed nucleus of stria terminalis, anterior amygdaloid area, medial nucleus of amygdala, central nucleus of amygdala, ventral pallidum, sublenticular sub s tantia innominata, dorsal, intermediate and ventral divisions of the lateral septum, septofimbrial nucleus, medial septum/diagonal band nuclei, lateral preoptic area, medial preoptic area magnocellular preoptic area, median preoptic area. |
| Diencephalon: |
| anterior hypothalamus, hypothalamic paraventricular nucleus, ventromedial hypothalamus, perifornical hypothalamus, lateral hypthalamus, dorsal hypothalamus, tuber cinereum parafascicular nucleus, zona incerta, thalamic paraventricular nucleus, lateral habenula medial habenula, supramammillary nucleus |
| Mesencephalon: |
| superior colliculus deep layers, periaqueductal gray, substantia nigra pars compacta and reticulata, deep mesencephalic field, anterior, dorsal and ventral tegmental nuclei |
| Pons and Medulla: |
| oral pontine reticular field, dorsal raphe, median raphe, paramedian raphe, raphe pontis pedunculopontine tegmental nucleus, laterodorsal tegmental nucleus, cuneiform nucleus, parabrachial nucleus, nucleus reticularis pontis, locus coeruleus, lateral reticular field, intermediate reticular field, gigantocellular reticular field |
| Cerebellum: |
| dentate nucleus |
robust inputs are bolded
The massive, convergent complex of VTA afferents essentially comprises three categories of neurons organized into three interconnected tiers. The most proximal of these comprises so-called ‘isodendritic’ neurons strung along the medial forebrain bundle (mfb) in the ventral pallidal-preoptic-hypothalamic continuum and brainstem. Isodendritic morphology was described by Ramon-Moliner and Nauta (1966) in reference to neurons in the so-called isodendritic core with very long, sparsely branched, sparsely to modestly innervated dendrites -consistent with function of a broadly integrative nature. First tier isodendritic VTA inputs (Geisler and Zahm, 2005) likely comprise an interconnected network that relays descending and ascending (see below) influences not only to the VTA, but to multiple forebrain, hypothalamic and brainstem effectors of somatomotor, autonomic and neuroendocrine function (Swanson and Mogenson, 1981). Most tier 1 neurons happen to lie squarely in the path of a second tier of VTA afferents, axons of which descend mainly within the mfb from densely packed medium size, densely spiny neurons (MdSNs) in the Acb, olfactory tubercle and lateral septum and, to a lesser extent, ventromedial caudate-putamen (CPu) and extended amygdala (EA). We use here the abbreviation MdSN in place of the more common MSN because the latter abbreviation has a traditional strong association with the caudate nucleus and putamen exclusively, whereas we would emphasize the broader distribution of MdSNs throughout an extensive, essentially continuous tier of subcortical GABA structures listed in the preceding sentence. MdSNs in these deep telencephalic structures have varyingly dense direct projections to the VTA and also provide input to tier 1 isodendritic afferents (Zahm, 1989; Loopuijt and Zahm, 2006), more densely to those in rostral parts of the basal forebrain, culminating in very dense projections from the Acb and olfactory tubercle to ‘pallidal’ neurons in the ventral pallidum (Heimer, 1972; Heimer et al., 1991; Groenewegen and Russchen, 1984). Ventral pallidal neurons project densely to the VTA (Zahm, 1989; Zahm and Heimer, 1990; Groenewegen et al., 1993) and we lump them here with the isodendritic category, although, according to Ramon-Moliner and Nauta (1966), they are not strictly isodendritic due to their dense striatopallidal input.
A number of papers have revealed a significant amount of specificity in connections conforming to the general organizational framework described above. For example, one subpopulation of Acb neurons expresses mainly D1 DA receptors and projects to ventral pallidum and the VTA (Robertson and Jian, 1995, Lu et al., 1998), where it is reported to target mainly GABA neurons (Xia et al., 2011; Bocklisch et al., 2013). Another expresses D2 receptors and projects to the ventral pallidum (Robertson et al., 1992), which, in turn, projects to the mediodorsal thalamic nucleus (Young et al., 1984). Such compartmentation of Acb connections may be a phylogenetic ancestor of the more complete ‘balkanization’ of CPu outputs that has substantial implications for afferent regulation of DA neurons (see below).
MdSN-containing, VTA-projecting structures, in turn, are massively innervated by a third tier of neurons, these located in the basal and cortical amygdala, subiculum of the hippocampus, and orbitofrontal cortex [i.e., Heimer and Van Hoesen’s (2006) cortical limbic lobe], which also have long descending projections to the vicinity of, and that likely are in contact with, first tier isodendritic VTA-projecting neurons. Moderate output from the orbitofrontal cortex even reaches the VTA itself (Sesack et al., 1989), including DA neurons (Carr and Sesack, 1999; 2000b; Sesack and Carr, 2002), although projections to the VTA from cortical and cortical-like structures otherwise is not the rule; i.e., cortex projects to the ventral midbrain mainly via relays in tiers 2 and 1 (for ‘cortical-like,’ see Heimer and Van Hoesen, 2006, and references therein). This aside, Carr and Sesack’s (2000b) elegant electron microscopic tract tracing study indicated that afferents originating in the medial (m) prefrontal cortex (PfC) project preferentially to [1] VTA DA neurons that project reciprocally to the PfC and [2] VTA GABA neurons that project to the Acb, but [3] not to VTA DA neurons that project to the Acb.
Much evidence indicates that tier 3 cortical and cortical-like outputs are exclusively GLU, whereas the outputs of tier 2 deep telencephalic nuclei are prominently GABA (Alheid and Heimer, 1988; Swanson, 2000; Fremeau et al., 2001; 2004; Geisler et al., 2007). In contrast, both GABA and GLU are represented among the important tier 1 VTA inputs, although, with few exceptions (e.g., see Jhou et al., 2009a; b), little is presently known about in which neurons specifically.
More is known about the distributions and functions of tier 1 peptidergic inputs to the VTA, e.g., the capacity of inputs to the VTA from orexinergic (Aston-Jones et al., 2009; Cason et al. 2010; Cason and Aston-Jones, 2013) and neurotensinergic (Rompre et al., 1992; St-Gelais et al., 2004; Reynolds et al., 2006; Geisler et al., 2006; Kortleven et al., 2012; Kempadoo et al., 2013) neurons in the lateral hypothalmo-preoptic-ventral pallidum continuum to facilitate DA-mediated locomotor and reward behaviors. Interestingly in this regard, Balcita-Pedicino and Sesack (2007), using electron microscopy, detected orexinergic axon terminals and synaptic contacts in the VTA that seemed to these authors inordinately few, in view of the robust functional orexigenic effects that had been reported (refs in preceding sentence). In general, the extent to which VTA neurons of various transmitter phenotypes are preferentially targeted anatomically and physiologically by subsets of neurons in all of the tiers remains to be resolved. Indeed, much detail remains to be learned about the configurations of connections within and between the various structures providing descending projections to the VTA.
VTA descending afferents in general are topographically organized, such that projections to more lateral parts of the VTA tend to come from more lateral parts of the forebrain and brainstem (Phillipson, 1979b; Geisler and Zahm, 2005; Geisler et al., 2007; Ikemoto, 2007). Mediolateral topography is particularly well represented in the ventral striatopallidal-VTA projections, which exhibit, in addition, a rostrocaudal and inverted dorsoventral topography, such that rostral parts of the Acb and ventral pallidum project to more rostral parts of the VTA (Heimer et al., 1991; Zahm and Heimer, 1993; Groenewegen et al., 1993), whereas neurons positioned more dorsally in the Acb project ventrally within the VTA (Deniau et al., 1996; Zahm et al., 2013), consistent with the well known inverted dorsoventral topography that characterizes striatal projections to the SN (Heimer et al., 1985; 1995; Deniau et al., 1996). Although abundant evidence exists that projections conveying functionally diverse information to the midbrain converge in several striatopallidal structures (Bevan et al., 1996; Smith et al., 1998), Deniau, Thierry and colleagues have argued equivalently forcefully that functional segregation is maintained in parallel basal ganglia circuits descending through the midbrain DA complex (Deniau et al., 1996; Deniau and Thierry, 1997; Maurice et al., 1998; Maurin et al., 1999). Within the midbrain DA complex, the generous spread and extensive overlap of afferents from manifold sources (Geisler and Zahm, 2005) and relatively meager evidence of connectional specificity suggests that VTA function must include a broadly integrative component.
Ascending projections to the VTA arise in numerous brainstem structures, including the superior colliculus (deep layer), RMTg, PAG, deep mesencephalic nucleus, dorsal raphe, pedunculopontine (PPTg) and laterodorsal tegmental (LDTg) nuclei, median raphe, parabrachial nucleus, cuneiform nucleus, oral and caudal pontine reticular nuclei and the nucleus of the solitary tract (Vertes, 1991; Oakman et al., 1995; Charara et al., 1996; Zahm et al., 2001; Geisler and Zahm, 2005; Geisler et al., 2006; Geisler et al., 2007; Mejias-Aponte et al., 2009; Omelchenko and Sesack, 2010; Miller et al., 2011). Neurons in a majority of the structures that give rise to ascending inputs to the VTA resemble basal forebrain tier 1 isodendritic afferents both morphologically and in that they are situated along the mfb and get descending input from forebrain tier 2 VTA afferents. Afferents that ascend to the midbrain DA complex from the brainstem, like the descending afferents, are broadly topographically organized and equivalently indifferent to imagined hard line boundaries between structures (Zahm et al., 2001; Geisler and Zahm, 2005; 2006; Geisler et al., 2007; Yetnikoff et al., 2013).
The LDTg and, to a lesser extent, PPTg, long regarded with particular interest as a source of ascending cholinergic inputs to VTA (Oakman et al., 1995; Winn et al., 1997; Mena-Segovia et al., 2008), are now recognized to also provide robust GABA and GLU afferents (Charara et al., 1996; Parent et al., 1999; Geisler et al., 2007; Wang and Morales, 2009). LDTg afferents are thought permissive to burst activity of VTA and SNc DA neurons (Lodge and Grace, 2006a), whereas the PPTg (Floresco et al., 2003), together with subiculum projections relayed through ventral striatopallidum, regulates the population activity and rate of bursting of VTA neurons (Floresco et al., 2003; Lodge and Grace, 2006b; Chen and Lodge, 2013). Apropos of this influence on burst firing, Lammel et al. (2012) determined that LDTg inputs to the VTA have synaptic contacts principally with neurons that project to the lateral Acb shell and subserve reward function. They contrasted these with projections thought to be related to aversive responding, e.g., from the lateral habenula (LHb) mainly to DA neurons projecting to the medial prefrontal cortex. Ascending projections from the PPTg and LDTg to the midbrain DA complex also are reported to be involved in non-DA reward mediated by opiates (Bechara et al., 1992; Ting-A-Kee et al., 2013) and concomitant behaviors that are dependent upon stimulation of M5 muscarinic receptors in the VTA and RMTg (Wasserman et al., 2013).
The dorsal raphe nucleus, which provides serotonin afferents to the VTA (Azmitia, 1978; Herve et al., 1987), now is known to also contain GABA (Charara and Parent, 1998) and GLU (Fremeau et al., 2002: Gras et al., 2002; Commons, 2009; Hioki et al., 2010) neurons of which the latter at least are a major source of input to the VTA (Geisler et al., 2007). A couple recent studies agree that optogenetic stimulation of dorsal raphe projections to the VTA produce a GLU mediated, rewarding activation of VTA neurons, but disagree about a rewarding vs aversive role of dorsal raphe-VTA serotonin (McDevitt et al., 2013; Wang and Morales., 2013).
RRF and PAGvl
The arrangement of inputs to the RRF and PAGvl much resembles that of the VTA, comprising sequential, interconnected isodendritic (tier 1), MdSN (tier 2) and cortical and cortical-like (tier 3) afferents (Zahm et al., 2011b). The list of brain structures that exhibit numerous retrogradely labeled neurons following injections of tracer into the RRF and PAGvl is even longer than observed after injections into the VTA, owing mainly to the addition of abundant, prominent projections from the central division (c) of the extended amygdala (EAc) (Zahm et al., 2011b), which includes the central amygdaloid nucleus (CeA), lateral part of the bed nucleus of stria terminalis (BST), dorsal part of the interstitial nucleus of the posterior limb of the anterior commissure and intervening parts of the sublenticular forebrain (Alheid and Heimer, 1988; de Olmos and Heimer, 1999; Shammah-Lagnado et al., 1999). Robust RRF-PAGvl inputs also arise in the hypothalamic paraventricular nucleus, lateral hypothalamus, parasubthalamic nucleus, parabrachial nucleus and nucleus of the tractus solitarius, of which all are structures with which the EAc is most extensively interconnected (Goto and Swanson, 2004; Mejias-Aponte et al., 2009; Geerling et al., 2010; Zahm et al., 2011b). Thus, the RRF and PAGvl together represent a sector of the midbrain DA complex serving prominently as a node receiving inputs from EAc.
Several investigators (Krettek and Price, 1978; Shinonaga et al., 1992; Zahm et al., 1999; Shammah-Lagnado et al., 2001; Gastard et al., 2002; Zahm et al., 2011b) have reported that deposits of anterogradely transported compounds in EAc structures, including the CeA and dorsolateral (dl) part of the BST produce labeled fibers in the VTA that largely lack the varicose “beaded” appearance of axon terminals with synaptic specializations. Such labeled fibers pass through the VTA and medial SNc, showing little evidence of synaptic differentiation until, as noted above, they reach a site in the lateral part of the SNc and suprajacent RRF, where the projection ramifies extensively and exhibits numerous varicosities. This region was emphasized by Gonzales and Chesselet (1990) as the main nigral recipient of a robust, functionally significant (Han et al., 1997; Lee et al., 2005; El-Amamy and Holland, 2007) projection from the CeA. Price and Amaral (1981), evaluating tritiated amino acid injections placed in the monkey CeA also illustrated little anterograde labeling in the VTA and a strong projection to the lateral SNc and RRF. Contrary evidence, i.e., that the CeA and BST project strongly to the VTA also has been provided (Fudge and Haber, 2000; 2001), but has been questioned on the basis of technical issues (Zahm, 2006; Heimer et al., 2008). Georges and Aston-Jones (2001; 2002; see also Dumont and Williams, 2004), however, demonstrated a short-latency excitation of the VTA following electrical andd chemical stimulation at a single, focal site in the BSTvl. Although the anatomy appears consistent with few direct projections from the EAc to the VTA, great numbers of VTA-projecting neurons form a densely packed column centered on the medial forebrain bundle that invades numerous forebrain structures, including the lateral and medial hypothalamus, sublenticular region, lateral and medial preoptic regions, diagonal band, septal nuclei, ventral pallidum and ventral parts of the BST (Geisler and Zahm, 2005). Many of the neurons in this column are thought to be GLU, i.e., excitatory, whereas the output from the MdSN-containing BST is mainly GABA (McDonald et al., 1989; Takayama and Miura, 1991; Sun and Cassell, 1993; Korotkova et al., 2004; Geisler et al., 2007). It is thus reasonable to suspect that excitatory responses in the VTA elicited by stimulation of the BSTvl are due to the activation of GLU neurons in the transition area between the BST and mfb. However, in the mean time, a number of groups have weighed in on a BST-VTA connection using modern chemico- and optogenetic methods (e.g., Kudo et al., 2012; Jennings et al., 2013; Kim et al., 2013) with the result of further complicating the issues of neural connectivity. Thus, there may be additional complexity in these relationships and, however they are resolved in future studies, it seems worth emphasizing that a comprehensive understanding of brain systems interactions cannot be realized in the absence of agreement, or logical basis for disagreement, among the various ways of looking at the problems.
SNc
The SNc contains a dense accumulation of A9 group DA neurons (Fig 1A–E) and extends lateralward from the VTA within the dorsalmost part of the substantia nigra reticulata (SNr) as a flat, wing-like mass (Fig. 1A and B), comprising a loosely organized dorsal tier of DA neurons with more or less horizontally oriented dendrites and a ventral cell-dense tier, from which vertically oriented dendrites of DA neurons descend far into the SNr (Gerfen, 1984). Modest to dense aggregations of A9 DA neurons also are scattered throughout the SNr, particularly in the rodent (Fig. 1C and D). Bolam and colleagues have reported that the density of GABA synapses as a proportion of total synapses is significantly greater for DA neurons and dendrites occupying the SNr beneath the ventral tier of DA neurons (Henny et al., 2012), which is consistent with the observed innervation of DA neurons by SNr GABA output neurons (Tepper et al., 1995) and which supports the notion that input to A9 DA neurons must in some way reflect that to the SNr. Consistent with its role as a basal ganglia output nucleus, the SNr and, presumably, SNc, get much afferent input from the CPu and intrinsic basal ganglia circuitry (Heimer et al., 1985; 1995), which has a fundamental organization similar to that of the descending afferents of the VTA. That is, SNr tier 1 input (Fig. 2) comes mainly from the globus pallidus (GP), entopeduncular nucleus (EPN, medial segment of the GP in primates) and subthalamic nucleus (STN), all comprising neurons with very long, sparely ramifying, mainly aspiny dendrites. Due to the dense striatopallidal and pallido-STN input to such neurons, however, they are not regarded as isodendritic (Ramon-Moliner and Nauta, 1966). The main second tier innervation of the SNr comprises MdSNs in the CPu, which, in turn, are recipient to a third tier of afferent innervation - a massive input from the neocortex (Künzle and Akert, 1977; Künzle, 1978; McGeorge and Faull, 1989). The mediolateral and dorsoventral topographies of these dorsal striatonigral projections are, respectively, preserved and inverted (Deniau et al., 1996).
As noted in a preceding section, a ‘balkanization’ (i.e., compartmentation) of inputs to the SNr and SNc from the CPu (dorsal striatum) is more differentiated than is that of the inputs to the VTA from ventral striatum. That is, cortico-striatonigral/striatopallidal-thalamocortical mechanisms in the classical basal ganglia have been thought to depend substantially on the interactions of distinct functionally opposed direct and indirect pathways (Albin et al., 1989; Gerfen, 1992; Smith et al., 1998; Gerfen and Surmeier, 2011) from the striatum to GABAergic basal ganglia output nuclei, i.e., the SNr, EPN and ventral pallidum. The direct pathway originates with a subset of GABAergic MdSNs (about half of striatal MdSNs) that co-expresses the peptides substance P and dynorphin and projects directly to the output nuclei, which, in turn, project to the cortex, via thalamus, and to brainstem motor nuclei (dir in Fig. 2A). Excitation of the direct pathway is thought to disinhibit its thalamocortical and brainstem targets, producing behavioral arousal, by inhibiting GABA projection neurons in the output nuclei. The indirect pathway originates with the other about half of striatal MdSNs, which co-express GABA and the neuropeptides leu- and/or met-enkephalin and project, via a relay in the GABA GP, to the STN, a GLU structure that projects to the output nuclei. Excitation of the indirect pathway results in disinhibition of the GLU projection from the STN to the output nuclei, which activates them, such that they, in turn, inhibit their thalamocortical and brainstem targets (indir in Fig. 2A), consistent with behavioral suppression. Modern optogenetic and chemicogenetic functional-anatomical studies have provided results consistent with the afore-mentioned theoretical functional roles of the direct and indirect pathways, showing that the direct pathway facilitates and indirect pathway interferes with acquisition and retention of reward-based learning (Hikida et al., 2010; Kravitz et al., 2012; Ferguson et al 2013), behavioral sensitization (Ferguson et al., 2011), movement and movement initiation (Kravitz et al., 2010; Tai et al., 2012; Agnoli et al., 2013; Farrell et al., 2013; Sano et al., 2013; Freeze et al., 2013), risk-based decision making (Stopper et al., 2013) and cocaine self-administration (Bock et al., 2013).
Excitation of the direct and indirect pathways is usually attributed to the cortical inputs, but may also (or instead) reflect activity in thalamostriatal projections from the midline-intralaminar thalamic nuclei (Pasupathy and Miller, 2005). Indeed, the midline-intralaminar thalamus has been much neglected as a potentially important source of input to MdSNs (Fig. 2B), insofar as it transmits informational content derived from the brainstem reticular formation (Ramon-Moliner, 1975; Groenewegen and Berendse, 1994; van der Werf et al., 2002; Geisler, 2009), presumably including interoceptive and nociceptive signaling (Peschanski and Besson, 1984; Bernard et al., 1996; Craig, 1996; Gariau and Bernard, 2002; Willis et al, 2004). In any event, a recent retrograde labeling investigation using a modified rabies virus showed that the overall patterns of afferent innervation of the two pathways differ (Wall et al., 2013).
Discovery of the direct and indirect pathways owed to differential visualization within them of peptides, immediate early genes and reporters of metabolic activity following administration of DA receptor subtype specific ligands (Trugman and Wooten, 1987; Albin et al., 1989; Mitchell et al., 1989; Robertson et al., 1990; Engber et al., 1990). MdSNs that give rise to the direct pathway express mainly DA D1 receptors, which mediate facilitatory responses to the binding of DA (Fig. 2A, green arrow), whereas those that give rise to the indirect pathway express DA D2 receptors that mediate DA-elicited inhibitory responses (Fig. 2A, yellow arrow). Consequently, increased striatal DA concentration should enhance behavioral activation associated with direct pathway-mediated inhibition and attenuate behavioral suppression associated with indirect pathway-mediated excitation of GABA neurons in the output nuclei. But, might not the direct pathway also suppress, and the indirect pathway also enhance, the activity of DA neurons in the SNc and SNr? Were this to occur, paradoxical behavioral correlates should ensue. What actually happens, of course, depends completely on how the axonal terminations of the direct and indirect pathways are distributed in the SNc and SNr relative to GABA and DA projection neurons, and, as it turns out, various data are discordant on this point. Whereas several functional studies indicate that the direct pathway acts preferentially on GABA neurons in the VTA and SNr (Grace and Bunney, 1979; 1985; Chuhma et al., 2011; Xia et al., 2011; Bocklisch et al., 2013) and negligibly affects the activity of nigrostriatal DA neurons, the available ultrastructural evidence shows, in contrast, that striatonigral (Smith et al., 1987; Nitsch and Riesenberg, 1988; Anderson et al., 1991; Pickel et al., 1993; Groenewegen et al., 1994) and pallidonigral (Hattori et al., 1975; Smith and Bolam, 1990) projections have abundant synaptic contacts with both DA and non-DA neurons. In addition, a recent chemicogenetic study designed to selectively demonstrate the afferents specifically of DA neurons in the VTA and SNc revealed that they receive modest to robust inputs from virtually all structures that project to the SNr (Watabe-Uchida et al., 2012). Of particular relevance to the present topic, this study showed particularly strong direct projections to DA neurons from MdSNs in the caudate-putamen, which is incongruous with the above-cited functional studies.
Thus, however well it has served as a heuristic framework, the theory of direct and indirect cortico-basal ganglia-thalamocortical pathways falls short of comprehensively modelling basal ganglia control of the activity of its DA and non-DA outputs, not only due to the aforementioned issues of connection specificity, but also because it neglects important connectivity (Fig. 2B). For example, Watabe-Uchida et al. (2012) also observed that SNc DA neurons are contacted by a strong projection from the EPN, which is a connection incongruous with the theory of direct and indirect pathways. Nor does the theory account for the so-called hyper direct projection (hyperdir in Fig. 2B) from the cortex to the STN (Nambu et al., 2002; Nambu, 2009; Jahfari et al., 2011; Nougaret et al., 2013) or a robust projection from one output nucleus, the EPN, to the other, the SNr. Possible preferential activation of the direct or indirect pathways by midline-intralaminar thalamic inputs is also not considered in the theory (Groenewegen and Berendse, 1994; van der Werf et al., 2002). Presumably, any of these connections could exert profound modulation on through-conduction of signals in the basal ganglia. Moreover, any theory concerned with this circuitry must take into account the effects of neruopeptides such as dynorphin, substance P, enkephalins and neurotensin, which co-exist with GABA in various subpopulations of MdSNs and tier 1 neurons (e.g., Deutch et al., 1985; Kalivas, 1985; Kelley and Cador, 1988; Steiner and Gerfen, 1998; Zahm et al., 1998; Geisler and Zahm, 2006; Reynolds et al. 2006).
None of this is meant to imply that the overall pattern of inputs to the SNc is not distinct from that to the SNr. Indeed, the ventral tier of the SNc is preferentially innervated by MdSNs in the CPu patch compartment, to which the ventral tier, in turn, preferentially projects (Gerfen, 1984; Jimenez-Castellanos and Graybiel, 1989a; b). In addition, abundant reports describe a lateralward spread through the SNc of numerous afferents that traverse the mfb, including ones originating in the Acb (Nauta and Domesick, 1978; Nauta et al., 1978; Nauta and Domesick, 1984; Zahm and Heimer, 1990; 1993; Heimer et al., 1991), ventral pallidum (Groenewegen et al., 1993), lateral hypothalamus (van der Kooy et al., 1984), paraventricular hypothalamic nucleus (Geerling et al., 2010); EAc (Krettek and Price, 1978; Gonzales and Chesselet,1990; Rosen et al., 1991; Zahm et al., 1999; Dong et al., 2001; Gastard et al., 2002) and parabrachial nucleus (Coizet et al., 2012). In this regard, Nauta and Domesick (1984) pointed out that “the limbic striatum’ seems enabled by its striatonigral efferents to modulate not only the source of its own dopamine innervation but also that of a large additional striatal region,” which they maintained is consistent with Mogenson’s ‘motivation to action’ conceptualization of the Acb-subpallidal-VTA axis (Mogenson et al., 1980). Haber and colleagues later picked up on this theme in describing a lateralward ‘spiraling’ of striato-nigro-striatal connectivity (Haber et al., 2000), which has generated interest in the drug abuse field. The ‘spirals’ are viewed as a presumptive substrate for a process by which early, rewarding, medially-mediated phases of drug self-administration later progress to a state thought to be more habitual, consistent with the role of lateral parts of the striatum in the formation and maintenance of motor habits (Everitt and Robbins, 2005; Balleine and O’Doherty, 2010, Belin-Rauschent et al., 2012).
Additional important SNc inputs
The RMTg provides a very robust GABA projection to the entire midbrain DA complex, including the SNc (Jhou et al. 2009a; b; Balcita-Pedicino et al., 2011). Activation of the RMTg has a strong suppressive effect on mesotelencephalic DA neurotransmission (Jhou et al., 2009a; Hong et al., 2011; Matsui and Williams, 2011) and thus is thought to have fundamental implications for neuropsychiatry and therapy (Barrot and Thome, 2011; Lavezzi and Zahm, 2011). The RMTg receives a very dense GLU input from the LHb, which is a presumptive integrator of negative hedonic information conveyed in afferent projections to the LHb from the basal forebrain, particularly the ventral pallidum (Haber et al., 1993; Tachibana and Hikosaka, 2012) and a site extending from the internal medullary lamina through the GPi and into the underlying sublenticular region that Hong and Hikosaka (2008; 2013) have called the GP border region (GPb). Contrary to expectations, projections from GPb to the LHb are excitatory (Shabel et al., 2012; Hong and Hikosaka; 2013). In addition, the lateral preoptic area has prominent neuroanatomical relationships with the RMTg, involving both direct projections (Zahm et al., 2011a) and a projection that relays in the LHb (Kowski et al., 2008). Thus, the RMTg is a final common site of through-conduction from widespread parts of brain for negative hedonic information to access DA neurons throughout the midbrain DA complex.
Redgrave and colleagues have promoted a projection to the SNc preferentially from the deep layers of the superior colliculus (Comoli et al., 2003; Coizet et al., 2003; McHaffie et al., 2006; Redgrave et al., 2008), which, consonant with its role in conveying short latency visual information, they suggest subserves the critical mental property of agency, i.e., an individual’s awareness of its own role in causation.
Presynaptic stimulation of DA release in forebrain structures
Afferents of DA neurons of the midbrain DA complex need not terminate within the complex itself, but rather may target the terminals of DA axons, particularly in forebrain structures. Howland et al. (2002) have demonstrated that DA is released into the Acb and PfC upon stimulation of GLU projections from the basal amygdala to those structures. Reverse microdialysis of DNQX, an antagonist of the AMPA-kainate GLU receptor, into the Acb or PfC, but not the VTA, blocked the effect, which thus is interpreted as a paracrine-like axo-axonal interaction, insofar as projections from the basal amygdala to the Acb and PfC do not have axo-axonic synapses with DA axon terminals. This mechanism of releasing DA in forebrain may have extraordinary behavioral significance, particularly in the primate, and especially human, brain, wherein mesocortical and mesothalamic DA projections are much more pervasive than in the rat (Lewis and Sesack, 1997; Lewis et al., 1998; Bentivoglio and Morelli, 2005).
Efferent connections
The VTA and SNc are widely regarded as having distinct efferent projections and, insofar as, respectively, about 60% and 80% of neurons comprising them are DA, the difference in part reflects the existence of distinct DA mesolimbic (A10) and nigrostriatal (A9) projection systems. This taxonomy, however, severely under-emphasizes extensive overlap of the terminal fields of VTA and SNc neurons (Beckstead et al., 1979; Nauta and Domesick, 1984; Lynd-Balta and Haber, 1994a; b; c; Bentivoglio and Morelli, 2005; Björklund and Dunnett, 2007). Indeed, nearly every telencephalic region that receives DA innervation has a medial aspect innervated by the VTA and a lateral aspect innervated by the SNc (Fallon and Moore, 1978), the two separated by a broad district in which VTA and SNc projections overlap (e.g., Nauta and Domesick, 1984). Nevertheless, SNc projects mainly to the CPu and in lesser abundance to cortical structures, amygdala and STN (Fallon and Moore, 1978; Fallon et al., 1978; Loughlin and Fallon, 1983; Gerfen et al., 1987; Jimenez-Castellano and Graybiel, 1987b; Lavoie et al., 1989; Smith and Villalba, 2008). It should be noted that the density of terminations of the mesostriatal DA projection as revealed with a modern promoter-driven expression of reporter molecule (Matsuda et al., 2009) appear to be much greater than demonstrated in any previous conventional tract tracing study. This may reflect a lesser capacity of axonally transported tracers such as PHA-L to reach all of the terminations of mesostriatal neurons, although it can be said that the photographed axonal terminations shown in Matsuda et al. (2009) appears less dense than the graphically illustrated ones (compare Figs. 1 and 3 – 7). Nonetheless, the data therein mainly are consistent with previous studies indicative of a specificity in these projections beyond gross topography, in that the ventral tier of the SNc projects preferentially to the striatal patch compartment (Gerfen et al., 1987; Jimenez-Castellanos and Graybiel, 1987).
The VTA projects most robustly to the Acb and olfactory tubercle, but also innervates the orbitofrontal and motor cortices, striatum, lateral septum, ventral pallidum, EA, subventricular zone, LHb, entorhinal cortex and hippocampus, including the ventral subiculum and stratum oriens of CA1 and CA3 (Fallon and Moore 1978; Fallon et al., 1978; Scatton et al., 1980; Swanson, 1982; Verney et al., 1985; Gasbarri et al., 1994, 1997; Goldsmith and Joyce, 1994; Gaykema and Zaborsky, 1996, 1997; Shammah-Lagnado et al., 1999; Hasue and Shammah- Lagnado, 2002; Santiago and Shammah-Lagnado, 2005; Del-Fava et al., 2007; Ikemoto, 2007; Hosp et al., 2011; Lennington et al., 2011). Whereas axonal projections originating from neurons in the VTA are mainly unbranched and thus target one structure, those from the SNc were reported to project more abundantly via axon collaterals to subcortical structures and the cortex (Fallon, 1981; Fallon and Loughlin, 1982; Swanson, 1982; Loughlin and Fallon, 1984; Sobel and Corbett, 1984; Takada and Hattori, 1986; Chandler et al., 2013). However, there has never been uniform agreement on the subject of the collateralization of mesofugal axons (e.g., Febvret et al., 1991) and a recent study with a modified, GFP-expressing virus injected into single SNc DA neurons revealed no significant collateralization of SNc DA axons (Matsuda et al., 2009), suggesting that collateralization observed in earlier studies may have involved non-DA neurons.
The RRF and the PAGvl (which contains the A10dc neurons) also project to numerous forebrain structures but most prominently to the EA. The issue of which outputs from these structures are DAergic is exacerbated by the substantial proportions of non-DA projection neurons in the RRF and PAGvl. The RRF projects to the olfactory tubercle, striatum, Acb, interstitial nucleus of the posterior anterior commissure, BST, amygdala, and pyriform and entorhinal cortices (Jimenez-Castellanos and Graybiel, 1987; Arts et al., 1996), but with particular density to EA structures (Deutch et al., 1988 Shammah-Lagnado et al., 1999; Hasue and Shammah-Lagnado, 2002). Outputs from the PAGvl terminate in the Acb, caudate putamen, medial prefrontal cortex, lateral septum, hippocampus, magnocellular basal forebrain, LHb, BST, and amygdala (Pohle et al., 1984; Descarries et al., 1986; Semba et al., 1988; Yoshida et al., 1989; Stratford and Wirtshafter, 1990; Li et al., 1993), but, again, most strongly in the EAc (Hasue and Shammah-Lagnado, 2002). Hasue and Shammah-Lagnado (2002) found that of all TH immunoreactive projections to the central amygdaloid nucleus (CeA) and lateral division of the BST almost half arise from neurons in the PAGvl, indicating that the A10dc group of neurons provides a main catecholaminergic input to the EAc. It is notable that A10dc neurons are reported to utilize L-DOPA rather than DA as a catecholamine neurotransmitter (Misu et al., 1996). An early report that the CeA (in cat) projects strongly to the SNl (Shinonaga et al., 1992), suggested that the SNl might, in turn, provide a main nigroamygdaloid projection. However, Moriizumi et al. (1992) demonstrated that the SNl projects mainly to the inferior colliculus via GABA neurons and to the superior colliculus, caudoventral CPu and, as later confirmed by Hasue and Shammah-Lagnado (2002), modestly to the amygdala with GABA and DA neurons.
A somewhat similar situation may exist vis a vis a dense projection from the supramammillary nucleus to the lateral septum (Shepard et al, 1988; Vertes, 1992), of which about 40% of the neurons of origin are dopaminergic neurons (Swanson, 1982) residing in Hökfelt’s et al. (1984b) A10rv group. Like the projection from A10dc to extended amgydala (Hasue and Shammah-Lagnado, 2002), that from A10rv to the lateral seputm supplements a substantial dopaminergic projection to the septum from the main body of the VTA (Lindall and Stenevi, 1978). A10rv neurons that give rise to this projection are reported to have several electrophysiological characteristics resembling nigral and VTA dopamine neurons (Shepard et al., 1988). Terminations of dopaminergic projections to the the lateral septum form pericellular baskets (Gall and Moore, 1984) around MdSNs that exhibit somatic spines (Jakab and Leranth, 1990).
Striatal DA-GLU interactions and the synaptic triad
DA and GLU inputs characterize all MdSN-containing structures and are particularly strong in the CPu, Acb and striatal districts of the olfactory tubercle. Very dense plexuses of DA (TH-imunoreactive) axons and axon varicosities are present in these striatal structures (Lindvall and Björkland, 1983; Voorn et al., 1989; Pickel et al., 1975; Bouyer et al., 1984), which also exhibit an abundance of VGLUT1-containing cortical and cortical-like inputs and equivalently dense, VGLUT2-containing thalamic inputs (e.g., Fremeau et al., 2001; Härtig et al., 2003). DA-GLU interactions thus play a major role in shaping functional potency in these structures (Wan et al., 1995; Morari et al., 1998), but in ways reflecting considerable mechanistic heterogeneity depending on the specific afferent systems involved (David et al., 2005). A so-called striatal synaptic triad, first described in neostriatum (Freund et al., 1984), has been the subject of considerable interest as regards striatal DA-GLU interactions. The triad comprises an MdSN dendritic spine with an asymmetrical synapse (excitatory, GLU), typically on the spine head, and a symmetrical synapse (DA), typically at the spine neck (Freund et al, 1984; Sesack and Pickel, 1990), an arrangement that may confer special capacity for the integration of DA and GLU influences in the striatum, possibly via gating of the GLU signal by the DA signal (Freund et al., 1984). However, Wilson et al. (1983) had estimated that in neostriatum only about 8% of spines get the symmetrical input and Bennett and Wilson (2000) later pointed out that only half of the symmetrical contacts are DA. Similar data are not available for ventral striatum, but one might anticipate similar numbers, or perhaps even fewer DA contributions to the spine innervation, particularly in the Acb shell (Zahm, 1992). These quantitative data suggest that the importance of the triad as a predominant substrate for MdSN DA-GLU interactions may be overstated.
Non-DA efferent connections
It has long been recognized that GABA cells are intermingled with DA neurons throughout the midbrain (Mugnaini and Oertel, 1985; Steffensen et al., 1998; Olson and Nestler, 2007); however, little is known regarding their efferent projections. One region known to receive VTA GABA input is the Acb (Van Bockstaele and Pickel, 1995; Carr and Sesack, 2000a; Van Zessen et al., 2012), wherein preferential VTA-derived GABA input to cholinergic interneurons has been reported (Brown et al., 2013). The mPfC is also innervated by VTA GABA neurons (Carr and Sesack, 2000a).
Interestingly, the rostral linear subnucleus of the VTA (VTArl), which is reported to contain few DA neurons (Ikemoto, 2007), has efferent connections that differ from the rest of the VTA. Specifically, VTArl projects heavily to a number of structures that receive modest DA inputs, including the ventral pallidal parts of the olfactory tubercle, magnocellular preoptic nucleus, lateral hypothalamus, central division of the mediodorsal thalamic nucleus, and the lateral part of the LHb (Del-Fava et al., 2007). Accordingly, a recent chemicogenetic study in mice revealed a general complementarity in the forebrain distributions of GABA and DA VTA projections (Taylor and Picciotto, 2013). In addition, Gorelova et al. (2012) remarked on the abundance of anterogradely labeled fibers that co-express the GLU marker VGLUT2 following injections of anterograde tracer into the rostral linear nucleus.
GLU neurons are present in all VTA subnuclei and project to both the mPfC and Acb (Yamaguchi et al., 2007, 2011; Gorelova et al., 2012; Hnasko et al., 2012). Whereas the Acb clearly gets heavier DA than GLU input, the opposite pattern is observed in the mPfC (Yamaguchi et al., 2011; Gorelova et al., 2012). Additional studies in mice have revealed that GLU neurons that reside in the medial part of the VTA and exhibit electrophysiological characteristics of medial VTA DA neurons preferentially target the Acb, mPfC, amygdala, ventral pallidum and LHb. Interestingly, those projections, like GABA input to the forebrain, are densest in areas of lesser DA input (Hnasko et al., 2012). These workers also noted that mice appear to have a lesser GLU VTA projection to the mPfC than rats.
Co-transmission in DA neurons
Longstanding evidence for co-release of GLU from midbrain DA complex DA neurons and axons (Kocsis and Kitai, 1977; Sulzer et al., 1998; Joyce and Rayport, 2000; Chuhma et al., 2004; Dal Bo et al., 2004; Descarries et al., 2008; Broussard, 2012) has been bolstered by recent technical advances. Optical stimulation of DA terminals in the Acb shell, but not dorsal striatum, of adult mice evokes GLU-mediated postsynaptic responses in MdSNs. The GLU signal is abolished by genetic deletion of VGLUT2 in mesoaccumbal but not mesostriatal DA axons (Stuber et al., 2010; Tecuapetla et al., 2010), which indicates that GLU is co-expressed by DA neurons in the VTA, but not SNc. Consistent with this, researchers have been able to detect little VGLUT2 expression in SNc DA neurons (Hnasko et al., 2010; Yamaguchi et al., 2013). It is unclear if DA and GLU are packaged together in the same synaptic vesicles of VTA DA neurons or even occupy the same axon terminals. That is, one study in rat found that VGLUT2 co-immunoprecipitates with the vesicular monoamine transporter 2 (VMAT2) from striatal synaptic vesicles (Hnasko et al., 2010), but no double-labeling of TH and VGLUT2 was detected in axon terminals in the mouse Acb in another study (Bérubé-Carrière et al., 2012).
It also seems that GABA is co-expressed in some ventral midbrain DA and GLU neurons (Rodriguez and Gonzalez-Hernandez, 1999). Remarkably, striatal DA terminals that co-labeled with VMAT2, but not the vesicular GABA transporter, can co-release GABA, which in turn postysnaptically inhibits striatal MdSNs (Tritsch et al, 2012). Blockade of VMAT2 prevented the inhibitions, suggesting that GABA is packaged by VMAT2 in these terminals. There also is emerging evidence that a unique population of LHb-projecting VTA neurons co-expresses GLU and GABA. Early reports indicated that VTA efferents to the LHb are mainly GABA (Swanson, 1982; Skagerberg et al., 1984; Del-Fava et al., 2007), but it was recently determined that about 50% of LHb-projecting VTA neurons are DA (Gruber et al., 2007). Stamatakis et al. (2013), however, observed that optogenetic stimulation of neurons targeted by their DA phenotype caused no detectable release of DA in the LHb, but instead produced a GABA inhibition of LHb neurons. Alternatively Root et al. (2013) demonstrated that close to 90% of meso-habenular neurons expresses both GAD and VGLUT2 and when VGLUT2 was targeted for optogenetic stimulation of these neurons, a GABA A receptor-mediated inhibitory response was elicited in the LHb (Mejias-Aponte et al., 2013). Although these reports are mutually supportive in spirit, the details need to be further ironed out. Incidentally, optical stimulation of VTA VGLUT2-expressing fibers in the ventral pallidum also elicits short latency inhibitory postsynaptic responses, indicative of GABA co-transmission in GLU, ventral pallidum-projecting VTA neurons (Hnasko et al., 2012).
As regards DA neurons that co-express neuropeptides, CCK is expressed by VTA DA neurons that project to the Acb, olfactory tubercle, septum and orbitofronal cortex, while SNc neurons that co-express CCK project to the striatum and anterior cingulate cortex (Hökfelt et al., 1980b; Seroogy and Fallon, 1989). Other VTA DA neurons projecting to the Acb and mPfC express NT (Hökfelt et al., 1984a; Kalivas and Miller 1984; Studler et al., 1988) and a subset of DA neurons that co-expresses both CCK and NT has been reported (Seroogy et al., 1987, 1988).
Heterogeneity of VTA DA neurons by projection target
Accumulating evidence indicates that VTA DA neurons are physiologically heterogeneous. A variety of morphological, electrophysiological and molecular biological characteristics of VTA DA neurons vary with their projection targets. Earlier studies showed that DA neurons in the medial part of the VTA projecting to medial Acb are smaller and have fewer and shorter dendritic branches as compared to lateral VTA neurons projecting to more lateral parts of the Acb (Tan et al., 1995). Lammel et al. (2008) extended these findings in a recent study that employed experimental neuroanatomical, electrophysiological, immunohistochemical and laser-dissection techniques to demonstrate one group of neurons centered in the medial VTA that projects to the mPfC, Acb medial shell and core, and basolateral amygdala and another positioned mainly in the lateral VTA that projects to the Acb lateral shell and ventrolateral caudate-putamen. Whereas medial VTA neurons are fast-firing and have low dopamine transporter (DAT)/TH mRNA ratios, lateral VTA neurons have a “classical” slow firing pattern reminiscent of SNc DA neurons and express DAT more robustly. DA neurons projecting to the mPfC were uniquely unresponsive to (normally inhibitory) stimulation of somatodendritic D2 autoreceptors, an effect apparently due to their low levels of D2 receptor and GIRK2 potassium channel expression (but see Margolis et al., 2008). Lammel et al. (2011) also found that the modulation of excitatory synaptic potentials elicited in VTA DA neurons by rewarding and aversive stimuli also varies as a function of projection target. Administration of cocaine increased AMPA/NMDA ratios in medial VTA DA neurons projecting to Acb medial shell, whereas infusion of formalin into the hindpaw (subchronic pain) increased AMPA/NMDA ratios in medial VTA DA neurons projecting to the mPfC. In contrast, both reward and pain increased AMPA/NMDA ratios in lateral VTA neurons projecting to the Acb lateral shell. Finally, Ford et al. (2006) showed in mice that VTA DA neurons projecting to the Acb have a greater inhibitory response to administration of kappa opioid agonist than amygdala-projecting VTA neurons. Interestingly, Margolis et al. (2006) observed the opposite, i.e., more kappa-mediated inhibition in amygdala-projecting VTA neurons, in the rat.
Rodent versus primate
Notable differences exist between rodents and primates in the patterns of midbrain DA projections to cortical and thalamic regions (Berger et al., 1991; Lewis and Sesack, 1997; Garcia-Cabezas et al., 2009). In the rat, robust DA innervation is observed in prefrontal, anterior cingulate, insular, piriform, entorhinal and perirhinal cortex, whereas there is only light innervation in posterior cingulate, motor, parietal and temporal regions (Berger et al., 1991). The functional impact of even a modest cortical DA innervation, however, may be profound (e.g., Bao et al., 2001). In contrast to the rodent, DA innervation in the primate targets all cortical regions, most densely in prefrontal and motor areas (Lewis et al., 1987; Gaspar et al., 1989; Berger et al., 1991; Williams and Goldman-Rakic 1993; Lewis and Sesack, 1997). Rat and monkey also exhibit regional differences in laminar density of DA inputs. In the rat, cortical DA innervation is limited mainly to deep layers, whereas both superficial and deep layers exhibit robust DA inputs in the primate (Descarries et al., 1987; Berger et al., 1991, Williams and Goldman-Rakic 1993; Lewis et al., 2001). The differences between rat and primate lead to speculation that DA innervation of cortical regions in the human brain may be even more expanded and dense. However, the results of a recent study comparing DA innervation in human and chimpanzee brain were not consistent with this expectation, insofar as DA projections to the cortex of humans were not obviously more extensive as compared to chimpanzees. Subtle differences in the patterns of DA innervation of the human as compared to chimpanzee cortex were evident, however, wherein humans show a sublaminar pattern of DA input in layer I of cortical areas 9 and 32 that is absent in the chimpanzee (Raghanti et al., 2008). It is also noteworthy that primates exhibit as much DA innervation of dorsal thalamic nuclei (including midline, mediodorsal, lateral posterior, and ventrolateral) as they do of cortical regions, whereas rodents have little DA innervation in comparable thalamic regions (Groenewegen 1988; Papadopoulos and Parnavelas 1990; Sanchez-Gonzalez et al., 2005; Garcia Cabezas et al., 2007, 2009). In contrast, related species differences involving the DA innervation of ventral thalamic nuclei and the epithalamus are not apparent (Garcia Cabezas et al., 2009).
Prospectus
The main purpose of investigating brain morphology and axonal connections is to provide clues about how to investigate brain function. The midbrain DA complex is famously uncharitable in this regard, insofar as its connections, including intrinsic, afferent and efferent, are so numerous and diverse and, in part, so apparently indiscriminate in distribution, as to tend to obscure rather than clarify mechanisms that may subserve its contribution to overall brain function. But this is the nature of reticular formation to which we would argue the midbrain DA complex belongs. About all that distinguishes structures comprising the midbrain DA complex from reticular formation are the dense projections of the midbrain DA complex to deep telencephalic nuclei and cortex. However, a variety of other structures that have been associated with, actually included within, reticular formation also have robust rostropetal projections. The midline-intralaminar thalamic nuclei (Ramon-Moliner, 1975) project massively to striatum and prefrontal cortex. The lateral hypothalamus-preoptic continuum (McMullen and Almli, 1981) has substantial projections to telencephalic deep nuclei and cortex. Basal forebrain cholinergic cell groups (Mesulam, 1995) project very strongly to cortex and, less so, thalamus. A common portrayal of reticular formation as ‘diffusely’ organized is falsified by the subtlety and sophistication with which it orchestrates the interactions of neuroendocrine, autonomic and somatomotor effectors (Swanson and Mogenson, 1981). Moreover, careful attention to structure that might have been regarded as diffuse, e.g., the projections of midline-intralaminar thalamic nuclei to cortex and striatum, clearly revealed that it is not (Groenewegen and Berendse, 1994; van der Werf et al., 2002). To the contrary, organization said to be ‘diffuse’, including that of the midbrain DA complex, despite the elusiveness of its underlying principles, undoubtedly ranks with the most elegant in brain.
The complexity of the local DA, GABA, GLU and neuropeptide network(s) of the midbrain DA complex in combination with uncertainty about the extent to which afferent influences are exerted on select neuron subpopulations versus extended functional-anatomical networks renders functional analysis of the midbrain DA complex a formidable undertaking. As regards outputs, an evidence-based impression that all DA neurons burst fire and inactivate in unison in response to presentations of reward predicting cues and omissions of expected rewards, respectively (Mirenowicz and Schultz, 1996; Schultz et al., 1997; Hollerman and Schultz, 1998; Schultz, 1998; Hong et al., 2011), is disputed by the work of Matsumoto and Hikosaka (2009) and Matumoto and Takada (2013), whose findings, in turn, are incongruous with other functional-anatomical evidence for a hedonic-aversive duality in midbrain DA complex input-output pathways (Lammel et al., 2008; 2011; 2012; 2014; Roeper, 2013). Despite the elegance and transformative impact of these and other studies afore-cited in this review, how the organization of the midbrain DA complex permits its contributions to all of the various functional realms that it is reported to modulate (see refs. in Introduction) remains a topic for continuing research.
We would argue that the depth of our understanding of the functional role of the midbrain DA complex will continue to increase only if accompanied by continued elucidation of its neuroanatomical relationships. Conventional experimental neuroanatomical studies continue to be valuable, having recently revealed, on one hand, important gross organizational principles, such as, e.g., the topographical relationships between sectors of the midbrain DA complex and various basal forebrain functional-anatomical macrosystems (Fig. 3), and, on the other hand, fine details of synaptic organization that yield only to determined ultrastructural investigation (e.g., Bayer and Pickel, 1990; Carr and Sesack, 2000a; b; Omelchenko et al., 2009; Balcita-Pedicino et al., 2011). In the same vein, it was a keen sense of neuroanatomical organization revealed with traditional methods that led to the discovery of the RMTg (Jhou, 2005; Perrotti et al., 2005), which has provided a new perspective from which to view the afferent regulation of the midbrain DA complex (Jhou et al., 2009a; Lavezzi and Zahm, 2011; Bourdy and Barrot, 2012).
Figure 3.
Diagram depicting topographic input-output relationships (inputs and outputs are same colors) that relate various sectors of the midbrain dopaminergic (DA) complex to basal forebrain functional-anatomical macrosystems. Dots (color coded respectively) indicate the regions of terminations of direct and transynaptic (e.g., striato-vental pallido-nigral) afferents of the macrosystems within the midbrain DA complex. Thus, the medial part of the ventral tegmental area (VTA) gets input from and gives output to the lateral septal-preoptic system. Much of the VTA receives from and projects to the medial accumbens (Acb), including the shell, and its ventral pallidal projection area. The Acb core and its ventral pallidal target area project to lateral parts of the VTA and much of the substantia nigra pars compacta (SNc) and gets output from them, where as the caudate-putamen and topographically related parts of the pallidum (dorsal striatopallidum) projects mainly to the SNc. The central division of the extended amygdala projects most strongly to lateral parts of the SNc, RRF and ventrolateral periaqueductal gray (PAGvl) and gets outputs from these regions. Note that the SNc has the most heterogeneous input of the entire complex, due to the circumstance that all of the macrosystems (except lateral septum-preoptic area) have projections that enter the complex in topographical relation, but diverge lateralward through the SNc enroute to the lateral retrorubral field (RRF) and PAGvl. Additional abbreviations: cp - cerebral peduncle; IPN -interpeduncular nucleus; lat. - lateral; med. - medial; ml - medial lemniscus; SNr - substantia nigra compacta; Tu - olfactory tubercle
More and more, however, it appears that future developments in our comprehension of the neurobiology of the midbrian DA complex will most depend upon continuing creative applications of modern chemicogenetic and optogenetic technology to reveal specificity of connections with the aid of viral delivery of promoter-specific, neuron-filling reporter molecules and provide optogenetic-electophysiological validations (Smedemark-Margulies and Trapani, 2013; Williams and Deiserroth, 2013). Roeper (2013), however, has pointed out some pitfalls and conflicing reports that have emerged in the interpretation of behavioral patterns elicited by optogenetic stimulations, which introduce into brains large neuronal activations and inactivations in unnatural, isolated spatial and temporal patterns that otherwise would never occur.
Indeed, one wonders if the role of the midbrain DA complex in the modulation of behavior can be captured in its essence by modern hyper-reductionist technology. A capacity to define circuit specificity does not diminish the reality of how abundant is the seemingly ‘irrational’ connectivity that characterizes the inputs and outputs of the midbrain DA complex. Even hints that occult specificity can underlie confusingly converged projections (MacAskill et al., 2012) do not deter our sense that the next leap in our understanding of functional-anatomical organization in the midbrain DA complex may come from an entirely different direction - something that helps us appreciate the neurobiological logic and adaptive value of mapping connectional specificity onto a background of connectional promiscuity.
Highlights.
-
-
Intrinsic organization and connections
-
-
Afferent connections
-
-
Efferent connections
-
-
Prospectus
Acknowledgement
The authors are grateful to Kenneth P. Parsley for providing superbly immunolabeled material shown in Figure 1.
Support: USPHS NIH grant NS-23805
List of Abbreviations
- Acb
accumbens
- BST
bed nucleus of the stria terminalis
- CeA
central nucleus of the amygdala
- CCK
cholecystokinin
- CPu
caudate-putamen
- DA
dopamine and dopaminergic
- DAT
dopamine transporter
- c
caudal
- dc
dorsal, caudal
- dl
dorsolateral
- EA
extended amygdala
- EAc
extended amygdala, central division
- EPN
entopeduncular nucleus
- GABA
gamma-aminobutyric acid and GABAerg
- GAD
glutamate decarboxylase
- GLU
glutamate and glutamatergic
- GP
globus pallidus
- GPb
globus pallidus, border region
- GPi
globus pallidus, internal segment
- LDTg
laterodorsal tegmental nucleus
- LHb
lateral habenula
- m
medial
- MdSN
medium densely spiny neuron
- MeA
medial nucleus of the amygdala
- mfb
medial forebrain bundle
- NT
neurotensin
- PAG
periaqueductal gray
- PfC
prefrontal cortex
- PHA-L
Phaseolus vulgaris leucoagglutinin
- PPTg
pedunculopontine tegmental nucleus
- RMTg
rostromedial tegmental nucleus
- RRF
retrorubral field
- SNc
substantia nigra pars compacta
- SNl
substantia nigra pars lateralis
- SNr
substantia nigra pars reticulata
- STN
subthalamic nucleus
- TH
tyrosine hydroxylase
- tVTA
tail of the ventral tegmental area
- VGLUT1
vesicular glutamate transporter 1
- VGLUT2
vesicular glutamate transporter 2
- vl
ventrolateral
- VMAT2
vesicular monoamine transporter 2
- VTA
ventral tegmental area
- VTArl
rostral linear nucleus of the VTA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. The J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnoli L, Mainolfi P, Invernizzi RW, Carli M. Dopamine D1-like and D2-like receptors in the dorsal striatum control different aspects of attentional performance in the five-choice serial reaction time task under a condition of increased activity of corticostriatal inputs. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:701–714. doi: 10.1038/npp.2012.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Allison DW, Ohran AJ, Stobbs SH, Mameli M, Valenzuela CF, Sudweeks SN, Ray AP, Henriksen SJ, Steffensen SC. Connexin-36 gap junctions mediate electrical coupling between ventral tegmental area GABA neurons. Synapse. 2006;60:20–31. doi: 10.1002/syn.20272. [DOI] [PubMed] [Google Scholar]
- Alsiö J, Nordenankar K, Arvidsson E, Birgner C, Mahmoudi S, Halbout B, Smith C, Fortin GM, Olson L, Descarries L, Trudeau LE, Kullander K, Levesque D, Wallen-Mackenzie A. Enhanced sucrose and cocaine self-administration and cue-induced drug seeking after loss of VGLUT2 in midbrain dopamine neurons in mice. J Neurosci. 2011;31:12593–12603. doi: 10.1523/JNEUROSCI.2397-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andén NE, Carlsson A, Dahlström A, Fuxe K, Hillarp NÅ, Larsson K. Demonstration and mapping out of nigro-neostriatal dopaminergic neurons. Life Sci. 1964;3:523–530. doi: 10.1016/0024-3205(64)90161-4. [DOI] [PubMed] [Google Scholar]
- Andén NE, Dahlström A, Fuxe K, Larsson K. Mapping out of catecholaminergic and 5-hydroxtryptamine neurons innervating the telencephalon and diencephalon. Life Sci. 1965;4:12751279. doi: 10.1016/0024-3205(65)90076-7. [DOI] [PubMed] [Google Scholar]
- Andén NE, Dahlström A, Fuxe K, Larsson K, Olson L, Ungerstedt U. Ascending monoamine neurons to the telencephalon and diencephalon. Acta Physiol. Scand. 1966a;67:313–326. [Google Scholar]
- Andén NE, Fuxe K, Hamberger B, Hökfelt T. A quantitative study on the nigro-striatal dopamine neuron system in the rat. Acta Physiol. Scand. 1966b;67:306–312. doi: 10.1111/j.1748-1716.1966.tb03317.x. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Karle EJ, Reiner A. Ultrastructural single- and double-label immunohistochemical studies of substance P-containing terminals and dopaminergic neurons in the substantia nigra in pigeons. The Journal of comparative neurology. 1991;309:341–362. doi: 10.1002/cne.903090305. [DOI] [PubMed] [Google Scholar]
- Arts MPM, Groenewegen HJ, Veening JG, Cools AR. Efferent projections of the retrorubral nucleus to the substantia nigra and ventral tegmental area in cats as shown by anterograde tracing. Brain Res Bull. 1996;40:219–228. doi: 10.1016/0361-9230(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;1(56 Suppl):112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC. The serotonin-producing neurons of the midbrain medial and dorsal raphe nuclei. In: Iversen LL, Iversen SD, Snyder SH, editors. Handbook of Psychopharmacology. Vol 9. New York: Chemcial pathways in the brain, Plenum Press; 1978. pp. 233–314. [Google Scholar]
- Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. The Journal of comparative neurology. 2011;519:1143–1164. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503:668–684. doi: 10.1002/cne.21420. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Barrot M, Calza L, Pozza M, Le Moal M, Piazza PV. Differential calbindin-immunoreactivity in dopamine neurons projecting to the rat striatal complex. Eur J Neurosci. 2000;12:4578–4582. [PubMed] [Google Scholar]
- Barrot M, Thome J. Discovering a new anatomical structure in the brain: implications for neuropsychiatry and therapy. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2011;(12 Suppl 1):19–22. doi: 10.3109/15622975.2011.598386. [DOI] [PubMed] [Google Scholar]
- Bayer Pickel. Ultrastructural localization of tyrosine hydroxylase in the rat ventral tegmental area: relationship between immunolabeling density and neuronal associations. J Neurosci. 1990;10(9):2996–3013. doi: 10.1523/JNEUROSCI.10-09-02996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Harrington F, Nader K, van der Kooy D. Neurobiology of motivation: double dissociation of two motivational mechanisms mediating opiate reward in drug-naive versus drug-dependent animals. Behav Neurosci. 1992;106:798–807. doi: 10.1037//0735-7044.106.5.798. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJH. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Belin-Rauscent A, Everitt BJ, Belin D. Intrastriatal shifts mediate the transition from drug-seeking actions to habits. Biological psychiatry. 2012;72:343–345. doi: 10.1016/j.biopsych.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Wilson CJ. In: Synaptology and physiology of neostriatal neurones. Miller R, Wickens JR, editors. Amsterdam: Brain Dynamics and the Striatal Complex, Harwood; 2000. pp. 111–140. pgs. [Google Scholar]
- Bentivoglio M, Morelli M. The organization and circuits of mesencephalic dopaminergic neurons and the distribution of dopamine receptors in the brain. In: Dunnett SB, Bentivoglio M, Björkland A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy: Dopamine. Vol. 21. Amsterdam: H Elsevier Press; 2005. pp. 1–107. [Google Scholar]
- Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Berman AC. A Cytoarchitectonic Atlas with Stereotaxic Coordinates. Madison WI: Univ Wisconsin Press; 1968. Thje brain STem of the Cat. [Google Scholar]
- Bernard JF, Bester H, Besson JM. Involvement of the spino-parabrachio -amygdaloid and -hypothalamic pathways in the autonomic and affective emotional aspects of pain. Progress in brain research. 1996;107:243–255. doi: 10.1016/s0079-6123(08)61868-3. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain research Brain research reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Smith AD, Bolam JP. The substantia nigra as a site of synaptic integration of functionally diverse information arising from the ventral pallidum and the globus pallidus in the rat. Neuroscience. 1996;75:5–12. doi: 10.1016/0306-4522(96)00377-6. [DOI] [PubMed] [Google Scholar]
- Bérubé-Carrière N, Guay G, Fortin GM, Kullander K, Olson L, Wallén-Mackenzie A, Trudeau L-E, Descarries L. Ultrastructural characterization of the mesostriatal dopamine innervation in mice, including two mouse lines of conditional VGLUT2 knockout in dopamine neurons. Eur J Neurosci. 2012;35:527–538. doi: 10.1111/j.1460-9568.2012.07992.x. [DOI] [PubMed] [Google Scholar]
- Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Björklund A, Lindvall O. Dopamine-containing systems in the CNS. In: Björklund A, Hökfelt T, editors. Classical Transmitters in the CNS. Handbook of Chemical Neuroanatomy. Volume 2. Amsterdam: Elsevier; 1984. pp. 55–122. Part I. [Google Scholar]
- Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, Gremel CM, Christensen CH, Adrover MF, Alvarez VA. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16:632–638. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocklisch C, Pascoli V, Wong JC, House DR, Yvon C, de Roo M, Tan KR, Luscher C. Science. Vol. 341. New York, NY: 2013. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area; pp. 1521–1525. [DOI] [PubMed] [Google Scholar]
- Bourdy R, Barrot M. A new control center for dopaminergic systems: pulling the VTA by the tail. Trends Neurosci. 2012;35(11):681–90. doi: 10.1016/j.tins.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Bouyer JJ, Joh TH, Pickel VM. Ultrastructural localization of tyrosine hydroxylase in rat nucleus accumbens. J Comp Neurol. 1984;227:92–103. doi: 10.1002/cne.902270110. [DOI] [PubMed] [Google Scholar]
- Broussard JI. Co-transmission of dopamine and glutamate. J Gen Physiol. 2012;139:93–96. doi: 10.1085/jgp.201110659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MTC, Tan KR, O’Connor EC, Nikonenko I, Muller D, Lüscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2013;492:452–456. doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Terminals from the rat prefrontal cortex synapse on mesoaccumbens VTA neurons. Ann N Y Acad Sci. 1999;877:676–678. doi: 10.1111/j.1749-6632.1999.tb09299.x. [DOI] [PubMed] [Google Scholar]
- Carr D, Sesack S. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000a;38:114–123. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000b;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking in rats. Psychopharmacology. 2013;226:155–165. doi: 10.1007/s00213-012-2902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol & Behav. 2010;100:419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DJ, Lamperski CS, Waterhouse BD. Location and distribution of projections from monoaminergic and cholinergic nuclei to functionally differentiated subregions of prefrontal cortex. Brain Res. 2013;1522:38–58. doi: 10.1016/j.brainres.2013.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charara A, Parent A. Chemoarchitecture of the primate dorsal raphe nucleus. Journal of chemical neuroanatomy. 1998;15:111–127. doi: 10.1016/s0891-0618(98)00036-2. [DOI] [PubMed] [Google Scholar]
- Charara A, Smith Y, Parent A. Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: Phaseolus vulgaris-Leucoagglutinin anterograde labeling combined with postembedding Glutamate and GABA immunohistochemistry. J Comp Neurol. 1996;364:254–266. doi: 10.1002/(SICI)1096-9861(19960108)364:2<254::AID-CNE5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Chen L, Lodge DJ. The lateral mesopontine tegmentum regulates both tonic and phasic activity of VTA dopamine neurons. Journal of neurophysiology. 2013;110:2287–2294. doi: 10.1152/jn.00307.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Choi WY, Mingote S, Rayport S. Dopamine neuron glutamate cotransmission: frequency-dependent modulation in the mesoventromedial projection. Neuroscience. 2009;164:1068–1083. doi: 10.1016/j.neuroscience.2009.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Tanaka KF, Hen R, Rayport S. Functional connectome of the striatal medium spiny neuron. J Neurosci. 2011;31:1183–1192. doi: 10.1523/JNEUROSCI.3833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Zhang H, Masson J, Zhuang X, Sulzer D, Hen R, Rayport S. Dopaminergic neurons mediate a fast excitatory signal via their glutamatergic synapses. J Neurosci. 2004;24:972–981. doi: 10.1523/JNEUROSCI.4317-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coizet V, Comoli E, Westby GW, Redgrave P. Phasic activation of substantia nigra and the ventral tegmental area by chemical stimulation of the superior colliculus: an electrophysiological investigation in the rat. Eur J Neurosci. 2003;17:28–40. doi: 10.1046/j.1460-9568.2003.02415.x. [DOI] [PubMed] [Google Scholar]
- Coizet V, Dommett EJ, Klop EM, Redgrave P, Overton PG. The parabrachial nucleus is a critical link in the transmission of short latency nociceptive information to midbrain dopaminergic neurons. Neuroscience. 2012;168:263–272. doi: 10.1016/j.neuroscience.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG. Locally collateralizing glutamate neurons in the dorsal raphe nucleus responsive to substance P contain vesicular glutamate transporter 3 (VGLUT3) J Chem Neuroanat. 2009;38:273–281. doi: 10.1016/j.jchemneu.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoli E, Coizet V, Boyes J, Bolam JP, Canteras NS, Quirk RH, Overton PG, Redgrave P. A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat Neurosci. 2003;6:974–980. doi: 10.1038/nn1113. [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Greenfield SA. Differential autoreceptor control of somatodendritic and axon terminal dopamine release in substantia nigra, ventral tegmental area, and striatum. J Neurosci. 1997;17:5738–5746. doi: 10.1523/JNEUROSCI.17-15-05738.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. An ascending general homeostatic afferent pathway originating in lamina I. Progress in brain research. 1996;107:225–242. doi: 10.1016/s0079-6123(08)61867-1. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Cholecystokinin-dopamine interactions. Trends Pharmacol Sci. 1991;12:232–236. doi: 10.1016/0165-6147(91)90558-a. [DOI] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Evidence for the existence of monoamine- containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand. 1964;62(Suppl 232):1–55. [PubMed] [Google Scholar]
- Dal Bo G, St-Gelais F, Danik M, Williams S, Cotton M, Trudeau LE. Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine. J Neurochem. 2004;88:1398–1405. doi: 10.1046/j.1471-4159.2003.02277.x. [DOI] [PubMed] [Google Scholar]
- David HN, Ansseau M, Abraini JH. Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of “intact” animals. Brain Res Rev. 2005;50:336–360. doi: 10.1016/j.brainresrev.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Del-Fava F, Hasue RH, Ferreira JGP, Shammah-Lagnado SJ. Efferent connections of the rostral linear nucleus of the ventral tegmental area in the rat. Neuroscience. 2007;145:1059–1076. doi: 10.1016/j.neuroscience.2006.12.039. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Menetrey A, Charpier S. The lamellar organization of the rat substantia nigra pars reticulata: segregated patterns of striatal afferents and relationship to the topography of corticostriatal projections. Neuroscience. 1996;73:761–781. doi: 10.1016/0306-4522(96)00088-7. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Thierry AM. Anatomical segregation of information processing in the rat substantia nigra pars reticulata. Adv Neurol. 1997;74:83–96. [PubMed] [Google Scholar]
- de Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci. 1999;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Maccari S, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. I. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7181–7188. doi: 10.1523/JNEUROSCI.15-11-07181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Berthelet F, Garcia S, Beaudet A. Dopaminergic projection from nucleus raphe dorsalis to neostriatum in the rat. J Comp Neurol. 1986;249:511–520. doi: 10.1002/cne.902490407. [DOI] [PubMed] [Google Scholar]
- Descarries L, Bérubé-Carrière N, Riad M, Dal Bo G, Mendez JA, Trudeau LE. Glutamate in dopamine neurons: synaptic versus diffuse transmission. Brain Res Rev. 2008;58:290–302. doi: 10.1016/j.brainresrev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Descarries L, Lemay B, Doucet G, Berger B. Regional and laminar density of the dopamine innervation in adult rat cerebral cortex. Neuroscience. 1987;21:807–824. doi: 10.1016/0306-4522(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Goldstein M, Baldino F, Roth RH. Telencephalic projections of the A8 dopamine cell group. Ann N Y Acad Sci. 1988;537:27–50. doi: 10.1111/j.1749-6632.1988.tb42095.x. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Maggio JE, Bannon MJ, Kalivas PW, Tam SY, Goldstein M, Roth RH. Substance K and substance P differentially modulate mesolimbic and mesocortical systems. Peptides 6 Suppl. 1985;2:113–122. doi: 10.1016/0196-9781(85)90143-3. [DOI] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Want HL, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci. 2010;30(1):218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H-W, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABA-A inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Amamy H, Holland PC. Dissociable effects of disconnecting amygdala central nucleus from the ventral tegmental area or substantia nigra on learned orienting and incentive motivation. The Eur J Neurosci. 2007;25:1557–1567. doi: 10.1111/j.1460-9568.2007.05402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engber TM, Susel Z, Kuo S, Chase TN. Chronic levodopa treatment alters basal and dopamine agonist-stimulated cerebral glucose utilization. J Neurosci. 1990;10:3889–3895. doi: 10.1523/JNEUROSCI.10-12-03889.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Fallon JH. Collateralization of monoamine neurons: mesotelencephalic dopamine projections to caudate, septum, and frontal cortex. J Neurosci. 1981;1:1361–1368. doi: 10.1523/JNEUROSCI.01-12-01361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH. Topographic association of ascending dopaminergic projections. Ann N Y Acad Sci. 1988;537:1–9. doi: 10.1111/j.1749-6632.1988.tb42093.x. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Loughlin SE. Monoamine innervation of the forebrain: collateralization. Brain Res Bull. 1982;9:295–307. doi: 10.1016/0361-9230(82)90143-5. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Loughlin SE. Monoamine innervation of cerebral cortex and a theory of the role of monoamines in cerebral cortex and basal ganglia. Cerebral Cortex. 1985;6:41–127. [Google Scholar]
- Fallon JH, Loughlin SE. Substantia nigra. In: Paxinos G, editor. The Rat Nervous System. 2nd Edition. Volume 1. Sydney: Academic Press; 1995. pp. 215–237. Forebrain and Midbrain. [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Riley JN, Moore RY. Substantia nigra dopamine neurons: separate populations project to neostriatum and allocortex. Neurosci Lett. 1978;7:157–162. doi: 10.1016/0304-3940(78)90160-x. [DOI] [PubMed] [Google Scholar]
- Farrell MS, Pei Y, Wan Y, Yadav PN, Daigle TL, Urban DJ, Lee HM, Sciaky N, Simmons A, Nonneman RJ, Huang XP, Hufeisen SJ, Guettier JM, Moy SS, Wess J, Caron MG, Calakos N, Roth BL. A Galphas DREADD mouse for selective modulation of cAMP production in striatopallidal neurons. Neuropsychopharmacol. 2013;38:854–862. doi: 10.1038/npp.2012.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febvret A, Berge B, Gaspar P, Verney C. Further indication that distinct dopaminergic subsets project to the rat cerebral cortex: lack of colocalization with neurotensin in the superficial dopaminergic fields of the anterior cingulate, motor, retrosplenial and visual cortices. Brain Res. 1991;547:37–52. doi: 10.1016/0006-8993(91)90572-d. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Ann Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Phillips PE, Roth BL, Wess J, Neumaier JF. Direct-pathway striatal neurons regulate the retention of decision-making strategies. J Neurosci. 2013;33:11668–11676. doi: 10.1523/JNEUROSCI.4783-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira JGP, Del-Fava F, Hause RH, Shammah-Lagnado SJ. Organisation of ventral tegmental area projections to the ventral tegmental area-nigral complex in the rat. Neuroscience. 2008;153:196–213. doi: 10.1016/j.neuroscience.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin GM, Bourque MJ, Mendez JA, Leo D, Nordenankar K, Birgner C, Arvidsson E, Rymar VV, Berube-Carriere N, Claveau AM, Descarries L, Sadikot AF, Wallen-Mackenzie A, Trudeau LE. Glutamate corelease promotes growth and survival of midbrain dopamine neurons. J Neurosci. 2012;32:17477–17491. doi: 10.1523/JNEUROSCI.1939-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC. Control of Basal Ganglia output by direct and indirect pathway projection neurons. J Neurosci. 2013;33:18531–18539. doi: 10.1523/JNEUROSCI.1278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr., Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci (USA) 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr., Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr., Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of T excitatory neurons and suggest novel roles for glutamate. TINS. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13:1189–215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Haber SN. The central nucleus of the amygdala projection to dopamine subpopulations in primates. Neuroscience. 2000;97:479–494. doi: 10.1016/s0306-4522(00)00092-0. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Haber SN. Bed nucleus of the stria terminalis and extended amygdala inputs to dopamine subpopulations in primates. Neuroscience. 2001;104:807–827. doi: 10.1016/s0306-4522(01)00112-9. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Gall C, Moore RY. Distribution of enkephalin, substance P, tyrosine hydroxylase, and 5-hydroxytryptamine immunoreactivity in the septal region of the rat. J Comp Neurol. 1984;225:212–227. doi: 10.1002/cne.902250207. [DOI] [PubMed] [Google Scholar]
- Garcia-Cabezas MA, Martinez-Sanchez P, Sanchez-Gonzalez MA, Garzón M, Cavada C. Dopamine innervation in the thalamus: monkey versus rat. Cerebral Cortex. 2009;19:424–434. doi: 10.1093/cercor/bhn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cabezas MÁ, Rico B, Sánchez-González MÁ, Cavada C. Distribution of the dopamine innervation in the macaque and human thalamus. NeuroImage. 2007;34:965–984. doi: 10.1016/j.neuroimage.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde t racing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res Bull. 1994;33:445–452. doi: 10.1016/0361-9230(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Packard MG. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1–22. doi: 10.1016/s0278-5846(96)00157-1. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvret A, Vigny A, Henry JP. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J Comp Neurol. 1989;279:249–271. doi: 10.1002/cne.902790208. [DOI] [PubMed] [Google Scholar]
- Gastard MC, Jensen SL, Martin JR, III, Williams EA, Zahm DS. The caudal sublenticular region/anterior amygdaloid area is the only part of the rat forebrain and mesopontine tegmentum occupied by magnocellular cholinergic neurons that receives outputs from the central division of extended amygdala. Brain Research. 2002;957:207–222. doi: 10.1016/s0006-8993(02)03513-8. [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Experimental physiology. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Záborszky L. Direct catecholaminergic-cholinergic interactions in the basal forebrain. II. Substantia nigra-ventral tegmental area projections to cholinergic neurons. J Comp Neurol. 1996;374:555–577. doi: 10.1002/(SICI)1096-9861(19961028)374:4<555::AID-CNE6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Záborszky L. Parvalbumin-containing neurons in the basal forebrain receive direct input from the substantia nigra-ventral tegmental area. Brain Res. 1997;747:173–179. doi: 10.1016/s0006-8993(96)01309-1. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol. 2010;518:1460–1499. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260:258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- Geisler S. Reticular Formation. In: Binder MD, Hirokawa N, Windhorst U, editors. Encyclopedia of Neuroscience. Vol 4. Springer; 2009. pp. 3482–3486. [Google Scholar]
- Geisler S, Bérod A, Zahm DS, Rostène W. Brain neurotensin, psychostimulants, and stress. Peptides. 2006;27:2364–2384. doi: 10.1016/j.peptides.2006.03.037. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Marinelli M, Degarmo B, Becker ML, Freiman AJ, Beales M, Meredith GE, Zahm DS. Prominent activation of brainstem and pallidal afferents of the ventral tegmental area by cocaine. Neuropsychopharmacol. 2008;33:2688–2700. doi: 10.1038/sj.npp.1301650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat - anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Neurotensin afferents of the ventral tegmental area in the rat: [1] re-examination of their origins and [2] responses to acute psychostimulant and antipsychotic drug administration. Eur J Neurosci. 2006;24:116–134. doi: 10.1111/j.1460-9568.2006.04928.x. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopaminergic neuron. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Ann Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Baimbridge KG, Miller JJ. The neostriatal mosaic: compartmenta l distribution of calcium-binding protein and parvalbumin in the basal ganglia of the rat and monkey. Proc Natl Acad Sci USA. 1985;82:8780–8784. doi: 10.1073/pnas.82.24.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Herkenham M, Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci. 1987;7:3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Ann Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith SK, Joyce JN. Dopamine D2 receptor expression in hippocampus and parahippocampal cortex of rat, cat, and human in relation to tyrosine hydroxylase-immunoreactive fibers. Hippocampus. 1994;4:354–373. doi: 10.1002/hipo.450040318. [DOI] [PubMed] [Google Scholar]
- Gonzales C, Chesselet M-F. Amygdalonigral pathway: an anterograde study in the rat with Phaseolus vulgaris Leucoagglutinin (PHA-L) J Comp Neurol. 1990;297:182–200. doi: 10.1002/cne.902970203. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Mulholland PJ, Chandler LJ, Seamans JK. Cerebral cortex. Vol. 22. New York, NY: 2012. The glutamatergic component of the mesocortical pathway emanating from different subregions of the ventral midbrain; pp. 327–336. 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. The dopamine system and the pathophysiology of schizophrenia: a basic science perspective. Int Rev Neurobiol. 2007;78:41–68. doi: 10.1016/S0074-7742(06)78002-3. [DOI] [PubMed] [Google Scholar]
- Goto M, Swanson LW. Axonal projections from the parasubthalamic nucleus. J Comp Neurol. 2004;469:581–607. doi: 10.1002/cne.11036. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Paradoxical GABA excitation of nigral dopaminergic cells: indirect median through reticulata inhibitory neurons. Eur J Pharmacol. 1979;59:211–218. doi: 10.1016/0014-2999(79)90283-8. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons. 3. Evidence for electrotonic coupling. Neuroscience. 1983;10:333–348. doi: 10.1016/0306-4522(83)90137-9. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Opposing effects of striatonigral feedback pathways on midbrain dopamine cell activity. Brain Res. 1985;333:271–284. doi: 10.1016/0006-8993(85)91581-1. [DOI] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Hirsch EC, Agid Y. The nigrostriatal system in Parkinson’s disease. Advances in neurology. 1990;53:17–29. [PubMed] [Google Scholar]
- Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW. The specificity of the ‘nonspecific’ midline and intralaminar thalamic nuclei. Trends Neurosci. 1994;17:52–57. doi: 10.1016/0166-2236(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57:113–142. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Wouterlood FG. In: Organization of the projections from the ventral striato-pallidal system to ventral mesencephalic dopaminergic neurons in the rat. Percheron G, editor. New York: Basal Ganglia IV. Plenum Press; 1994. pp. 81–93. [Google Scholar]
- Groenewegen HJ, Russchen FT. Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: a tracing and immunohistochemical study in the cat. J Comp Neurol. 1984;223:347–367. doi: 10.1002/cne.902230303. [DOI] [PubMed] [Google Scholar]
- Gruber C, Kahl A, Lebenheim L, Kowski A, Dittgen A, Veh RW. Dopaminergic projections from the VTA substantially contribute to the mesohabenular pathway in the rat. Neurosci Lett. 2007;427:165–170. doi: 10.1016/j.neulet.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. Journ al of chemical neuroanatomy. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL. The primate substantia nigra and VTA: integrative circuitry and function. Crit Rev Neurobiol. 1997;11:323–342. doi: 10.1615/critrevneurobiol.v11.i4.40. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Lynd-Balta E, Mitchell SJ. The organization of the descending ventral pallidal projections in the monkey. J Comp Neurol. 1993;329:111–128. doi: 10.1002/cne.903290108. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacol. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Ryoo H, Cox C, Lu W. Subsets of midbrain dopaminergic neurons in monkeys are distinguished by different levels of mRNA for the dopaminergic transporter: comparison with the mRNA for the D2 receptor, tyrosine hydroxylase and calbindin immunoreactivity. J Comp Neurol. 1995;362:400–410. doi: 10.1002/cne.903620308. [DOI] [PubMed] [Google Scholar]
- Han J-S, McMahan RW, Holland P, Gallagher M. The role of an amygdalo-nigrostriatal pathway in associative learning. J Neurosci. 1997;17:3913–3919. doi: 10.1523/JNEUROSCI.17-10-03913.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtig W, Riedel A, Grosche J, Edwards RH, Fremeau RT, Jr., Harkany T, Brauer K, Arendt T. Complementary distribution of vesicular glutamate transporters 1 and 2 in the nucleus accumbens of rat: Relationship to calretinin-containing extrinsic innervation and calbindin-immunoreactive neurons. J Comp Neurol. 2003;465:1–10. doi: 10.1002/cne.10789. [DOI] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: A combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- Hattori T, Fibiger HC, McGeer PL. Demonstration of a pallido-nigral projection innervating dopaminergic neurons. J Comp Neurol. 1975;162:487–504. doi: 10.1002/cne.901620406. [DOI] [PubMed] [Google Scholar]
- Heimer L. The olfactory connections of the diencephalon in the rat. An experimental light- and electron-microscopic study with special emphasis on the problem of terminal degeneration. Brain, behavior and evolution. 1972;6:484–523. doi: 10.1159/000123728. [DOI] [PubMed] [Google Scholar]
- Heimer L, Alheid GF, Zaborszky L. Basal Ganglia. In: Paxinos G, editor. The Rat Nervous System. 2nd Edition. Volume 1. Sydney: Academic Press; 1985. pp. 37–86. Forebrain and Midbrain. [Google Scholar]
- Heimer L, Alheid GF, Zahm DS. Basal Ganglia. In: Paxinos G, editor. The Rat Nervous System. 2nd Edition. Volume 1. Sydney: Academic Press; 1995. pp. 579–628. pgs Forebrain and Midbrain. [Google Scholar]
- Heimer L, Van Hoesen GW. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW, Trimble M, Zahm DS. Anatomy of Neuropsychiatry: The New Anatomy of the Basal Forebrain and its Implications for Neuropsychiatric Disease. Amsterdam- New York - San Diego: Elsevier; 2008. [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Henny P, Brown MTC, Northrop A, Faunes M, Ungless MA, Magill PJ, Bolam JP. Structural correlates of heterogeneous in vivo activity of midbrain dopaminergic neurons. Nature Neuroscience. 2012;15:613–619. doi: 10.1038/nn.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve D, Pickel VM, Joh TH, Beaudet A. Serotonin axon terminals in the ventral tegmental area of the rat: fine structure and synaptic input to dopaminergic neuro ns. Brain research. 1987;435(1–2):71–83. doi: 10.1016/0006-8993(87)91588-5. [DOI] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66(6):896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Hioki H, Nakamura H, Ma YF, Konno M, Hayakawa T, Nakamura KC, Fujiyama F, Kaneko T. Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J Comp Neurol. 2010;518:668–686. doi: 10.1002/cne.22237. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH. Ventral tegmental area glutamate neurons: electrophysiological properties and projections. J Neurosci. 2012;32:15076–15085. doi: 10.1523/JNEUROSCI.3128-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T, Everitt BJ, Theodorsson-Norheim E, Goldstein M. Occurrence of neurotensinlike immunoreactivity in subpopulations of hypothalamic, mesencephalic, and medullary catecholamine neurons. J Comp Neurol. 1984a;222:543–559. doi: 10.1002/cne.902220407. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Mårtensson R, Björklund A, Kleinau S, Goldstein M. Distribution maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain. In: Björklund A, Hökfelt T, editors. Classical Transmitters in the CNS. Handbook of Chemical Neuroanatomy. Volume 2. Amsterdam, Netherlands: Elsevier Press; 1984b. pp. 277–379. Part I. [Google Scholar]
- Hökfelt T, Rehfeld JF, Skirboll L, Ivemark B, Goldstein M, Markey K. Evidence for coexistence of DA and CCK in mesolimbic neurones. Nature. 1980a;285:476–478. doi: 10.1038/285476a0. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Skirboll L, Rehfeld JF, Goldstein M, Markey K, Dann O. A subpopulation of mesencephalic dopamine neurons projecting to limbic areas contains a cholecystokinin-like peptide: evidence from immunohistochemistry combined with retrograde tracing. Neuroscience. 1980b;5:2093–2124. doi: 10.1016/0306-4522(80)90127-x. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1(4):304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Hong S. Dopamine system: manager of neural pathways. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron. 2008;60:720–729. doi: 10.1016/j.neuron.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. Diverse sources of reward value signals in the basal ganglia nuclei transmitted to the lateral habenula in the monkey. Front Hum Neurosci. 2013;7:778. doi: 10.3389/fnhum.2013.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith MK, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci. 2011;31(32):11457–11471. doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR. Dopaminergic Projections from Midbrain to Primary Motor Cortex Mediate Motor Skill Learning. J Neurosci. 2011;31:2481–2487. doi: 10.1523/JNEUROSCI.5411-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland JG, Taepavarapruk P, Phillips AG. Glutamate receptor-dependent modulation of dopamine efflux in the nucleus accumbens by basolateral, but not central, nucleus of the amygdala in rats. J Neurosci. 2002;22:1137–1145. doi: 10.1523/JNEUROSCI.22-03-01137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Bonci A. Neurocircuitry of drug reward. Neuropharmacology. 2014;76(Pt B):329–341. doi: 10.1016/j.neuropharm.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs KR, Jacobowitz DM. Mapping of the colocalization of calretinin and tyrosine hydroxylase in the rat substantia nigra and ventral tegmental area. Exp Brain Res. 1994;99:34–42. doi: 10.1007/BF00241410. [DOI] [PubMed] [Google Scholar]
- Jahfari S, Waldorp L, van den Wildenberg WP, Scholte HS, Ridderinkhof KR, Forstmann BU. Effective connectivity reveals important roles for both the hyperdirect (fronto-subthalamic) and the indirect (fronto-striatal-pallidal) fronto-basal ganglia pathways during response inhibition. J Neurosci. 2011;31:6891–6899. doi: 10.1523/JNEUROSCI.5253-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, Leranth C. Catecholaminergic, GABAergic, and hippocamposeptal innervation of GABAergic “somatospiny” neurons in the rat lateral septal area. J Comp Neurol. 1990;302:305–321. doi: 10.1002/cne.903020209. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC. Neural mechanisms of freezing and passive aversive behaviors. J Comp Neurol. 2005;493:111–114. doi: 10.1002/cne.20734. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009a;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009b;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Castellanos J, Graybiel AM. Subdivisions of the dopamine-containing A8-A9-A10 complex identified by their differential mesostriatal innervation of striosomes and extrastriosomal matrix. Neuroscience. 1987;23:223–242. doi: 10.1016/0306-4522(87)90285-5. [DOI] [PubMed] [Google Scholar]
- Jimenez-Castellanos J, Graybiel AM. Compartmental origins of striatal efferent projections in the cat. Neuroscience. 1989a;32:297–321. doi: 10.1016/0306-4522(89)90080-8. [DOI] [PubMed] [Google Scholar]
- Jimenez-Castellanos J, Graybiel AM. Evidence that histochemically distinct zones of the primate substantia nigra pars compacta are related to patterned distributions of nigrostriatal projection neurons and striatonigral fibers. Exp Brain Res. 1989b;74:227–238. doi: 10.1007/BF00248855. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: An analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- Joyce MP, Rayport S. Mesoaccumbens dopamine neuron synapses reconstructed in vitro are glutamatergic. Neuroscience. 2000;99:445–456. doi: 10.1016/s0306-4522(00)00219-0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Interactions between neuropeptides and dopamine neurons in the ventromedial mesencephalon. Neursosci Biobehav Rev. 1985;9:573–587. doi: 10.1016/0149-7634(85)90004-1. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Bourdelais A, Abhold R, Abbott L. Somatodendritic release of endogenous dopamine: in vivo dialysis in the A10 dopamine region. Neurosci Lett. 1989;100:215–220. doi: 10.1016/0304-3940(89)90687-3. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. A comparison of axonal and somatodendritic DA release using in vivo dialysis. J Neurochem. 1991;56:961–967. doi: 10.1111/j.1471-4159.1991.tb02015.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Miller JS. Neurotensin neurons in the ventral tegmental area project to the medial nucleus accumbens. Brain Res. 1984;300:157–160. doi: 10.1016/0006-8993(84)91351-9. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H, Hisano S. Particular subpopulations of midbrain and hypothalamic dopamine neurons expr e ss vesicular glutamate transporter 2 in the rat brain. J comp Neurol. 2006;498:581–592. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Cador M. Behavioral evidence for differential neuropeptide modulation of the mesolimbic dopamine system. Ann N Y Acad Sci. 1988;537:415–434. doi: 10.1111/j.1749-6632.1988.tb42124.x. 45. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger GM, Stuber GD, Zhang F, Myers MG, Deisseroth K, de Lecea L, Bonci A. Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J Neurosci. 2013;33:7618. doi: 10.1523/JNEUROSCI.2588-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, Malenka RC, Warden MR, Neve R, Tye KM, Deisseroth K. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496:219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirouac GJ, Li S, Mabrouk G. GABAergic projection from the ventral tegmental area and substantia nigra to the periaqueductal gray region and the dorsal raphe nucleus. J Comp Neurol. 2004;469:170–184. doi: 10.1002/cne.11005. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Kitai ST. Dual excitatory inputs to caudate spiny neurons from substantia nigra stimulation. Brain research. 1977;138:271–283. doi: 10.1016/0006-8993(77)90745-4. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Ponomarenko AA, Brown RE, Haas HL. Functional diversity of ventral midbrain dopamine and GABAergic neurons. Molec Neurobiol. 2004;29:243–259. doi: 10.1385/MN:29:3:243. [DOI] [PubMed] [Google Scholar]
- Kortleven C, Bruneau LC, Trudeau LE. Neurotensin inhibits glutamate-mediated synaptic inputs onto ventral tegmental area dopamine neurons through the release of the endocannabinoid 2-AG. Neuropharmacol. 2012;63:983–991. doi: 10.1016/j.neuropharm.2012.07.037. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K, Hataguchi Y, Nagatsu I, Wu JY, Ottersen OP, Storm-Mathisen J, Hama K. Catecholaminergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res. 1987;66:191–210. doi: 10.1007/BF00236215. [DOI] [PubMed] [Google Scholar]
- Kowski AB, Geisler S, Krauss M, Veh RW. Differential projections from subfields in the lateral preoptic area to the lateral habenular complex of the rat. J Comp Neurol. 2008;507:1465–1478. doi: 10.1002/cne.21610. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15(6):816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the forebrain and brainstem in the rat and cat. J Comp Neurol. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- Krzywkowski P, Jacobowitz DM, Lamour Y. Calretinin-containing pathways in the rat forebrain. Brain Res. 1995;705:273–294. doi: 10.1016/0006-8993(95)01167-6. [DOI] [PubMed] [Google Scholar]
- Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y, Minami M, Watanabe M. Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. J Neurosci. 2012;32:18035–18046. doi: 10.1523/JNEUROSCI.4057-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzle H. An autoradiographic analysis of the efferent connections from premotor and adjacent prefrontal regions (areas 6 and 9) in macaca fascicularis. Brain Behav Evol. 1978;15:185–234. doi: 10.1159/000123779. [DOI] [PubMed] [Google Scholar]
- Kunzle H, Akert K. Efferent connections of cortical, area 8 (frontal eye field) in Macaca fascicularis. A reinvestigation using the autoradiographic technique. J Comp Neurol. 1977;173:147–164. doi: 10.1002/cne.901730108. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RN. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol (Lond) 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacol. 2014;76(Pt B):351–359. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson’s disease. First of two parts. New Eng J Med. 1998a;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson’s disease. Second of two parts. New Eng J Med. 1998b;339:1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- Langer LF, Jimenez-Castellanos J, Graybiel AM. The substantia nigra and its relations with the striatum in the monkey. Progress in brain research. 1991;87:81–99. doi: 10.1016/s0079-6123(08)63048-4. [DOI] [PubMed] [Google Scholar]
- Lau CI, Wang HC, Hsu JL, Liu ME. Does the dopamine hypothesis explain schizophrenia? Rev Neurosci. 2013;24:389–400. doi: 10.1515/revneuro-2013-0011. [DOI] [PubMed] [Google Scholar]
- Lavezzi H, Zahm DS. The mesopontine tegmental nucleus: an integrative modulator of the reward system. Basal Ganglia. 2011;1:191–200. doi: 10.1016/j.baga.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie B, Smith Y, Parent A. Dopaminergic innervation of the basal ganglia in the squirrel monkey as revealed by tyrosine hydroxylase immunohistochemistry. J Comp Neurol. 1989;289:36–52. doi: 10.1002/cne.902890104. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Groshek F, Petrovich GD, Cantalini JP, Gallagher M, Holland PC. Role of amygdalo-nigral circuitry in conditioning of a visual stimulus paired with food. J Neurosci. 2005;25:3881–3888. doi: 10.1523/JNEUROSCI.0416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, Jones JC, Rhodes CJ, Chua S, Jr., Diano S, Horvath TL, Seeley RJ, Becker JB, Munzberg H, Myers MG., Jr. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennington JB, Pope S, Goodhear AE, Drozdowicz L, Daniels SB, Salamone JD, Conove JC. Midbrain Dopamine Neurons Associated with Reward Processing Innervate the Neurogenic Subventricular Zone. J Neurosci. 2011;31:13078–13087. doi: 10.1523/JNEUROSCI.1197-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH. The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J Neurosci. 1987;7:279–290. doi: 10.1523/JNEUROSCI.07-01-00279.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol. 2001;432:119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Sesack SR. Dopamine systems in the primate brain. In: Bloom FE, Bjorklund A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy, The Primate Nervous System. Amsterdam: Elsevier; 1997. pp. 261–373. Part I. [Google Scholar]
- Lewis DA, Sesack SR, Levey AI, Rosenberg DR. Adv Pharmacol. Vol. 42. San Diego, Calif; 1998. Dopamine axons in primate prefrontal cortex: specificity of distribution, synaptic targets, and development; pp. 703–706. [DOI] [PubMed] [Google Scholar]
- Li Y-Q, Takada M, Shinonaga Y, Mizuno N. The sites of origin of dopaminergic afferent fibers to the lateral habenular nucleus in the rat. J Comp Neurol. 1993;333:118–133. doi: 10.1002/cne.903330110. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Björkland A. Dopamine- and norepinephrine-containing neuron systems: Their anatomy in the rat brain. In: Emson PC, editor. Chemical Neuroanatomy. New York: Raven Press; 1983. pp. 229–256. [Google Scholar]
- Lindvall O, Stenevi U. Dopamine and noradrenaline neurons projecting to the septal area in the rat. Cell Tiss Res. 1978;190:383–407. doi: 10.1007/BF00219554. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Shin R, Ikemoto S. Dual role of medial A10 dopamine neurons in affective encoding. Neuropsychopharmacol. 2008;33:3010–3020. doi: 10.1038/npp.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci (USA) 2006a;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacol. 2006b;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Loopuijt LD, Zahm DS. Synaptologic and fine structural features distinguishing a subset of basal forebrain cholinergic neurons embedded in the dense intrinsic fiber network of the caudal extended amygdala. The Journal of comparative neurology. 2006;498:93–111. doi: 10.1002/cne.21044. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Fallon JH. Dopaminergic and non-dopaminergic projections to amygdala from substantia nigra and ventral tegmental area. Brain Res. 1983;262:334–338. doi: 10.1016/0006-8993(83)91029-6. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Fallon JH. Substantia nigra and ventral tegmental area projections to cortex: topography and collateralization. Neuroscience. 1984;11:425–435. doi: 10.1016/0306-4522(84)90034-4. [DOI] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Lynd-Balta E, Haber SN. The organization of midbrain projections to the ventral striatum in the primate. Neuroscience. 1994a;59:609–623. doi: 10.1016/0306-4522(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Lynd-Balta E, Haber SN. The organization of midbrain projections to the striatum in the primate: sensorimotor-related striatum versus ventral striatum. Neuroscience. 1994b;59:625–640. doi: 10.1016/0306-4522(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Lynd-Balta E, Haber SN. Primate striatonigral projections: a comparison of the sensorimotor-related striatum and the ventral striatum. J Comp Neurol. 1994c;345:562–578. doi: 10.1002/cne.903450407. [DOI] [PubMed] [Google Scholar]
- MacAskill AF, Little JP, Cassel JM, Carter AG. Subcellular connectivity underlies pathway-specific signaling in the nucleus accumbens. Nat Neurosci. 2012;15:1624–1626. doi: 10.1038/nn.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis E, Lock H, Chefer V, Shippenberg T, Hjelmstad G, Fields H. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D2 receptor inhibition. J Neurosci. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Toy B, Himmels P, Morales M, Fields HL. Identification of rat ventral tegmental area GABAergic neurons. PloS One. 2012;7:e42365. doi: 10.1371/journal.pone.0042365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Rudick CN, Hu X-T, White FJ. Excitability of dopamine neurons: modulation and physical consequences. CNS & Neurol Disorders - Drug Targets. 2006;5:79–97. doi: 10.2174/187152706784111542. [DOI] [PubMed] [Google Scholar]
- Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29:444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Williams JT. Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J Neurosci. 2011;31:17729–17735. doi: 10.1523/JNEUROSCI.4570-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Takada M. Distinct representations of cognitive and motivational signals in midbrain dopamine neurons. Neuron. 2013;79:1011–1024. doi: 10.1016/j.neuron.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Maurice N, Deniau JM, Menetrey A, Glowinski J, Thierry AM. Synapse. Vol. 29. New York, NY: 1998. Prefrontal cortex-basal ganglia circuits in the rat: involvement of ventral pallidum and subthalamic nucleus; pp. 363–370. [DOI] [PubMed] [Google Scholar]
- Maurin Y, Banrezes B, Menetrey A, Mailly P, Deniau JM. Three-dimensional distribution of nigrostriatal neurons in the rat: relation to the topography of striatonigral projections. Neuroscience. 1999;91:891–909. doi: 10.1016/s0306-4522(98)00681-2. [DOI] [PubMed] [Google Scholar]
- McDevitt RA, Chung SL, Bonci A. Non- serotonin neurons in the dorsal raphe nucleus mediate reward via excitation of ventral tegmental area dopamine neurons. Soc Neurosci Abstr. 2013;776:11. [Google Scholar]
- McDonald AJ, Beitz AJ, Larson AA, Kuriyama R, Sellito C, Madl JE. Co-localization of glutamate and tubulin in putative excitatroy neurons of the hippocampus and amygdala: an immunohistochemical study using monoclonal antibodies. Neuroscience. 1989;30:405–421. doi: 10.1016/0306-4522(89)90261-3. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Jiang H, May PJ, Coizet V, Overton PG, Stein BE, Redgrave P. A direct projection from superior colliculus to substantia nigra pars compacta in the cat. Neuroscience. 2006;138:221–234. doi: 10.1016/j.neuroscience.2005.11.015. [DOI] [PubMed] [Google Scholar]
- McMullen NT, Almli CR. Cell types within the medial forebrain bundle: a Golgi study of preoptic and hypothalamic neurons in the rat. Am J Anat. 1981;161:323–340. doi: 10.1002/aja.1001610306. [DOI] [PubMed] [Google Scholar]
- Mejias-Aponte CA, Drouin C, Aston-Jones G. Adrenergic and noradrenergic innervation of the midbrain ventral tegmental area and retrorubral field: prominent inputs from medullary homeostatic centers. J Neurosci. 2009;29:3613–3626. doi: 10.1523/JNEUROSCI.4632-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejias-Aponte C, Root DH, Ayorinde M, Morales M. Ventral tegmental area VGLUT2- expressing neurons inhibit lateral habenula neurons. Soc Neurosci Abstr. 2013;803:15. [Google Scholar]
- Mena-Segovia J, Winn P, Bolam JP. Cholinergic modulation of midbrain dopaminergic systems. Brain Res Rev. 2008;58:265–271. doi: 10.1016/j.brainresrev.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Mendez JA, Bourque MJ, Dal Bo G, Bourdeau ML, Danik M, Williams S, Lacaille JC, Trudeau LE. Developmental and target-dependent regulation of vesicular glutamate transporter expression by dopamine neurons. J Neurosci. 2008;28:6309–6318. doi: 10.1523/JNEUROSCI.1331-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Cholinergic pathways and the ascending reticular activating system of the human brain. Annals of the New York Academy of Sciences. 1995;757:169–179. doi: 10.1111/j.1749-6632.1995.tb17472.x. [DOI] [PubMed] [Google Scholar]
- Miller RL, Stein MK, Loewy AD. Serotonergic inputs to FoxP2 neurons of the pre-locus coeruleus and parabrachial nuclei that project to the ventral tegmental area. Neuroscience. 2011;193:229–240. doi: 10.1016/j.neuroscience.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Misu Y, Goshima Y, Ueda H, Okamura H. Neurobiology of L-DOPAergic systems. Prog Neurobiol. 1996;49:415–454. doi: 10.1016/0301-0082(96)00025-1. [DOI] [PubMed] [Google Scholar]
- Mitchell IJ, Clarke CE, Boyce S, Robertson RG, Peggs D, Sambrook MA, Crossman AR. Neural mechanisms underlying parkinsonian symptoms based upon regional uptake of 2-deoxyglucose in monkeys exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neuroscience. 1989;32(1):213–226. doi: 10.1016/0306-4522(89)90120-6. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Moore H, West AR, Grace AA. The regulation of forebrain dopamine transmission: relevance to the pathophysiology and psychopathology of schizophrenia. Biol Psychiat. 1999;46:40–55. doi: 10.1016/s0006-3223(99)00078-5. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the dopamine systems. Ann Rev Neurosci. 1978;1:129–169. doi: 10.1146/annurev.ne.01.030178.001021. [DOI] [PubMed] [Google Scholar]
- Morari M, Marti M, Sbrenna S, Fuxe K, Bianchi C, Beani L. Reciprocal dopamine- glutamate modulation of release in the basal ganglia. Neurochem Int. 1998;33:383–397. doi: 10.1016/s0197-0186(98)00052-7. [DOI] [PubMed] [Google Scholar]
- Moriizumi T, Leduc-Cross B, Wu JY, Hattori T. Separate neuronal populations of the rat substantia nigra pars lateralis with distinct projection sites and transmitter phenotypes. Neuroscience. 1992;46:711–720. doi: 10.1016/0306-4522(92)90157-w. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Paladini CA. Dynamic regulation of midbrain dopamine neuron activity: intrinsic, synaptic, and plasticity mechanisms. Neuroscience. 2011;198:95–111. doi: 10.1016/j.neuroscience.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnaini E, Oertel WH. Atlas of the distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunohistochemistry. In: Björklund A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy. GABA and neuropeptides in the CNS. Amsterdam: Elsevier; 1985. pp. 436–595. [Google Scholar]
- Nambu A. Functions of direct, indirect and hyperdirect pathways. Brain and Nerve. 2009;61:360–372. [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo- pallidal ‘hyperdirect’ pathway. Neurosci Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Nauta WJH, Domesick VB. Cross roads of limbic and striatal circuitary: hypothalamonigral connections. In: Livingston KE, Hornykiewicz O, editors. Limbic Mechanisms. New York: Plenum Press; 1978. pp. 75–93. [Google Scholar]
- Nauta WJH, Domesick VB. Afferent and efferent relationships of the basal ganglia. In: Evered D, O’Connor M, editors. Functions of the Basal Ganglia; Ciba Foundation symposium; New York: Raven Press; 1984. pp. 3–29. [DOI] [PubMed] [Google Scholar]
- Nauta WJ, Smith GP, Faull RL, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3:385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- Nagai T, McGeer PL, McGeer EG. Distribution of GABA-T-intensive neurons in the rat forebrain and midbrain. J Comp Neurol. 1983;218:220–238. doi: 10.1002/cne.902180209. [DOI] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, Gabaergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch C, Riesenberg R. Immunocytochemical demonstration of GABAergic synaptic connections in rat substantia nigra after different lesions of the striatonigral projection. Brain Research. 1988;461:127–142. doi: 10.1016/0006-8993(88)90731-7. [DOI] [PubMed] [Google Scholar]
- Nougaret S, Meffre J, Duclos Y, Breysse E, Pelloux Y. First evidence of a hy perdirect prefrontal pathway in the primate: precise organization for new insights on subthalamic nucleus functions. Front Comp Neurosci. 2013;7:135. doi: 10.3389/fncom.2013.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Research. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson V, Nestler E. Topographical organization of GABAergic neurons within the ventral tegmental area of the rat. Synapse. 2007;61:87–95. doi: 10.1002/syn.20345. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci. 2009;30:1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol. 2005;483:217–235. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons. J Comp Neurol. 2006;494:863–875. doi: 10.1002/cne.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience. 2007;146:1259–1274. doi: 10.1016/j.neuroscience.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko NV, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 2009;63:895–906. doi: 10.1002/syn.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko NV, Sesack SR. Periaqueductal gray afferents synapse onto dopamine and GABA neurons in the rat ventral tegmental area. J Neurosci Res. 2010;88:981–991. doi: 10.1002/jnr.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett CL, Lalive AL, Tan KR, Terunuma M, Munoz MB, Pangalos MN, Martinez-Hernandez J, Wantanabe M, Moss SJ, Lujan R, Luscher C, Slessinger PA. Methamphetamine-evoked depression of GABAB receptor signaling in GABA neurons of the VTA. Neuron. 2012;73:978–989. doi: 10.1016/j.neuron.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos GC, Parnavelas JG. Distribution and synaptic organization of dopaminergic Faxons in the lateral geniculate nucleus of the rat. J Comp Neurol. 1990;294:356–361. doi: 10.1002/cne.902940305. [DOI] [PubMed] [Google Scholar]
- Parent A, Cote PY, Lavoie B. Chemical anatomy of primate basal ganglia. Prog Neurobiol. 1995;46:131–197. [PubMed] [Google Scholar]
- Parent A, Parent M, Charara A. Glutamatergic inputs to midbrain dopaminergic neurons in primates. Parkinsonism & Related Disorders. 1999;5:193–201. doi: 10.1016/s1353-8020(99)00037-1. [DOI] [PubMed] [Google Scholar]
- Parent A, Cote PY, Lavoie B. Chemical anatomy of primate basal ganglia. Prog Neurobiol. 1995;46:131–197. [PubMed] [Google Scholar]
- Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–6. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Bolanos CA, Choi KH, Russo SJ, Edwards S, Ulery PG, Wallace DL, Self DW, Nestler EJ, Barrot M. DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur J Neurosci. 2005;21:2817–2824. doi: 10.1111/j.1460-9568.2005.04110.x. [DOI] [PubMed] [Google Scholar]
- Peschanski M, Besson JM. A spino-reticulo-thalamic pathway in the rat: an anatomical study with reference to pain transmission. Neuroscience. 1984;12:165–178. doi: 10.1016/0306-4522(84)90145-3. [DOI] [PubMed] [Google Scholar]
- Phillipson OT. The cytoarchitecture of the interfascicular nucleus and ventral tegmental area of Tsai in the rat. J Comp Neurol. 1979a;187:85–98. doi: 10.1002/cne.901870106. [DOI] [PubMed] [Google Scholar]
- Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979b;187:117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Philpot KB, Dallvechia-Adams S, Smith Y, Kuhar MJ. A cocaine-and-amphetamine- regulated-transcript peptide projection from the lateral hypothalamus to the ventral tegmental area. Neuroscience. 2005;135:915–925. doi: 10.1016/j.neuroscience.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Sesack SR. Cellular substrates for interactions between dynorphin terminals and dopamine dendrites in rat ventral tegmental area and substantia nigra. Brain research. 1993;602:275–289. doi: 10.1016/0006-8993(93)90693-h. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Joh TH, Reis DJ. Immunohistochemical localization of tyrosine hydroxylase in brain by light and electron microscopy. Brain Res. 1975;85:295–300. doi: 10.1016/0006-8993(75)90084-0. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Born B, Adams M, Kalivas PW. Repeated intra-ventral tegmental area administration of SKF-38393 induces behavioral and neurochemical sensitization to a subsequent cocaine challenge. J Pharmacol Exp Therap. 1996;278:384–392. [PubMed] [Google Scholar]
- Pohle W, Ott T, Muller-Welde P. Identification of neurons of origin providing the dopaminergic innervation of the hippocampus. J Hirnforsch. 1984;25:1–10. [PubMed] [Google Scholar]
- Prensa L, Gimenez-Amaya JM, Parent A, Bernacer J, Cebrian C. The nigrostriatal pathway: axonal collateralization and compartmental specificity. J Neural Trans Suppl. 2009:49–58. doi: 10.1007/978-3-211-92660-4_4. [DOI] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC. Cortical dopaminergic innervation among humans, chimpanzees, and macaque monkeys: A comparative study. Neuroscience. 2008;155:203–220. doi: 10.1016/j.neuroscience.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon-Moliner E. Specialized and generalized dendritic patterns. In: Santini M, editor. Golgi Centennial Symposium Proceedings. New York: Raven Press; 1975. pp. 87–100. pgs. [Google Scholar]
- Ramon-Moliner E, Nauta WJ. The isodendritic core of the brain stem. J Comp Neurol. 1966;126:311–335. doi: 10.1002/cne.901260301. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K, Reynolds J. What is reinforced by phasic dopamine signals? Brain Res Rev. 2008;58:322–339. doi: 10.1016/j.brainresrev.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Geisler S, Berod A, Zahm DS. Neurotensin antagonist acutely and robustly attenuates locomotion that accompanies stimulation of a neurotensin-containing pathway from rostrobasal forebrain to the ventral tegmental area. Eur J Neurosci. 2006;24:188–196. doi: 10.1111/j.1460-9568.2006.04791.x. [DOI] [PubMed] [Google Scholar]
- Rice ME, Richards CD, Nedergaard S, Hounsgaard J, Nicholson C, Greenfield SA. Direct monitoring of dopamine and 5-HT release in substantia nigra and ventral tegmental area in vitro. Exp Brain Res. 1994;100:395–406. doi: 10.1007/BF02738400. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ, Greenfield SA. Characteristics of electrically evoked somatodendrtic dopamine release in substantia nigra and ventral tegmental area in vitro. J Neurophysiol. 1997;77:853–862. doi: 10.1152/jn.1997.77.2.853. [DOI] [PubMed] [Google Scholar]
- Rice ME, Patel JC, Cragg SJ. Dopamine release in the basal ganglia. Neurosci. 2011;198:112–137. doi: 10.1016/j.neuroscience.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson GS, Jian M. D1 and D2 dopamine receptors differentially increase Fos-like immunoreactivity in accumbal projections to the ventral pallidum and midbrain. Neuroscience. 1995;64:1019–1034. doi: 10.1016/0306-4522(94)00426-6. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Vincent SR, Fibiger HC. Striatonigral projection neurons contain D1 dopamine receptor-activated c-fos. Brain Res. 1990;523:288–290. doi: 10.1016/0006-8993(90)91498-6. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Vincent SR, Fibiger HC. D1 and D2 dopamine receptors differentially regulate c-fos expression in striatonigral and striatopallidal neurons. Neuroscience. 1992;49:285–296. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Gonzalez-Hernandez T. Electrophysiological and morphological evidence for a GABAergic nigrostriatal pathway. J Neurosci. 1999;19:4682–4694. doi: 10.1523/JNEUROSCI.19-11-04682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeper J. Dissecting the diversity of midbrain dopamine neurons. Trends Neurosci. 2013;36:336–342. doi: 10.1016/j.tins.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Rogers JH. Immunohistochemical markers in rat brain: colocalization of calretinin and calbindin-D28k with tyrosine hydroxylase. Brain Res. 1992;587:203–210. doi: 10.1016/0006-8993(92)90998-o. [DOI] [PubMed] [Google Scholar]
- Rompre PP, Bauco P, Gratton A. Facilitation of brain stimulation reward by mesencephalic injections of neurotensin-( 1–13) Eur J Pharmacol. 1992;211:295–303. doi: 10.1016/0014-2999(92)90384-g. [DOI] [PubMed] [Google Scholar]
- Root DH, Zhang S, Mejias-Aponte CA, Morales M. A novel glutamate-GABA neuronal subpopulation within the ventral tegmental area projects to the lateral habenula. Soc Neurosci Abstr. 2013;803:06. [Google Scholar]
- Rosen JB, Hitchcock JM, Sananes CB, Miserendino MJ, Davis M. A direct projection from the central nucleus of the amygdala to the acoustic startle pathway: anterograde and retrograde tracing studies. Behav Neurosci. 1991;105:817–825. doi: 10.1037/0735-7044.105.6.817. [DOI] [PubMed] [Google Scholar]
- Rougé-Pont F, Marinelli M, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. II. Sensitization of the increase in extracellular dopamine induced by cocaine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7189–7195. doi: 10.1523/JNEUROSCI.15-11-07189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gonzalez MA, Garcia-Cabezas MA, Rico B, Cavada C. The primate thalamus is a key target for brain dopamine. J Neurosci. 2005;25:6076–6083. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Chiken S, Hikida T, Kobayashi K, Nambu A. Signals through the striatopallidal indirect pathway stop movements by phasic excitation in the substantia nigra. J Neurosci. 2013;33:7583–7594. doi: 10.1523/JNEUROSCI.4932-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago AC, Shammah-Lagnado SJ. Afferent connections of the amygdalopiriform transition area in the rat. J Comp Neurol. 2005;489:349–371. doi: 10.1002/cne.20637. [DOI] [PubMed] [Google Scholar]
- Scatton B, Simon H, Le Moal M, Bischoff S. Origin of dopaminergic innervation of the rat hippocampal formation. Neurosci Lett. 1980;18:125–131. doi: 10.1016/0304-3940(80)90314-6. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple functions of dopamine neurons. F1000 Biol Rep. 2010;2:2. doi: 10.3410/B2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. Science. Vol. 275. New York, NY: 1997. A neural substrate of prediction and reward; pp. 1593–1599. [DOI] [PubMed] [Google Scholar]
- Semba K, Reiner PB, McGee EG, Fibiger HC. Brainstem afferents to the magnocellular basal forebrain studied by axonal transport, immunohistochemistry, and electrophysiology in the rat. J Comp Neurol. 1988;267:433–453. doi: 10.1002/cne.902670311. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Ceccatelli S, Schalling M, Hokfelt T, Frey P, Walsh J, Dockray G, Brown J, Buchan A, Goldstein M. A subpopulation of dopaminergic neurons in rat ventral mesencephalon contains both neurotensin and cholecystokinin. Brain Res. 1988;455:88–98. doi: 10.1016/0006-8993(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Fallon JH. Forebrain projections from cholecystokininlike-immunoreactive neurons in the rat midbrain. J Comp Neurol. 1989;279:415–435. doi: 10.1002/cne.902790307. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Mehta A, Fallon JH. Neurotensin and cholecystokinin coexistence within neurons of the ventral mesencephalon: projections to forebrain. Exp Brain Res. 1987;68:277–289. doi: 10.1007/BF00248793. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in apposition to each other. Brain Res. 1990;527:266–279. doi: 10.1016/0006-8993(90)91146-8. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J:Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Carr DB. Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiol Behav. 2002;77:513–517. doi: 10.1016/s0031-9384(02)00931-9. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacol. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ, Proulx CD, Trias A, Murphy RT, Malinow R. Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012;74:475–481. doi: 10.1016/j.neuron.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L. Afferent connections of the interstitial nucleus of the posterior limb of the anterior commissure and adjacent amygdalostriatal transition area in the rat. Neuroscience. 1999;94:1097–1123. doi: 10.1016/s0306-4522(99)90280-4. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L. Striatal and central extended amygdala parts of the interstitial nucleus of the posterior limb of the anterior commissure: evidence from tract-tracing techniques in the rat. J Comp Neurol. 2001;439:104–126. doi: 10.1002/cne.1999. [DOI] [PubMed] [Google Scholar]
- Shepard PD, Mihailoff GA, German DC. Anatomical and electrophysiological characterization of presumed dopamine-containing neurons within the supramammillary region of the rat. Brain Res Bull. 1988;20:307–314. doi: 10.1016/0361-9230(88)90060-3. [DOI] [PubMed] [Google Scholar]
- Shinonaga Y, Takada M, Mizuno N. Direct projections from the central amygdaloid nucleus to the globus pallidus and substantia nigra in the cat. Neuroscience. 1992;51:691–703. doi: 10.1016/0306-4522(92)90308-o. [DOI] [PubMed] [Google Scholar]
- Skagerberg G, Lindvall O, Björklund A. Origin, course and termination of the mesohabenular dopamine pathway in the rat. Brain Res. 1984;307:99–108. doi: 10.1016/0006-8993(84)90465-7. [DOI] [PubMed] [Google Scholar]
- Smedemark-Margulies N, Trapani JG. Tools, methods, and applications for optophysiology in neuroscience. Front mMolec Neurosci. 2013;6:18. doi: 10.3389/fnmol.2013.00018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bolam JP. The output neurones and the dopaminergic neurones of the substantia nigra receive a GABA-containing input from the globus pallidus in the rat. J Comp Neurol. 1990;296:47–64. doi: 10.1002/cne.902960105. [DOI] [PubMed] [Google Scholar]
- Smith DE, Saji M, Joh TH, Reis DJ, Pickel VM. Ibotenic acid-induced lesions of striatal target and projection neurons: ultrastructural manifestations in dopaminergic and non-dopaminergic neurons and in glia. Histol Hisopathol. 1987;2:251–263. [PubMed] [Google Scholar]
- Smith Y, Villalba R. Striatal and extrastriatal dopamine in the basal ganglia: An overview of its anatomical organization in normal and Parkinsonian brains. Mov Disord. 2008;23:S534–S547. doi: 10.1002/mds.22027. [DOI] [PubMed] [Google Scholar]
- Sobel E, Corbett D. Axonal branching of ventral tegmental and raphe projections to the frontal cortex in the rat. Neurosci Lett. 1984;48:121–125. doi: 10.1016/0304-3940(84)90006-5. [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, Jennings JH, Ung RL, Blair GA, Weinberg RJ, Neve RL, Boyce F, Mattis J, Ramakrishnan C, Deisseroth K, Stuber GD. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80:1039–1053. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Bradley KD, Hansen DM, Wilcox JD, Wilcox RS, Alison DW, Merrill CB, Edwards JG. The role of connexin-36 gap junctions in alcohol intoxication and consumption. Synapse. 2011;65:695–707. doi: 10.1002/syn.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Taylor SR, Horton ML, Barber EN, Lyle LT, Stobbs SH, Allison DW. Cocaine disinhibits dopamine neurons in the ventral tegmental area via use-dependent blockade of GABA neuron voltage-sensitive sodium channels. Eur J Neurosci. 2008;28:2028–2040. doi: 10.1111/j.1460-9568.2008.06479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- St-Gelais F, Legault M, Bourque MJ, Rompre PP, Trudeau LE. Role of calcium in neurotensin-evoked enhancement in firing in mesencephalic dopamine neurons. J Neurosci. 2004;24:2566–2574. doi: 10.1523/JNEUROSCI.5376-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. Ascending dopaminergic projections from the dorsal raphe nucleus in the rat. Brain Res. 1990;511:173–176. doi: 10.1016/0006-8993(90)90239-8. [DOI] [PubMed] [Google Scholar]
- Stobbs SH, Ohran AJ, Lassen MB, Allison DW, Brown JE, Steffensen SC. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves N- methyl-D-aspartate receptors. J Pharmacol Exp Ther. 2004;311:282–289. doi: 10.1124/jpet.104.071860. [DOI] [PubMed] [Google Scholar]
- Stopper CM, Khayambashi S, Floresco SB. Receptor-specific modulation of risk-based decision making by nucleus accumbens dopamine. Neuropsychopharmacol. 2013;38:715–728. doi: 10.1038/npp.2012.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studler JM, Kitabgi P, Tramu G, Herve D, Glowinski J, Tassin P. Extensive colocalization of neurotensin with dopamine in rat mesocortico-frontal dopaminergic neurons. Neuropeptides. 1988;11:95–100. doi: 10.1016/0143-4179(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattor T, Rayport S. Dopamine neurons make glutamatergic synapses in vitro. J Neurosci. 1998;18:4588–4602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol. 1993;330:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Research. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Mogenson GJ. Neural mechanisms for the functional coupling of autonomic, endocrine and somatomotor responses in adaptive behavior. Brain research. 1981;228:1–34. doi: 10.1016/0165-0173(81)90010-2. [DOI] [PubMed] [Google Scholar]
- Taber E. The cytoarchitecture of the brain stem of the cat. I. Brain stem nuclei of cat. The J Comp Neurol. 1961;116:27–69. doi: 10.1002/cne.901160104. [DOI] [PubMed] [Google Scholar]
- Tachibana Y, Hikosaka O. The primate ventral pallidum encodes expected reward value and regulates motor action. Neuron. 2012;76:826–837. doi: 10.1016/j.neuron.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai LH, Lee AM, Benavidez N, Bonci A, Wilbrecht L. Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nat Neurosci. 2012;15:1281–1289. doi: 10.1038/nn.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada M, Hattori T. Collateral projections from the substantia nigra to the cingulate cortex and striatum in the rat. Brain Res. 1986;380:331–335. doi: 10.1016/0006-8993(86)90230-1. [DOI] [PubMed] [Google Scholar]
- Takayama K, Miura M. Glutamate immunoreactive neurons of the central amygdaloid nucleus projecting to the subretrofactial nucleus of SHR and WKY rats: A double-labeling study. Neurosci Lett. 1991;134:62–22. doi: 10.1016/0304-3940(91)90509-r. [DOI] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouebe G, Deisseroth K, Tye KM, Luscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Brog JS, Williams ES, Zahm DS. Morphometric analysis of ventral mesencephalic neurons retrogradely labeled with fluoro-gold following injections in the shell, core and rostral pole of the rat nucleus accumbens. Brain Res. 1995;689:151–156. doi: 10.1016/0006-8993(95)00556-6. [DOI] [PubMed] [Google Scholar]
- Tan Y, Williams ES, Zahm DS. Negative correlation of calbindin-D 28kD immunofluorescence and vulnerability to neurotoxins in ventral mesencephalic neurons labeled following injections of Fluro-Gold in nucleus accumbens subterritories. Brain Res. 1999;844:67–77. doi: 10.1016/s0006-8993(99)01890-9. [DOI] [PubMed] [Google Scholar]
- Taylor SR, Picciotto MR. Ventral tegmental area GABA projections. Soc Neurosci bstr. 2013;393:09. [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Martin LP, Anderson DR. GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J Neurosci. 1995;15:3092–3103. doi: 10.1523/JNEUROSCI.15-04-03092.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting-A-Kee R, Vargas-Perez H, Mabey JK, Shin SI, Steffensen SC, van der Kooy D. Ventral tegmental area GABA neurons and opiate motivation. Psychopharmacol. 2013;227:697–709. doi: 10.1007/s00213-013-3002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torack RM, Morris JC. Mesolimbocortical dementia. A clinicopathologic case study of a putative disorder. Arch Neurol. 1986;43:1074–1078. doi: 10.1001/archneur.1986.00520100078018. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trugman JM, Wooten GF. Selective D1 and D2 dopamine agonists differentially alter basal ganglia glucose utilization in rats with unilateral 6-hydroxydopamine substantia nigra Ilesions. J Neurosci. 1987;7:2927–2935. doi: 10.1523/JNEUROSCI.07-09-02927.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand [Suppl] 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pickel VM. GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res. 1995;682:215–221. doi: 10.1016/0006-8993(95)00334-m. [DOI] [PubMed] [Google Scholar]
- Vandecastelle M, Glowinski J, Venance L. Electrical synapses between dopaminergic neurons of the substantia nigra pars compacta. J Neurosci. 2005;25:291–298. doi: 10.1523/JNEUROSCI.4167-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- van Domburg PH, ten Donkelaar HJ. The human substantia nigra and ventral tegmental area. A neuroanatomical study with notes on aging and aging diseases. Adv Anat Embryol Cell Biol. 1991;121:1–132. [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA Neurons Disrupts Reward Consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verity MA, Roitberg B, Kepes JJ. Mesolimbocortical dementia: clinico-pathological studies on two cases. J Neurol Neurosurg Psych. 1990;53:492–495. doi: 10.1136/jnnp.53.6.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney C, Baulac M, Berger B, Alvarez C, Vigny A, Helle KB. Morphological evidence for a dopaminergic terminal field in the hippocampal formation of young and adult rat. Neuroscience. 1985;14:1039–1052. doi: 10.1016/0306-4522(85)90275-1. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vertes RP. PHA-L analysis of projections from the supramammillary nucleus in the rat. J Comp Neurol. 1992;326:595–622. doi: 10.1002/cne.903260408. [DOI] [PubMed] [Google Scholar]
- Vianna DM, Brandao ML. Anatomical connections of the periaqueductal gray: specific neural substrates for different kinds of fear. Braz J Biol Med Res. 2003;36:557–566. doi: 10.1590/s0100-879x2003000500002. [DOI] [PubMed] [Google Scholar]
- Voorn P, Gerfen CR, Groenewegen HJ. Compartmental organization of the ventral striatum of the rat: immunohistochemical distribution of enkephalin, substance P, dopamine, and calcium-binding protein. J Comp Neurol. 1989;289:189–201. doi: 10.1002/cne.902890202. [DOI] [PubMed] [Google Scholar]
- Wall NR, De La Parra M, Callaway EM, Kreitzer AC. Differential innervation of direct-and indirect-pathway striatal projection neurons. Neuron. 2013;79:347–360. doi: 10.1016/j.neuron.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DM, Magnuson DJ, Gray TS. The amygdalo-brainstem pathway: selective innervation of dopaminergic, noradrenergic and adrenergic cells in the rat. Neurosci Lett. 1989;97:252–258. doi: 10.1016/0304-3940(89)90606-x. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Magnuson DJ, Gray TS. Organization of amygdaloid projections to brainstem dopaminergic, noradrenergic and adrenergic cell groups in the rat. Brain Res Bull. 1992;28:447–454. doi: 10.1016/0361-9230(92)90046-z. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Geyer MA, Swerdlow NR. Presynaptic dopamine-glutamate interactions in the nucleus accumbens regulate sensorimotor gating. Psychopharmacol (Berl) 1995;120:433–441. doi: 10.1007/BF02245815. [DOI] [PubMed] [Google Scholar]
- Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, Glutamatergic and gabaergic neurons in the rat. The Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, Morales M. Role of dorsal raphe serotonin neurons and serotoninergic Projecton to ventral tegmental area in reward: An optogenetic behavioral study. Soc Neurosci Abstr. 2013;816:14. [Google Scholar]
- Wasserman DI, Wang HG, Rashid AJ, Josselyn SA, Yeomans JS. Cholinergic control of morphine-induced locomotion in rostromedial tegmental nucleus versus ventral tegmental area sites. Eur J. Neurosci. 2013;38:2774–2785. doi: 10.1111/ejn.12279. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Williams SC, Deisseroth K. Optogenetics. PNAS USA. 2013;110:16287. doi: 10.1073/pnas.1317033110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Characterization of the dopaminergic innervation of the primate frontal cortex using a dopamine-specific antibody. Cereb Cortex. 1993;3:199–222. doi: 10.1093/cercor/3.3.199. [DOI] [PubMed] [Google Scholar]
- Willis WD, Westlund KN, Carlton SM. Pain system. In: Paxinos G, editor. The Rat Nervous System. Amsterdam: Elsevier Academic Press; 2004. pp. 853–890. [Google Scholar]
- Wilson CJ, Groves PM, Kitai ST, Linder JC. Three-dimensional structure of dendritic spines in the rat neostriatum. J Neurosci. 1983;3:383–388. doi: 10.1523/JNEUROSCI.03-02-00383.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn P, Brown VJ, Inglis WL. On the relationships between the striatum and the pedunculopontine tegmental nucleus. Crit Rev Neurobiol. 1997;11:241–261. doi: 10.1615/critrevneurobiol.v11.i4.10. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nature Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacol. 2014;39:254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO. Nuc leus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. J Neurosci. 2011;31:7811–7816. doi: 10.1523/JNEUROSCI.1504-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Morales M. Glutamate neurons in the substantia nigra compacta and retrorubral field. Eur J Neurosci. 2013;38:3602–3610. doi: 10.1111/ejn.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. J Neurosci. 2011;31:8476–8490. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetnikoff L, Reichard RA, Schwartz ZM, Parsely KP, Zahm DS. Protracted maturation of forebrain afferent connections of the ventral tegmental area in the rat. J Comp Neurol [Epub ahead of print] 2013 doi: 10.1002/cne.23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science. 1975;187:547–549. doi: 10.1126/science.1114313. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Shirouzu M, Tanaka M, Semba K, Fibiger HC. Dopaminergic neurons in the nucleus raphe dorsalis innervate the prefrontal cortex in the rat: a combined etrograde tracing and immunohistochemical study using anti-dopamine serum. Brain Res. 1989;496:373–376. doi: 10.1016/0006-8993(89)91091-3. [DOI] [PubMed] [Google Scholar]
- Young WS, Alheid GF, Heimer L. The ventral pallidal projection to the mediodorsal thalamus: a study with fluorescent retrograde tracers and immunohistofluorescence. J Neurosci. 1984;4:1626–1638. doi: 10.1523/JNEUROSCI.04-06-01626.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung KKL, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience. 1995;65:709–730. doi: 10.1016/0306-4522(94)00536-e. [DOI] [PubMed] [Google Scholar]
- Zahm DS. The ventral striatopallidal parts of the basal ganglia in the rat-II. Compartmentation of ventral pallidal efferents. Neuroscience. 1989;30:33–50. doi: 10.1016/0306-4522(89)90351-5. [DOI] [PubMed] [Google Scholar]
- Zahm DS. An electron microscopic morphometric comparison of tyrosine hydroxylase immunoreactive innervation in the neostriatum and the nucleus accumbens core and shell. Brain Res. 1992;575:341–6. doi: 10.1016/0006-8993(92)90102-f. [DOI] [PubMed] [Google Scholar]
- Zahm DS. The evolving theory of basal forebrain functional-anatomical “macrosystems. Neurosci Biobehav Rev. 2006;30:148–172. doi: 10.1016/j.neubiorev.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Zahm DS. Pharmacotherapeutic approach to the treatment of addiction: persistent challenges. Missouri Med. 2010;107:276–280. [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Arends JJA, Parsley KP, Geisler S. Projection to the mesopontine rostromedial tegmental nucleus from the lateral preoptic area. Soc Neurosic Abstr. 2011a 79.09. [Google Scholar]
- Zahm DS, Cheng AY, Lee TJ, Ghobadi CW, Schwartz ZM, Geisler S, Parsely KP, Gruber C, Veh RW. Inputs to the midbrain dopaminergic complex in the rat, with emphasis on extended amygdala-recipient sectors. J Comp Neurol. 2011b;519:3159–3188. doi: 10.1002/cne.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Grosu S, Williams EA, Qin S, Berod A. Neurons of origin of the neurotensinergic plexus enmeshing the ventral tegmental area in rat: retrograde labeling and in situ hybridization combined. Neuroscience. 2001;104:841–851. doi: 10.1016/s0306-4522(01)00118-x. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Two transpallidal pathways originating in rat nucleus accumbens. J Comp Neurol. 1990;302:437–446. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. J Comp Neurol. 1993;327:220–232. doi: 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Jensen SL, Williams ES, Martin JR., III Direct comparison of projections from the central amygdaloid region and nucleus accumbens shell. Eur J Neurosci. 1999;11:1119–11126. doi: 10.1046/j.1460-9568.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Parsley KP, Schwartz ZM, Cheng AY. On lateral septum-like characteristics of outputs from the accumbal hedonic “hotspot” of Pecina and Berridge with commentary on the transitional nature of basal forebrain “boundaries”. J Comp Neurol. 2013;521(1):50–68. doi: 10.1002/cne.23157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Williams ES, Krause JE, Welch MA, Grosu DS. Distinct and interactive effects of d-amphetamine and haloperidol on levels of neurotensin and its mRNA in subterritories in the dorsal and ventral striatum of the rat. J Comp Neurol. 1998;400:487–503. doi: 10.1002/(sici)1096-9861(19981102)400:4<487::aid-cne4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]