Abstract
Background & Aims
Pediatric functional abdominal pain has been linked to functional gastrointestinal disorders (FGID) in adulthood, but little is known about patient characteristics in childhood that increase risk for FGID in young adulthood. We investigated the contribution of GI symptoms, extra-intestinal somatic symptoms, and depressive symptoms in pediatric patients with functional abdominal pain and whether these predicted FGIDs later in life.
Methods
In a longitudinal study, consecutive new pediatric patients, diagnosed with functional abdominal pain in a subspecialty clinic, completed a comprehensive baseline evaluation of the severity of their physical and emotional symptoms. They were contacted 5–15 years later and evaluated, based on Rome III symptom criteria, for abdominal pain-related FGIDs, including irritable bowel syndrome (IBS), functional dyspepsia, functional abdominal pain syndrome, and abdominal migraine. Controlling for age, sex, baseline severity of abdominal pain, and time to follow-up evaluation, multivariable logistic regression was used to evaluate the association of baseline GI, extra-intestinal somatic, and depressive symptoms in childhood with FGID in adolescence and young adulthood.
Results
Of 392 patients interviewed an average of 9.2 years after initial evaluation, 41% (n=162) met symptom criteria for FGID; most met the criteria for IBS. Extra-intestinal somatic and depressive symptoms at the initial pediatric evaluation were significant predictors of FGID later in life, after controlling for initial levels of GI symptoms. Age, sex, and abdominal pain severity at initial presentation were not significant predictors of FGID later in life.
Conclusions
In pediatric patients with functional abdominal pain, assessment of extra-intestinal and depressive symptoms may be useful in identifying those at risk for FGID in adolescence and young adulthood.
Keywords: Functional gastrointestinal disorders, somatic symptoms, depression, irritable bowel syndrome, prospective
BACKGROUND
Chronic or recurrent abdominal pain is common in childhood, affecting 8–25% of otherwise healthy school-age children. In the majority of cases, medical evaluation yields no evidence of organic disease and the pain is considered “functional”.1–3 A review of the literature estimated that abdominal pain persisted at long-term follow-up in 29.1% (95% CI 28.1–30.2) of youth with pediatric functional abdominal pain (Ped-FAP).4 Indeed, it has been suggested that Ped-FAP in childhood may be a precursor to functional gastrointestinal disorders (FGIDs) such as irritable bowel syndrome (IBS) in adulthood.5–7
Little is known, however, about characteristics of symptom presentation in childhood that may predict outcomes in adolescence and young adulthood. The empirical literature has shown that, in addition to their gastrointestinal symptoms, many patients with Ped-FAP experience high rates of extra-intestinal somatic complaints8–11 and psychological symptoms such as depression.12–17 Whether these co-existing symptoms are relevant to clinical outcomes of Ped-FAP and therefore merit inclusion in the clinical evaluation is unclear.
A recent review of the literature on prognostic factors for Ped-FAP found insufficient evidence to determine whether extra-intestinal somatic symptoms predicted persistence of abdominal pain and conflicting evidence regarding the relation of psychological symptoms to pain persistence.18 No studies to date have evaluated whether extra-intestinal and depressive symptoms increment the prediction of the prognosis of Ped-FAP over and above abdominal symptoms alone. This is an important limitation of the literature, as abdominal symptoms correlate with both extra-intestinal and depressive symptoms, raising the concern that assessing these latter symptoms may not add to the prognostication beyond the value of assessing abdominal symptoms, and therefore might not be necessary at all. Although other patient characteristics such as attentional bias to bodily symptoms and parental factors may predict clinical outcomes of Ped-FAP,19–21 we focused here on extra-intestinal and depressive symptoms as these can be reliably and efficiently assessed in the clinic setting without appreciably extending the clinic visit. Specifically, the current study assessed the extent to which extra-intestinal somatic symptoms and depressive symptoms prospectively predicted FGID in adulthood, over and above the baseline severity of abdominal pain and other GI symptoms evaluated at the time of initial subspecialty evaluation for Ped-FAP.
MATERIALS AND METHODS
Sample
Participants were drawn from a large database of consecutive new patients with Ped-FAP who had participated in studies conducted by Walker and colleagues between 1993 and 200422–24 and agreed to be contacted for follow-up. They were contacted by mail or telephone and invited to participate in the follow-up. Eligibility criteria at the time of initial study enrollment in childhood included evaluation at a single center pediatric gastroenterology clinic for abdominal pain of at least three months’ duration and consistent with Apley’s definition of pediatric recurrent abdominal pain12, age between 8 and 16 years, living with parent(s) or parent figure, capable of consent/assent, and no chronic illness or developmental delay. Patients who had minor histologic findings of esophagitis (with normal endoscopy on visualization at initial pediatric evaluation) were eligible for the follow-up study as histologic findings alone are neither sensitive nor specific for reflux esophagitis or other organic disorder.25 Additional eligibility criteria for the follow-up study included: age 12 years or older at follow-up, at least four years elapsed since the initial pediatric evaluation, and no current chronic or life-threatening disease.
Procedure
At the time of initial study enrollment, validated patient report symptom questionnaires were administered to Ped-FAP patients and their parents before the child’s medical evaluation. Results of the initial evaluation in childhood have been reported previously.22–24 At the time of study enrollment, participants gave consent to be contacted in the future regarding participation in additional studies. Data for the present study were collected as part of a follow-up evaluation of long-term health outcomes of Ped-FAP; other aspects of the evaluation have been reported elsewhere.26, 27
The protocol for the follow-up study included a structured interview conducted by telephone by an interviewer who was unaware of the participant’s original symptom presentation. The interviewer elicited demographic information and administered patient-report measures of current health status and functioning. Informed consent/assent was obtained by telephone prior to conducting the interview. All procedures were approved by our center’s Institutional Review Board.
Baseline Measures
Depressive Symptoms
Depressive symptoms in childhood were evaluated using the Children’s Depression Inventory (CDI), a validated self-report measure for children ranging from 7 to 17 years of age.28, 29 This questionnaire was completed by the child at the baseline pediatric evaluation. A total score is computed with higher scores indicating greater severity of depressive symptoms; scores above 12 indicate clinically significant depressive symptoms in children evaluated in a medical setting.30, 31
Gastrointestinal and Extra-intestinal Somatic Symptoms
Gastrointestinal (GI) and extra-intestinal (non-GI) symptoms were assessed at the initial pediatric evaluation with the Children’s Somatization Inventory (CSI), a validated self-report questionnaire for children and adolescents.32 The CSI includes 9 GI symptoms (e.g., abdominal pain, nausea, constipation, diarrhea, bloating) and 26 extra-intestinal somatic symptoms (e.g., dizziness, back pain, headaches, sore muscles). Participants rate the extent to which they have experienced each symptom in the past two weeks using a 5-point scale ranging from not at all (0) to a whole lot (4). Separate scores were calculated to reflect the total number of GI symptoms (range: 0 – 9) and extra-intestinal symptoms (range: 0 – 26), with symptoms rated 3 or 4 considered present at the initial evaluation.
Abdominal pain severity
Abdominal pain severity in childhood was evaluated using the Abdominal Pain Index (API), a validated questionnaire completed by the child.33 The API total score is derived from items assessing the intensity, duration, and frequency of abdominal pain episodes in the previous two weeks.
Measures obtained at Follow up
Functional Gastrointestinal Disorders (FGID)
Presence of Rome III FGID symptom criteria at follow up was determined using the Rome III Questionnaire, a measure developed by the Rome Foundation Board to identify individuals who endorse the Rome III symptom criteria associated with various FGIDs.34–36 We administered the 24 items that assess symptom criteria for FGIDs associated with abdominal pain, including IBS, FD, functional abdominal pain syndrome, and abdominal migraine. Participants’ responses were scored according to the pediatric Rome III criteria (for participants < 18 years of age) or the adult Rome III criteria (for participants ≥ 18 years).
Demographics
Age, sex, and time elapsed since initial pediatric evaluations were recorded at follow up.
Statistical Methods
Baseline demographic characteristics were compared between participants with (FGID-Pos) and without (FGID-Neg) Rome III symptom criteria at follow-up using either Chi square analysis for proportions or Wilcoxon sum rank tests. Multivariable logistic regression evaluated the relationship between predictor variables and FGID outcome, with restricted cubic splines on continuous predictors allowing for potential nonlinear relationships. Regression analyses adjusted for age, sex, baseline severity of abdominal pain, and time from initial evaluation to follow-up.
RESULTS
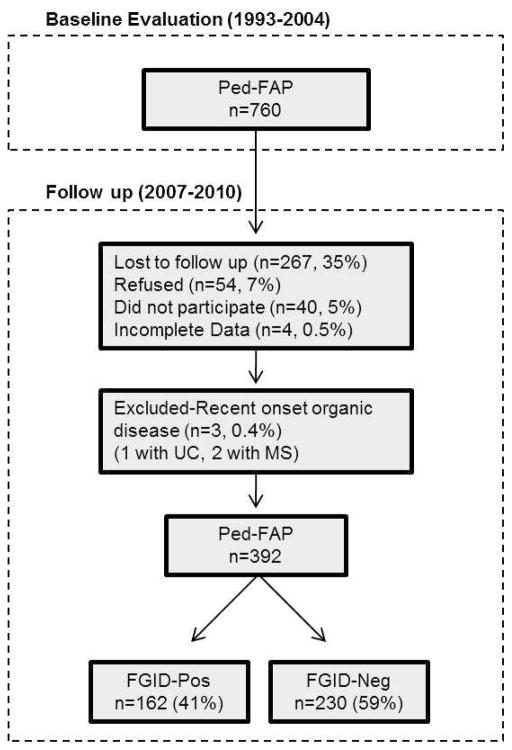
Of patients who participated in the initial pediatric evaluation, 760 individuals were eligible for the follow-up study. They were contacted by mail or telephone and invited to participate in the follow-up. As shown in Figure 1, some participants were lost to follow-up because they could not be located (n = 267), refused (n = 54), agreed to participate but could not be scheduled (n = 40), or had incomplete data (n = 4). In addition, 3 participants were excluded due to self-reported autoimmune disease at follow-up (1 participant reported ulcerative colitis and was taking balsalazide at the follow-up evaluation, and 2 participants reported multiple sclerosis). Thus, the final sample included 392 participants constituting 51.7% of eligible patients from the initial pediatric study. Age, sex, and abdominal pain severity at the baseline evaluation did not differ significantly for those who did versus did not participate in the follow-up.
Figure 1.
Study design. Ped-FAP = Pediatric functional abdominal pain. FGID-Neg = does not meet Rome III symptom criteria for functional gastrointestinal disorder at follow-up. FGID-Pos = meets Rome III symptom criteria for functional gastrointestinal disorder at follow-up.
Incidence and characteristics of FGIDs at Follow-up
Forty-one percent (n= 162) of participants met Rome III symptom criteria for FGID at follow-up and were classified as FGID-Pos. Age and gender distributions are presented in Table 1. Among those in the FGID-Pos group, the majority met criteria for IBS (n = 69), FD (n = 36) or both (n = 41) at follow-up. Functional abdominal pain syndrome (n=8) and abdominal migraine (n = 8) were relatively rare. (Table 2)
Table 1.
Demographic characteristics of individuals with (FGID-POS) and without (FGID-NEG) Rome III symptom criteria for FGID at follow-up
| FGID-NEG n=230 |
FGID-POS n=162 |
p value | |
|---|---|---|---|
| Sex, % female | 60.0% | 71.0% | p=0.03 |
| Age at Initial Evaluation (years), Mean age ± SD | 11.8 ± 2.6 | 11.9 ± 2.5 | p=0.82 |
| Follow-up period (years), Mean interval ± SD | 9.2 ± 3.5 | 9.0 ± 3.5 | p=0.84 |
Note. FGID = functional gastrointestinal disorder associated with abdominal pain.
Table 2.
Number and percentage of each type of functional gastrointestinal disorder (FGID) at follow-up in adolescence and young adulthood
| Presence of Functional Gastrointestinal Disorder (FGID) at Follow-up | N (%) |
|---|---|
| No FGID at follow-up (FGID-Neg) | 230 (58.6%) |
| Any FGID at follow-up (FGID-Pos) | 162 (41.4%) |
| Irritable Bowel Syndrome (IBS) only | 69 (17.6%) |
| Functional Dyspepsia (FD) only | 36 (9.2%) |
| Irritable Bowel Syndrome and Functional Dyspepsia (IBS+FD) | 41 (10.5%) |
| Functional Abdominal Pain only | 8 (2.0%) |
| Abdominal Migraine only | 8 (2.0%) |
Within the FGID-Pos group, the FGID subtypes did not differ significantly on any baseline measure including abdominal pain severity, number of gastrointestinal or extra-intestinal symptoms, or severity of depressive symptoms. Therefore, for analyses evaluating the utility of baseline variables in predicting FGID outcomes in adulthood, participants were retained in two FGID outcome groups -- those meeting criteria for one or more FGIDs at follow up (FGID-Pos) and those not meeting criteria for any FGID at follow-up (FGID-Neg).
Baseline Predictors of FGID at Follow-up
Based on a multivariable logistic regression model, baseline measures of gastrointestinal symptoms, extra-intestinal somatic symptoms, and depressive symptoms were each significant predictors of FGID in adolescence and young adulthood in analyses controlling for age, sex, baseline abdominal pain severity, and time to follow up. (Table 3) The multivariable model tested the significance of each baseline variable after accounting for the other variables included in the model. Measures of extra-intestinal somatic symptoms and depressive symptoms remained significant predictors in the multivariable model, suggesting that each had unique predictive value for subsequent presence of FGID in adolescence and young adulthood. The relationship of extra-intestinal somatic symptoms at baseline to FGID at follow up was linear; that is, each additional gastrointestinal or extra-intestinal somatic symptom reported in childhood increased the likelihood of meeting symptom criteria for FGID at follow-up in adolescence and young adulthood.
Table 3.
Results of multivariable cubic spline logistic regression model evaluating the association of each childhood factor with the presence of FGID in adolescence and young adulthood
| Childhood factor | X2 | p value |
|---|---|---|
| Depressive symptoms | 12.38 | 0.006 |
| Extra-intestinal somatic symptoms | 6.00 | 0.049 |
| Gastrointestinal symptoms | 7.87 | 0.049 |
| Abdominal pain severity | 0.69 | 0.88 |
| Age at initial evaluation | 2.08 | 0.55 |
| Sex | 2.57 | 0.11 |
| Follow-up period (years) | 4.05 | 0.26 |
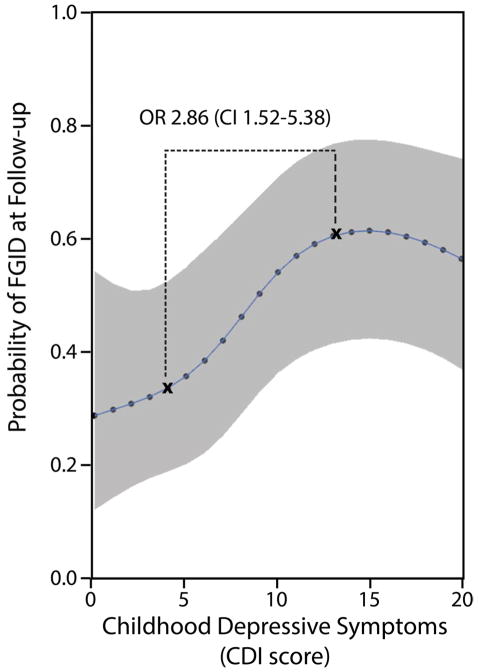
Higher levels of depressive symptoms in childhood also were associated with greater likelihood of FGID in adolescence and young adulthood. For example, when adjusting for age, sex, baseline abdominal pain severity, time to follow up, baseline gastrointestinal and extra-intestinal somatic symptoms, a child with a CDI score above the screening cut-off for risk of depression (CDI score = 12) at the initial pediatric evaluation was nearly three times as likely (OR 2.86, CI 1.52, 5.38) to meet FGID symptom criteria at follow-up as compared to a child with a CDI score below the 25% percentile (CDI score = 4). Of note, the test for nonlinearity of the association between childhood depressive symptoms (CDI score) and FGID in adolescence and young adulthood was significant (p < 0.05). This nonlinear relation is illustrated in Figure 2, which shows that the probability of FGID at follow-up increased with each increase in CDI score up to a CDI score of 13, which is a cut-point often used when screening for depression in children.30, 31 At CDI scores higher than 13, the probability of FGID remained fairly constant.
Figure 2.
The probability of Functional Gastrointestinal Disorder (FGID) at follow-up in adolescence or young adulthood based on baseline scores on the Children’s Depression Inventory (CDI), fixing all other variables to constant values (using the average value of each variable, or if a categorical variable, using the model-based reference group). Odds ratio between the 25% and 75% interquartile values of the CDI scores is shown.
Discussion
This prospective study of pediatric patients with functional abdominal pain found that a large proportion of these patients still had frequent abdominal pain at follow up in adolescence and young adulthood, even though organic gastrointestinal disease was rare (<1%). In contrast to studies that have relied on a measure of current abdominal pain severity to index the persistence of Ped-FAP, we applied the symptom criteria for Rome III FGID which include the severity, duration, and location of abdominal pain as well as associated bowel symptoms. Results showed that 41% of Ped-FAP patients had clinically significant abdominal pain at follow-up, as defined by the Rome III FGID criteria. The most common FGID at follow-up was IBS. This finding underscores the clinical significance of Ped-FAP for subsequent FGID.
Controlling for baseline level of gastrointestinal symptoms, the level of extra-intestinal somatic symptoms in childhood predicted FGID in young adulthood. Prior research has shown that children with functional abdominal pain report more somatic symptoms as compared to pain-free peers8–11 and a similar pattern has been found in adults.37, 38 Patients with chronic pain conditions, including FGIDs, may have a heightened sensory responsiveness throughout the body.39 For example, elsewhere we have shown that individuals with a history of Ped-FAP have enhanced responsiveness to laboratory pain testing.40 On the other hand, some studies of patients with IBS have not shown enhanced CNS responses to pain and have suggested that these patients may be hypervigilant and that psychiatric comorbidity may influence and enhance these perceptions.38 Other factors not evaluated in this study such as social learning within the family also may contribute to the continued manifestation of extra-intestinal somatic symptoms.8, 19, 20
Although it is well known that psychological symptoms such as depression are common in both children and adults with functional abdominal pain and/or FGID, it has been unclear whether higher levels of depressive symptoms in Ped-FAP were a significant marker for persistence of abdominal pain and FGIDs into early adulthood. Our prospective study provides evidence that the presence of depressive symptoms in childhood significantly predicts FGID in adolescence and young adulthood. This association is particularly noteworthy, as it was significant even when controlling for sex, age, time to follow up, baseline severity of abdominal pain, and the number of gastrointestinal and extra-intestinal somatic symptoms at baseline.
Interestingly, the association between childhood depressive symptoms and subsequent FGID was nonlinear. That is, the probability of FGID at follow up increased with each increase in CDI total score up to a score of 13, and then remained fairly constant. Previous studies have suggested a cut-point score of 12 or 13 when using the CDI to screen for depression in children in a clinical setting.30, 31 Our findings indicate that once the level of depressive symptoms is in the clinical range, higher levels of depressive symptoms do not further increase the likelihood of FGID at follow up. The mechanisms underlying the relation of depressive symptoms to the long-term persistence of functional abdominal pain are unknown. Others have suggested that the association of psychological factors with GI symptoms may be related to central nervous system modulation of GI function, including motility and visceral pain.41, 42
Strengths of this study include the large sample size, prospective design with long-term follow-up, and evaluation of outcomes with psychometrically sound measures. Additionally, use of the Rome III criteria for FGIDs as the primary outcome measure was considerably more informative than a measure of abdominal pain alone, as abdominal pain is common in the general population and may not reflect a clinically significant disorder.17, 43, 44 Thus evaluation of Rome III symptom criteria may be critical for assessing the clinical significance of abdominal discomfort as an outcome in studies of Ped-FAP. Moreover, use of the Rome III criteria for FGIDs facilitates comparison of results across studies.
The present study is limited in that the Ped-FAP cohort was recruited from a pediatric tertiary care center and may differ from youth with Ped-FAP seen in primary care or in the general population. A recent review of the literature, however, reported that abdominal pain outcomes were similar in Ped-FAP patients with and without a tertiary care evaluation.4 Participant attrition is another study limitation, although nonparticipants did not differ significantly from participants on baseline symptom severity. Our baseline evaluation of emotional symptoms focused on depressive symptoms and did not specifically assess symptoms of anxiety, which are common in pediatric functional abdominal pain patients.16, 45 Nonetheless, because symptoms of depression and anxiety are known to be highly correlated, patients with high levels of depressive symptoms likely also had elevated anxiety.46, 47 Finally, although no standard treatments have demonstrated efficacy for Ped-FAP, many treatment approaches are available (e.g., medication, dietary modification, behavioral therapies) and the study is limited in that the possible use of such treatments by some patients during the many years between study enrollment and follow-up was not assessed.
In summary, at long-term follow-up of Ped-FAP patients, the presence of FGIDs was common, and was predicted not only by severity of gastrointestinal symptoms in childhood, but also by extra-intestinal somatic symptoms and depressive symptoms in childhood. An important clinical implication is that extra-intestinal and depressive symptoms could be assessed in the clinical setting to aid physicians in identifying children with increased risk for FGID in adolescence and young adulthood. Identification of characteristics of pediatric patients at risk for FGID later in development might aid in further understanding the etiology of FGID and in formulating treatment plans, including the need for follow-up and monitoring of Ped-FAP. Future research should explore whether treating depressive symptoms in patients with Ped-FAP reduces their risk for FGIDs in adolescence and young adulthood.
Acknowledgments
Grant support:
This research was supported by R01 HD23264 (Lynn S. Walker, P.I.) from the National Institute on Child Health and Development (NICHD) and does not necessarily represent the official views of the NICHD or the National Institutes of Health (NIH). Support also was provided by the Vanderbilt Kennedy Center (P30 HD15052), the Vanderbilt Digestive Disease Research Center (DK058404), and the Vanderbilt CTSA grant 1 UL1 RR024975 from the National Center for Research Resources, NIH.
Footnotes
Disclosures: None of the authors has a conflict of interest.
Author Contribution:
Study concept and design: Sara Horst, Grace Shelby, Sari Acra, D. Brent Polk, Judy Garber, Lynn S. Walker
Acquisition of data: Sara N. Horst, Grace Shelby, Lynn S. Walker
Analysis and interpretation of data: All authors
Drafting of the manuscript: Sara N. Horst, Lynn S. Walker
Critical revision of the manuscript: All authors
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hyams JS, Burke G, Davis PM, et al. Abdominal pain and irritable bowel syndrome in adolescents: a community-based study. J Pediatr. 1996;129:220–6. doi: 10.1016/s0022-3476(96)70246-9. [DOI] [PubMed] [Google Scholar]
- 2.Chitkara DK, Rawat DJ, Talley NJ. The epidemiology of childhood recurrent abdominal pain in Western countries: a systematic review. Am J Gastroenterol. 2005;100:1868–75. doi: 10.1111/j.1572-0241.2005.41893.x. [DOI] [PubMed] [Google Scholar]
- 3.Apley J, Hale B. Children with recurrent abdominal pain: how do they grow up? Br Med J. 1973;3:7–9. doi: 10.1136/bmj.3.5870.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gieteling MJ, Bierma-Zeinstra SM, Passchier J, et al. Prognosis of chronic or recurrent abdominal pain in children. J Pediatr Gastroenterol Nutr. 2008;47:316–26. doi: 10.1097/MPG.0b013e31815bc1c1. [DOI] [PubMed] [Google Scholar]
- 5.Walker LS, Guite JW, Duke M, et al. Recurrent abdominal pain: a potential precursor of irritable bowel syndrome in adolescents and young adults. J Pediatr. 1998;132:1010–5. doi: 10.1016/s0022-3476(98)70400-7. [DOI] [PubMed] [Google Scholar]
- 6.Howell S, Poulton R, Talley NJ. The natural history of childhood abdominal pain and its association with adult irritable bowel syndrome: birth-cohort study. Am J Gastroenterol. 2005;100:2071–8. doi: 10.1111/j.1572-0241.2005.41753.x. [DOI] [PubMed] [Google Scholar]
- 7.Chitkara DK, Talley NJ, Schleck C, et al. Recollection of childhood abdominal pain in adults with functional gastrointestinal disorders. Scand J Gastroenterol. 2009;44:301–7. doi: 10.1080/00365520802555975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy RL, Whitehead WE, Walker LS, et al. Increased somatic complaints and health-care utilization in children: effects of parent IBS status and parent response to gastrointestinal symptoms. Am J Gastroenterol. 2004;99:2442–51. doi: 10.1111/j.1572-0241.2004.40478.x. [DOI] [PubMed] [Google Scholar]
- 9.Mulvaney S, Lambert EW, Garber J, et al. Trajectories of symptoms and impairment for pediatric patients with functional abdominal pain: a 5-year longitudinal study. J Am Acad Child Adolesc Psychiatry. 2006;45:737–44. doi: 10.1097/10.chi.0000214192.57993.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Little CA, Williams SE, Puzanovova M, et al. Multiple somatic symptoms linked to positive screen for depression in pediatric patients with chronic abdominal pain. J Pediatr Gastroenterol Nutr. 2007;44:58–62. doi: 10.1097/01.mpg.0000243423.93968.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker LS, Garber J, Greene JW. Somatization symptoms in pediatric abdominal pain patients: relation to chronicity of abdominal pain and parent somatization. J Abnorm Child Psychol. 1991;19:379–94. doi: 10.1007/BF00919084. [DOI] [PubMed] [Google Scholar]
- 12.Apley J, Naish N. Recurrent abdominal pains: a field survey of 1,000 school children. Arch Dis Child. 1958;33:165–70. doi: 10.1136/adc.33.168.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campo JV, Bridge J, Ehmann M, et al. Recurrent abdominal pain, anxiety, and depression in primary care. Pediatrics. 2004;113:817–24. doi: 10.1542/peds.113.4.817. [DOI] [PubMed] [Google Scholar]
- 14.Ramchandani PG, Hotopf M, Sandhu B, et al. The epidemiology of recurrent abdominal pain from 2 to 6 years of age: results of a large, population-based study. Pediatrics. 2005;116:46–50. doi: 10.1542/peds.2004-1854. [DOI] [PubMed] [Google Scholar]
- 15.Walker LS, Greene JW. Children with recurrent abdominal pain and their parents: more somatic complaints, anxiety, and depression than other patient families? J Pediatr Psychol. 1989;14:231–43. doi: 10.1093/jpepsy/14.2.231. [DOI] [PubMed] [Google Scholar]
- 16.Dufton LM, Dunn MJ, Compas BE. Anxiety and somatic complaints in children with recurrent abdominal pain and anxiety disorders. J Pediatr Psychol. 2009;34:176–86. doi: 10.1093/jpepsy/jsn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saps M, Seshadri R, Sztainberg M, et al. A prospective school-based study of abdominal pain and other common somatic complaints in children. J Pediatr. 2009;154:322–6. doi: 10.1016/j.jpeds.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 18.Gieteling MJ, Bierma-Zeinstra SM, Lisman-van Leeuwen Y, et al. Prognostic factors for persistence of chronic abdominal pain in children. J Pediatr Gastroenterol Nutr. 2011;52:154–61. doi: 10.1097/MPG.0b013e3181e82a28. [DOI] [PubMed] [Google Scholar]
- 19.Walker LS, Zeman JL. Parental response to child illness behavior. J Pediatr Psychol. 1992;17:49–71. doi: 10.1093/jpepsy/17.1.49. [DOI] [PubMed] [Google Scholar]
- 20.Garber J, Van Slyke DA, Walker LS. Concordance between mothers’ and children’s reports of somatic and emotional symptoms in patients with recurrent abdominal pain or emotional disorders. J Abnorm Child Psychol. 1998;26:381–91. doi: 10.1023/a:1021955907190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck JE, Lipani TA, Baber KF, et al. Attentional bias to pain and social threat in pediatric patients with functional abdominal pain and pain-free youth before and after performance evaluation. Pain. 2011;152:1061–7. doi: 10.1016/j.pain.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker LS, Smith CA, Garber J, et al. Testing a model of pain appraisal and coping in children with chronic abdominal pain. Health Psychol. 2005;24:364–74. doi: 10.1037/0278-6133.24.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker LS, Garber J, Smith CA, et al. The relation of daily stressors to somatic and emotional symptoms in children with and without recurrent abdominal pain. J Consult Clin Psychol. 2001;69:85–91. [PMC free article] [PubMed] [Google Scholar]
- 24.Walker LS, Baber KF, Garber J, et al. A typology of pain coping strategies in pediatric patients with chronic abdominal pain. Pain. 2008;137:266–75. doi: 10.1016/j.pain.2007.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandenplas Y, Rudolph CD, Di Lorenzo C, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) J Pediatr Gastroenterol Nutr. 2009;49:498–547. doi: 10.1097/MPG.0b013e3181b7f563. [DOI] [PubMed] [Google Scholar]
- 26.Shelby GD, Shirkey KC, Sherman AL, et al. Functional Abdominal Pain in Childhood and Long-term Vulnerability to Anxiety Disorders. Pediatrics. 2013;132:475–82. doi: 10.1542/peds.2012-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker LS, Sherman AL, Bruehl S, et al. Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain. 2012;153:1798–806. doi: 10.1016/j.pain.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovacs M. The Children’s Depression, Inventory (CDI) Psychopharmacol Bull. 1985;21:995–8. [PubMed] [Google Scholar]
- 29.Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatr. 1981;46:305–15. [PubMed] [Google Scholar]
- 30.Kazdin AE, Colbus D, Rodgers A. Assessment of depression and diagnosis of depressive disorder among psychiatrically disturbed children. J Abnorm Child Psychol. 1986;14:499–515. doi: 10.1007/BF01260519. [DOI] [PubMed] [Google Scholar]
- 31.Lobovits DA, Handal PJ. Childhood depression: prevalence using DSM-III criteria and validity of parent and child depression scales. J Pediatr Psychol. 1985;10:45–54. doi: 10.1093/jpepsy/10.1.45. [DOI] [PubMed] [Google Scholar]
- 32.Walker LS, Garber J, Greene JW. Somatization symptoms in pediatric abdominal pain patients: relation to chronicity of abdoinal pain and parent somatizaiton. J Abnorm Child Psychol. 2001;19:379–394. doi: 10.1007/BF00919084. [DOI] [PubMed] [Google Scholar]
- 33.Walker LS, Smith CA, Garber J, et al. Development and Validation of a Pain Response Inventeroy for Children. Psychol Assess. 1997;9:392–405. [Google Scholar]
- 34.Clouse RE, Mayer EA, Aziz Q, et al. Functional abdominal pain syndrome. Gastroenterology. 2006;130:1492–7. doi: 10.1053/j.gastro.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 35.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 36.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–79. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 37.Brown WH, Chey WD, Elta GH. Number of responses on a review of systems questionnaire predicts the diagnosis of functional gastrointestinal disorders. J Clin Gastroenterol. 2003;36:222–7. doi: 10.1097/00004836-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Whitehead WE, Palsson OS, Levy RR, et al. Comorbidity in irritable bowel syndrome. Am J Gastroenterol. 2007;102:2767–76. doi: 10.1111/j.1572-0241.2007.01540.x. [DOI] [PubMed] [Google Scholar]
- 39.Geisser ME, Strader Donnell C, Petzke F, et al. Comorbid somatic symptoms and functional status in patients with fibromyalgia and chronic fatigue syndrome: sensory amplification as a common mechanism. Psychosomatics. 2008;49:235–42. doi: 10.1176/appi.psy.49.3.235. [DOI] [PubMed] [Google Scholar]
- 40.Dengler-Crish CM, Bruehl S, Walker LS. Increased wind-up to heat pain in women with a childhood history of functional abdominal pain. Pain. 2011;152:802–8. doi: 10.1016/j.pain.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–9. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drossman DA, Camilleri M, Mayer EA, et al. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 43.Stanford EA, Chambers CT, Biesanz JC, et al. The frequency, trajectories and predictors of adolescent recurrent pain: a population-based approach. Pain. 2008;138:11–21. doi: 10.1016/j.pain.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 44.Brun Sundblad GM, Saartok T, Engstrom LM. Prevalence and co-occurrence of self-rated pain and perceived health in school-children: Age and gender differences. Eur J Pain. 2007;11:171–80. doi: 10.1016/j.ejpain.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Garber J, Zeman J, Walker LS. Recurrent abdominal pain in children: psychiatric diagnoses and parental psychopathology. J Am Acad Child Adolesc Psychiatry. 1990;29:648–56. doi: 10.1097/00004583-199007000-00021. [DOI] [PubMed] [Google Scholar]
- 46.Ford T, Goodman R, Meltzer H. The British Child and Adolescent Mental Health Survey 1999: the prevalence of DSM-IV disorders. J Am Acad Child Adolesc Psychiatry. 2003;42:1203–11. doi: 10.1097/00004583-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Wichstrom L, Berg-Nielsen TS, Angold A, et al. Prevalence of psychiatric disorders in preschoolers. J Child Psychol Psychiatry. 2012;53:695–705. doi: 10.1111/j.1469-7610.2011.02514.x. [DOI] [PubMed] [Google Scholar]