Abstract
Compared to normal cells, cancer cells strongly upregulate glucose uptake and glycolysis to give rise to increased yield of intermediate glycolytic metabolites and the end product pyruvate. Moreover, glycolysis is uncoupled from the mitochondrial tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS) in cancer cells. Consequently, the majority of glycolysis-derived pyruvate is diverted to lactate fermentation and kept away from mitochondrial oxidative metabolism. This metabolic phenotype is known as the Warburg effect. While it has become widely accepted that the glycolytic intermediates provide essential anabolic support for cell proliferation and tumor growth, it remains largely elusive whether and how the Warburg metabolic phenotype may play a role in tumor progression. We hereby review the cause and consequence of the restrained oxidative metabolism, in particular in tumor metastasis. Cells change or lose their extracellular matrix during the metastatic process. Inadequate/inappropriate matrix attachment generates reactive oxygen species (ROS) and causes a specific type of cell death, termed anoikis, in normal cells. Although anoikis is a barrier to metastasis, cancer cells have often acquired elevated threshold for anoikis and hence heightened metastatic potential. As ROS are inherent byproducts of oxidative metabolism, forced stimulation of glucose oxidation in cancer cells raises oxidative stress and restores cells’ sensitivity to anoikis. Therefore, by limiting the pyruvate flux into mitochondrial oxidative metabolism, the Warburg effect enables cancer cells to avoid excess ROS generation from mitochondrial respiration and thus gain increased anoikis resistance and survival advantage for metastasis. Consistent with this notion, pro-metastatic transcription factors HIF and Snail attenuate oxidative metabolism, whereas tumor suppressor p53 and metastasis suppressor KISS1 promote mitochondrial oxidation. Collectively, these findings reveal mitochondrial oxidative metabolism as a critical suppressor of metastasis and justify metabolic therapies for potential prevention/intervention of tumor metastasis.
1. Introduction: the Warburg effect in cancer
Altered metabolism is a universal property of most, if not all, cancer cells [1] [2]. One of the first identified and most common biochemical characteristics of cancer cells is aberrant glucose metabolism. Glucose is a main source of energy and carbon for mammalian cells, providing not only energy (ATP) but also metabolites for various anabolic pathways [3]. Glucose is taken up into the cell by glucose transporters and metabolized to pyruvate in the cytosol through a multi-step process known as glycolysis, which also yields a small amount of ATP. In normal (quiescent) cells, the glycolysis-derived pyruvate is predominantly imported into the mitochondrial matrix where it is oxidized to acetyl coenzyme A (CoA) by the pyruvate dehydrogenase (PDH) complex. Acetyl CoA is then fed into the tricarboxylic acid (TCA) cycle, followed by oxidative phosphorylation (OXPHOS) for high-efficiency ATP generation. The full oxidation of one molecule of glucose produces up to 38 ATP molecules (including 2 ATP generated by glycolysis).
By contrast, most cancer cells show conspicuous alterations in glucose metabolism (Fig. 1): (i) Compared to normal cells, cancer cells typically exhibit drastically increased glucose uptake and glycolytic rates. Increased glucose consumption generates more intermediate glycolytic metabolites and significant amount of ATP from glycolysis. (ii) Moreover, a substantial fraction of glucose carbon, in the form of assorted glycolytic intermediates, is shunted into multiple biosynthetic pathways instead of giving rise to pyruvate. (iii) Finally, following glycolysis, most pyruvate is converted to lactate in the cytoplasm by the action of lactate dehydrogenase (LDH) and secreted, rather than being oxidized through mitochondrial metabolism. This occurs even in the presence of sufficient oxygen to support mitochondrial respiration. The metabolic phenomenon was first described by Otto Warburg and is referred to as aerobic glycolysis or the “Warburg effect” [4]. Although human cancers display a diverse range of metabolic profiles [5], the Warburg metabolic phenotype is a widespread cancer-associated trait. Indeed, enhanced glucose uptake by cancer cells has become the basis for positron emission tomography (PET) with 18-fluorodeoxyglucose (FDG), which preferentially accumulates in tumor cells as a result of their rapid uptake of glucose. Because of the prevalence of this phenotype, PET is an effective clinical imaging technique to detect most cancers and monitor therapeutic responses.
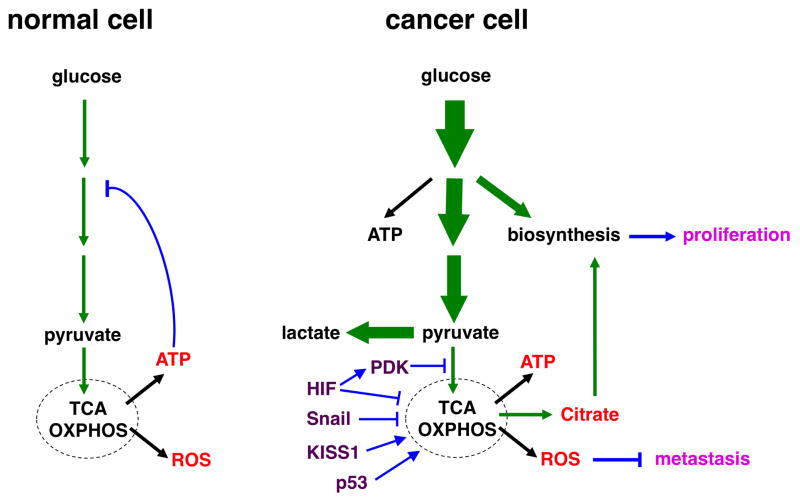
Fig. 1. Schematic illustration of glucose metabolism in normal and cancer cells under normoxia.
(left) In normal (quiescent) cells, glucose is converted to pyruvate through glycolysis, and most pyruvate enters mitochondrial oxidative metabolism for efficient energy generation (in the form of ATP). Glucose is predominantly used for energy production. High levels of ATP attenuate glycolysis via feedback inhibition.
(right) Cancer cells dramatically increase glucose uptake and glycolysis (indicated by bold arrows). A significant portion of glucose carbon is diverted to biosynthetic pathways to fuel cell proliferation. Pyruvate is preferentially shunted to lactate, resulting in increased lactate production. Oxidative metabolism persists, but is uncoupled from increased glycolysis. The respiration byproducts ROS exhibit anti-metastasis activity, which may explain why cancer cells keep glucose oxidation in check.
The flux of glucose carbon is indicated by green arrows (The thickness of arrows reflects the relative amount of the flow). Major mitochondrial products are depicted in red; metastatic regulators are in purple; regulatory steps are in blue; and the metabolic effects on cancer are in pink.
It is noteworthy that mitochondrial function in most cancer cells is intact. Warburg observed that the absolute rate of mitochondrial respiration in cancer cells remains comparable to that of normal cells [4]. Oxidative metabolism indeed persists in the vast majority of tumors and remains a major source for ATP generation [6], [7]. Nevertheless, while there is a profoundly elevated flux of glucose in cancer cells, it is predominantly directed to lactate fermentation, and the flow to oxidative metabolism does not increase proportionally. Simply put, increased glucose consumption in cancer cells is devoted to lactate conversion and biosynthesis, but is uncoupled from oxidative metabolism. In fact, there exist regulatory mechanisms downregulating oxidative metabolism of glucose in cancer cells (see below). Since a considerable portion of observed oxygen consumption in cancer cells may be attributed to oxidation of alternate fuels such as glutamine [8], [9], the actual glucose oxidation in cancer cells is probably even lower.
The cause and consequence of the Warburg effect have been at the center of cancer metabolism study. Cancer is a disease arising from genetic and epigenetic alterations in oncogenes and tumor suppressors, many of which are also able to reprogram metabolism. The metabolic changes occurring in cancer were thus considered a secondary effect to the transformation process. However, as rapid cell proliferation requires accelerated production of the basic cellular building blocks for assembling new cells, it has now become well recognized that alterations in cellular metabolism in turn fuel tumor growth by maximally producing substrates for biosynthesis [3]. Glycolytic breakdown of glucose produces various intermediate metabolites, which as precursors can be diverted into anabolic pathways including the pentose phosphate pathway (PPP), serine and triacylglycerol synthesis pathways for the de novo synthesis of nucleotides, amino acids, and lipids [10]. While normal cells metabolize glucose almost exclusively for maximal energy production (though full energy extraction deprives cells from biosynthesis of building blocks), cancer cells boost glucose consumption primarily to provide a constant supply of glycolytic intermediates to satisfy the anabolic need of dividing cells. In this regard, glycolytic intermediates seem to be more important than the final product pyruvate. Cancer cells indeed use a variety of strategies to slow down the last step of glycolysis that is catalyzed by pyruvate kinase (PKM) [11], allowing buildup of glycolytic intermediates for biosynthesis. The altered glucose metabolism thus favors the conversion of glucose into biomass and sustains the highly proliferative nature of cancer [3]. Taken together, while alterations in oncogenes and tumor suppressors drive inappropriate cell proliferation, they also concomitantly rewire and coordinate cellular metabolism to meet the biosynthetic demands of continuous cell division.
Although the proliferative advantage offered by the Warburg metabolic phenotype is well established, its significance in metastatic progression has been much less clear. Moreover, if the principal goal of aerobic glycolysis is to produce glycolytic intermediates for anabolic support for cell proliferation, it remains elusive why after glycolysis, pyruvate is preferentially disposed of as secreted lactate rather than utilized through the TCA cycle in the mitochondria [12]. This appears to be an inefficient use of carbon resources because acetyl-CoA is a major carbon provider for fatty acid synthesis. TCA cycle reactions generate biosynthetic precursors in a process termed cataplerosis [13]. When uncoupled from OXPHOS, intermediates of the TCA cycle (e.g. citrate) are diverted into anabolic pathways and are essential for rapid tumor growth [1]. To compensate for the limited supply from glucose/pyruvate, cancer cells increase consumption of glutamine to replenish the TCA cycle [8]. Therefore, it appears that cancer cells purposefully restrain pyruvate from entry into mitochondrial oxidative metabolism.
2. Is glucose oxidation incompatible with high glycolysis in cancer cells?
One reason that cancer cells limit oxidative metabolism of glucose may be that it is not compatible with high rates of glycolysis. The TCA cycle is a hub of metabolism, with central importance in both energy production and biosynthesis. Cells must control the TCA cycle to regulate energy balance and TCA metabolite levels in the mitochondria. This regulation is fulfilled by feedback inhibition. As an important part of metabolism, metabolic flux through the glycolytic pathway is tightly regulated to respond to environment and to meet the bioenergetic and anabolic needs.
A primary control point in glycolysis is the conversion of fructose-6-phosphate (F6P) to fructose 1,6-bisphosphate (F1,6BP), a rate-limiting and irreversible early reaction in the pathway. This step is catalyzed by phosphofructokinase-1 (PFK1), the prominent pacemaker of glycolysis. PFK1 is an allosteric enzyme and is subject to extensive regulation by various metabolites in the glucose metabolism pathways. PFK1 is controlled through allosteric activation or inhibition, and in this way, cell can increase or decrease the rate of glycolysis. A total of six ligand binding sites are found in PFK1: the substrate-binding sites for ATP and F6P, activator-binding sites for AMP and fructose-2,6-bisphosphate (F2,6BP), and inhibitor-binding sites for ATP and citrate. ATP is both a substrate and an allosteric inhibitor. Binding of ATP to the allosteric modulation site, which is separated from the substrate binding site, facilitates the formation of a conformation that lowers the affinity for substrate F6P, thereby inhibiting the enzyme activity. Binding of citrate enhances the inhibitory effect of ATP. During the evolution of metazoans, eukaryotic PFK1 enzymes develop increased sensitivity to citrate [14], suggesting the importance of citrate in downregulating PFK1 and hence the glycolytic flux. Complete oxidation of glucose through the TCA cycle produces high levels of ATP. Truncated TCA cycles result in intermediate metabolites, notably citrate. When the TCA cycle is highly active, ATP and citrate accumulate, indicating an adequate supply of energy. Through negative feedback, these metabolites allosterically suppress PFK1 activity and glycolysis. Therefore, active glucose oxidation is generally not compatible with a high rate of glycolysis.
However, accumulating evidence suggests that cancer cells may have acquired ability to evade this feedback regulation. PFK1 is often hyperactivated in cancer cells and becomes resistant to the feedback inhibition. PFK1 enzymes from cancer cells are less sensitive to allosteric inhibitors, and are more potently activated by F2,6BP than that from normal cells [15] [16]. F2,6BP is the most potent allosteric activator of PFK1. The binding of F2,6BP to PFK1 increases the affinity of the enzyme for F6P and overrides ATP-mediated inhibition. F2,6BP levels thus critically regulate the glycolytic rate.
F2,6BP is generated from F6P through phosphorylation by phosphofructokinase-2 (PFK2). Increased glucose uptake and hexokinase activity leads to elevated levels of F6P, and consequently yielding more F2,6BP. F2,6BP in turn allosterically stimulates PFK1 and glycolysis. This represents a feedforward mechanism as glycolysis is enhanced when glucose is abundant. The steady-state concentrations of F2,6BP depend on the enzymatic activity of PFK2. PFK2 is a bifunctional enzyme that catalyzes either the phosphorylation of F6P to F2,6BP (kinase activity) or the dephosphorylation of F2,6BP to F6P (phosphatase activity). The enzyme exists in multiple isozymic forms, encoded by different genes, which differ in kinetics and regulatory properties. Among all PFK2 enzymes, PFKFB3 has high kinase activity and almost no phosphatase activity, thereby exhibiting the highest kinase/phosphatase activity ratio, and preferentially driving the synthesis of F2,6BP. PFKFB3 is commonly overexpressed in human cancers and sustains high-rate glycolysis [17]. PFK1 and glycolysis in such cancer cells are expected to be largely refractory to negative feedback by the TCA cycle. There is also argument that citrate generated by mitochondrial TCA cycle is mainly used for lipid synthesis after exported into the cytosol, and never builds up to a sufficient concentration to inhibit PFK1. As a result of these mechanisms, cancer cells can potentially overcome the feedback inhibition by glucose oxidation and maintain high levels of PFK1 activity and glycolysis.
3. Is glucose oxidation limited because high glycolysis relies on NADH generated from increased lactate conversion?
Glycolysis is a catabolic pathway that consumes nicotinamide adenine dinucleotide (NAD+). In glycolysis, oxidation of glyceraldehyde 3-phosphate requires NAD+ as an electron acceptor--it converts NAD+ to NADH. This step is catalyzed by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and NAD+ is a mandatory coenzyme. A recent study shows that reduced availability of NAD+ attenuates glycolysis at the GAPDH step, resulting in the accumulation of glycolytic intermediates before this step and a decrease of glycolytic intermediates after the step [18]. Cells contain a limited supply of NAD+. Constant high-rate glycolysis will use up the NAD+ pool. Glycolysis cannot sustain unless NAD+ is regenerated. LDH-mediated reduction of pyruvate into lactate (fermentation) is coupled to the oxidation of NADH into NAD+, thus efficiently replenish NAD+. Therefore, cancer cells may limit glucose oxidation in order to maximally increase lactate conversion and NAD+ recycling, allowing the continuation of high-rate glycolysis.
However, lactate fermentation alone is not sufficient to restore all NAD+ consumed in glycolysis. Since a significant portion of glucose carbon flows into anabolic pathways (e.g. serine synthesis), the more diversion of glycolytic flux into biosynthesis, the less NAD+ can be recovered by lactate fermentation. Lactate fermentation cannot supply enough NAD+ to maintain glycolysis self-sufficiency.
Cellular NAD+ levels are maintained at stable levels via equilibrium between NAD+ consumption and NAD+ synthesis. There exist multiple resources to produce NAD+. NAD+ can be synthesized from nicotinamide through the “salvage pathway”. Although there are alternate biosynthetic routes for NAD+ biosynthesis, virtually all cells depend on nicotinamide to maintain adequate intracellular NAD+ levels. Biochemical and physiological studies have established nicotinamide as the most important and widely available NAD+ precursor in mammals [19]. Nicotinamide phosphoribosyltransferase (NAMPT) is the rate-limiting enzyme catalyzing the first step in the salvage synthesis of NAD+ from nicotinamide. It plays a central role in the regulation of NAD+ homeostasis. Inhibition of NAMPT reduces the total intracellular NAD+ levels, resulting in blockade of glycolysis at the GAPDH step [18]. Conversely, increased dosage of NAMPT increases cellular NAD+ levels. NAMPT is indeed overexpressed in a number of cancers [20], [21], suggesting elevated salvage synthesis of NAD+ is the primary source for NAD+ generation in cancer cells to support glycolysis.
Collectively, these findings suggest cancer cells may be able to maintain high-rate glycolysis irrespective to the fate of pyruvate. Theoretically, cancer cells may simultaneously keep both glycolysis and glucose oxidation at high levels. This conflicts with the prevalent Warburg effect.
4. Control of glucose oxidation by PDH and PDKs
At the center of glucose oxidative metabolism lies the multisubunit PDH complex. PDH complex commits pyruvate into the TCA cycle by catalyzing the rate-limiting oxidative decarboxylation of pyruvate into acetyl-CoA. It interconnects glycolysis and the TCA cycle, thus representing a key regulatory step in glucose metabolism. The activity of PDH is tightly regulated by a variety of allosteric effectors and by reversible phosphorylation. PDH complex E1α subunit can be phosphorylated by PDH kinases (PDKs) and dephosphorylated by PDH phosphatases (PDPs) [22]. Phosphorylation of PDH inhibits the enzyme and is the primary determinant of its activity. By phosphorylation and inactivation of PDH, PDKs are a major gatekeeper of pyruvate entry into the TCA cycle.
There are four different PDKs (PDK1-4) in human that are differentially expressed in tissues [22]. Different PDKs respond to various hormonal and nutritional stimuli, including hypoxia and nutrient levels, to critically control glucose metabolism [23], [24]. Among the 4 PDKs, PDK4 is a principal isoenzyme responsible for the modulation of PDH activity and fuel selection between glucose and fatty acid for energy utilization [25]. PDK4 is highly expressed in metabolically active tissues including heart, skeletal muscle, liver, pancreas, and kidney. Selective fuel utilization depending on the fed-fast cycle is a crucial metabolic control in all mammals. In the fed state, increased plasma glucose stimulates PDH activity, glucose oxidation, and fatty acid synthesis. In the starved state, PDH activity is downregulated to limit the use of glucose. Instead, free fatty acids released from adipose tissue are used for oxidative ATP generation. Perturbation of the selection of glucose or fatty acids as energy source is a key part of diabetes and metabolic syndrome. PDK4 expression is selectively induced in most tissues and organs in response to metabolic stress conditions [25]. Starvation, fasting, and glucose deprivation markedly upregulate PDK4 levels. The abundance of PDK4 is also stimulated by a variety of dietary lipids and hormones, and is negatively regulated by insulin. Transcription of PDK4 is activated by transcription factors FOXOs, ERRs, PPARs, and coactivator PGC1, all of which are well-established key global metabolic regulators [26]. Increased PDK4 expression reduces PDH activity, thereby conserving glucose and preventing hypoglycemia. The importance of PDK4 in glucose homeostasis has indeed been validated in PDK4 knockout mice [27]. The PDK4 mutant mice exhibit elevated PDH activity and increased glucose utilization. Therefore, PDK4 is a pivotal regulator in nutrient sensing and control of PDH activity.
5. Metabolic control of anoikis in normal cells
Metabolism is intrinsically linked to cell death, as mitochondria play a central role in both energy metabolism and apoptosis [28]. Mitochondrial intermembranous space (IMS) contains key pro-apoptotic factors, such as cytochrome c, which trigger apoptosis if released into the cytosol. A specific type of apoptosis, termed anoikis, is induced by loss of cell-matrix interaction [29]. Survival of normal cells relies on integrin-mediated attachment to the extracellular matrix that elicits anti-apoptotic and pro-survival signals. Matrix detachment in anoikis-competent cells activates both extrinsic and intrinsic apoptotic pathways. Cell detachment leads to upregulation and activation of several BH3-only pro-apoptotic proteins (e.g., BMF, BIM, and BID) [30]. These proteins then activate the pro-apoptotic members of the BCL-2 family of proteins (BAK and BAX), resulting in mitochondrial outer membrane permeabilization (MOMP) that facilitates the release of IMS apoptogenic factors, and consequently downstream caspase activation and apoptosis [31].
Furthermore, matrix detachment markedly stimulates the generation of reactive oxygen species (ROS) specifically in the mitochondria of detached cells, such as endothelial cells and mammary epithelial cells [32] [33]. The mitochondrial origin of increased ROS was based on the mitochondrial indicator MitoSOX. ROS have dual functions. Low levels of ROS can activate various signaling pathways to stimulate cell proliferation and survival, whereas excess ROS irreversibly damage cellular macromolecular components (proteins, lipids, nucleic acids) and cause cell death (including apoptosis). ROS may activate intrinsic mitochondrial apoptotic pathway in part through lipid peroxidation. Cytochrome c is normally retained to the mitochondrial inner membrane due to its association with cardiolipin, a mitochondria-specific anionic phospholipid. ROS peroxidate cardiolipin, resulting in the liberation of cytochrome c [34], which may translocate through damaged mitochondrial outer membrane (as a consequence of MOMP) into cytosol, and activate the caspase cascade and apoptosis. ROS play an essential role in anoikis induction. Treatment of detached cells with antioxidants suppresses anoikis [32] [35] [36].
ROS are constantly generated as byproducts of normal mitochondrial oxidative metabolism under physiological conditions [37]. Mitochondrial respiration is indeed the major source of intracellular ROS. The electron transport chain (ETC) respiratory complexes produce superoxide anion (the first member in a plethora of ROS) when single electrons are transferred to O2 during electron transport. Approximately 1–5% of the total oxygen consumed during normal respiration is converted to superoxide radicals. To counter the deleterious effect of ROS, cells express anti-oxidants including anti-oxidant enzymes to detoxify ROS and prevent them from accumulating at high concentrations [38]. The mitochondria-located manganese superoxide dismutase (MnSOD, or SOD2) efficiently converts superoxide to the less reactive hydrogen peroxide that breaks down further into water and dioxygen by other enzymatic and non-enzymatic antioxidants. The balance between the production and elimination of ROS leads to redox homeostasis.
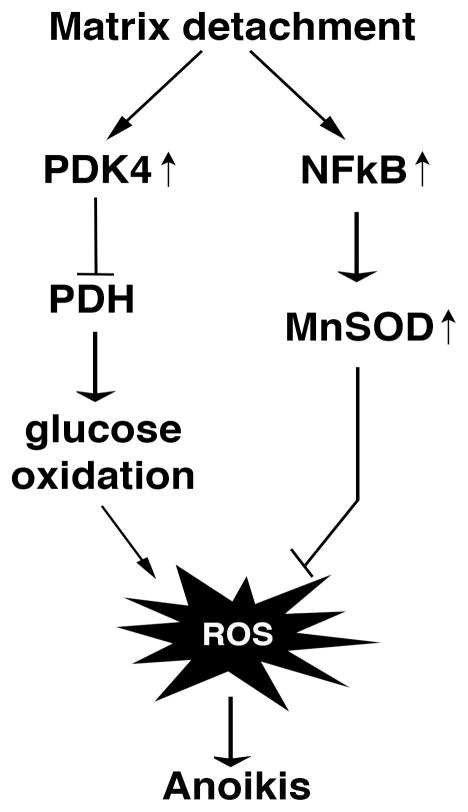
The metabolic root of ROS raised the possibility that metabolism is implicated in anoikis regulation. In fact, cell detachment causes dramatic global metabolic changes characterized by reduced uptake of nutrients (including glucose) and consequently decreased glycolysis and the TCA cycle [35] [39] [36]. In particular, when detached from the matrix, untransformed mammary epithelial cells robustly upregulate PDK4, thereby inhibiting PDH and disproportionally decreasing the flux of glucose carbon into the TCA cycle [39] [36]. Depletion of PDK4 or forced activation of PDH increases mitochondrial respiration in suspended cells, further exacerbating induction of ROS and anoikis [36]. Conversely, overexpression of PDKs antagonizes anoikis by prolonging survival of cells in suspension [36]. Moreover, cell detachment also strongly activate expression of MnSOD, the principal mitochondrial antioxidant enzyme, to detoxify mitochondrial ROS resulting from detachment [33]. Cells depleted of MnSOD are hypersensitive to matrix detachment. Taken together, although normal cells generate ROS and undergo anoikis when detached from matrix, detached cells manage to keep ROS under control by (i) shutting down glucose oxidation to evade excess ROS production and (ii) increasing antioxidant capacity to alleviate oxidative stress (Fig. 2). Such metabolic reprogramming renders cells survive longer in the absence of anchorage and mitigate anoikis. Without these metabolic endeavors, detached cells would die munch faster. These findings highlight the metabolic control of anoikis and also reinforce the vital role of ROS in anoikis.
Fig. 2. Normal cells reprogram metabolism and redox regulation to counter oxidative stress induced by matrix detachment.
Although they undergo anoikis, detached normal cells shut down glucose oxidation and increase antioxidant capacity by upregulating of PDK4 and MnSOD, respectively, to contain ROS and delay anoikis.
6. The Warburg effect contributes to anoikis resistance and metastasis
The development of metastasis is a complex process including detachment of tumor cells from the primary site, local invasion and migration, intravasation, survival in the circulation, extravasation, and colonization of the secondary sites. During the process, cells are displaced from their natural matrix niche or completely deprived of matrix support (during circulation). Therefore, resistance to anoikis is a prerequisite for tumor metastasis.
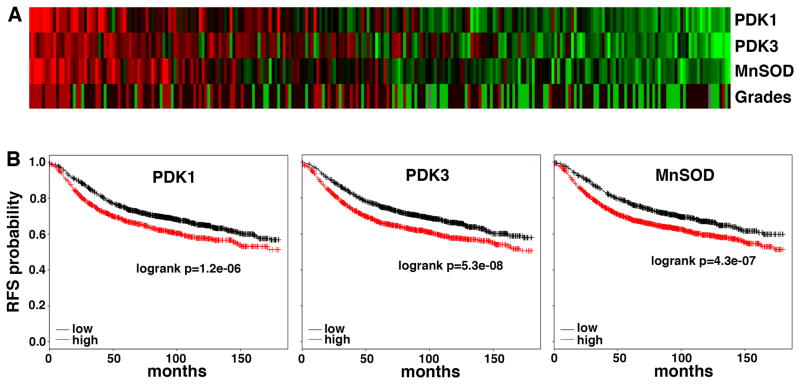
In contrast to normal cells that are sensitive to matrix detachment, cancer cells have attained increased resistance to anoikis. This is in part because apoptotic pathway is often compromised in cancer cells. In addition, given the relationship between metabolism and anoikis, one may wonder whether cell’s metabolic profile may impact its anoikis sensitivity and metastatic potential. While normal cells attenuate glucose oxidation upon detachment, many cancer cells already limit glucose oxidation prior to detachment thanks to the Warburg effect, and may hence inherently possess a survival advantage when detached. Normal cells activate PDK4 to inhibit PDH activity in response to detachment to reprogram glucose metabolism, cancer cells express high levels of PDKs (such as PDK1) even under attached conditions [36]. Depletion of PDK or activation of PDH in cancer cells stimulates glucose oxidation and ROS production, and restores their susceptibility to anoikis, leading to decreased metastatic potential [36]. Therefore, the Warburg effect allows cancer cells to evade excess ROS that would be generated by glucose oxidation. It has previously been reported that glycolysis indeed diminishes cellular oxidative stress [41]. Thus, reducing ROS levels and protecting against ROS-mediated anoikis, which promotes metastasis, may represent an advantage conferred by a Warburg metabolic phenotype. Consistently, expression of PDK1 and PDK3 in human cancer positively correlates with tumor histological grades and negatively with disease-free survival [42], [43] (Fig. 3).
Fig. 3. Expression of PDKs and MnSOD in breast cancer correlates with tumor grades and unfavorable clinical outcome.
A. Correlation between expression of PDK1, PDK3, and MnSOD, and tumor histological grades in breast cancer based on the dataset GSE3494 (n=251). B. Higher levels of PDK1, PDK3, and MnSOD predict worse relapse-free survival (RFS) in a large cohort of breast cancer patients (n=3455). Analysis was performed using the webtool developed by [40].
Cancerous tissues were reported to produce increased amounts of ROS [44]. However, cancer cells also develop heightened antioxidant systems to cope with the high oxidative stress [45]. While MnSOD is induced following matrix detachment in normal cells [33], many antioxidants, including MnSOD, are constitutively overexpressed in cancer. Elevated expression of MnSOD is associated with worse clinical outcomes [46][47] [33] (Fig. 3). Consistent with these findings, increased glucose consumption by cancer cells diverts more glucose carbon into the oxidative branch of PPP, a principal mechanism to generate NADPH [45]. NADPH is an essential cofactor for replenishing reduced glutathione (GSH)--cell’s most critical antioxidant. Cancer cells can further enhance this antioxidant generation pathway by inhibiting PKM when oxidative stress rises [48]. Enhanced antioxidant capacity allows cancer cells to better survive detachment-induced oxidative stress and metastasize. Consistently, in a lung cancer mouse model, treatment with antioxidants reduced oxidative stress and accelerated lung cancer progression (as evidenced by development of more tumors and at more advanced stages) [49]. In our hands, normal cells (MCF10A human mammary epithelial cells) apparently accumulate more ROS than highly glycolytic and metastatic cancer cells (MDA-MB-231 human breast cancer cells) (not shown). This might be attributed partly to higher antioxidants in cancer cells.
Taken together, the Warburg effect brings multiple benefits to cancer cells: increased glucose consumption provides anabolic support for proliferation and enhances antioxidant capacity, whereas the shift of pyruvate away from mitochondrial oxidation allows cells to avoid excess ROS generation. Therefore, cancer cells are better equipped to tolerate oxidative stress generated by matrix detachment, and thus acquire anoikis resistance and metastatic potential. In essence, the Warburg effect promotes tumor growth and importantly, metastasis.
7. Metastasis regulators impact oxidative metabolism
As discussed in many excellent reviews [1][3] [50], many well-established oncogenes and tumor suppressors, such as hypoxia-inducible factor (HIF), Akt, Myc, and p53, exert direct impact on metabolism, most notably on glucose uptake and glycolysis. However, increased glycolysis does not evenly increase downstream metabolic pathways (Fig. 1). In particular, pyruvate is preferentially diverted to lactate fermentation, with very little to the mitochondrial TCA cycle. The regulatory mechanisms for oxidative metabolism of glucose are distinct from those for glycolysis. For instance, Akt stimulates glucose uptake and glycolysis without affecting the rate of oxidative phosphorylation [51]. Because oxidative metabolism antagonizes metastasis, here we discuss several metastasis regulators, namely HIF, Snail, p53, and KISS1, for their effects on mitochondrial metabolism.
HIF promotes metastasis and suppresses oxidative metabolism
Development of intratumoral hypoxia is a nearly universal feature of rapidly growing solid tumors. Hypoxia occurs when cancer cells outgrow the blood vessels or as a result of unstable tumor vasculature. Hypoxia is a negative prognostic factor because it enhances invasiveness, stemness, metabolic shift, angiogenesis, and metastatic potential of tumor cells [52]. Cells pretreated with hypoxia gain increased metastatic ability than their normoxic counterparts [53]. The adaptive changes are primarily mediated by the transcription factor HIF, a master regulator to balance oxygen supply and demand [54]. HIF is stabilized and activated under hypoxic environment, leading to activation of its target genes. HIF is also activated by loss of tumor suppressor (e.g., VHL), activation of oncogenic signaling, and increased ROS levels [52], [55]. Therefore, many tumors may have constitutively activated the HIF-dependent program even under normoxia. Higher levels of HIF are associated with higher cancer mortality [56].
HIF is a prominent determinant of the metabolic shift from glucose oxidation to aerobic glycolysis in cancer. Besides stimulating glucose uptake and glycolysis, HIF transcriptionally activates PDK1 and PDK3 [23] [24] [57]. These PDKs phosphorylate and inactivate PDH, prevent pyruvate from fueling the mitochondrial TCA cycle, and hence reduce mitochondrial oxygen consumption and prevent the excessive production of ROS.
HIF also enhances LDH-mediated pyruvate-to-lactate conversion. LDH is a tetrameric enzyme consisting of LDH-H subunit (encoded by ubiquitously expressed LDHB gene) and/or LDH-M subunit (encoded by LDHA). Generally, LDH-catalyzed reaction is reversible. However, LDH enzyme comprising “pure” LDH-M subunits preferentially converts pyruvate into lactate. LDHA is a direct transcriptional target of HIF and highly inducible by hypoxia. Therefore, HIF promotes the formation of LDH enzyme made of only LDH-M and more efficiently turns pyruvate into lactate. This may indirectly decrease the pyruvate flux into mitochondria by competition.
HIF cooperates with other transcriptional regulators, such as Myc. Myc is an oncogenic transcription factor involved in the regulation of cell metabolism and cell proliferation, and is commonly deregulated in cancer [58]. Acting alone (under normoxia) or in conjunction with HIF, Myc activates PDK1 and LDHA [59]. Estrogen-related receptors (ERRs), normally controlling the fuel selection between glucose and fatty acids by suppressing glucose oxidation via upregulation of PDK4, are associated with tumor progression [60] [61]. Our previous studies suggest that ERRs enhance HIF and Myc activity and contribute to the Warburg effect [62] [63]. Collectively, HIF-dependent transcriptional program decreases PDH activity (via upregulation of PDKs) and increases LDH-mediated pyruvate-to-lactate conversion, thereby efficiently diverting pyruvate away from mitochondrial oxidation.
PDKs only control the flow of glucose carbons, and do not directly regulate metabolites derived from fatty acid oxidation and glutaminolysis, which may also fuel the TCA cycle. Recently, it was shown that HIF shifts glutamine metabolism from oxidation to reductive carboxylation as well [64]. In fact, HIF is able to broadly attenuate mitochondrial respiration and ROS generation. There are multiple ROS production sites in the ETC, which consists of four complexes. The ubiquinone reduction site of Complex I and the outer quinone-binding site of the Q cycle in Complex III possess the greatest maximum capacity of ROS production [65]. Hypoxia upregulates microRNA-210 (miR-210), which targets iron-sulfur cluster assembly proteins (ISCU1/2) [66]. These proteins facilitate the assembly of iron-sulfur clusters (including those in Complex I and Complex III). Iron-sulfur clusters are critical for electron transport and mitochondrial redox reactions. Consequently, miR-210 disrupts the integrity of iron-sulfur clusters and represses mitochondrial respiration [66]. Furthermore, Complex I (NADH:ubiquinone oxidoreductase) catalyzes the first step in the ETC. NADH dehydrogenase (ubiquinone) 1α subcomplex subunit 4-like 2 (NDUFA4L2), considered as an inhibitory component of Complex I, was identified as a direct transcriptional target of HIF [67]. HIF induces expression of NDUFA4L2, which in turn inhibits ETC Complex I activity, thereby attenuating oxygen consumption and mitochondrial ROS production [67].
Together, as a master commander, HIF stimulates glycolysis, switches glucose metabolism from oxidative phosphorylation to lactate fermentation, and attenuates overall mitochondrial respiration. These metabolic effects protect cells against oxidative stress, and may impart cancer cells with a selective advantage during metastasis.
EMT inducer Snail inhibits mitochondrial respiration
Epithelial cells are able to acquire mesenchymal properties through epithelial-to-mesenchymal transition (EMT). EMT is a characteristic of embryonic development, but also contributes to tumor progression by bestowing cancer cells with increased metastatic potential, cancer stem cell (CSC)-like traits, and therapeutic resistance [68][69]. A defining feature of EMT is resistance to anoikis, which contributes to metastasis [70]. The mechanistic coupling between EMT and resistance to anoikis remains poorly understood [70]. The transcriptional repressor Snail is a central driver of EMT and its expression correlates with metastasis and poor clinical prognosis [71] [72].
As part of ETC Complex IV, cytochrome c oxidase (COX) is the last enzyme in the respiratory chain that transfers the electrons to oxygen during mitochondrial respiration. COX is an oligomeric protein complex that is composed of 13 different subunits encoded by 3 mitochondrial genes and 10 nuclear genes. Three COX subunits, COX6c, COX7a, and COX7c, were identified as direct targets of Snail [73]. Snail binds to their promoters and inhibits their expression. Increased Snail expression inhibits COX expression and activity, and consequently represses oxygen consumption and mitochondrial respiration [73].
Snail also regulates glucose metabolism. Fructose-1,6-biphosphatase 1 (FBP1) is a rate-limiting enzyme in the pathway of gluconeogenesis. FBP1 catalyzes the hydrolysis of F1,6BP to F6P, which is opposite to the rate-limiting glycolytic reaction catalyzed by PFK1. Therefore, FBP1 functions to antagonize glycolysis. In cancer cells, expression of FBP1 is often downregulated due to promoter hypermethylation [74]. Restoration of FBP1 expression reduces glucose uptake, glycolysis, and lactate generation, meanwhile increases mitochondrial oxygen consumption as well as ROS production [74] [75]. These metabolic effects suppress anchorage-independent growth and tumorigenecity [74]. FBP1 was recently discovered as a direct target of Snail [75]. Snail binds to the FBP1 promoter and represses FBP1 expression, thereby enhancing glycolysis. Together with its ability to suppress mitochondrial respiration [73], Snail facilitates the metabolic shift towards aerobic glycolysis.
Snail not only induces EMT but also suppresses mitochondrial oxidative metabolism. As EMT confers resistance to anoikis, it is conceivable that Snail-mediated metabolic changes may contribute to anoikis resistance and metastasis.
p53 suppresses the Warburg effect
As one of the most frequently mutated genes in cancer, mutations in the p53 tumor suppressor occur in the majority of human cancers. p53 suppresses tumorigenesis primarily by orchestrating cell cycle arrest and apoptosis in response to cellular stress [76]. Accumulating evidence suggests that p53 functions at multiple stages of cancer, including metastasis. Tumor-derived mutant p53 can drive tumor survival, invasion, and metastasis [77]. p53 has also been discovered to be an important regulator of metabolic pathways. p53 has been shown to control glycolysis, oxidative phosphorylation, glutaminolysis, fatty acid oxidation, and redox [50] [78]. p53-deficient cells exhibit higher rates of glycolysis, more lactate production, and decreased mitochondrial respiration compared with wild-type cells [79], suggesting p53 suppresses the Warburg effect.
Mechanistically, p53 regulates genes involved in the balance between the utilization of respiratory and glycolytic pathways. Basal levels of p53 can promote oxidative metabolism through transcriptional activation of SCO2 [79]. SCO2 is critical for regulating the assembly of the COX complex in the ETC, the major site of oxygen consumption in the mitochondria. Cells with mutant p53 have compromised oxidative phosphorylation. Disruption of the SCO2 gene in cells with wild-type p53 recapitulates the aerobic glycolysis phenotype that is exhibited by p53-deficient cells. In addition, p53 downregulates PDK2 to promote the entry of pyruvate into mitochondria for oxidative metabolism [80]. Moreover, p53 activates transcription of glutaminase 2 (GLS2) to promote glutaminolysis to fuel the TCA cycle and also facilitates fatty acid oxidation (FAO) as an alternative energy source [78].
In coordination with activation of oxidative metabolism, p53 restricts glycolytic flux through a number of mechanisms (Berkers et al. 2013), which include transcriptional activation of TIGAR. TIGAR acts as a fructose-2,6-bisphosphatase that lowers F2,6BP levels and thereby reduces the activity of PFK1. Hence, p53, via TIGAR, can decrease the rate of glycolysis [81]. Collectively, p53 counteracts the Warburg effect by favoring oxidative phosphorylation and minimizing the glycolytic phenotype. p53 suppresses tumor development and progression perhaps in part through its function at the level of metabolism.
KISS1 promotes oxidative metabolism
KISS1 is a member of metastasis suppressor genes, which are defined by their ability to block metastasis without affecting primary tumor development [82]. KISS1 encodes for Kisspeptin, which is a peptide ligand for G protein-coupled receptor KISS1R. KISS1 exhibits anti-metastatic effects in numerous human cancers, including melanoma, thyroid, ovarian, bladder, gastric, esophageal, pancreatic, lung, and pituitary cancers [83] [84]. Clinically, reduced KISS1 expression is associated with poor prognosis in cancer patients. It is proposed that KISS1 suppresses metastasis through inhibition of cancer cell migration and invasion [84]. A recent study links KISS1-dependent metastatic suppression to metabolism [85]. Expression of KISS1 shifts metabolism from aerobic glycolysis to oxidative phosphorylation, apparently via pathways that boost mitochondrial respiration and/or biogenesis through stabilization of PGC1α. PGC1α is a transcriptional co-activator for most genes in the TCA cycle and ETC, and thus is a master regulator of mitochondrial respiration and oxidative metabolism [86]. Importantly, PGC1α seems to be essential for KISS1-mediated metabolic changes and suppression of metastasis [85], suggesting the pro-oxidative metabolic effect of KISS1 is important for its metastasis-suppressing activity.
8. Metabolic modulation for anti-metastasis therapy
The Warburg effect not only fuels tumor growth but also facilitates metastatic progression. Current studies position the Warburg effect as a central player in malignancy. Metastasis is the primary cause of cancer mortality. Discovery of its root in altered metabolism may expose its vulnerability, making metabolic modulation a viable therapeutic anti-metastasis approach. An important step towards metabolic therapy is to identify and manipulate critical biochemical nodes that are deregulated in cancer metabolism. The Warburg effect is a metabolic hallmark of cancer cells and is generally reversible, therefore may represent an attractive target for therapy. As part of the Warburg phenotype, glucose oxidation is restrained in cancer cells. Forced activation of oxidative metabolism may curtail cancer cell’s anoikis resistance and metastatic potential.
PDH is a key determinant of pyruvate entry into the mitochondria. PDH activity is diminished in cancer cells partly due to increased PDK expression. The small molecule Dichloroacetate (DCA) is a pyruvate mimetic and can compete with pyruvate for binding to PDKs [87]. DCA inhibits PDKs and was initially used in clinical treatment for lactic acidosis [88]. Inhibition of PDK by DCA enhances pyruvate entry into the TCA cycle and shifts metabolism from glycolysis to glucose oxidation. This metabolic normalization increases mitochondrial respiration and ROS generation, and induces apoptosis in certain cancer cells even under attached conditions [89] [90]. In our study of breast cancer cells, ectopic activation of PDH increases oxidative stress, restores anoikis sensitivity, and reduces metastasis, but has little effect on the viability of attached cells [91]. The discrepancy may originate from the difference in the antioxidant capacity of different cancer cells. In the mouse brain tumor model, DCA is able to cross brain blood barrier and induce regression of metastatic tumor mass [90]. DCA has been on several clinical trials, including for glioma and refractory metastatic breast cancer (ClinicalTrials.gov Identifier: NCT01111097, NCT01386632, and NCT01029925) [92] [93].
Several other regulators of glycolysis are also amenable for anticancer therapy. Modulation of such metabolic targets may indirectly enhance oxidative metabolism as well [92] [94]. Inhibition of LDH re-directs pyruvate into mitochondrial oxidation [95]. Glycolysis inhibition by 2-deoxy-D-glucose (2-DG) increases oxygen utilization and decreases lactate production, shifting metabolism toward OXPHOS and reverting the metastatic phenotype in vitro and in vivo [96]. Overall, these approaches reverse the Warburg glycolytic phenotype, boost oxidative metabolism, heighten oxidative stress, and may restore anoikis sensitivity and suppress metastasis. Furthermore, because commonly used radiation and chemotherapy depend on the induction of free radicals to kill cancer cells, the pro-oxidative metabolic modulation may enhance their therapeutic efficacy. However, the currently available metabolic modulators are very limited. The potency and specificity of DCA are quite low. All these justify the quest for new reagents targeting aerobic glycolysis for anti-metastasis treatment.
Acknowledgments
This work was supported by NIH grants R01CA137021 (to J.L.) and R01CA149646 (to M.T.).
Abbreviations
- 2-DG
2-deoxy-D-glucose
- CoA
coenzyme A
- COX
cytochrome c oxidase
- DCA
Dichloroacetate
- EMT
epithelial-to-mesenchymal transition
- ERR
Estrogen-related receptor
- ETC
electron transport chain
- F1
6BP, fructose 1,6-bisphosphate
- F2
6BP, fructose-2,6-bisphosphate
- F6P
fructose-6-phosphate
- FAO
fatty acid oxidation
- FBP1
Fructose-1,6-biphosphatase 1
- FDG
18-fluorodeoxyglucose
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GSH
reduced glutathione
- HIF
hypoxia-inducible factor
- IMS
Mitochondrial intermembranous space
- ISCU
iron-sulfur cluster assembly protein
- LDH
lactate dehydrogenase
- MnSOD
manganese superoxide dismutase
- MOMP
mitochondrial outer membrane permeabilization
- NAD+
nicotinamide adenine dinucleotide
- NAMPT
Nicotinamide phosphoribosyltransferase
- OXPHOS
oxidative phosphorylation
- PDH
pyruvate dehydrogenase
- PDK
pyruvate dehydrogenase kinases
- PDP
pyruvate dehydrogenase phosphatases
- PET
positron emission tomography
- PFK
phosphofructokinase
- PKM
pyruvate kinase
- PPP
pentose phosphate pathway
- ROS
reactive oxygen species
- TCA
tricarboxylic acid
Footnotes
Conflict of Interest Statement
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008 Jun;13(6):472–82. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008 Sep;134(5):703–7. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 3.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009 May;324(5930):1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011 May;11(5):325–37. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 5.Jose C, Bellance N, Rossignol R. Choosing between glycolysis and oxidative phosphorylation: a tumor’s dilemma? Biochim Biophys Acta. 2011 Jun;1807(6):552–61. doi: 10.1016/j.bbabio.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang XL, Rajagopalan KN, Maddie M, Vemireddy V, Zhao Z, Cai L, Good L, Tu BP, Hatanpaa KJ, Mickey BE, Matés JM, Pascual JM, Maher EA, Malloy CR, Deberardinis RJ, Bachoo RM. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012 Jun;15(6):827–37. doi: 10.1016/j.cmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guppy M, Leedman P, Zu X, Russell V. Contribution by different fuels and metabolic pathways to the total ATP turnover of proliferating MCF-7 breast cancer cells. Biochem J. 2002 May;364(Pt 1):309–15. doi: 10.1042/bj3640309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007 Dec;104(49):19345–50. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008 Dec;105(48):18782–7. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011 Jan;27:441–64. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 11.Vander Heiden MG, Lunt SY, Dayton TL, Fiske BP, Israelsen WJ, Mattaini KR, Vokes NI, Stephanopoulos G, Cantley LC, Metallo CM, Locasale JW. Metabolic pathway alterations that support cell proliferation. Cold Spring Harb Symp Quant Biol. 2011 Jan;76:325–34. doi: 10.1101/sqb.2012.76.010900. [DOI] [PubMed] [Google Scholar]
- 12.McKnight SL. On getting there from here. Science. 2010 Dec;330(6009):1338–9. doi: 10.1126/science.1199908. [DOI] [PubMed] [Google Scholar]
- 13.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008 Jan;7(1):11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Usenik A, Legiša M. Evolution of allosteric citrate binding sites on 6-phosphofructo-1-kinase. PLoS One. 2010 Jan;5(11):e15447. doi: 10.1371/journal.pone.0015447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vora S, Halper JP, Knowles DM. Alterations in the activity and isozymic profile of human phosphofructokinase during malignant transformation in vivo and in vitro: transformation- and progression-linked discriminants of malignancy. Cancer Res. 1985 Jul;45(7):2993–3001. [PubMed] [Google Scholar]
- 16.Staal GE, Kalff A, Heesbeen EC, van Veelen CW, Rijksen G. Subunit composition, regulatory properties, and phosphorylation of phosphofructokinase from human gliomas. Cancer Res. 1987 Oct;47(19):5047–51. [PubMed] [Google Scholar]
- 17.Atsumi T, Chesney J, Metz C, Leng L, Donnelly S, Makita Z, Mitchell R, Bucala R. High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res. 2002 Oct;62(20):5881–7. [PubMed] [Google Scholar]
- 18.Tan B, Young DA, Lu ZH, Wang T, Meier TI, Shepard RL, Roth K, Zhai Y, Huss K, Kuo MS, Gillig J, Parthasarathy S, Burkholder TP, Smith MC, Geeganage S, Zhao G. Pharmacological inhibition of nicotinamide phosphoribosyltransferase (NAMPT), an enzyme essential for NAD+ biosynthesis, in human cancer cells: metabolic basis and potential clinical implications. J Biol Chem. 2013 Feb;288(5):3500–11. doi: 10.1074/jbc.M112.394510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallí M, Van Gool F, Rongvaux A, Andris F, Leo O. The nicotinamide phosphoribosyltransferase: a molecular link between metabolism, inflammation, and cancer. Cancer Res. 2010;70:8–11. doi: 10.1158/0008-5472.CAN-09-2465. [DOI] [PubMed] [Google Scholar]
- 20.Bi T, Che X. Nampt/PBEF/visfatin and cancer. Cancer Biol Ther. 2010 Jul;10(2):119–25. doi: 10.4161/cbt.10.2.12581. [DOI] [PubMed] [Google Scholar]
- 21.Garten A, Petzold S, Körner A, Imai SI, Kiess W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009 Apr;20(3):130–8. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. 2006 Apr;34(Pt 2):217–22. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Kwon HS, Harris RA. Mechanisms responsible for regulation of pyruvate dehydrogenase kinase 4 gene expression. Adv Enzyme Regul. 2004 Jan;44:109–21. doi: 10.1016/j.advenzreg.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Jeong JY, Jeoung NH, Park KG, Lee IK. Transcriptional regulation of pyruvate dehydrogenase kinase. Diabetes Metab J. 2012 Oct;36(5):328–35. doi: 10.4093/dmj.2012.36.5.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeoung NH, Wu P, Joshi MA, Jaskiewicz J, Bock CB, Depaoli-Roach AA, Harris RA. Role of pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) in glucose homoeostasis during starvation. Biochem J. 2006 Aug;397(3):417–25. doi: 10.1042/BJ20060125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen JL, Kornbluth S. The tangled circuitry of metabolism and apoptosis. Mol Cell. 2013 Feb;49(3):399–410. doi: 10.1016/j.molcel.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997 Oct;9(5):701–6. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 30.Gilmore AP. Anoikis. Cell Death Differ. 2005 Nov;12(Suppl 2):1473–7. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- 31.Llambi F, Moldoveanu T, Tait SWG, Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP, Green DR. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011 Nov;44(4):517–31. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li AE, Ito H, Rovira II, Kim KS, Takeda K, Yu ZY, Ferrans VJ, Finkel T. A role for reactive oxygen species in endothelial cell anoikis. Circ Res. 1999 Aug;85(4):304–10. doi: 10.1161/01.res.85.4.304. [DOI] [PubMed] [Google Scholar]
- 33.Kamarajugadda S, Cai Q, Chen H, Nayak S, Zhu J, He M, Jin Y, Zhang Y, Ai L, Martin SS, Tan M, Lu J. Manganese superoxide dismutase promotes anoikis resistance and tumor metastasis. Cell Death Dis. 2013;4:e504. doi: 10.1038/cddis.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007 Jan;47:143–83. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 35.Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, Gao S, Puigserver P, Brugge JS. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009 Sep;461(7260):109–13. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamarajugadda S, Stemboroski L, Cai Q, Simpson NE, Nayak S, Tan M, Lu J. Glucose oxidation modulates anoikis and tumor metastasis. Mol Cell Biol. 2012 May;32(10):1893–907. doi: 10.1128/MCB.06248-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stowe DF, Camara AKS. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009 Jun;11(6):1373–414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009 Jul;8(7):579–91. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 39.Grassian AR, Metallo CM, Coloff JL, Stephanopoulos G, Brugge JS. Erk regulation of pyruvate dehydrogenase flux through PDK4 modulates cell proliferation. Genes Dev. 2011 Aug;25(16):1716–33. doi: 10.1101/gad.16771811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Györffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010 Oct;123(3):725–31. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 41.Brand KA, Hermfisse U. Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J. 1997 Apr;11(5):388–95. doi: 10.1096/fasebj.11.5.9141507. [DOI] [PubMed] [Google Scholar]
- 42.Wigfield SM, Winter SC, Giatromanolaki A, Taylor J, Koukourakis ML, Harris AL. PDK-1 regulates lactate production in hypoxia and is associated with poor prognosis in head and neck squamous cancer. Br J Cancer. 2008 Jun;98(12):1975–84. doi: 10.1038/sj.bjc.6604356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu CW, Lin SC, Chien CW, Lin SC, Lee CT, Lin BW, Lee JC, Tsai SJ. Overexpression of pyruvate dehydrogenase kinase 3 increases drug resistance and early recurrence in colon cancer. Am J Pathol. 2011 Sep;179(3):1405–14. doi: 10.1016/j.ajpath.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haklar G, Sayin-Ozveri E, Yüksel M, Aktan AO, Yalçin AS. Different kinds of reactive oxygen and nitrogen species were detected in colon and breast tumors. Cancer Lett. 2001 Apr;165(2):219–24. doi: 10.1016/s0304-3835(01)00421-9. [DOI] [PubMed] [Google Scholar]
- 45.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011 Feb;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 46.Pani G, Colavitti R, Bedogni B, Fusco S, Ferraro D, Borrello S, Galeotti T. Mitochondrial superoxide dismutase: a promising target for new anticancer therapies. Curr Med Chem. 2004 May;11(10):1299–308. doi: 10.2174/0929867043365297. [DOI] [PubMed] [Google Scholar]
- 47.Landriscina M, Maddalena F, Laudiero G, Esposito F. Adaptation to oxidative stress, chemoresistance, and cell survival. Antioxid Redox Signal. 2009 Nov;11(11):2701–16. doi: 10.1089/ars.2009.2692. [DOI] [PubMed] [Google Scholar]
- 48.Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang J, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, Cantley LC. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011 Dec;334(6060):1278–83. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014 Jan;6(221):221ra15. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- 50.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010 Dec;330(6009):1340–4. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 51.Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, Thompson CB. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004 Jun;64(11):3892–9. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 52.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664–71. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rofstad EK, Danielsen T. Hypoxia-induced metastasis of human melanoma cells: involvement of vascular endothelial growth factor-mediated angiogenesis. Br J Cancer. 1999 Aug;80(11):1697–707. doi: 10.1038/sj.bjc.6690586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaelin WG, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008 May;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 55.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010 Feb;29(5):625–34. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu CW, Lin SC, Chen KF, Lai YY, Tsai SJ. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J Biol Chem. 2008;283:28106–28114. doi: 10.1074/jbc.M803508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dang CV. MYC on the Path to Cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dang CV. The interplay between MYC and HIF in the Warburg effect. Ernst Schering Found Symp Proc. 2007:35–53. doi: 10.1007/2789_2008_088. [DOI] [PubMed] [Google Scholar]
- 60.Deblois G, Giguère V. Oestrogen-related receptors in breast cancer: control of cellular metabolism and beyond. Nat Rev Cancer. 2013 Jan;13(1):27–36. doi: 10.1038/nrc3396. [DOI] [PubMed] [Google Scholar]
- 61.Chang C, McDonnell DP. Molecular pathways: the metabolic regulator estrogen-related receptor α as a therapeutic target in cancer. Clin Cancer Res. 2012 Nov;18(22):6089–95. doi: 10.1158/1078-0432.CCR-11-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ao A, Wang H, Kamarajugadda S, Lu J. Involvement of estrogen-related receptors in transcriptional response to hypoxia and growth of solid tumors. Proc Natl Acad Sci U S A. 2008;105:7821–7826. doi: 10.1073/pnas.0711677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cai Q, Lin T, Kamarajugadda S, Lu J. Regulation of glycolysis and the Warburg effect by estrogen-related receptors. Oncogene. 2013 Apr;32(16):2079–86. doi: 10.1038/onc.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun RC, Denko NC. Hypoxic Regulation of Glutamine Metabolism through HIF1 and SIAH2 Supports Lipid Synthesis that Is Necessary for Tumor Growth. Cell Metab. 2014 Feb;19(2):285–92. doi: 10.1016/j.cmet.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10:273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tello D, Balsa E, Acosta-Iborra B, Fuertes-Yebra E, Elorza A, Ordóñez Á, Corral-Escariz M, Soro I, López-Bernardo E, Perales-Clemente E, Martínez-Ruiz A, Enríquez JA, Aragonés J, Cadenas S, Landázuri MO. Induction of the Mitochondrial NDUFA4L2 Protein by HIF-1α Decreases Oxygen Consumption by Inhibiting Complex I Activity. Cell Metabolism. 2011;14:768–779. doi: 10.1016/j.cmet.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 68.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009 Apr;9(4):265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 69.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 70.Frisch SM, Schaller M, Cieply B. Mechanisms that link the oncogenic epithelial-mesenchymal transition to suppression of anoikis. J Cell Sci. 2013;126:21–9. doi: 10.1242/jcs.120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007 Jun;7(6):415–28. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 72.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013 Feb;13(2):97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 73.Lee SY, Jeon HM, Ju MK, Kim CH, Yoon G, Han SI, Park HG, Kang HS. Wnt/Snail signaling regulates cytochrome C oxidase and glucose metabolism. Cancer Res. 2012;72:3607–17. doi: 10.1158/0008-5472.CAN-12-0006. [DOI] [PubMed] [Google Scholar]
- 74.Liu X, Wang X, Zhang J, Lam EKY, Shin VY, Cheng ASL, Yu J, Chan FKL, Sung JJY, Jin HC. Warburg effect revisited: an epigenetic link between glycolysis and gastric carcinogenesis. Oncogene. 2010;29:442–450. doi: 10.1038/onc.2009.332. [DOI] [PubMed] [Google Scholar]
- 75.Dong C, Yuan T, Wu Y, Wang Y, Fan TWM, Miriyala S, Lin Y, Yao J, Shi J, Kang T, Lorkiewicz P, St Clair D, Hung MC, Evers BM, Zhou BP. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23:316–31. doi: 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009 May;137(3):413–31. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 77.Muller PAJ, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013 Jan;15(1):2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 78.Berkers CR, Maddocks ODK, Cheung EC, Mor I, Vousden KH. Metabolic regulation by p53 family members. Cell Metab. 2013 Nov;18(5):617–33. doi: 10.1016/j.cmet.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. p53 regulates mitochondrial respiration. Science. 2006 Jun;312(5780):1650–3. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 80.Contractor T, Harris CR. p53 negatively regulates transcription of the pyruvate dehydrogenase kinase Pdk2. Cancer Res. 2012 Jan;72(2):560–7. doi: 10.1158/0008-5472.CAN-11-1215. [DOI] [PubMed] [Google Scholar]
- 81.Bensaad K, Tsuruta A, Selak MA, Vidal MNC, Nakano K, Bartrons R, Gottlieb E, Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006 Jul;126(1):107–20. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 82.Smith SC, Theodorescu D. Learning therapeutic lessons from metastasis suppressor proteins. Nat Rev Cancer. 2009;9:253–264. doi: 10.1038/nrc2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beck BH, Welch DR. The KISS1 metastasis suppressor: a good night kiss for disseminated cancer cells. Eur J Cancer. 2010;46:1283–1289. doi: 10.1016/j.ejca.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cvetković D, Babwah AV, Bhattacharya M. Kisspeptin/KISS1R System in Breast Cancer. J Cancer. 2013 Jan;4(8):653–661. doi: 10.7150/jca.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu W, Beck BH, Vaidya KS, Nash KT, Feeley KP, Ballinger SW, Pounds KM, Denning WL, Diers AR, Landar A, Dhar A, Iwakuma T, Welch DR. Metastasis suppressor KISS1 appears to reverse the Warburg effect by enhancing mitochondrial biogenesis. Cancer Res. 2013 Dec; doi: 10.1158/0008-5472.CAN-13-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006 Dec;27(7):728–35. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 87.Knoechel TR, Tucker AD, Robinson CM, Phillips C, Taylor W, Bungay PJ, Kasten SA, Roche TE, Brown DG. Regulatory roles of the N-terminal domain based on crystal structures of human pyruvate dehydrogenase kinase 2 containing physiological and synthetic ligands. Biochemistry. 2006 Jan;45(2):402–15. doi: 10.1021/bi051402s. [DOI] [PubMed] [Google Scholar]
- 88.Stacpoole PW, Lorenz AC, Thomas RG, Harman EM. Dichloroacetate in the treatment of lactic acidosis. Ann Intern Med. 1988 Jan;108(1):58–63. doi: 10.7326/0003-4819-108-1-58. [DOI] [PubMed] [Google Scholar]
- 89.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007 Jan;11(1):37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 90.Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D, Abdulkarim B, McMurtry MS, Petruk KC. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010 May;2(31):31ra34. doi: 10.1126/scitranslmed.3000677. [DOI] [PubMed] [Google Scholar]
- 91.Kamarajugadda S, Lu J, Stemboroski L, Nayak S, Simpson NE, Cai Q, Tan M. Glucose Oxidation Modulates Anoikis and Tumor Metastasis. Molecular and Cellular Biology. 2012;32:1893–1907. doi: 10.1128/MCB.06248-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Porporato PE, Dhup S, Dadhich RK, Copetti T, Sonveaux P. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front Pharmacol. 2011 Jan;2:49. doi: 10.3389/fphar.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Papandreou I, Goliasova T, Denko NC. Anticancer drugs that target metabolism: Is dichloroacetate the new paradigm? Int J Cancer. 2011 Mar;128(5):1001–8. doi: 10.1002/ijc.25728. [DOI] [PubMed] [Google Scholar]
- 94.Butler EB, Zhao Y, Muñoz-Pinedo C, Lu J, Tan M. Stalling the engine of resistance: targeting cancer metabolism to overcome therapeutic resistance. Cancer Res. 2013;73:2709–17. doi: 10.1158/0008-5472.CAN-12-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sottnik JL, Lori JC, Rose BJ, Thamm DH. Glycolysis inhibition by 2-deoxy-D-glucose reverts the metastatic phenotype in vitro and in vivo. Clin Exp Metastasis. 2011 Dec;28(8):865–75. doi: 10.1007/s10585-011-9417-5. [DOI] [PubMed] [Google Scholar]