Abstract
Eating behavior is guided by a complex interaction between signals conveying information about energy stores, food availability, and palatability. How peripheral signals regulate brain circuits that guide feeding during sensation and consumption of a palatable food is poorly understood. We used fMRI to measure brain response to a palatable food (milkshake) when n=32 participants were fasted and fed with either a fixed-portion or ad libitum meal. We found that larger post-prandial reductions in ghrelin and increases in triglycerides were associated with greater attenuation of response to the milkshake in brain regions regulating reward and feeding including the midbrain, amygdala, pallidum, hippocampus, insula and medial orbitofrontal cortex. Satiation-induced brain responses to milkshake were not related to acute changes in circulating insulin, glucose, or free fatty acids. The impact of a meal on the response to milkshake in the midbrain and dorsolateral prefrontal cortex differed depending upon whether meal termination was fixed or volitional, irrespective of the amount of food consumed. We conclude that satiation-induced changes in brain response to a palatable food are strongly and specifically associated with changes in circulating ghrelin and triglycerides and by volitional meal termination.
Keywords: Satiation, Satiety, Feeding, Ghrelin, Triglycerides, fMRI, Gut-brain axis
1. Introduction
All organisms must consume food to maintain homeostasis and survive. However, eating beyond homeostatic need can be elicited by external factors, such as the pleasurable sensory properties of food [1–4] or the presence of conditioned cues that predict food availability [5,6]. Importantly, there is evidence that overweight and obese individuals are more susceptible to the influence of external stimuli than are their healthy weight counterparts [4,7]. This raises the possibility that propensity to eat in the absence of hunger contributes to vulnerability for weight gain in the modern food environment [8]. It is therefore of critical importance to define neural circuits that are responsive to food and food cues in the absence of hunger.
Prior work using positron emission tomography (PET) [9–16] and fMRI [17–29] demonstrates that while variability exists, greater brain response is observed in reward circuits when fasted compared to when fed. Many of these responses correlate with ratings of meal pleasantness, reflecting the known influence of satiation on the hedonic impact of food – a phenomenon termed alliesthesia [30]. Increased responses during hunger are also observed in brain networks important for attentional orienting. These findings are consistent with food cues being more salient and rewarding when the body is experiencing an energy deficit [11,31,32]. Notably, overweight and obese individuals tend to show attenuated influences of satiation on brain response to food cues [12,13,25], which has supported the idea that obesity is related to the chronic failure of central homeostatic circuits to recognize and respond to internal satiation signals. An alternative possibility is that the abnormal hormonal signaling that accompanies obesity subsequently disrupts feeding and satiation-related signaling to the brain [33]. Unreliable internal cues may therefore contribute to a shift of reliance on internal to external cues to govern feeding behavior.
Peripherally circulating internal indicators of an organism’s metabolic state can be roughly characterized based on whether they are orexigenic (stimulating food intake) or anorexigenic (inhibiting food intake). Plasma concentrations of ghrelin, an orexigenic peptide of mostly gastric origin, decrease after a meal [34] and infusions of ghrelin stimulate appetite and food intake in humans [35]. Conversely, the anorexigenic signals glucose and insulin rise during a meal and fall in the between-meal interim [36]; These fluctuations have been associated with subjective appetite sensations and subsequent energy intake [37]. Markers of lipid metabolism are also influenced by food intake: For example, plasma concentrations of non-esterified fatty acids, or free fatty acids (FFA), drop in response to a meal [38] while levels of triglycerides are increased [39]. The majority of studies that have measured such peripheral feeding and satiation-related markers concomitant to assessing brain response in fasted vs. fed states did so using designs where subjects were scanned while resting with no food cues present [9,12–15,40,41]. Such experiments may assess more broad state-specific effects of satiation rather than how satiation impacts the processing of food-related environmental cues. Morris and Dolan measured both plasma hormone levels as well as how brain response to visual food cues correlated with recognition for food images in fed vs. fasted states, but did not test for relationships between blood and brain measures [11]. Malik et al. found that intravenous infusions of ghrelin, mimicking the physiological hungry state, increased neural response to viewing pictures of food compared to scenery in the amygdala, orbitofrontal cortex (OFC), midbrain, caudate, hippocampus, insula and visual areas [42]. Likewise a recent study by Karra et al. showed that human homozygotes of the at-risk vs. low-risk allele of the fat mass and obesity-associated gene (FTO) show differential modulatory effects of ghrelin on brain response to viewing pictures of foods. Specifically, homozygotes of the low-risk allele showed a positive relationship between BOLD response to food vs. nonfood images and post-meal ghrelin suppression in the fusiform gyrus, postcentral gyrus, and cuneus, while homozygotes of the high-risk allele exhibited a negative relationship between BOLD response and post-meal ghrelin suppression in those areas [43]. These studies highlight a role for ghrelin in regulating brain response to palatable food cues but leave unanswered the role of other peripheral factors and how these factors influence responses to palatable food consumption. This is an important distinction since differential effects of satiation and caloric deprivation have been observed in response to taste compared to visual cues [24,44]. To our knowledge, only one previous study has reported peripheral feeding metabolites obtained via blood sampling while measuring the effect of internal state on brain response to taste cues [24]. Uher et al. found greater brain response when hungry vs. sated to both sweet and savory food tastes in insula and frontal operculum. However, the authors did not test for an interaction between peripheral signals and brain response.
This study sought to take an integrative approach towards examining central and peripheral mechanisms of satiation. We used fMRI to measure brain response to a palatable food (milkshake) when fasted and after being acutely satiated with a lunchtime meal. We predicted greater brain response to milkshake in reward areas when individuals are hungry compared to fed, and that the magnitude of this effect would be associated with individual differences in circulating plasma concentrations of feeding-related hormones and nutrients. Importantly, we included two different fed conditions, one in which subjects ate a fixed-portion meal and the other in which they were instructed to eat as much as they wanted. Both approaches have been used in the past and we wanted to test whether they would yield differential effects; especially in prefrontal regions posited to be critical for food-related decision making and meal termination [10,45].
2. Materials and methods
2.1 Subjects
32 right-handed subjects (Age range 18-39, M=25.5, SD=5.7; BMI range 19.5-37.0, M=25.3, SD=4.4; 14 male) were recruited from the greater New Haven area through the Yale University Interdisciplinary Research Consortium on Stress, Self-Control and Addiction (IRCSSA) P30 Subject’s core as well as via flyer advertisement. Subjects were screened over the phone to be less than 40 years of age, free of psychiatric disorders, eating disorders, current dieting behavior, alcoholism, use of tobacco or drugs other than alcohol, history of head injury with loss of consciousness, use of daily medication other than monophasic birth control, chemosensory impairments, lactose intolerance or food allergies. We did not impose a BMI upper-limit. Individuals were included so long as they felt comfortable while inserted in the scanner bore. Females provided the date of their last period to ensure that they were not scanned during menstruation or ovulation. All subjects provided written informed consent at their first lab visit and the study was approved by the Yale Human Investigations Committee.
2.2 Stimuli
Milkshake stimuli consisted of two different flavors (chocolate and strawberry) that were presented in an interleaved fashion during the scan in order to minimize sensory adaptation. The chocolate milkshake was made by combining 12 fl oz each of whole milk, Garelick Farms brand Chug Chocolate Milkshake, and Garelick Farms brand Chug Cookies and Cream Milkshake. The strawberry milkshake consisted of 32 fl oz of whole milk to which 6 fl oz of Hershey’s brand strawberry syrup was added. One subject disliked the chocolate milkshake, so for this subject only, a vanilla milkshake consisting of 24 fl oz Garelick Farms brand Chug Vanilla Milkshake and 12 fl oz of whole milk was substituted for the chocolate. The tasteless solution consisted of each subject’s choice of whichever solution tasted “the most like nothing” out of 25mM KCl /2.5mM NaHCO3, 18.75mM KCl/1.875mM NaHCO3, 12.5mM KCl/1.25mM NaHCO3, 6.25mM KCl/0.625mM NaHCO3, or 3.13mM KCl/0.313mM NaHCO3 in distilled water [46].
The standardized breakfast consisted of commercially available prepackaged granola bars (190 kcal per package). For lunch, subjects received apple slices (approximately 25 kcal of apple per bag) and their choice of sandwich from the options of tuna, ham, turkey, or avocado served on white bread with cheese, tomato, and mayonnaise. Each sandwich was designed to contain approximately 400 kcal and was cut into quarters before serving to discourage subjects from interpreting each entire sandwich as one large “portion”.
2.3 Stimulus delivery
Liquid tastes were delivered to the subjects through a portable gustometer system as 0.5ml of solution infused into the mouth over 4s. Detailed description of the gustometer system can be found in a previous publication [47]. In brief, tastes are held in 60 ml syringes loaded into BS-8000 syringe pumps (Braintree Scientific, Braintree, MA). Each syringe infuses liquids into 25 feet of Tygon beverage tubing (Saint-Gobain Performance Plastics, Akron, OH) that connects to a custom-designed Teflon manifold mounted on the MRI headcoil. The manifold has a series of ports that funnel the tastes from individually machined channels into a silicon tube that sits comfortably in the mouth and allows the tastes to drip onto the tongue.
2.4 Experimental procedures
2.4.1 Overview
Subjects took part in one training session, three fMRI scanning sessions (Hungry, Fixed meal and Ad Lib meal conditions), and one behavioral test session. All sessions were conducted on separate days and scan order was counterbalanced.
2.4.2 Training session
Subjects were instructed to refrain from eating or drinking anything other than water for at least an hour before the session. Upon arrival, subjects were trained to make computerized ratings of their internal state as well as the perceptual qualities of our stimuli on computerized scales. Internal state ratings were indicated on a series of adapted cross-modal general Labeled Magnitude Scales (gLMS) consisting of a 100mm vertical line scale with the labels “no sensation” at the lower anchor point and “strongest imaginable sensation” at the upper anchor point [48–50]. Subjects rated the intensity of their feelings of hunger, fullness, thirst, anxiety, and need to urinate. Perceptual qualities consisted of ratings of each stimulus’s intensity, liking, familiarity, edibility, and wanting to eat. Intensity was measured with the gLMS. Liking was measured using a labeled hedonic scale (LHS) consisting of a 100mm vertical line scale with the labels “most disliked sensation imaginable” at the lower anchor point, “most liked sensation imaginable” at the upper anchor point, and “neutral” in the middle [51]. Edibility, familiarity, and wanting to eat were rated on 200mm visual analogue scales labeled at the left (−10), center (0) and right (+10) anchor points. Edibility labels were “not edible at all” at (−10), neutral at (0) and “very edible” at (+10). Familiarity labels were “not familiar at all” (−10), “neutral” (0) and “very familiar” (+10). Wanting to eat labels were “I would never want to consume this” (−10), “neutral” (0) and “I would want to consume this more than anything” (+10).
Subjects were then brought to the fMRI simulator and outfitted with the stimulus delivery system. They first made internal state ratings as well as perceptual ratings of each of the stimuli using a mouse on a computer monitor viewed via back projection on a headcoil-mounted mirror. After completing the ratings, subjects were inserted into the bore of the mock scanner and underwent simulated fMRI runs. The taste run was 6m32s long, and consisted of the uncued delivery of two different types of stimuli: (1) a 4s delivery of milkshake, followed by a 6-13s rest period where the subject could swallow, a 4s tasteless rinse, and another 6-13s rest period; or (2) a 4s delivery of tasteless solution, followed by a 6-13s rest period (Fig. 1a). There were ten repetitions of each of the two events of interest (milkshake and tasteless). The subjects were instructed to swallow and exhale through their nose after receiving each liquid.
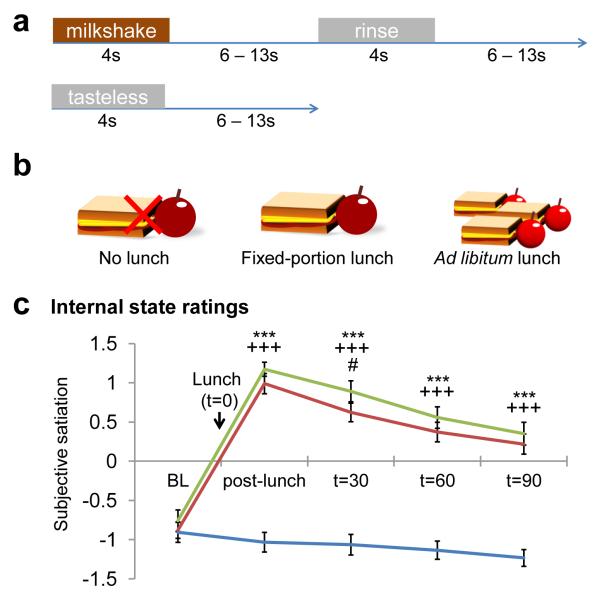
Figure 1.
Experimental design and manipulation. a) Protocol for milkshake and tasteless stimulus presentation during the functional runs. b) Schematic of the three internal state conditions. c) Internal state ratings of subjective satiation over the time course of the three fMRI scan sessions. Blue=Hungry, Green=Fixed, Red=Ad Lib. Error bars represent the standard error of the mean. BL=baseline, *=p<.05, **=p<.01, ***=p<.005; *=Hungry vs. Fixed, +=Hungry vs. Ad Lib, #=Fixed vs. Ad Lib.
Following the simulation runs, subjects were removed from the bore and asked to make a second round of internal state and stimulus ratings. The LHS liking ratings of the milkshake stimuli were then examined to ensure that subjects found the milkshakes palatable (both flavors of milkshake rated above “neutral” in the scale). They were then provided with breakfast bars and told to eat them for breakfast the morning before each fMRI scan session.
2.4.3 fMRI scanning sessions
A schematic of each test session can be found in Fig. 1b. Subjects ate the breakfast bars (1 package for women, 1.5 packages for men) in the morning at home and then refrained from eating or drinking, with the exception of water, until their session. Subjects arrived at the scanning center at 11:45 AM. Starting at 12:15 PM, a Teflon catheter was inserted into an antecubital vein for blood sampling and a series of three baseline internal state ratings were obtained at 15 minute increments. Baseline blood samples were obtained concomitant to the second and third internal state ratings. Five minutes after the third internal state rating, subjects ate either a fixed-portion lunch (Fixed scan; 1 sandwich and 1 serving of apple slices for women, 1.5 sandwiches and 1 serving of apple slices for men), an ad lib lunch (Ad Lib scan; 3 sandwiches and 4 servings of apple slices for both women and men and instructed to eat as much as they’d like), or nothing (Hungry scan). The amount and type of foods consumed at Fixed and Ad Lib lunches were recorded without the subjects’ knowledge.
After making a fourth set of internal state ratings, the subjects were then taken to the scanner at approximately 1 PM. Meal duration during Fixed and Ad Lib sessions and time of scan start was not standardized, however subsequent internal state ratings and blood samples were collected inside the scanner at 30, 60, and 90m from time of meal (or no meal) onset to minimize temporal variability between the different internal state conditions.
Imaging data were acquired on a Siemens 3.0 Tesla TIM Trio Scanner at the Yale University Magnetic Resonance Research Center. High-resolution T1-weighted structural scans were acquired for each subject with the following parameters: TR=2230ms, TE=1.73 ms, flip angle=9°, matrix=256x256, 1mm thick slices, FOV=250x250, 176 slices.
For fMRI taste runs, a susceptibility-weighted single-shot echo-planar sequence was used to image regional distribution of the blood oxygenation level dependent (BOLD) signal. At the beginning of each functional run, the MR signal was allowed to equilibrate over 6 scans for a total of 12 seconds, which were subsequently excluded from the analyses. Acquisition parameters were: TR=2000ms, TE=20ms, flip angle=80°, FOV=220, matrix=64×64, slice thickness=3mm. Forty contiguous slices were acquired in an interleaved method to reduce the cross-talk of the slice selection pulse.
Two taste runs as described at the training session were collected at each scan. The taste runs were interspersed with additional anatomical and functional scans using odors that will discussed in a future manuscript. Perceptual ratings of the stimuli were collected at the beginning and end of each scan session; Ratings of chocolate and strawberry milkshake pre- and post-scan were averaged to obtain mean intensity, pleasantness, edibility, familiarity and wanting to consume for milkshake under each internal state condition.
2.4.4 Behavioral test session
The behavioral test session took place on a separate day concomitant to the fMRI scan sessions. Upon arrival at the laboratory anthropometric measurements were taken. Body weight and height was measured with jackets and shoes removed at the beginning of the session using a Detecto 439 balance beam scale with stadiometer. Body Mass Index (BMI) was calculated as weight (in kilograms) divided by the squared height (in meters) of the subject (BMI=kg/m2). Subjects also filled out surveys and engaged in computer tasks as a part of a complimentary study that will not be discussed in this manuscript.
2.5 Data analysis
2.5.1 Behavior
Internal state ratings for each subject were log transformed and an index of satiation created by subtracting hunger from fullness ratings. One subject’s internal state ratings from the Hungry scan and another’s from the Ad Lib scan were omitted due to technical malfunction of equipment during collection. The amount of kilocalories consumed by each subject at Fixed and Ad Lib lunches were calculated based on information provided on the nutrition facts label. Planned comparisons of behavioral data were analyzed in PASW Statistics 18 (SPSS Inc., Chicago, IL) using repeated measures analyses of variance (ANOVA).
2.5.2 Blood
Sufficient blood samples for processing at all three scans were obtained from n=25 out of the 32 total subjects. Samples were centrifuged immediately and kept on ice for the duration of each session, then frozen at either −80°C (FFA, ghrelin, insulin) or −20°C (glucose, triglycerides). Plasma levels of feeding-related hormones were measured with commercially available materials. Insulin and total ghrelin levels were measured with radioimmunoassay kits that utilize the double antibody technique with 125I-labeled hormone and hormone antiserum (Ghrelin: Cat. # GHRT-89HK; Insulin: Cat. # HI-14K, EMD Millipore Corporation, Billerica MA). Glucose, FFA and triglyceride concentrations were measured using enzymatic colorimetric techniques (FFA: Cat. # 999-34691, 991034891, 993-35191, Wako Pure Chemical Industries, Ltd., Osaka, Japan. Triglycerides: Cat. # SA1023, RX1023; Glucose: Cat. # SA1014, RX1014, Alfa Wassermann Diagnostic Techniques, West Caldwell NJ).
2.5.3 Neuroimaging
Neuroimaging data were analyzed using the SPM8 software (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, London, UK) in MATLAB R2010b 7.11.0 (Mathworks, Inc., Sherborn, MA). Functional images were time-acquisition corrected to the slice obtained at 50% of the TR and realigned to the mean image. Anatomical and functional images were normalized to the standard MNI template brain implemented in SPM8, resulting in voxel sizes of 3 mm3 and 1 mm3 respectively. Functional time-series data was detrended [52] and then smoothed using a 6 mm FWHM isotropic Gaussian kernel. The Artifact Detection Tools (ART) toolbox for MATLAB was used to detect global mean and motion outliers in the functional data (Gabrieli Lab, McGovern Institute for Brain Research, Cambridge MA). Motion parameters were included as regressors in the design matrix at the single-subject level. Additionally, image volumes where the z-normalized global brain activation exceeded 3 standard deviations from the mean of the run or showed greater than 1mm of composite (linear plus rotational) movement were flagged as outliers and deweighted during SPM estimation.
One design matrix was created for each subject’s taste runs across all scan days, specifying the onset and duration of the two events of interest: Milkshake (chocolate plus strawberry) and Tasteless control. Event onsets were defined as the beginning of liquid delivery and event durations were defined as the four seconds of taste delivery. The rinse was modeled as an event of no interest. A 270 Hz high-pass filter, adapted to 1.5 times the period of stimulus presentation, was applied to the time-series data with the aim to remove low-frequency noise and slow signal drifts. The general linear model was employed to estimate condition-specific effects at each model. A canonical hemodynamic response function (HRF), including a temporal derivative, was used to model neural response to events of interest.
All second-level fMRI analyses included BMI and sex as covariates in order to evaluate the effect of internal state independently of influences of BMI and gender. Data collection is ongoing to achieve the necessary power to test the influence of these variables. To assess brain response to milkshake consumption under different scan conditions, a 3×2 flexible factorial ANOVA was used where each subject is designated as an independent factor with equal variance; each condition (Hungry, Fixed, and Ad Lib) as a dependent factor of unequal variance; and each stimulus type (Milkshake and Tasteless) as a dependent factor of unequal variance. To test associations between BOLD response and plasma hormone concentrations, one-session t-test random effects analyses were performed that regressed brain response to Milkshake-Tasteless against change in hormones from baseline. For hormones that were collected at multiple post-manipulation time points (ghrelin, glucose, and insulin), the time point at which maximum change from baseline occurred was used for analysis. Additionally, extreme outliers (defined as greater than three times the interquartile range) in hormone concentrations were identified using PASW Statistics 18 and excluded from analysis on a case-by-case basis. The t-map threshold was set at puncorrected<.005 and a 5 voxel cluster size. Unpredicted responses were considered significant at p<.05 Family Wise Error (FWE) corrected across the entire brain for multiple comparisons. For predicted responses a region of interest (ROI) approach was used. 11 ROIs were selected based on previous literature on feeding and reward [16,19,26,29,45,53]. ROIs of the insula, hippocampus, amygdala, caudate, putamen, midbrain, pallidum, nucleus accumbens, and hypothalamus were anatomically defined with masks from the WFU PickAtlas software for SPM (ANSIR Laboratory, Wake Forest University, Winston-Salem NC). ROIs of the medial orbitofrontal cortex (mOFC) and dorsolateral prefrontal cortex (DLPFC) were constructed as spheres with 10mm radiuses centered at the coordinates MNI x,y,z=[−2, 54, −6] and [−48, 15,24], respectively, and duplicated so as to be bilateral [45,54]. For all ROIs, left and right hemispheres were tested simultaneously. Peaks in ROI analyses were considered significant at p<.05 FWE corrected across the total number of voxels across the individual ROI.
3. Results
3.1 Effect of internal state on behavioral measures
A time course of internal state ratings in all three scans can be found in Fig. 1c. Baseline internal state ratings did not differ between Hungry, Fixed and Ad Lib scans. There was also no statistically significant difference in the amount of kilocalories eaten during Fixed and Ad Lib lunches (Fixed M=523.9 kcal, SD=102.6; Ad Lib M=576.8 kcal, SD=262.0), which was not surprising as the fixed-portion meal was designed to be satiating. Also as expected, subjects felt subjectively more satiated following lunch at Fixed and Ad Lib sessions compared to the Hungry session (Hungry vs. Fixed F(1,30)=182.81, p<.001; Hungry vs. Ad Lib F(1,29)=369.14, p<.001). This difference in satiation between conditions persisted at every time point after lunch (t=30m Hungry vs. Fixed F(1,30)=236.55, p<.001, Hungry vs. Ad Lib F(1,29)=193.91,p<.001; t=60m Hungry vs. Fixed F(1,30)=218.65, p<.001, Hungry vs. Ad Lib F(1,29)=169.22, p<.001; t=90m Hungry vs. Fixed F(1,30)=145.88, p<.001; Hungry vs. Ad Lib F(1,29)=116.81, p<.001). There was no significant difference in subjective ratings of satiation between Fixed and Ad Lib scans except at t=60m (F(1,30)=6.10, p<.05), resulting from greater reported satiation in Fixed vs. Ad Lib. There was no effect of condition on perceptual ratings of milkshake pleasantness, intensity, edibility or familiarity. However, there was a significant effect of condition on ratings of wanting to consume milkshake (F(2,62)=4.12, p<.05), where subjects’ desire to consume the milkshake was higher when hungry than when sated (Hungry vs. Fixed F(1,31)=4.90, p<.05; Hungry vs. Ad Lib F(1,31)=7.56, p=.01; Fixed vs. Ad Lib n.s.).
3.2 Effect of internal state on peripheral feeding-related markers
Repeated measures ANOVAs on the effect of time on peripheral feeding-related markers at each session revealed that ghrelin levels did not change during the Hungry scan, but decreased after both Fixed and Ad Lib meals and continued to decrease through scanning (Fixed F(3,72)=11.39, p<.001; Ad Lib F(3,72)=8.10, p<.001). Levels of glucose and insulin during the Hungry scan experienced an initial dip at t=30 but recovered as milkshake was received over the course of scanning (Glucose Hungry F(3,72)=4.66, p<.05; Insulin Hungry F(3,72)=12.65, p<.001). Levels of glucose and insulin during the Fixed and Ad Lib scans spiked at t=30 after the meal and decreased through scanning (Glucose Fixed F(3,72)=20.20, p<.001; Glucose Ad Lib F(3,72)=4.49, p <.05; Insulin Fixed F(3,72)=19.57; Insulin Ad Lib F(3,72)=17.41, p<.001). Triglycerides and FFAs were only measured at baseline and t=60. Triglyceride levels decreased from baseline at the Hungry scan, and increased from baseline at the Fixed scan (Hungry F(1,24)=25.82, p<.001; Fixed F(1,24)=7.99, p<.005). Triglyceride levels also increased from baseline at the Ad Lib scan at trend level (Ad Lib F(1,24)=3.99, p=.057). Levels of FFA increased from baseline at the Hungry scan and decreased from baseline at Fixed and Ad Lib scans (Hungry F(1,24)=25.15, p<.001; Fixed F(1,24)=11.27, p<.005; Ad Lib F(1,24)=17.63, p<.001). Table 1 shows the change in plasma concentration of feeding-related markers from baseline at Fixed and Ad Lib scans minus the change from baseline at Hungry scan, to take into account differences introduced by receiving the milkshake stimuli over the course of the scan.
Table 1.
Average change in plasma hormone concentrations from baseline at each time point in minutes from onset of lunch manipulation, for Fixed and Ad Lib minus values at Hungry scan.
| Plasma hormone concentration (±SEM) | |||
|---|---|---|---|
|
| |||
| Fixed (vs. Hungry) | t=30 | t=60 | t=90 |
| Ghrelin pg/ml | −72.0 (35.6) | −143.3 (42.8) | −186.5 (36.3) |
| Glucose mg/dl | 18.2 (2.5) | 9.1 (2.8) | 1 (2.1) |
| Insulin uU/ml | 28.3 (3.2) | 27.5 (2.4) | 13.3 (2.1) |
| Free Fatty Acids mM | -- | −0.3 (0.1) | -- |
| Triglycerides mg/dl | -- | 16.9 (3.9) | -- |
|
| |||
| Ad Lib (vs. Hungry) | t=30 | t=60 | t=90 |
|
| |||
| Ghrelin pg/ml | −91.6 (41.3) | −120.2 (36.8) | −193.1 (46.7) |
| Glucose mg/dl | 10.3 (2.3) | 6.3 (3.3) | 1.9 (2.3) |
| Insulin uU/ml | 23.2 (3.0) | 20.6 (4.0) | 10.9 (2.4) |
| Free Fatty Acids mM | -- | −0.4 (0.1) | -- |
| Triglycerides mg/dl | -- | 11.9 (3.1) | -- |
3.3 Brain response to milkshake and influence of internal state
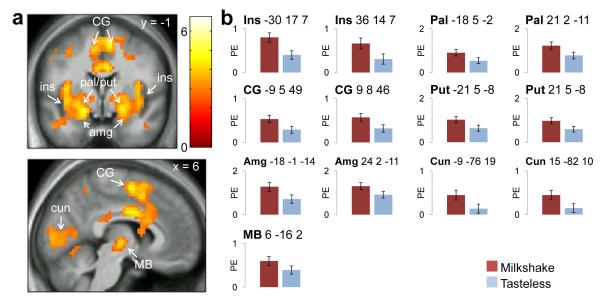
Consistent with prior work, a comparison of Milkshake > Tasteless (collapsed across condition) produced responses in the insula, pallidum, putamen, amygdala, cingulate gyrus, cuneus, and midbrain (Fig. 2a, Tables 2 & 3).
Figure 2.
Main effect of stimulus. CG=cingulate gyrus, ins=insula, pal=pallidum, put=putamen, amg=amygdala, cun=cuneus, MB=midbrain. Color bar depicts t-values. a) Preferential responses to Milkshake>Tasteless (Hungry, Fixed, and Ad Lib). b) Parameter estimates for Milkshake and Tasteless stimuli at the peak voxel in those regions.
Table 2.
Milkshake>Tasteless All: Whole brain analyses
| Milkshake>Tasteless All: Whole brain | |||||||
|---|---|---|---|---|---|---|---|
| Size (voxels) | MNI coordinates | Region | L/R | Z | pFWE-peak | ||
| x | y | z | |||||
|
|
|||||||
| 5020 | −30 | 17 | 7 | Insula | L | 6.29 | <.001 |
| −30 | 23 | 7 | Insula | L | 6.17 | <.001 | |
| −36 | −7 | 10 | Insula | L | 5.70 | <.001 | |
| 9 | 8 | 46 | Cingulate gyrus | R | 4.95 | .011 | |
| −36 | 5 | −11 | Insula | L | 4.89 | .014 | |
| −9 | 5 | 49 | Cingulate gyrus | L | 4.89 | .014 | |
| 33 | 17 | 10 | Insula | R | 4.76 | .025 | |
| 36 | 14 | 7 | Insula | R | 4.69 | .034 | |
| 21 | 2 | −11 | Pallidum | R | 4.66 | .037 | |
| −9 | −76 | 19 | Cuneus | L | 4.65 | .039 | |
| 15 | −82 | 10 | Cuneus | R | 4.64 | .041 | |
Table 3.
Milkshake>Tasteless All: ROI analyses
| Milkshake>Tasteless All | |||||||
|---|---|---|---|---|---|---|---|
| ROI | L/R | Size (voxels) | MNI coordinates | Z | pFWE-peak | ||
| x | y | z | |||||
|
|
|||||||
| Insula | L | 331 | −30 | 17 | 7 | 6.29 | <.001 |
| −30 | 23 | 7 | 6.17 | <.001 | |||
| −36 | −7 | 10 | 5.70 | <.001 | |||
| −36 | 5 | −11 | 4.89 | <.001 | |||
| R | 253 | 36 | 14 | 7 | 4.69 | <.001 | |
| 39 | 8 | −11 | 4.23 | .006 | |||
| 39 | −4 | 13 | 4.14 | .009 | |||
| 30 | 8 | −17 | 3.95 | .018 | |||
| 33 | 11 | −14 | 3.83 | .026 | |||
| Amygdala | L | 44 | −18 | −1 | −14 | 4.55 | <.001 |
| −30 | 2 | −17 | 3.73 | .007 | |||
| R | 53 | 24 | 2 | −11 | 4.40 | <.001 | |
| Putamen | L | 91 | −21 | 5 | −8 | 4.42 | .001 |
| −27 | −4 | −5 | 3.93 | .009 | |||
| −24 | 14 | 1 | 3.53 | .036 | |||
| R | 80 | 21 | 5 | −8 | 4.66 | <.001 | |
| 21 | 2 | 1 | 3.75 | .017 | |||
| Pallidum | L | 38 | −18 | 5 | −2 | 4.12 | .001 |
| −24 | −1 | −5 | 3.84 | .003 | |||
| −15 | −1 | −11 | 3.70 | .006 | |||
| −21 | 2 | 1 | 3.61 | .008 | |||
| R | 66 | 21 | 2 | −11 | 4.66 | <.001 | |
| 18 | 8 | −5 | 4.27 | <.001 | |||
| 21 | 2 | 1 | 3.75 | .005 | |||
| Midbrain | R | 67 | 6 | −16 | −2 | 3.87 | ,013 |
| 0 | −16 | −8 | 3.73 | .021 | |||
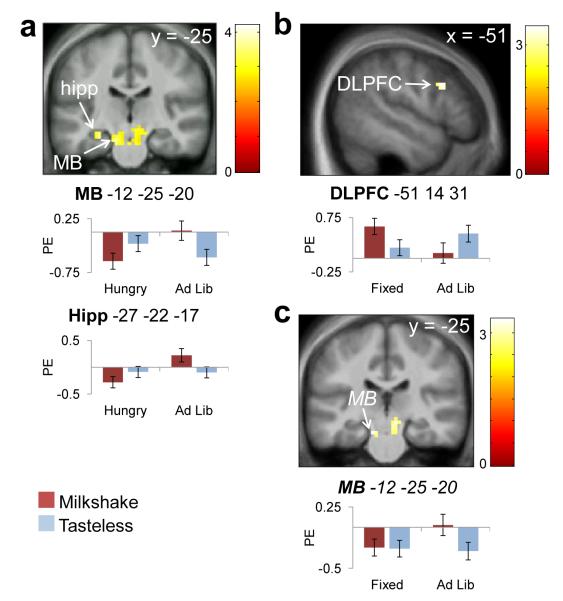
Contrary to our predictions we did not observe greater response to Milkshake > Tasteless when subjects were hungry compared to when they had consumed either a fixed or ad lib meal (Fig. 3 a-c, Table 4). The only significant differential response observed as a function of fed vs. fasted conditions was greater response in the midbrain and hippocampus to Milkshake > Tasteless during the Ad Lib scan as compared to the Hungry scan (Fig. 3a). There was also a trend towards greater response in midbrain to Milkshake > Tasteless in Ad Lib > Fixed conditions (p=.090, Fig 3c). Differential response to Milkshake > Tasteless between the two fed conditions was also observed in DLPFC, which was greater in Fixed compared to Ad Lib conditions (Fig. 3b). These between-scan differences persisted when differences in caloric intake were included as covariates.
Figure 3.
Effect of internal state on preferential responses to milkshake. Bar graphs show parameter estimates for Milkshake and Tasteless stimuli at the peak voxel in those regions. Panels masked with combined a priori ROIs for display purposes. MB=midbrain, hipp=hippocampus, DLPFC=dorsolateral prefrontal cortex. Color bar depicts t-values. a) Milkshake>Tasteless, Ad Lib>Hungry. b) Milkshake>Tasteless, Fixed>Ad Lib. c) Milkshake>Tasteless, Ad Lib>Fixed (trend).
Table 4.
ROI analyses of between-scan effects.
| Milkshake>Tasteless Ad Lib>Hungry | |||||||
|---|---|---|---|---|---|---|---|
| ROI | L/R | Size (voxels) | MNI coordinates | Z | pFWE-peak | ||
| x | y | z | |||||
|
|
|||||||
| Midbrain | L | 150 | −12 | −25 | −20 | 4.08 | .006 |
| −3 | −37 | −20 | 3.49 | .045 | |||
| Hippocampus | L | 9 | −27 | −22 | −17 | 3.58 | .008 |
| Milkshake>Taste less Fixed>Ad Lib | |||||||
| ROI | L/R | Size (voxels) | MNI coordinates | Z | pFWE-peak | ||
|
| |||||||
| x | y | z | |||||
|
|
|||||||
| DLPFC | L | 12 | −51 | 14 | 31 | 3.36 | .036 |
| Milkshake>Tasteless Ad Lib>Fixed | |||||||
| ROI | L/R | Size (voxels) | MNI coordinates | Z | pFWE-peak | ||
|
| |||||||
| x | y | z | |||||
|
|
|||||||
| Midbrain * | L | 53 | −12 | −25 | −20 | 3.25 | .090 |
= trend.
3.4 Effect of internal state on brain response is related to peripheral feeding-related markers
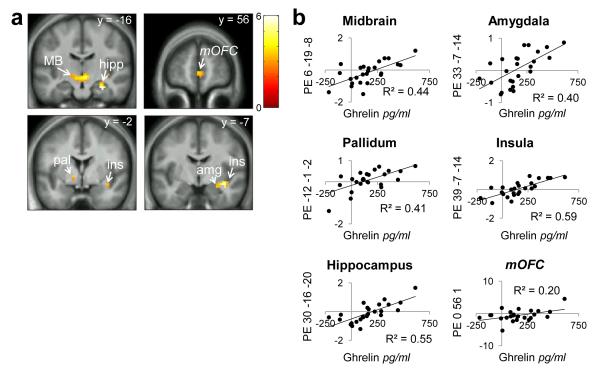
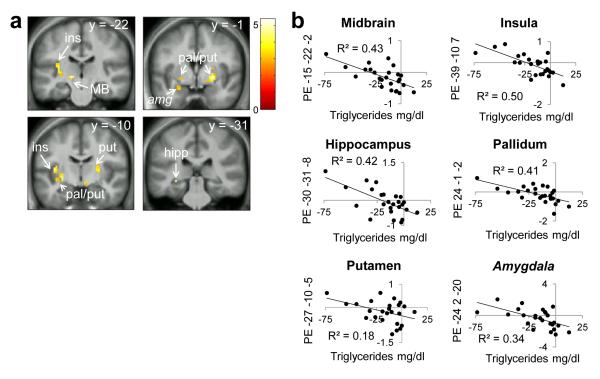
To test whether peripherally circulating feeding-related markers might influence the effect of internal state on brain response we next regressed changes in glucose, insulin, ghrelin, triglycerides and FFA against differential response to Milkshake > Tasteless in Hungry > Fixed and Hungry > Ad Lib conditions. Maximal change in ghrelin occurred at t=90m and maximal change in glucose and insulin occurred at t=30m. There were no significant correlations between brain response to Milkshake > Tasteless and levels of FFA, insulin, or glucose. However, there was a significant positive relationship between brain response to Milkshake > Tasteless and change in ghrelin in most of our ROIs including the midbrain, amygdala, pallidum, hippocampus and insula, and a trend (p=.054) in mOFC. In these regions stronger differential brain responses to Milkshake > Tasteless were associated with more robust reductions in ghrelin levels after the fixed-portion lunch (Figs. 4a, b; Table 5). We also observed a negative relationship between ROI response to Milkshake > Tasteless and change in triglyceride levels after the fixed-portion lunch. Subjects with greater increases in circulating triglycerides showed greater differential responses to Milkshake > Tasteless in midbrain, insula, hippocampus, pallidum, putamen, with a trend (p=.064) in the amygdala (Figs.5a,b; Table 6). Similar positive correlations in insula, putamen, pallidum, caudate and midbrain were observed when regressing change in ghrelin against the comparison of Milkshake > Tasteless in Hungry > Ad Lib conditions but they failed to reach statistical significance. Likewise, there was a trend towards a positive correlation in mOFC response to Milkshake > Tasteless with change in triglycerides in Hungry > Ad Lib conditions (p=.051).
Figure 4.
Ghrelin and preferential responses to milkshake in Hungry vs. Fixed sessions. MB=midbrain, hipp=hippocampus, pal=putamen, ins=insula, amg=amygdala, mOFC=medial orbitofrontal cortex (trend). Panels masked with combined a priori ROIs for display purposes. Color bar depicts t-values. a) Milkshake>Tasteless Hungry>Fixed positively correlated with change in ghrelin levels at 90 minutes from baseline Hungry-Fixed. b) Parameter estimates from the peak voxel in those regions.
Table 5.
Areas where Milkshake>Tasteless Hungry>Fixed positively correlated with change in ghrelin at 90 minutes from baseline Hungry–Fixed.
| Milkshake>Tasteless Hungry>Fixed | |||||||
|---|---|---|---|---|---|---|---|
| Positive correlation with ghrelin | |||||||
| Region | L/R | Size (voxels) | MNI coordinates | Z | pFWE-peak | ||
| x | y | z | |||||
|
|
|||||||
| Hippocampus | R | 24 | 30 | −16 | −20 | 4.53 | <.001 |
| Insula | R | 24 | 39 | −7 | −14 | 4.40 | .005 |
| Midbrain | R | 267 | 6 | −19 | −8 | 4.26 | .005 |
| 3 | −34 | −11 | 3.95 | .015 | |||
| −6 | −22 | −11 | 3.89 | .018 | |||
| Amygdala | R | 5 | 33 | −7 | 14 | 3.52 | .017 |
| Pallidum | L | 7 | −12 | −1 | −2 | 3.11 | .045 |
| Medial orbitofrontal * | -- | 32 | 0 | 56 | 1 | 3.09 | .054 |
= trend.
Figure 5.
Triglyceride and preferential responses to milkshake in Hungry vs. Fixed sessions. Ins=insula, MB=midbrain, pal=pallidum, put=putamen, hipp=hippocampus, amg=amygdala (trend). Panels masked with combined a priori ROIs for display purposes. Color bar depicts t-values. a) Milkshake>Tasteless Hungry>Fixed negatively correlated with change in triglyceride levels at 90 minutes from baseline Hungry-Fixed. b) Parameter estimates from the peak voxel in those regions.
Table 6.
Areas where Milkshake>Tasteless Hungry>Fixed negatively correlated with change in triglyceride at 60 minutes from baseline Hungry–Fixed.
| Milkshake>Tasteless Hungry>Fixed | |||||||
|---|---|---|---|---|---|---|---|
| Negative correlation with triglyceride | |||||||
| Region | L/R | Size (voxels) | MNI coordinates | Z | pFWE-peak | ||
| x | y | z | |||||
|
|
|||||||
| Midbrain | L | 6 | −15 | −22 | −2 | 4.23 | .006 |
| Insula | L | 45 | −39 | −10 | −7 | 3.83 | .038 |
| Hippocampus | L | 5 | −30 | −31 | −8 | 3.58 | .010 |
| Putamen | L | 55 | −27 | −10 | −5 | 3.85 | .018 |
| R | 27 | 24 | −1 | −2 | 4.20 | .005 | |
| 22 | 27 | −16 | 7 | 3.93 | .013 | ||
| Pallidum | L | 12 | −21 | −7 | −5 | 3.65 | .001 |
| R | 37 | 24 | −1 | −2 | 4.20 | .009 | |
| Amygdala * | L | 25 | −24 | 2 | −20 | 3.08 | .064 |
= trend.
Finally, to determine if the effects of ghrelin and triglycerides were independent we reran the analyses using these measures as covariates. Specifically, we included change in triglycerides as a covariate in our model correlating ghrelin and brain response, and vice versa. The positive relationship between change in ghrelin and Milkshake > Tasteless in Hungry > Fixed remained in the hippocampus, insula, midbrain, amygdala and a trend was observed in mOFC. The association in the pallidum did not survive. A negative relationship between change in triglycerides and Milkshake > Tasteless in Hungry > Fixed remained in the midbrain, putamen and pallidum, and the trend in amygdala survived. Effects were no longer significant in the hippocampus, and the insula response was reduced to a trend (p=.069). For the positive relationship between ghrelin and Milkshake > Tasteless in Hungry > Ad Lib the correlations that did not reach significance in the insula, putamen, pallidum and midbrain remained but the response in the caudate did not. The positive trend in mOFC between change in triglycerides and Milkshake > Tasteless Hungry > Ad Lib remained a trend. This pattern of results indicates that the modulatory effects of ghrelin and triglycerides are largely independent.
4. Discussion
In the current study we demonstrate that the effect of satiation on brain response to palatable and caloric milkshake is specifically associated with the efficacy of a meal to reduce the levels of ghrelin and to increase the levels of triglycerides circulating in the periphery. Participants who display larger post-prandial reductions in ghrelin and increases in triglycerides show greater attenuation of response to a palatable milkshake in brain regions involved in feeding. These associations were largely independent, and stronger when comparing Hungry and Fixed, as opposed to Hungry and Ad Lib sessions. We also found that choice over meal termination influenced the effect of the meal on milkshake-induced response in the midbrain and DLPFC.
4.1 Central effects of satiation depend upon hormonal and metabolic responses to a meal
Prior studies consistently find that hunger potentiates brain responses to food cues [19,20,22,31] and that responses to food and food cues are attenuated by satiation [10,18,27,28]. In contrast, in the current study we did not observe an attenuated response to milkshake in the fasted compared to either of the two fed conditions. There are several possible explanations for our discrepant findings. First, whereas robust reductions in response to distal food cues, such as food pictures, are observed following a meal [17–19,22,42], studies examining the influence of internal state on response to milkshake tend to report weaker effects [24,31]. This raises the possibility that feeding is more effective at modulating responses to external cues of food availability compared to responses during food consumption, a concept that warrants further investigation. A second possibility, supported by our data, is that the influence of satiety on response to a palatable food depends upon peripheral satiety signals, which varied considerably in our sample, characterized by a wide range of BMI, but not likely in prior samples that included only lean participants [24,31]. Here we rule out differences in BMI as accounting for the discrepant results by including it as a covariate of no interest in our model. However, BMI is associated with alterations in hormonal and metabolic response and a regression analyses clearly indicated that the magnitude of the reduction in circulating ghrelin and increase in circulating triglycerides following the meal were associated with meal-induced reductions in brain response to milkshake in the Hungry compared to the Fixed condition. These associations were robust in that they occurred in most of our ROIs (amygdala, midbrain, pallidum, insula, hippocampus, and putamen) and specific, as no correlations were found with insulin, glucose, or FFA. Similar but weaker associations were observed in the Hungry versus Ad Lib condition, serving to internally validate our findings. We therefore suggest that brain responses to calorie dense palatable foods following a satiating meal are influenced by both ghrelin signaling and triglyceride metabolism. By extension, it follows that the absence of typical neural signatures associated with a satiated state reflects deregulated ghrelin responses and triglyceride metabolism.
Our results are consistent with prior work demonstrating satiation-induced modulation of response to food cues in the midbrain, amygdala, pallidum, hippocampus, insula and medial OFC [9,10,12,13,18–22,24,26–29,40,41] and with findings highlighting a critical role for ghrelin in regulating these responses. Ghrelin infusions, which artificially induce hunger, increase BOLD response to images of food compared to scenery in the amygdala, insula, OFC, hippocampus, and midbrain [42]. Here we show similar effects with naturally occurring shifts in ghrelin levels in response to meal consumption, and demonstrate an additional role for lipid metabolism. Regulation of response to food cues by ghrelin signaling has also recently been linked to genetic risk for obesity. Single nucleotide polymorphisms within the first intron of the fat mass and obesity related gene (FTO) are robustly associated with increased BMI [55]. This relationship is thought to be mediated primarily by increased energy intake [56–58] with individuals carrying the at-risk allele showing increased obesity promoting behaviors such as increased food cue reactivity [59] and decreased satiation [60]. Karra and colleagues recently showed that healthy weight male participants homozygous for the at-risk vs. low-risk alleles of FTO, but matched for BMI, showed differences in post-prandial acyl-ghrelin suppression as well as differential neural response to images of high>low calorie food in a region of insula that extended into OFC and putamen [43]. Likewise, we found that BMI-independent individual differences in ghrelin signaling are associated with the influence of satiation on food cue reactivity. Thus there is emerging support that deregulated ghrelin signaling, independent of BMI, may be linked with blunted influences of satiation on central responses to food and food cues.
A key feature of our study is that we assessed brain response to milkshake, which is a typical treat or dessert, directly after subjects consumed typical lunch foods; a situation commonly encountered in everyday life. In contrast, most prior studies that have assessed brain response to the taste or smell of food do so before and after subjects are satiated on the same food represented by the stimuli in the scanner [10,16,21,27,28]. Indeed, many of the attenuated responses (particularly in insula, amygdala and OFC) reported in such studies have been shown to be specific to the satiating food, reflecting sensory specific satiation [21,27,28]. It is therefore possible that this paradigmatic difference accounts for our failure to observe a main effect of satiation on brain response to milkshake. However, two prior studies have also probed brain response to milkshakes following a meal and found satiation-induced attenuations in brain response. Uher et al. found increased preferential response to milkshake in the insula, operculum and DLPFC when hungry than after having eaten breakfast [24], and Stice et al. found that response to milkshake and anticipated intake of milkshake in the DLPFC and putamen positively correlated with the number of hours (ranging from 3 to 22.5) since last dietary intake [31]. Thus, the fact that we did not investigate sensory-specific satiation likely does not account for the lack of main effect seen in our study.
One important limitation of the current work is that we only tested the influence of one type of meal. It is, for example, possible that other signals such as glucose and insulin would be associated with central effects of satiation following consumption of a higher glycemic meal. Likewise it is possible that the associations we have identified might not extend to brain response to other types of food cues such as aromas or pictures, or to the consumption of foods or beverages comprised of different macronutrients than our milkshake stimuli. Additionally, it is important to keep in mind that our peripheral blood measures obtained at discrete time points do not allow us to infer how and when the hormones and fuels quantified are trafficked into, or act within, the brain. Therefore it remains an open question whether the relationships we observed between blood and brain measures are due to the direct action of ghrelin and triglycerides centrally, or indirectly through feedback from peripheral sensors. Finally, while we included BMI as a covariate in our fMRI analyses, we did not test for insulin sensitivity. As insulin is known to inhibit ghrelin secretion [61], it is possible that individual differences in insulin sensitivity (rather than the insulin secretion measured) mediates ghrelin response.
4.2 Differential effects of fixed versus volitional meal termination on brain response to milkshake
Compared to the hungry condition, response to milkshake in the hippocampus and midbrain was significantly greater following the ad libitum meal, but not the fixed-portion meal. Hippocampal involvement in the signaling of satiation has been previously reported. Rats with neurotoxic lesions of the hippocampus exhibit excessive intake and body weight gain [62]. The famous patient HM, with bilateral hippocampal resection for the treatment of intractable epilepsy, never reported being hungry and failed to alter self-reported fullness ratings following a meal [63]. Additionally, rats with hippocampal lesions fail to exhibit feature negative discrimination, where they show deficits in learning that an unconditioned stimulus (food) will not be delivered when a different “feature negative” cue (tone) follows a previously acquired conditioned stimulus (light) [64]. This may also be generalizable to appetitive behavior, where satiation is the feature negative cue that should indicate when the unconditioned stimulus, rewarding post-ingestive effects, will not occur in response to eating [65]. In line with this hypothesis, rats with hippocampal lesions show impaired ability to use interoceptive satiety cues to control appetitive behavior, and persist in responding for food despite being sated [66].
Intriguingly, there was also a trend for the midbrain response to be stronger after the ad libitum versus the fixed-portion meal, while DLPFC showed the opposite effect with greater response to milkshake after the fixed versus the ad libitum meal. These effects occurred despite subjects having consumed similar amounts of kilocalories during both meals, and persisted when the difference in kilocalories consumed was included as a covariate. Midbrain response has been shown to correlate with subjective pleasantness ratings of chocolate [10] and sucrose [67], as well as to predict behavioral preference for food [68]. DLPFC has been implicated in exerting self-control during food-related decisions [45] and is less activated after a meal in men who are obese [14]. This suggests that inhibitory and reward processing of milkshake in DLPFC and midbrain circuits respectively may be influenced by control over meal termination.
It is important, however, to note that this interpretation carries several important caveats. First, we did not measure macronutrient content. Although average caloric intake did not differ between the two fed conditions, participants sometimes made alterations to the sandwiches at the Ad Lib lunch to their taste—For example, removing cheese or wiping off the mayonnaise. While we adjusted our estimates of caloric intake when these changes occurred, we did not quantify macronutrient content. Thus we cannot rule out the possibility that systematic differences in, for example, fat ingestion contributed to the differential responses observed following the fixed versus the ad lib meals. Such unaccounted-for variability in macronutrients may also have contributed to the difference in subjective feelings of satiation between Fixed and Ad Lib scans at the 60-minute time point, as it has been demonstrated that isocaloric breakfasts differing in macronutrient composition exert differential effects on subjective hunger following the meal [69]. Second, we did not assess liking of the lunch meal, and therefore it is possible that differences in affective state (e.g. unhappiness at having to consume a fixed portion of a disliked sandwich) subsequently influenced the rewarding properties of the milkshake. Finally, as our paradigm did not actively engage subjects in a decision-making task in the scanner, the data only offer indirect evidence that these processes are engaged as a result of volitional versus fixed meal termination. Further studies that directly test the relationship between control over meal termination and self-control in making food-related decisions are needed.
5. Conclusions
In summary, we report that satiation-induced changes in brain response to a palatable and energy dense food are influenced by meal-induced alterations in circulating ghrelin and triglycerides. Specifically, attenuated peripheral responses are associated with attenuated central responses, indicating that blunted ghrelin and lipid signaling may reduce the impact of energy intake on brain coding of palatable foods. Our findings also suggest that the impact of a meal on brain response to a palatable food may differ depending upon whether meal termination is fixed or volitional.
Highlights.
Meal-induced changes in ghrelin and triglycerides are associated with meal-induced attenuation of brain response to milkshake.
Abnormalities in ghrelin and lipid signaling may reduce the impact of energy intake on neural encoding of palatable foods.
Meal-induced changes in brain response to milkshake differ following volitional vs. fixed meal termination.
Acknowledgements
Funded by NIH R01 DK085579 and also supported by NIH PL1 DA024859.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This manuscript is based on work presented during the 2013 Annual Meeting of the Society for the Study of Ingestive Behavior, July 30 – August 3, 2013.
References
- [1].Kenny PJ. Reward mechanisms in obesity: New insights and future directions. Neuron. 2011;69:664–79. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zheng H, Lenard NR, Shin AC, Berthoud H-R. Appetite control and energy balance regulation in the modern world: Reward-driven brain overrides repletion signals. Int J Obes. 2009;33:S8–13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schachter S. Obesity and eating. Science. 1968;161:751–6. doi: 10.1126/science.161.3843.751. [DOI] [PubMed] [Google Scholar]
- [4].Herman CP, Polivy J. External cues in the control of food intake in humans: The sensory-normative distinction. Physiol Behav. 2008;94:722–8. doi: 10.1016/j.physbeh.2008.04.014. [DOI] [PubMed] [Google Scholar]
- [5].Weingarten HP. Conditioned cues elicit feeding in sated rats: A role for learning in meal initiation. Science. 1983;220:431–3. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- [6].Ridley-Siegart T, Crombag H, Yeomans M. Conditioned sweet-paired stimuli elicit potentiated feeding in humans. Talk Given at: Society for the Study of Ingestive Behavior 21st Annual Meeting 2013; New Orleans, LA. [Google Scholar]
- [7].Jansen A, Theunissen N, Slechten K, Nederkoorn C, Boon B, Mulkens S, et al. Overweight children overeat after exposure to food cues. Eat Behav. 2003;4:197–209. doi: 10.1016/S1471-0153(03)00011-4. [DOI] [PubMed] [Google Scholar]
- [8].Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009;90:1453–6. doi: 10.3945/ajcn.2009.28595. [DOI] [PubMed] [Google Scholar]
- [9].Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, et al. Neuroanatomicalcorrelates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci U S A. 1999;96:4569–74. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain. 2001;124:1720–33. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- [11].Morris JS, Dolan RJ. Involvement of human amygdala and orbitofrontal cortex in hunger-enhanced memory for food stimuli. J Neurosci. 2001;21:5304–10. doi: 10.1523/JNEUROSCI.21-14-05304.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gautier JF, Chen K, Salbe AD, Bandy D, Pratley RE, Heiman M, et al. Differential brain responses to satiation in obese and lean men. Diabetes. 2000;49:838–46. doi: 10.2337/diabetes.49.5.838. [DOI] [PubMed] [Google Scholar]
- [13].Gautier JF, Del Parigi A, Chen K, Salbe AD, Bandy D, Pratley RE, et al. Effect of satiation on brain activity in obese and lean women. Obes Res. 2001;9:676–84. doi: 10.1038/oby.2001.92. [DOI] [PubMed] [Google Scholar]
- [14].Le DSNT, Pannacciulli N, Chen K, Del Parigi A, Salbe AD, Reiman EM, et al. Less activation of the left dorsolateral prefrontal cortex in response to a meal: A feature of obesity. Am J Clin Nutr. 2006;84:725–31. doi: 10.1093/ajcn/84.4.725. [DOI] [PubMed] [Google Scholar]
- [15].Del Parigi A, Chen K, Gautier J-F, Salbe AD, Pratley RE, Ravussin E, et al. Sex differences in the human brain’s response to hunger and satiation. Am J Clin Nutr. 2002;75:1017–22. doi: 10.1093/ajcn/75.6.1017. [DOI] [PubMed] [Google Scholar]
- [16].Smeets P a M, de Graaf C, Stafleu A, van Osch MJP, Nievelstein RAJ, van der Grond J. Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr. 2006;83:1297–305. doi: 10.1093/ajcn/83.6.1297. [DOI] [PubMed] [Google Scholar]
- [17].Frank S, Laharnar N, Kullmann S, Veit R, Canova C, Hegner YL, et al. Processing of food pictures: influence of hunger, gender and calorie content. Brain Res. 2010;1350:159–66. doi: 10.1016/j.brainres.2010.04.030. [DOI] [PubMed] [Google Scholar]
- [18].Führer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: An exploratory visually stimulated fMRI study. Obesity. 2008;16:945–50. doi: 10.1038/oby.2008.33. [DOI] [PubMed] [Google Scholar]
- [19].Goldstone AP, Prechtl de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009;30:1625–35. doi: 10.1111/j.1460-9568.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- [20].LaBar KS, Gitelman DR, Parrish TB, Kim Y-H, Nobre AC, Mesulam M-M. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- [21].O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G, et al. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport. 2000;11:893–7. doi: 10.1097/00001756-200003200-00046. [DOI] [PubMed] [Google Scholar]
- [22].Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: An fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2009;198:149–58. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- [23].Noseworthy MD, Alfonsi J, Bells S. Attenuation of brain BOLD response following lipid ingestion. Hum Brain Mapp. 2003;20:116–21. doi: 10.1002/hbm.10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: Effects of fasting and gender. Behav Brain Res. 2006;169:111–9. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- [25].Martens MJI, Born JM, Lemmens SGT, Karhunen L, Heinecke A, Goebel R, et al. Increased sensitivity to food cues in the fasted state and decreased inhibitory control in the satiated state in the overweight. Am J Clin Nutr. 2013;97:471–9. doi: 10.3945/ajcn.112.044024. [DOI] [PubMed] [Google Scholar]
- [26].Fletcher PC, Napolitano A, Skeggs A, Miller SR, Delafont B, Cambridge VC, et al. Distinct modulatory effects of satiety and sibutramine on brain responses to food images in humans: A double dissociation across hypothalamus, amygdala, and ventral striatum. J Neurosci. 2010;30:14346–55. doi: 10.1523/JNEUROSCI.3323-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–71. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- [28].Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–7. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- [29].Haase L, Cerf-Ducastel B, Murphy C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage. 2009;44:1008–21. doi: 10.1016/j.neuroimage.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cabanac M. Physiological role of pleasure. Science. 1971;173:1103–7. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- [31].Stice E, Burger K, Yokum S. Caloric deprivation increases responsivity of attention and reward brain regions to intake, anticipated intake, and images of palatable foods. Neuroimage. 2013;67:322–30. doi: 10.1016/j.neuroimage.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mohanty A, Gitelman DR, Small DM, Mesulam MM. The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cereb Cortex. 2008;18:2604–13. doi: 10.1093/cercor/bhn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Palouzier-Paulignan B, Lacroix M-C, Aimé P, Baly C, Caillol M, Congar P, et al. Olfaction under metabolic influences. Chem Senses. 2012;37:769–97. doi: 10.1093/chemse/bjs059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tschöp M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, et al. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24:RC19–C21. doi: 10.1007/BF03351037. [DOI] [PubMed] [Google Scholar]
- [35].Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, et al. Ghrelin enhances appetite and increases food intake in humans. 2001. [DOI] [PubMed]
- [36].Grossman SP. The role of glucose, insulin and glucagon in the regulation of food intake and body weight. Neurosci Biobehav Rev. 1986;10:295–315. doi: 10.1016/0149-7634(86)90015-1. [DOI] [PubMed] [Google Scholar]
- [37].Flint A, Møller BK, Raben A, Sloth B, Pedersen D, Tetens I, et al. Glycemic and insulinemic responses as determinants of appetite in humans. Am J Clin Nutr. 2006;84:1365–73. doi: 10.1093/ajcn/84.6.1365. [DOI] [PubMed] [Google Scholar]
- [38].Dole VP. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956;35:150–4. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Paglialunga S, Cianflone K. Regulation of postprandial lipemia: an update on current trends. Appl Physiol Nutr Metab. 2007;32:61–75. doi: 10.1139/h06-100. [DOI] [PubMed] [Google Scholar]
- [40].Batterham RL, Ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–9. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- [41].Wang G-J, Tomasi D, Backus W, Wang R, Telang F, Geliebter A, et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage. 2008;39:1824–31. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- [42].Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–9. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- [43].Karra E, Daly OGO, Choudhury AI, Yousseif A, Millership S, Neary MT, et al. A link between FTO , ghrelin , and impaired brain food-cue responsivity. J Clin Invest. 2013;123:3539–51. doi: 10.1172/JCI44403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–52. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–8. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- [46].O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85:1315–21. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- [47].Veldhuizen MG, Bender G, Constable RT, Small DM. Trying to detect taste in a tasteless solution: Modulation of early gustatory cortex by attention to taste. Chem Senses. 2007;32:569–81. doi: 10.1093/chemse/bjm025. [DOI] [PubMed] [Google Scholar]
- [48].Green BG, Shaffer GS, Gilmore MM. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem Senses. 1993;18:683–702. [Google Scholar]
- [49].Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the “Labeled Magnitude Scale” for measuring sensations of taste and smell. Chem Senses. 1996;21:323–34. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- [50].Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko C-W, Lucchina L a, et al. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 2004;82:109–14. doi: 10.1016/j.physbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- [51].Lim J, Wood A, Green BG. Derivation and evaluation of a labeled hedonic scale. Chem Senses. 2009;34:739–51. doi: 10.1093/chemse/bjp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Macey PM, Macey KE, Kumar R, Harper RM. A method for removal of global effects from fMRI time series. Neuroimage. 2004;22:360–6. doi: 10.1016/j.neuroimage.2003.12.042. [DOI] [PubMed] [Google Scholar]
- [53].O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–26. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- [54].Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35:1219–36. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–94. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CNA. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359:2558–66. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- [57].Speakman JR, Rance KA, Johnstone AM. Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity. 2008;16:1961–5. doi: 10.1038/oby.2008.318. [DOI] [PubMed] [Google Scholar]
- [58].Wardle J, Llewellyn C, Sanderson S, Plomin R. The FTO gene and measured food intake in children. Int J Obes. 2009;33:42–5. doi: 10.1038/ijo.2008.174. [DOI] [PubMed] [Google Scholar]
- [59].Velders FP, De Wit JE, Jansen PW, Jaddoe VWV, Hofman A, Verhulst FC, et al. FTO at rs9939609, food responsiveness, emotional control and symptoms of ADHD in preschool children. PLoS One. 2012;7:e49131. doi: 10.1371/journal.pone.0049131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wardle J, Carnell S, Haworth CMA, Farooqi IS, O’Rahilly S, Plomin R. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab. 2008;93:3640–3. doi: 10.1210/jc.2008-0472. [DOI] [PubMed] [Google Scholar]
- [61].Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Oikawa S. Effects of insulin, leptin, and glucagon on ghrelin secretion from isolated perfused rat stomach. Regul Pept. 2004;119:77–81. doi: 10.1016/j.regpep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- [62].Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19:235–52. doi: 10.1002/hipo.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hebben N, Corkin S, Eichenbaum H, Shedlack K. Diminished ability to interpret and report internal states after bilateral medial temporal resection: Case H.M. Behav Neurosci. 1985;99:1031–9. doi: 10.1037//0735-7044.99.6.1031. [DOI] [PubMed] [Google Scholar]
- [64].Holland PC, Lamoureux JA, Han JS, Gallagher M. Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus. 1999;9:143–57. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- [65].Davidson TL, Kanoski SE, Schier L a, Clegg DJ, Benoit SC. A potential role for the hippocampus in energy intake and body weight regulation. Curr Opin Pharmacol. 2007;7:613–6. doi: 10.1016/j.coph.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: Links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103:59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Frank GKW, Oberndorfer TA, Simmons AN, Paulus MP, Fudge JL, Yang TT, et al. Sucrose activates human taste pathways differently from artificial sweetener. Neuroimage. 2008;39:1559–69. doi: 10.1016/j.neuroimage.2007.10.061. [DOI] [PubMed] [Google Scholar]
- [68].O’Doherty JP, Buchanan TW, Seymour B, Dolan RJ. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49:157–66. doi: 10.1016/j.neuron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- [69].Stubbs RJ, van Wyk MC, Johnstone AM, Harbron CG. Breakfasts high in protein, fat or carbohydrate: effect on within-day appetite and energy balance. 1996. [PubMed]