Abstract
Polarized epithelial cells create tightly packed arrays of microvilli in their apical membrane, but the fate of these microvilli is relatively unknown when epithelial cell polarity is lost during wound healing. In this issue, Klingner et al. (2014. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201402037) show that, when epithelial cells become subconfluent, actomyosin contractions locally within the apical cortex cause their microvilli to become motile over the dorsal/apical surface. Their unexpected observations may have implications for epithelial responses in wound healing and disease.
The cell cortex of animal cells is a thin, interconnected network of actin filaments and myosin motors directly underneath the plasma membrane (Clark et al., 2013). This network controls cell shape and provides mechanical stiffness to the cell surface (Salbreux et al., 2012). Unlike other mechanical elements of the cell such as intermediate filaments and the microtubule cytoskeleton, which are both arrayed throughout the cytoplasm, the close association of the actin cortex with the membrane allows the cell to resist hydrostatic pressure (Stewart et al., 2011). Superimposed on the cell cortex are many other types of actin-rich structures with important and specific cellular functions. Lamellipodia and filopodia are protrusive and adhesive structures with rapidly polymerizing actin that provide the force to distend the cell membrane (Pollard and Borisy, 2003; Gupton and Gertler, 2007) and may be anchored into the actin cortex (Bornschlögl et al., 2013). Microvilli, found in tightly packed arrays on the apical surfaces of polarized epithelial cells, increase the surface area of the cell exposed to the lumen to enhance absorption and secretion. Microvilli contain parallel bundles of actin filaments and exhibit exquisite length control at their bases (Wayt and Bretscher, 2014), which are connected into a specialized domain of the cell cortex, the myosin-rich “terminal web” (Mooseker and Tilney, 1975; Mooseker, 1983).
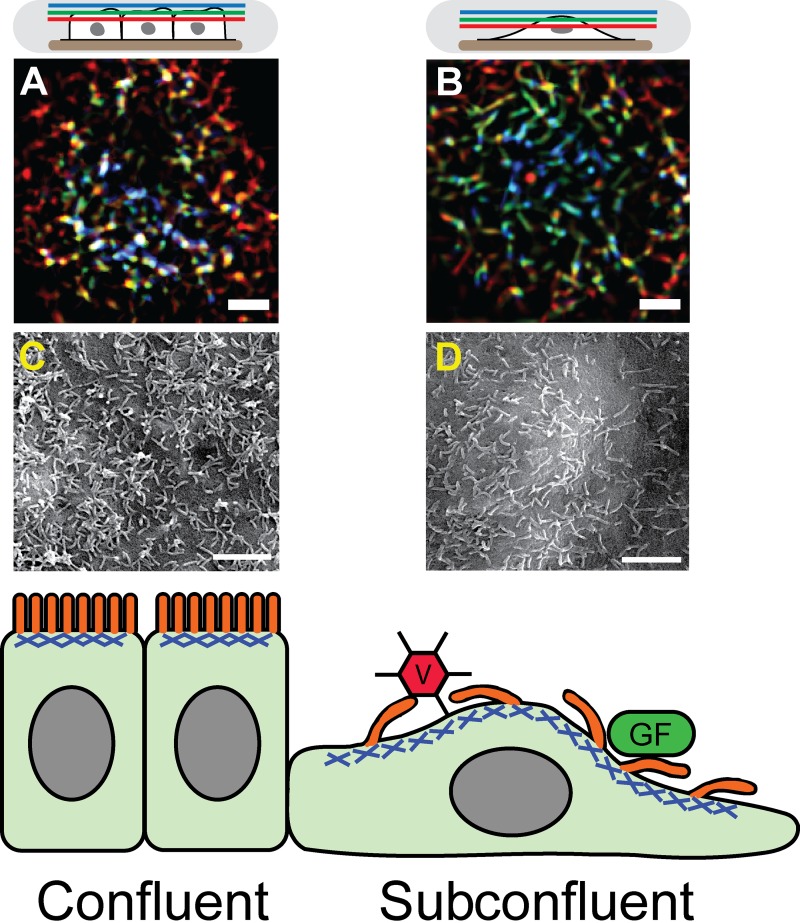
Although this stereotypical arrangement of microvilli is common in fully polarized, confluent layers of epithelial cells, many physiological conditions cause epithelia to break down or undergo epithelial–mesenchymal transitions (EMT). During embryogenesis, wound healing, and other tissue repair, EMT is accompanied by reorganization of the cytoskeleton and cell polarity to generate motility (Nelson, 2009; Lamouille et al., 2014). Under such conditions, the activity or fate of the microvilli array are largely unknown. In this issue, Klingner et al. demonstrate that, when epithelia cells become subconfluent, their microvilli become longer and more sparsely spaced (Fig. 1, B and D) when compared with the shorter, more densely packed microvilli observed in confluent cells (Fig. 1, A and C). Interestingly, the authors found that microvilli on subconfluent cells became much more motile and exhibited dynamic and coordinated movements within the plane of the apical membrane, yet still were enriched in characteristic microvilli proteins such as ERM proteins and EBP50 (Morales et al., 2004; Fehon et al., 2010). These motions were powered by an isotropic array of myosin II motors at the apical cell membrane. Like the cortex found in dividing cells (Clark et al., 2014), Klingner et al. (2014) find that the apical actomyosin cortex is under apparently isotropic tension. The authors demonstrated that the microvilli morphology and kinetic behavior were induced by stimuli such as wound healing or hepatocyte growth factor–induced migration, which suggests that increased microvilli dynamics may be a normal physiological response during EMT or cell migration.
Figure 1.
Transition to subconfluence in epithelial cells alters microvilli morphology and activity. Confluent cell microvilli are more vertically oriented than those in subconfluent cells. (A and B) Color-coded height projections of confocal images of GFP-LifeAct (labels actin) in the apical microvilli of epithelial cells either in confluent (A) or subconfluent (B) conditions. As shown in the schematic on top, blue colors indicate distal z planes, green intermediate z planes, and red apical membrane proximal z planes, so that combined colors represent a vertical structure found in multiple z planes, with white showing structures spanning all three planes. Microvilli are more sparsely spaced on subconfluent cells than on confluent cells. (C and D) Scanning electron micrographs of epithelial cells either confluent (C) or subconfluent (D) to show microvilli morphology and density. A–D have been reproduced from Klingner et al. (2014). See the article for further details. Bars, 2 µm. The bottom panel shows a diagram of epithelial cells in the confluent (left) or subconfluent (right) wound edge. Microvilli, shown in orange, are bound into the apical acto-myosin cortex, shown in blue. In subconfluent cells, the longer, more motile microvilli may enable enhanced binding and uptake of virus (V) or growth factors (GF).
The obvious question that remains is: what effects do increased microvilli dynamics have on the epithelial cells? Other types of actin-rich protrusions exhibit active movement important to their functions for the cell. For example, filopodia dynamics enable cells to sense both soluble and matrix-bound signals for directional guidance and migration (Gupton and Gertler, 2007; Heckman and Plummer, 2013). Similar to microvilli in their actin organization, stereocilia undergo deformation in response to mechanical stimuli to transmit signals required for mechanosensation and hearing (Fettiplace and Kim, 2014), and active movement by the stereocilia themselves may be involved in hearing adaptation in some species (Strimbu et al., 2012). In the case of microvilli, Klingner et al. (2014) find that microvilli on subconfluent cells can specifically bind to and move collagen-coated beads in an integrin-dependent manner, whereas the microvilli of confluent cells could not. Even more intriguing, epithelial growth factor (EGF) was trapped and/or bound by the dynamic microvilli of subconfluent epithelial cells (Fig. 1, bottom), whereas confluent epithelial cells bound EGF more poorly. These observations raise the interesting possibility that, as epithelial cells undergo EMT, they may become even more sensitive to additional signaling events that amplify their migratory response, which could aid in wound healing or developmental organization. Alternatively, amplification of EMT signals may also play deleterious roles in kidney fibrosis (Zeisberg et al., 2003) or tumor metastasis via “hybrid cells,” which can incorporate both mesenchymal and epithelial cell characteristics (Lu et al., 2013). Another potential consequence for enhanced microvilli dynamics in wounded epithelia could be enhanced virus uptake by the apical microvilli (Fig. 1, bottom), which has also been shown to be myosin II dependent (Lehmann et al., 2005). Whether these broad-reaching speculations turn out to be true or not, the data from Klingner et al. (2014) demonstrate that dynamic microvilli and the associated apical cortex in subconfluent cells likely have a role distinct from the function of the static microvilli array in confluent epithelia.
“Descriptive” has become an epithet in modern biology, carrying with it the implication that a descriptive investigation lacks a testable hypothesis. However, much of our understanding of biology has been founded in visualization and careful description of biological processes. One area of cell biology that remains ripe for careful quantitative description is the investigation of the actin cortex in various cell contexts. While we have appreciated the role of this contractile network in cell migration and division for many decades (Bray and White, 1988), we still lack a detailed understanding of its organization in the context of most nondividing cells. Klingner et al. (2014) demonstrate that careful and quantitative descriptions can still be used to provide new insights, here into the dynamic apical cortex and associated microvilli of epithelial cells during cell migration or wound healing.
Acknowledgments
R.S. Fischer is supported by the National Heart Lung and Blood Institute Division of Intramural Research.
The author declares no competing financial interests.
References
- Bornschlögl, T., Romero S., Vestergaard C.L., Joanny J.F., Van Nhieu G.T., and Bassereau P.. 2013. Filopodial retraction force is generated by cortical actin dynamics and controlled by reversible tethering at the tip. Proc. Natl. Acad. Sci. USA. 110:18928–18933 10.1073/pnas.1316572110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray, D., and White J.G.. 1988. Cortical flow in animal cells. Science. 239:883–888 10.1126/science.3277283 [DOI] [PubMed] [Google Scholar]
- Clark, A.G., Dierkes K., and Paluch E.K.. 2013. Monitoring actin cortex thickness in live cells. Biophys. J. 105:570–580 10.1016/j.bpj.2013.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, A.G., Wartlick O., Salbreux G., and Paluch E.K.. 2014. Stresses at the cell surface during animal cell morphogenesis. Curr. Biol. 24:R484–R494 10.1016/j.cub.2014.03.059 [DOI] [PubMed] [Google Scholar]
- Fehon, R.G., McClatchey A.I., and Bretscher A.. 2010. Organizing the cell cortex: the role of ERM proteins. Nat. Rev. Mol. Cell Biol. 11:276–287 10.1038/nrm2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace, R., and Kim K.X.. 2014. The physiology of mechanoelectrical transduction channels in hearing. Physiol. Rev. 94:951–986 10.1152/physrev.00038.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton, S.L., and Gertler F.B.. 2007. Filopodia: the fingers that do the walking. Sci. STKE. 2007:re5 10.1126/stke.4002007re5 [DOI] [PubMed] [Google Scholar]
- Heckman, C.A., and Plummer H.K. III. 2013. Filopodia as sensors. Cell. Signal. 25:2298–2311 10.1016/j.cellsig.2013.07.006 [DOI] [PubMed] [Google Scholar]
- Klingner, C., Cherian A.V., Fels J., Diesinger P.M., Aufschnaiter R., Maghelli N., Keil T., Beck G., Tolić-Nørrelykke I.M., Bathe M., and Wedlich-Soldner R.. 2014. Isotropic actomyosin dynamics promote organization of the apical cell cortex in epithelial cells. J. Cell Biol. 207:107–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille, S., Xu J., and Derynck R.. 2014. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15:178–196 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, M.J., Sherer N.M., Marks C.B., Pypaert M., and Mothes W.. 2005. Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells. J. Cell Biol. 170:317–325 10.1083/jcb.200503059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, M., Jolly M.K., Levine H., Onuchic J.N., and Ben-Jacob E.. 2013. MicroRNA-based regulation of epithelial-hybrid-mesenchymal fate determination. Proc. Natl. Acad. Sci. USA. 110:18144–18149 10.1073/pnas.1318192110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker, M.S.1983. Actin binding proteins of the brush border. Cell. 35:11–13 10.1016/0092-8674(83)90202-7 [DOI] [PubMed] [Google Scholar]
- Mooseker, M.S., and Tilney L.G.. 1975. Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J. Cell Biol. 67:725–743 10.1083/jcb.67.3.725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales, F.C., Takahashi Y., Kreimann E.L., and Georgescu M.M.. 2004. Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc. Natl. Acad. Sci. USA. 101:17705–17710 10.1073/pnas.0407974101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, W.J.2009. Remodeling epithelial cell organization: transitions between front-rear and apical-basal polarity. Cold Spring Harb. Perspect. Biol. 1:a000513 10.1101/cshperspect.a000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard, T.D., and Borisy G.G.. 2003. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 112:453–465 10.1016/S0092-8674(03)00120-X [DOI] [PubMed] [Google Scholar]
- Salbreux, G., Charras G., and Paluch E.. 2012. Actin cortex mechanics and cellular morphogenesis. Trends Cell Biol. 22:536–545 10.1016/j.tcb.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Stewart, M.P., Helenius J., Toyoda Y., Ramanathan S.P., Muller D.J., and Hyman A.A.. 2011. Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding. Nature. 469:226–230 10.1038/nature09642 [DOI] [PubMed] [Google Scholar]
- Strimbu, C.E., Fredrickson-Hemsing L., and Bozovic D.. 2012. Coupling and elastic loading affect the active response by the inner ear hair cell bundles. PLoS ONE. 7:e33862 10.1371/journal.pone.0033862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayt, J., and Bretscher A.. 2014. Cordon Bleu serves as a platform at the basal region of microvilli, where it regulates microvillar length through its WH2 domains. Mol. Biol. Cell. 25:2817–2827 10.1091/mbc.E14-06-1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg, M., Hanai J., Sugimoto H., Mammoto T., Charytan D., Strutz F., and Kalluri R.. 2003. BMP-7 counteracts TGF-β1–induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 9:964–968 10.1038/nm888 [DOI] [PubMed] [Google Scholar]