Abstract
Children with attention-deficit/hyperactivity disorder (ADHD) are at increased risk for developing depression. The neurobiological substrates that convey this risk remain poorly understood. On the basis of considerable data implicating hippocampal abnormalities in depressive disorders, we aimed to explore the relationship between the hippocampus and levels of depressive symptomatology in ADHD. We used structural magnetic resonance imaging (MRI) to examine the functional magnetic resonance imaging (fMRI) to assess the resting state functional connectivity (rs-fcMRI) of the hippocampus in a sample of 32 medication-naive children with ADHD (ages 6–13) and 33 age- and sex-matched healthy control (HC) participants. Compared with the HC participants, the participants with ADHD had (i) reduced volumes of the left hippocampus and (ii) reduced functional connectivity (rs-fcMRI) between the left hippocampus and the left orbitofrontal cortex (OFC); these hippocampal effects were associated with more severe depressive symptoms, even after controlling for the severity of inattentive and hyperactive/impulsive symptoms. Altered hippocampal structure and connectivity were not associated with anxiety or more general internalizing symptoms. Though preliminary, these findings suggest that the relationship between hippocampal anomalies and ADHD youth's susceptibility to developing depression and other mood disorders may merit further investigation with follow-up longitudinal research.
Keywords: Attention-deficit/hyperactivity disorder, Hippocampus, Depression, Functional connectivity, Orbitofrontal cortex
1. Introduction
The increased risk for depression conveyed by a diagnosis of attention deficit hyperactivity disorder (ADHD) has previously been ascribed to demoralization (Daviss, 2008). Children with ADHD are more prone to receiving negative feedback in the form of academic setbacks, social rejection, and familial tensions. With time, it is thought that this onslaught of negative feedback results in negative self-esteem, demoralization, and finally, depression. Though intuitively appealing, this hypothesized relationship between ADHD and depression is not well supported by empirical evidence. Longitudinal research suggests that hyperactive children with the most severe ADHD symptoms (and presumably the ones most likely to receive negative feedback) are not, in fact, the ones most affected by depression (Biederman et al., 1998). An alternative hypothesis regarding the relationship between ADHD and depression suggests that shared neurobiological anomalies may link the two disorders. In other words, neurobiological anomalies associated with ADHD, but not necessarily contributing to the diagnostic symptoms of the disorder (i.e., hyperactivity, impulsivity, and inattention), may explain, at least in part, an ADHD-associated vulnerability to depression in some hyperactive children. A related possibility is an interaction between neurobiological vulnerabilities and environmental effects, such that depressogenic inputs from the environment have inordinately strong effects in hyperactive children who carry neurobiological vulnerabilities for depression. To date, neurobiological vulnerabilities for depression have not been examined in ADHD.
Structural and functional magnetic resonance imaging (MRI) studies have repeatedly associated hippocampal anomalies with major depressive disorder (MDD) (MacQueen et al., 2003, Sheline et al., 2003) as well as anxiety disorders (Bremner et al., 1997). Depressed adults tend to have reduced hippocampal volumes (Sheline et al., 2003), impaired hippocampal functioning (MacQueen et al., 2003), and hippocampal hyperperfusion (Videbech et al., 2001), as determined by positron emission tomography. Conversely, hippocampal volumes partially normalize following successful treatment with antidepressants (Vermetten et al., 2003), and animal models suggest that neurogenesis within the hippocampus is a crucial element in the responsiveness of an individual to pharmacological and electroconvulsive therapies for depression (Santarelli et al., 2003; Sahay and Hen, 2007). Similar findings are reported in pediatric depression (Hulvershorn et al., 2011); likewise, childhood trauma and chronic developmental stress are associated with hippocampal atrophy and adult-onset depression (Kaufman and Charney, 2001).
Few prior MRI studies have examined hippocampal volumes in ADHD, and the findings from these studies have been inconsistent. A region of interest (ROI) analysis of hippocampal and amygdalar volumes in 51 children with ADHD demonstrated enlarged hippocampal volumes bilaterally in ADHD (Plessen et al., 2006); however, prior studies conducting whole brain analyses found that hippocampal volumes have no ADHD-related effects (Castellanos et al., 1996; Filipek et al., 1997). There are, however, a number of limitations to the extant studies of hippocampal volumes in ADHD. First, no prior studies of ADHD have examined hippocampal volumes in a sample of medicationnaïve individuals with the disorder or taken account of variations in long-term medication use. Given the neurotrophic effects of psychostimulants on the hippocampus (Griesbach et al., 2008), differences between study findings relating to the hippocampus may reflect differences in the extent and chronicity of exposure to psychostimulants (Frodl and Skokauskas, 2012). Second, no studies have taken account of levels of depression within samples or examined the relationship between hippocampal volumes and depressive symptoms in individuals with ADHD. We reasoned that because reduced hippocampal volumes are associated with depression, hippocampal abnormalities may also underlie the susceptibility that children with ADHD have for developing depression – that is, the extent of an association between altered hippocampal structure and ADHD will vary as a function of the level of depression in a sample.
A relatively novel approach for examining neural organization is resting state functional connectivity MRI (rs-fcMRI), which examines the temporal coherence of neural activity across disparate brain regions. When two brain regions exhibit neural activity that is highly correlated over time, these regions are termed “functionally connected” (Posner et al., 2013). Whereas several rs-fcMRI studies have examined connectivity within neural circuits underlying cognitive, motor, and attentional control in individuals with ADHD (Konrad and Eickhoff, 2010; Posner et al., 2014), no prior studies have examined hippocampal connectivity in this population. More specifically, no prior studies have examined the connectivity between the hippocampus and the orbitofrontal cortex (OFC), a circuit thought to subserve emotion regulation (Milad et al., 2007), and whether altered connectivity within this circuit underlies depressive symptomology in children with ADHD.
We conducted a multimodal MRI study consisting of volumetric and rs-fcMRI analyses of the hippocampus in medication-naïve children with ADHD and age-matched healthy controls. Our primary hypothesis for this analysis was that children with ADHD would have altered hippocampal volumes relative to age-matched controls. Our second hypothesis was hippocampal-orbitofrontal (OFC) connectivity would also be anomalous in children with ADHD, as determined by rs-fcMRI. Lastly, we examined the relationship between hippocampal volumes and connectivity with depressive symptoms in our sample of children with ADHD. We explored whether hippocampal anomalies would correlate with depressive symptoms in the ADHD group, while controlling for diagnostic symptoms of ADHD (i.e., hyperactivity, impulsivity, and inattention). Such a finding would suggest that hippocampal anomalies convey risk for depression irrespective of ADHD symptom severity.
2. Methods
Study procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute (NYSPI). Child participants provided informed assent, and a legal guardian provided written informed consent. Data collected for these analyses were part of a larger MRI study designed to examine emotional functioning in children with ADHD (Posner et al., 2013).
2.1. Participants
Our sample comprised 32 children with ADHD and 33 healthy control (HC) participants between the ages of 6 and 13. The ADHD participants fulfilled DSM-IV criteria for ADHD-Combined Type, ADHD-Predominantly Hyperactive-Impulsive Type, or ADHD-Predominantly Inattentive Type. HC participants were free of DSM-IV Axis I psychiatric disorders and were group-matched to the ADHD patients by age and gender (Table 1). In both the ADHD and HC groups, no study participant had prior exposure to psychotropic medication. Diagnoses were made using the parent and child versions of the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS) (Kaufman et al., 1996) and confirmed by a board-certified child psychiatrist. ADHD participants were excluded if found to have a diagnosis of a pervasive development disorder, bipolar disorder, psychotic disorder, or substance use disorder. Other comorbid disorders such as depressive and/or anxiety disorders were not exclusionary, but were recorded and covaried for in subsequent analyses (Table 1). Additional exclusion criteria for both groups included the following: neurological illness or significant head trauma (i.e., loss of consciousness > 2 min), serious medical problems, and MRI contraindications (e.g., braces).
Table 1.
Demographic and clinical characteristics of study participants with structural data
| ADHD (n=30) | HC (n=31) | Test statistic p value | |
|---|---|---|---|
| Age, years | 9.83±2.12 | 10.77±1.98 | t(59) = 1.79 0.08 |
| FS-IQ | 99.12±15.82 | 109.10±15.74 | t(59) = 2.45 0.02* |
| Gender | 24 boys, 6 girls | 21 boys, 10 girls | χ2(1,61)= 1.18 0.38 |
| ADHD-IV Rating Scale | |||
| Hyperactive/Impulsive | 22.1±5.1 | ||
| Inattentive | 23.8±4.9 | ||
| CDI | |||
| Total | 11.2±9.1** | ||
| ADHD Subtypes | 24 ADHD-C; 5 ADHD-PI; | ||
| 1 ADHD-PH | |||
| DSM-IV Axis I comorbidity | |||
| No comorbid disorder | 19 | ||
| ODD/CD | 4 | ||
| ODD/CD, MDD | 2 | ||
| ODD/CD, SAD | 1 | ||
| ODD/CD, enuresis | 1 | ||
| ODD/CD, MDD, GAD | 1 | ||
| MDD | 1 | ||
| Enuresis | 1 | ||
Note. ADHD, Attention-deficit/hyperactivity disorder; ADHD-C, ADHD-Combined Type; ADHD-PI, ADHD-Predominantly Inattentive Type; ADHD-PH, ADHD-Predominantly Hyperactive-Impulsive Type; CDI, Children's Depressive Inventory; FS-IQ, Full scale IQ estimated by the Wechsler Abbreviated Scale of Intelligence (WASI); ODD/CD, Oppositional Defiant Disorder/ Conduct Disorder; MDD, Major Depressive Disorder; SAD, Separation Anxiety Disorder; SES, Socioeconomic status. Values are mean ± SD unless specified.
Statistical significance.
Four participants with ADHD had CDI summary scores in the clinical significant range and co-morbid diagnoses of MDD.
Parents completed the ADHD Rating Scale–IV (DuPaul, 1991), Conners’ Parent Rating Scales Revised (Conners et al., 1998), the Child Behavior Checklist (Achenbach and Rescorla, 2001), and the Hollingshead Index of Social Status (Hollingshead, 1975). Participants were administered the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), Children's Depression Inventory (CDI) (Kovacs, 1985), the Revised Multidimensional Anxiety Scale for Children (RCMAS) (March et al., 1997), and the Tanner scale. Compared with the HC participants, the participants with ADHD had a significantly lower estimated mean IQ (Table 1), which was controlled for in hypothesis testing.
2.2. MRI pulse sequences
Anatomical images were acquired at the New York State Psychiatric Institute on a GE Signa 3.0 Tesla whole-body scanner. High-resolution T1-weighted images were acquired using a fast spoiled gradient-recall sequence with 11° flip angle; 256 × 256 matrix; 25 cm field of view; and 1 mm isotropic acquisition. Axial echoplanar images (repetition time = 2200 ms, echo time = 30 ms, 90° flip angle, receiver bandwidth = 62.5 kHz, single excitation per image, slice thickness = 3.5 mm, no gaps, 24 × 24 cm field of view, 64 × 64 matrix) were obtained to provide an effective resolution of 3.75 × 3.75 × 3.5 mm and whole-brain coverage. For resting state image acquisition, participants were instructed to remain still with their eyes closed and to let their minds wander freely. Two 5-min resting-state scans were obtained for each participant.
2.3. MRI volumetric analysis
Hippocampal volumes were measured with the fully automated FreeSurfer volume-based processing stream (Fischl et al., 2004), which consists of Talairach coordinate transformation, normalization of MRI signal intensity, segregation of brain from skull and dura (i.e., skull stripping), delineating gray matter–white matter boundaries, and segmentation of subcortical gray matter. The Freesurfer processing stream conducts segmentation of several subcortical structures including, but not limited to, the hippocampus. For the subsequent analyses, we restricted our consideration to the hippocampus.
2.4. Functional connectivity analysis
Image preprocessing used SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/) with the conn_toolbox (http://www.nitrc.org/projects/conn) for functional connectivity analysis. Functional images were slice time and motion corrected, coregistered with a high-resolution anatomical scan, normalized into template space, resampled at 2 mm3, and smoothed with a Gaussian kernel of 6 mm3 full width at half-maximum. Connectivity procedures followed the component-based noise-correction method described elsewhere (Behzadi et al., 2007; Whitfield-Gabrieli and Nieto-Castanon, 2012) to minimize non-neural influences on functional magnetic resonance imaging (fMRI) signal. Quantitative measurement of head motion did not differ by group, and did not correlate with ADHD or depressive symptom severity. To further minimize the likelihood that the connectivity findings were confounded by subjects’ head motion during scanning, we used the Artifact Detection Toolbox (www.nitrc.org/projects/artifact_detect) to “scrub” each scan with regressors to covary for images with excessive motion. Four participants (2 with ADHD and 2 HC) were removed from the structural MRI analysis because of excessive head motion, leaving a sample of 30 children with ADHD and 31 HC participants for the structural MRI analysis. Of these participants, an additional seven participants (3 with ADHD and 4 HC) were excluded from the rs-fcMRI analysis either because of excessive head motion or technical problems during scanning acquisition.
Following preprocessing, the resting state blood oxygen level dependent (BOLD) time series were correlated voxel by voxel for each participant across the complete resting time series. Fisher z transformation was applied. Given our hypothesis that children with ADHD would have altered hippocampal-OCF connectivity, we generated connectivity maps for each subject with the seed region within the right and left anterior hippocampus. Masks for the hippocampus were obtained from the SPM8 Anatomy toolbox (Eickhoff et al., 2005); an analogous approach is described elsewhere (Chen and Etkin, 2013). We selected the anterior hippocampus as the seed region because, unlike the posterior hippocampus, the anterior hippocampus has reciprocal connectivity with the orbitofrontal cortex, forming a circuit underlying emotion regulation (Kalisch et al., 2006). We used the WakeForest PickAtlas (Maldjian et al., 2004) to create masks of the left and right OFC (Brodmann area 11). Clusters within the OFC demonstrating significant ADHD vs. HC group differences in hippocampal-OFC connection strength based family-wise error corrections were extracted, and further hypothesis testing was conducted with the Statistical Program for the Social Sciences (SPSS) Edition 20.0 (IBM, SPSS, Inc.). Whole-brain voxelwise comparisons of anterior hippocampal connectivity are presented in the supplemental material.
2.5. Hypothesis testing
For the right and left hippocampal volumes, we began our hypothesis testing with a mixed-model, omnibus F-test with a between-subjects factor: group (2 levels: ADHD and HC); and one within-subject factor: hemisphere (2 levels: left hippocampus and right hippocampus). We then conducted post hoc testing to examine volumetric differences between the ADHD and HC participants in the left and right hippocampus separately. We used multiple regression with the hippocampal volumes as the dependent variable and group (2 levels: ADHD and HC participants) as the predictor variable. Each model contained the following covariates: total intracranial volume (ICV), IQ, ADHD subtype, comorbid diagnoses, sex, age, and socioeconomic status (SES). Non-significant terms were removed via backward stepwise regression conducted in SPSS. Analogous regression models were constructed for the left and right hippocampal-OFC connection strengths.
2.6. Correlations with symptom severity
We conducted exploratory analyses examining associations of hippocampal volumes and hippocampal-OFC connectivity with depressive symptom severity in the ADHD participants using partial correlations to control for ICV (for volumetric analysis), IQ, age, gender, and ADHD symptom severity. ADHD and depressive symptom severity were based on the ADHD rating scale score and the Children's Depressive Inventory summary score, respectively. To examine the specificity of this association, we calculated analogous partial correlations but replaced depressive symptoms with anxiety and internalizing symptoms, as determined by the RCMAS and CBCL internalized subscales, respectively. These additional partial correlations were calculated to determine whether the relationship between altered hippocampal structure and connectivity was specific to depressive symptoms, or conversely, non-specifically associated with mood symptoms more broadly.
3. Results
3.1. Structural MRI
Our omnibus F-test demonstrated a significant main effect of group (F(1,58) = 6.8, p = 0.01). We then conducted post hoc testing of the left and right hippocampal volumes separately.
Compared with the HC participants, the participants with ADHD had significantly reduced volumes of the left hippocampus (ADHD participants = 4068.7±362.3 mm3 vs. HC participants = 4353.6±396.7 mm3; t(1,58) = 2.3, p = 0.02). A trend suggested reduced volumes in the right hippocampus in ADHD participants, as well (ADHD participants = 4053.5±611.8 mm3 vs. HC participants = 4383.3±360.3 mm3; t(1,58) = 1.9, p = 0.06; Table 2). Controlling for total ICV, IQ, ADHD subtype, comorbid diagnoses, sex, age, and SES did not meaningfully influence the results.
Table 2.
Mean measures, mm3 ± SD
| Volumes, mm3 | Test statistic | p value | ||
|---|---|---|---|---|
| Brain region | ADHD (n=30) | HC (n=31) | ||
| Left hippocampus | 4068.7±362.4 | 4363.9±396.7 | t(1,58) = 2.3 | 0.02 |
| Right hippocampus | 4053.4±611.8 | 4383.3±360.3 | t(1,58) = 1.9 | 0.06 |
| Total ICV | 1338470.7±166528.3 | 1407286.7±154728.6 | t(59) = 1.7 | 0.1 |
ADHD, attention-deficit/hyperactivity disorder; HC, healthy control participants; ICV, intracranial volume.
3.2. Functional connectivity
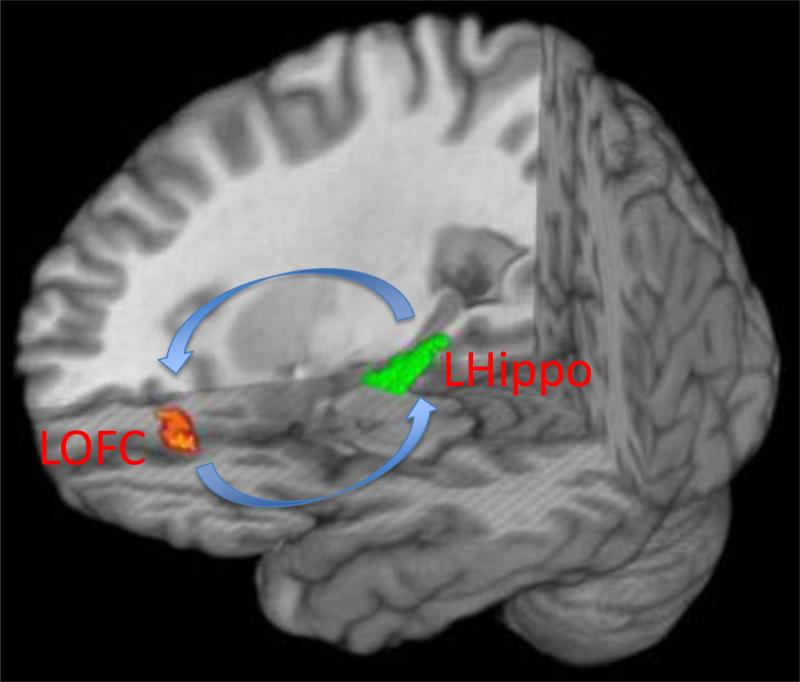
Compared with healthy controls, children with ADHD had significantly reduced left hippocampal – left OFC connectivity (mean connection strength, z, ADHD participants = 0.10±0.2 vs. HC participants = 0.25±0.1, t(59)=3.5, pfwe<0.05; Fig. 1). Controlling for IQ, ADHD subtype, comorbid diagnoses, sex, age, and SES did not meaningfully influence the results. Connectivity between the right hippocampal and right OFC did not differ significantly between children with ADHD and HC participants. Whole brain voxelwise comparisons are presented in the supplemental material.
Fig. 1.
Connection strength between the left anterior hippocampus (LHippo) and left orbitofrontal cortex (LOFC) was significantly weaker in participants with ADHD vs. healthy control participants (mean connection strength, z, ADHD participants = 0.10±0.2 vs. HC participants = 0.25±0.1, t=3.5, pfwe<0.05).
3.3. Correlations with symptom severity
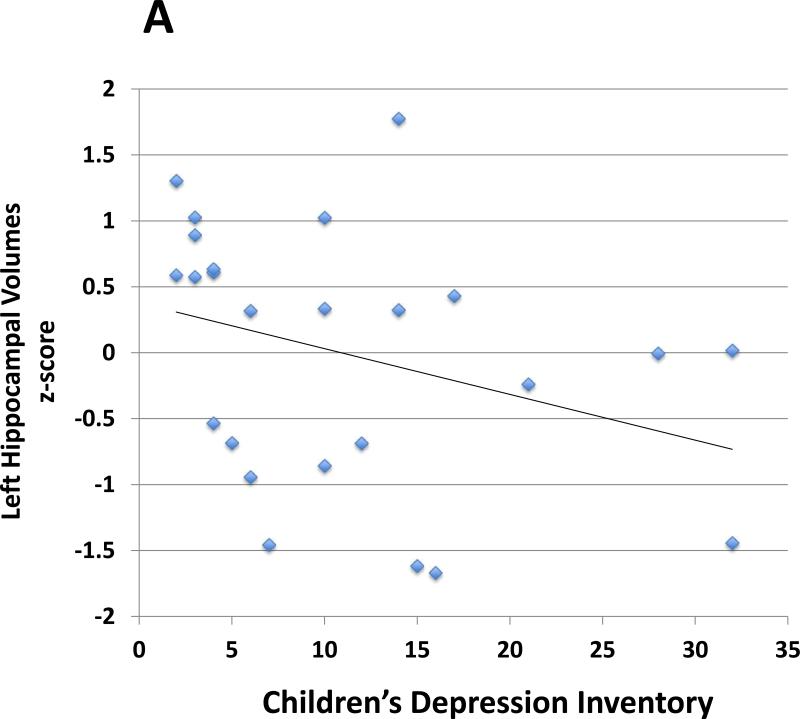
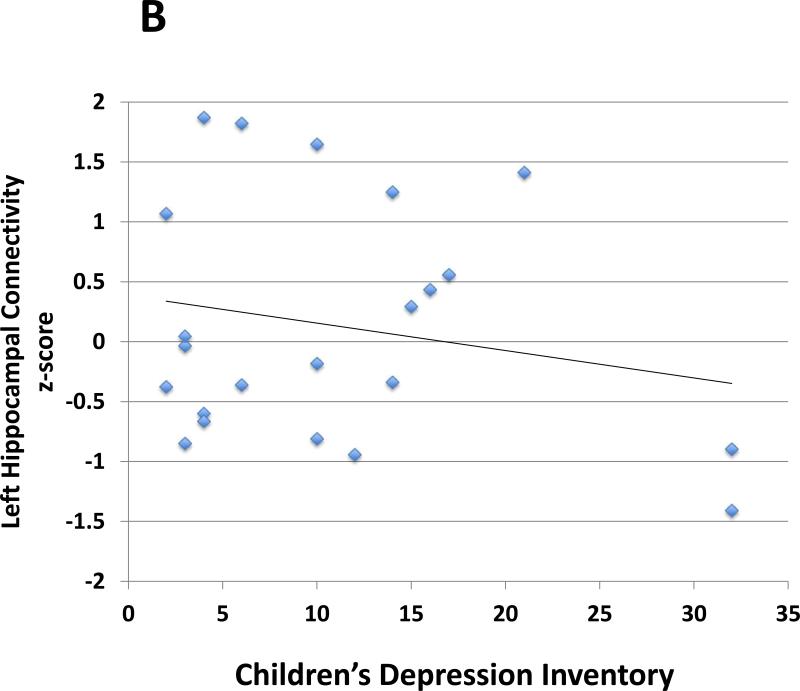
In analyses controlling for ICV, sex, age, IQ, SES, and ADHD symptoms (inattention and hyperactivity/impulsivity), left hippocampal volumes in the ADHD group were inversely correlated with depressive symptoms (r = −0.5, p = 0.01; Table 3, Fig. 2) based on the CDI summary score. Likewise, in analyses controlling for sex, age, IQ, SES, and ADHD symptoms, left hippocampal – left OFC connectivity in the ADHD group was inversely correlated with depressive symptoms (r = −0.4, p = 0.04; Table 3, Fig. 2). Reduced left hippocampal volumes and reduced left hippocampal - left OFC connectivity did not correlate significantly with anxiety, internalizing, inattentive or hyperactive/impulsive symptoms (Table 3).
Table 3.
Correlations of left hippocampal structure and connectivity with symptom severity
| Symptom (rating scale) | Left hippocampal volumes | Left hippocampal – Left OFC connectivity | ||
|---|---|---|---|---|
| r | p | r | p | |
| CDI | ||||
| Depressive symptoms | −0.5 | 0.013* | −0.4 | 0.04* |
| RCMAS | ||||
| Anxiety symptoms | −0.1 | 0.2 | −0.1 | 0.4 |
| CBCL | ||||
| Internalizing symptoms | −0.3 | 0.1 | −0.2 | 0.2 |
| ADHD-IV | ||||
| Hyperactive/impulsive symptoms | −0.02 | 0.5 | −0.03 | 0.4 |
| Inattentive symptoms | 0.3 | 0.1 | 0.2 | 0.2 |
Note. OFC, orbitofrontal cortex. CDI, Children's Depression Inventory; RCMAS, Revised Children's Manifest Anxiety Scale; CBCL, Child Behavior Checklist; ADHD-IV, ADHD Rating Scale IV. Statistical calculations (r and p values) are based on partial correlations controlling for intracranial volume (for volumetric analyses), sex, age, IQ, socioeconomic status, and ADHD symptoms.
Statistical significance.
Fig. 2.
Scatterplot demonstrates associations between: A. Left hippocampal volumes and depressive symptom severity (r = −0.5, p = 0.01) and B. Left hippocampus and left orbitofrontal cortex (OFC) connection strength and depressive symptom severity (r = −0.4, p = 0.04). Partial correlations were calculated controlling for sex, age, IQ, socioeconomic status (SES), intracranial volume (for the volumetric analysis), and ADHD symptom severity. The Children's Depression Inventory (CDI) summary score determined depressive symptom severity. Two ADHD participants had depressive symptoms far exceeding the group mean (z-score > 2; CDI summary score = 32 for both participants). After exclusion of these participants, we continued to find that left hippocampal volumes in the ADHD group were inversely correlated with depressive symptoms (p<0.05). The correlation between left hippocampal – left OFC connectivity and depressive symptoms was no longer significant.
We conducted an additional analysis to ensure that ADHD participants with depressive symptoms that far exceeded the group mean (we used a cutoff of z-score > 2; the highest z-score was 2.3) were not unduly influencing the correlations detected between the MRI findings and depressive symptoms (i.e., to exclude the possibility of correlations driven by statistical outliers). After excluding these subjects (n=2), we continued to find that left hippocampal volumes in the ADHD group were inversely correlated with depressive symptoms (p<0.05). The correlation was also detected using the nonparametric measure Spearman's rho (p<0.05). The correlation between left hippocampal – left OFC connectivity and depressive symptoms was no longer significant.
3.4. Sensitivity analysis
Hippocampal volumes were derived from automated brain segmentation conducted using FreeSurfer. To verify our structural findings, we re-examined hippocampal volumes using FSL (Smith et al., 2004), which implements a different brain segmentation algorithm. The anatomical MRI results were replicated. Second, seven of the children with ADHD had comorbid diagnoses such as oppositional defiant/conduct disorder and/or an anxiety or depressive disorder. Excluding these participants from the structural and rs-fcMRI analyses did not meaningfully affect the hypothesis testing. Third, our sample included a relatively wide age range (ages 6-13). We did not find significant group × age interactions in our analyses. Therefore, it is unlikely that disproportionate effects in certain age groups unduly influenced our findings (e.g., it is unlikely that younger, but not older, children with ADHD had reduced hippocampal volumes relative to controls). Fourth, the OFC is susceptible to signal loss during fMRI data acquisition. In theory, group differences in signal loss could confound hippocampal-OFC connectivity findings. To exclude this possibility, we extracted, on a subject-by-subject basis, the mean fMRI signal from the OFC and compared mean OFC signal intensity across the ADHD and HC groups. Mean OFC signal intensity did not differ significantly across the two groups, making it unlikely that group differences in hippocampal-OFC connectivity were confounded by disparate OFC signal loss.
4. Discussion
This is the first MRI study to examine hippocampal volumes in a sample of medication-naïve children with ADHD and the first study to examine hippocampal connectivity in this population regardless of medication status. Several points merit further discussion: First, children with ADHD were found to have significantly reduced left hippocampal volumes. Second, children with ADHD were also found that have significantly reduced connectivity between the left hippocampus and the left OFC. Third, in analyses controlling for the severity of ADHD symptoms, reduced left hippocampal volumes correlated with the severity of depressive symptoms in the ADHD group, as did reduced connectivity between the left hippocampus and the left OFC. Taken together, these findings suggest that altered hippocampal structure and connectivity may represent an important association between ADHD and depression, and potentially other mood disorders as well. Follow-up studies might examine whether altered hippocampal anomalies suggest indices of ADHD individuals who are vulnerable to depression.
Our finding of reduced volumes of the hippocampus in children with ADHD differs from prior studies. Prior studies have either shown enlarged hippocampal volumes (Plessen et al., 2006) or nonsignificant differences in hippocampal volumes between children with and without ADHD (Castellanos et al., 1996; Filipek et al., 1997). One potential explanation for why our findings diverge from those of prior studies is that the sample in our study, unlike earlier ones, was medication-naïve. Chronic administration of psychostimulants leads to up-regulation of brain-derived neurotrophic factor (BNDF), which is associated with synaptic proliferation, neurogenesis within the hippocampus, and dendritic arborization—all of which could increase hippocampal volumes and thus confound volumetric measures derived from medicated ADHD subjects (Lee et al., 2012). Our finding of reduced hippocampal volumes in ADHD thus underscores the potentially confounding influence of medication exposure on volumetric MRI measures. Other explanations for the divergent findings, such as differences in age ranges, comorbid disorders, and imaging methodologies, are also possible.
In addition to reduced left hippocampal volumes, we also found that children with ADHD have reduced connectivity between the left hippocampal and the left OFC. This finding is consistent with studies reporting reduced connectivity in ADHD within the affective resting state network, which encompasses the hippocampus, amygdala, nucleus accumbens, and orbitrofrontal cortex (Posner et al., 2013). Within this network, interactions between the hippocampus and the OFC mediate the top-down regulation of inordinately strong negative affects potentially through interactions with the hypothalamic-pituitary-adrenocortical (HPA) axis (Jacobson and Sapolsky, 1991) and through gating of amygdala-dependent responses (Quirk and Beer, 2006; Posner et al., 2011). Reduced connectivity between the hippocampus and OFC in ADHD may therefore have an important role in the disproportionally high rates of depressive, anxiety, and bipolar disorders seen in these children (Pliszka, 2009).
Reduced hippocampal volumes have been detected in several studies of individuals with major depressive disorder including two meta-analyses (Campbell et al., 2004; Videbech and Ravnkilde 2004). Reduced hippocampal volumes, as well as altered hippocampal function, have also been reported in other mood disorders, including post-traumatic stress disorder (Bremner et al., 1995) and obsessive-compulsive disorder (Atmaca et al., 2008). In addition, altered hippocampal connectivity has been reported in depressive (Sheline et al., 2010) and anxiety disorders (Etkin et al., 2009). Taken together, these findings suggest that altered hippocampal structure and connectivity may underlie a liability for depressive symptoms, and potentially anxiety, that cuts across multiple diagnoses, rather than conveying a specific diagnostic association. This suggestion is in keeping with our findings that anomalous hippocampal structure and connectivity appear to convey risk for depression in children with ADHD regardless of a diagnosis of a comordid mood disorder. At the same time, because depressive and anxiety symptoms and disorders are so often comorbid, it is difficult to determine whether hippocampal anomalies convey a specific vulnerability to depression, or rather a more general vulnerability toward affective symptoms. Our findings suggest the former (altered hippocampal structure and connectivity correlate significantly with depressive, but not anxiety or internalizing, symptoms); however, subsequent research with more detailed characterization of affective symptoms and emotion regulation might better differentiate these effects.
Longitudinal research has examined the relationship between ADHD and depression (Biederman et al., 1998). This longitudinal research has challenged the perception that children with more severe ADHD symptoms are also more affected by depression (Biederman et al., 1998). Instead of depression's being a consequence of ADHD symptoms (i.e., depression as an epiphenomenon), shared neurobiological anomalies associated with both ADHD and depression may predispose children with ADHD to depression. In other words, neurobiological anomalies associated with ADHD may increase the risk for depression without contributing to ADHD symptom severity. Our findings support this hypothesis by suggesting potential neurobiological substrates that convey risk for depression in children with ADHD without contributing to ADHD symptoms. That is, reduced hippocampal volumes and connectivity were both associated with depressive symptoms independent of ADHD symptom severity. Based on our findings, we would hypothesize that children with ADHD who have the greatest reductions in hippocampal volumes and connectivity are likewise the most likely to develop depressive disorders (Biederman et al., 1991)—this interpretation is speculative, but the hypothesis could be tested in a longitudinal study.
Another area relating ADHD and depression is that neurobiological vulnerabilities may interact with environmental contributions. ADHD youth with putative neurobiological vulnerabilities may be more susceptible to depressogenic environmental factors, such as chronic stress and/or trauma (Caspi et al., 2003). Without a longitudinal design, it is difficult to differentiate whether hippocampal effects alone, or only in conjunction with environmental contributions, convey risk for depression in children with ADHD. Chronic stress can, for example, lead to elevated glucocorticoids. Over time, glucocorticoids lead to hippocampal apoptosis and inhibit neurogenesis, both of which depress hippocampal volumes (McEwen, 1999). Importantly, whether it is neurobiological vulnerabilities alone or only in conjunction with environmental factors that convey risk for depression in ADHD, hippocampal integrity appears more central to identifying at-risk children than the severity of ADHD symptoms, as is often believed.
Several study limitations are worth noting. First, the optimal approach for diagnosing ADHD in children should include school-based observations, such as teacher reports of hyperactivity and inattention. Though our participant characterizations did not include school-based assessments, a child psychiatrist evaluated all participants with ADHD. Second, Freesurfer, as with other automated brain segmentation pipelines, may overestimate subcortical volumes (Tae et al., 2008). However, the sensitivity to group differences of Freesurfer is well established (Groen et al., 2010; Lehmann et al., 2010), and we replicated our findings using another imaging platform. Third, as mentioned previously, the specificity of hippocampal effects on mood symptoms is difficult to establish. Follow-up research with more detailed characterization of mood symptomology could test other hypotheses about the relationship between hippocampal integrity and clinical correlates. Fourth, the levels of depressive symptoms in our sample tended to be low. Some caution is thus warranted in interpreting whether the relationship between depressive symptoms and hippocampal anomalies would be present across the full range of depressive symptoms in children with ADHD. Follow-up research specifically designed to examine the relationship between depression and hippocampal anomalies in youth with ADHD might focus on a sample of ADHD youth with a broader range of depressive symptoms. Fifth, our sample included participants from a somewhat wide developmental range (ages 6–13), and thus developmental effects could be confounding the study findings. Such an effect is, however, unlikely because the groups were matched on age and Tanner stage. Moreover, we not find significant group × age interactions. Finally, replication with a larger sample is needed to help establish the reliability of the study findings.
In conclusion, our findings demonstrate that relative to HC participants, medication-naïve children with ADHD have reduced volumes of the left hippocampus, as well as reduced connectivity between the left hippocampus and the left OFC, and that these hippocampal abnormalities are associated with depressive symptoms. The findings point to the importance of longitudinal studies to examine the relationship between hippocampal anomalies and the susceptibility to affective disorders in children with ADHD. Additionally, future studies might examine pharmacological effects on hippocampal structure and connectivity in children with ADHD and whether normalization of the hippocampus curtails the likelihood of these children developing a depressive disorder.
Supplementary Material
Highlights.
We used MRI to examine hippocampal volumes and connectivity in children with ADHD
Hippocampal volumes were reduced in ADHD
Hippocampal – orbitofrontal cortex connectivity was also reduced in ADHD
Hippocampal effects were associated with depressive, but not ADHD, symptoms
Hippocampal anomalies in ADHD may confer risk for mood disorders
Acknowledgments
This study was supported in part by National Institute of Mental Health grants: K23-MH091249 (JP) and R01-MH101172 (JP) and by funding from the Edwin S. Webster Foundation. Dr. Posner is a principal investigator on an investigator-initiated grant from Shire Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The remaining authors have no conflicts to disclose.
References
- Achenbach TM, Rescorla LA. Manual for ASEBA School-Age Forms & Profiles. Research Center for Children, Youth, & Families, University of Vermont; Burlington, VT.: 2001. [Google Scholar]
- Atmaca M, Yildirim H, Ozdemir H, Ozler S, Kara B, Ozler Z, Kanmaz E, Mermi O, Tezcan E. Hippocampus and amygdalar volumes in patients with refractory obsessive–compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32(5):1283–1286. doi: 10.1016/j.pnpbp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV. Depression in attention deficit hyperactivity disorder (ADHD) children. Journal of Affective Disorders. 1998;47(1-3):113–122. doi: 10.1016/s0165-0327(97)00127-4. [DOI] [PubMed] [Google Scholar]
- Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. American Journal of Psychiatry. 1991;148(5):564–577. doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. American Journal of Psychiatry. 1995;152(7):973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse, a preliminary report. Biological Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. American Journal of Psychiatry. 2004;161(4):598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science Signaling. 2003;301(5631):386. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Lange N, Kaysen D, Krain AL, Ritchie GF, Rajapakse JC, Rapoport JL. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Archives of General Psychiatry. 1996;53(7):607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- Chen AC, Etkin A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology. 2013;38(10):1889–1898. doi: 10.1038/npp.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C, Sitarenios G, Parker J, Epstein J. The revised Conners' Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998;26(4):257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Daviss WB. A review of co-morbid depression in pediatric ADHD: etiologies, phenomenology, and treatment. Journal of Child and Adolescent Psychopharmacology. 2008;18(6):565–571. doi: 10.1089/cap.2008.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ. Parent and teacher ratings of ADHD symptoms: psychometric properties in a community-based sample. Journal of Clinical Child Psychology. 1991;20:245–253. [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry. 2009;66(12):1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek PA, Semrud-Clikeman M, Steingard R, Renshaw P, Kennedy D, Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48(3):589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatrica Scandinavica. 2012;125(2):114–126. doi: 10.1111/j.1600-0447.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- Griesbach G, Hovda D, Gomez-Pinilla F, Sutton R. Voluntary exercise or amphetamine treatment, but not the combination, increases hippocampal brain-derived neurotrophic factor and synapsin I following cortical contusion injury in rats. Neuroscience. 2008;154(2):530–540. doi: 10.1016/j.neuroscience.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen W, Teluij M, Buitelaar J, Tendolkar I. Amygdala and hippocampus enlargement during adolescence in autism. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(6):552–560. doi: 10.1016/j.jaac.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four factor index of social status. Yale University; New Haven, CT.: 1975. Unpublished manuscript. [Google Scholar]
- Hulvershorn LA, Cullen K, Anand A. Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging and Behavior. 2011;5(4):307–328. doi: 10.1007/s11682-011-9134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocrine Reviews. 1991;12(2):118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. The Journal of Neuroscience. 2006;26(37):9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. Kiddie-SADS-Present and Lifetime Version (K-SADS-PL) Version 1.0. New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Kaufman J, Charney D. Effects of early stress on brain structure and function: Implications for understanding the relationship between child maltreatment and depression. Development and psychopathology. 2001;13(3):451–471. doi: 10.1017/s0954579401003030. [DOI] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Human Brain Mapping. 2010;31(6):904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. The Children's Depression Inventory (CDI). Psychopharmacology Bulletin. 1985;21(4):995–998. [PubMed] [Google Scholar]
- Lee TH, Lee CH, Kim IH, Yan BC, Park JH, Kwon S-H, Park OK, Ahn JH, Cho JH, Won M-H. Effects of ADHD therapeutic agents, methylphenidate and atomoxetine, on hippocampal neurogenesis in the adolescent mouse dentate gyrus. Neuroscience Letters. 2012;524(2):84–8. doi: 10.1016/j.neulet.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Douiri A, Kim LG, Modat M, Chan D, Ourselin S, Barnes J, Fox NC. Atrophy patterns in Alzheimer's disease and semantic dementia: a comparison of FreeSurfer and manual volumetric measurements. Neuroimage. 2010;49(3):2264–2274. doi: 10.1016/j.neuroimage.2009.10.056. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(3):1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21(1):450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(4):554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annual Review of Neuroscience. 1999;22(1):105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, Martin L, Durkin K, Blair C, Royal J, Hugdahl K, Peterson BS. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2006;63(7):795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka S. Treating ADHD and Comorbid Disorders: Psychosocial and Psychopharmacological Interventions. Guilford Press; New York: 2009. [Google Scholar]
- Posner J, Hellerstein DJ, Gat I, Mechling A, Klahr K, Wang Z, McGrath PJ, Stewart JW, Peterson BS. Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry. 2013;70(4):373–382. doi: 10.1001/jamapsychiatry.2013.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Nagel B, Maia T, Mechling A, Oh M, Wang Z, Peterson B. Abnormal amygdalar activation and connectivity in adolescents with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50(8):828–837. doi: 10.1016/j.jaac.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Park C, Wang Z. Connecting the dots: a review of resting connectivity MRI studies in attention-deficit/hyperactivity disorder. Neuropsychology Review. 2014;24(1):3–15. doi: 10.1007/s11065-014-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Rauh V, Gruber A, Gat I, Wang Z, Peterson BS. Dissociable attentional and affective circuits in medication-naïve children with attention-deficit/hyperactivity disorder. Psychiatry Research: Neuroimaging. 2013;213(1):24–30. doi: 10.1016/j.pscychresns.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16(6):723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nature Neuroscience. 2007;10(9):1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. American Journal of Psychiatry. 2003;160(8):1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Tae WS, Kim SS, Lee KU, Nam E-C, Kim KW. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology. 2008;50(7):569–581. doi: 10.1007/s00234-008-0383-9. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biological Psychiatry. 2003;54(7):693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. American Journal of Psychiatry. 2004;161(11):1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B, Pedersen A, Egander A, Landbo B, Rasmussen N, Andersen F, Stodkilde-Jorgensen H, Gjedde A, Rosenberg R. The Danish PET/depression project: PET findings in patients with major depression. Psychological Medicine. 2001;31(7):1147–1158. doi: 10.1017/s0033291701004469. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; San Antonio, TX.: 1999. [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. CONN: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.