Abstract
Purpose
Risk of cancer progression is reduced for patients with human papillomavirus (HPV) –positive oropharynx cancer (OPC) relative to HPV-negative OPC, but it is unknown whether risk of death after progression is similarly reduced.
Patients and Methods
Patients with stage III-IV OPC enrolled onto Radiation Therapy Oncology Group trials 0129 or RTOG 0522 who had known tumor p16 status plus local, regional, and/or distant progression after receiving platinum-based chemoradiotherapy were eligible for a retrospective analysis of the association between tumor p16 status and overall survival (OS) after disease progression. Rates were estimated by Kaplan-Meier method and compared by log-rank; hazard ratios (HRs) were estimated by Cox models. Tests and models were stratified by treatment protocol.
Results
A total of 181 patients with p16-positive (n = 105) or p16-negative (n = 76) OPC were included in the analysis. Patterns of failure and median time to progression (8.2 v 7.3 months; P = .67) were similar for patients with p16-positive and p16-negative tumors. After a median follow-up period of 4.0 years after disease progression, patients with p16-positive OPC had significantly improved survival rates compared with p16-negative patients (2-year OS, 54.6% v 27.6%; median, 2.6 v 0.8 years; P < .001). p16-positive tumor status (HR, 0.48; 95% CI, 0.31 to 0.74) and receipt of salvage surgery (HR, 0.48; 95% CI; 0.27 to 0.84) reduced risk of death after disease progression whereas distant versus locoregional progression (HR, 1.99; 95% CI, 1.28 to 3.09) increased risk, after adjustment for tumor stage and cigarette pack-years at enrollment.
Conclusion
Tumor HPV status is a strong and independent predictor of OS after disease progression and should be a stratification factor for clinical trials for patients with recurrent or metastatic OPC.
INTRODUCTION
Human papillomavirus (HPV) is the cause of a subset of oropharyngeal squamous cell carcinomas (OPCs), and tumor HPV status is a strong and independent biomarker for prognosis. The risk of cancer progression for patients with HPV-positive OPC is significantly reduced compared with patients with HPV-negative OPC.1–2 Nevertheless, 10% to 25% of patients with HPV-positive OPC experience disease progression within 3 years of completing primary therapy.1,3–7 Whether the survival benefit experienced by patients with HPV-positive versus HPV-negative OPC continues after disease progression is currently unknown.
Data from prospective clinical trials indicate that patients with HPV-positive OPC have reduced rates of locoregional failure, but not distant metastases, relative to patients with HPV-negative OPC.1,4 Retrospective analyses and case reports have reported unusual patterns of disease progression for HPV-positive OPC, including late failures, unusual anatomic site distributions or a “disseminated” pattern of distant metastases.8–11 Because of the potential for ascertainment or reporting bias, these findings should be confirmed in a prospective clinical trial.
HPV-positive OPC is a newly identified and unique clinical entity with increasing incidence in the United States.12 Because of the strong prognostic advantage associated with HPV-positive OPC, disease progression may be unexpected. Currently, few data are available to counsel patients regarding expectations for survival after disease progression. Such data will also have important implications for clinical trial design in this patient population. Here, we examine the influence of tumor HPV status on patterns of failure and survival after disease progression within prospective clinical trials conducted by the Radiation Therapy Oncology Group (RTOG).
PATIENTS AND METHODS
Protocol and Treatment
RTOG trials 0129 and RTOG 0522 were phase III clinical trials designed to evaluate whether accelerated fractionation by concomitant boost (AFX-C) in comparison to standard fractionation (SFX) radiotherapy improves overall survival (OS) rates of head and neck cancer patients treated with concurrent high-dose cisplatin, and whether adding cetuximab to cisplatin with AFX-C radiotherapy could improve progression-free survival, respectively. The primary results of both trials have been published.1,13–14
Eligible patients for RTOG 0129 and RTOG 0522 had untreated, pathologically confirmed, stage III-IV15 squamous cell carcinoma of the oral cavity (RTOG 0129 only), oropharynx, hypopharynx, or larynx; Zubrod performance status 0 to 1; age ≥ 18 years; and adequate bone marrow, hepatic, and renal function. Patients were stratified by tumor site (larynx v other), nodal stage (N0 v N1-N2b v N2c-N3), and Zubrod performance status (0 v 1). Patients in RTOG 0522 only were also stratified by use of intensity-modulated radiotherapy (IMRT; yes v no) and receipt of pretreatment positron emission tomography/computed tomography scan (yes v no).
Patients in RTOG 0129 were randomly assigned to receive cisplatin concurrent with either SFX (70 Gy in 35 fractions [fx], 2 Gy/fx, over 7 weeks) or AFX-C (72 Gy delivered in 42 fx over 6 weeks, inclusive of twice-per-day irradiation for 12 treatment days). Patients in RTOG 0522 were randomly assigned to receive cisplatin concurrent with or without cetuximab with AFX-C (as in the case of patients in RTOG 0129, except with IMRT 70 Gy in 35 fx, 2 Gy/fx, over 6 weeks, 6 fx/week). Chemotherapy consisted of intravenous cisplatin 100 mg/m2 of body-surface area for SFX (days 1, 22, and 43) and AFX-C (days 1 and 22). Cetuximab dose was intravenous 400 mg/m2 the week before radiotherapy, then 250 mg/m2 weekly during radiotherapy.16
History of cigarette smoking in pack-years was obtained at enrollment via interviewer-administered questionnaire. To assess disease status, follow-up examinations and imaging studies were performed four times per year for 2 years, twice per year through year 5, and once per year thereafter.
Patients eligible for this analysis included patients with OPC who were enrolled onto RTOG trials 0129 or RTOG 0522 with evaluable p16 expression status (a surrogate of HPV tumor status) and disease progression (local, regional, or distant) during the follow-up period.
Laboratory Analysis
Tumor p16 expression was evaluated by immunohistochemistry using a mouse monoclonal antibody (MTM Laboratories, Heidelberg, Germany) and was visualized with the Ventana XT autostainer using the 1-view secondary detection kit (Ventana, Tuscon, AZ).17 p16 expression was scored as positive if strong and diffuse nuclear and cytoplasmic staining was present in at least 70% of the tumor cells.18–19 Testing and interpretation were centralized.
Statistical Analysis
Disease progression was defined as evidence of local, regional, or distant disease related to OPC. Salvage surgery was defined as resection of local, regional, or distant disease within 6 weeks of documented progression (independent of radiotherapy completion date). To evaluate potential biases introduced by missing data, we compared patient and tumor characteristics plus survival rates after progression for OPC patients with known and unknown p16 expression status. We also compared categorical variables using Fisher's exact test and ordinal or continuous variables using the Wilcoxon rank-sum test. We compared time from randomization to progression between the p16-positive and p16-negative groups using the Wilcoxon rank-sum test.
The principal outcome of interest was OS after disease progression, calculated as time from first disease progression event to death or last follow-up. Survival rates were estimated using the Kaplan-Meier method20 and were compared using a two-sided stratified (by trial) log-rank test.21 Hazard ratios were estimated by Cox proportional hazards model stratified22 by trial. Multivariable models were compared using Akaike information criterion (AIC). Models with AIC within two of the minimum AIC were considered sufficiently similar to warrant consideration as the final model. p16 status, age, sex, race, Zubrod performance status, anemia, pack-years, T stage, N stage, protocol therapy, salvage surgery, and site of progression were considered for inclusion in the multivariable model. A sensitivity analysis evaluated salvage surgery at any time after progression as a time-dependent covariate. Potential interactions between p16 tumor status and both progression type and surgical salvage were explored with multivariable modeling. To investigate potential bias in estimates owing to missing pack-years, we repeated analysis of the final model using values imputed with the Markov chain Monte Carlo algorithm with a noninformative prior distribution. Twenty data sets were created, and the resulting analyses were combined per Rubin's formula.23
RESULTS
Characteristics of the Study Population
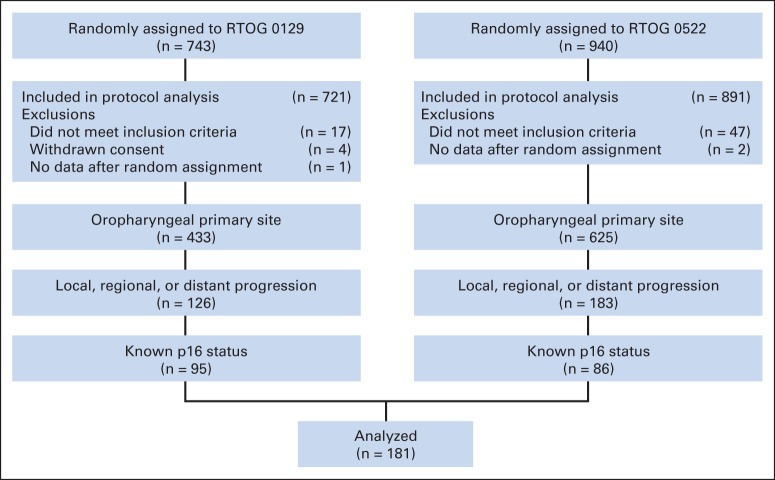
A total of 1,058 eligible patients with OPC were randomly assigned to receive protocol therapy in RTOG 0129 (n = 433) from July 2002 to June 2005 and RTOG 0522 (n = 625) from November 2005 to March 2009. Of these, 637 patients had evaluable p16 tumor status. Disease progression occurred among 105 (23.3%) of 450 p16-positive patients versus 76 (40.6%) of 187 p16-negative patients (P < .001; Fig 1). These 181 patients with disease progression and available tumor p16 status comprised the study population. The characteristics and treatment outcomes for patients with OPC (n = 309) who experienced disease progression with evaluable p16 tumor status (n = 181) and without (n = 128) were similar (Appendix Table A1; Appendix Fig A1 [online-only]).
Fig 1.
CONSORT diagram. RTOG, Radiation Therapy Oncology Group.
The study population included 95 patients from RTOG 0129 and 86 patients from RTOG 0522. At enrollment in the original protocols, patients' median age was 56 years (interquartile range, 51 to 62). The majority of the patients were men (87.8%) and had American Joint Committee on Cancer stage IV disease (93.4%; T4, 35.4%; N2b-N3, 77.3%). Median pack-years of cigarette smoking at enrollment was 23.8 pack-years (IQR, 4.5 to 45 pack-years).
Of the 181 patients, 105 (58%) patients had p16-positive tumors and 76 (42%) had p16-negative tumors. Despite disease progression, patients with p16-positive tumors were significantly more likely than patients with p16-negative tumors to be younger, of white race, report less cumulative cigarette exposure, and present with a smaller (ie, earlier tumor stage) tumor of the tonsil or base of tongue at enrollment (Table 1). p16-positive patients received a greater number of cycles of cisplatin during primary therapy, although this was not statistically significant.
Table 1.
Patient and Tumor Characteristics by p16 Tumor Status
| Characteristic | p16 Negative (n = 76) |
p16 Positive (n = 105) |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Protocol | .07* | ||||
| RTOG 0129 | 46 | 60.5 | 49 | 46.7 | |
| RTOG 0522 | 30 | 39.5 | 56 | 53.3 | |
| Age at enrollment, years | .01† | ||||
| Median | 58.5 | 53 | |||
| Range | 37-79 | 36-75 | |||
| Sex | .11* | ||||
| Male | 63 | 82.9 | 96 | 91.4 | |
| Female | 13 | 17.1 | 9 | 8.6 | |
| Race | .002* | ||||
| Asian | 2 | 2.6 | 0 | 0.0 | |
| Black or African American | 15 | 19.7 | 6 | 5.7 | |
| White | 59 | 77.6 | 98 | 93.3 | |
| Unknown | 0 | 0.0 | 1 | 1.0 | |
| Zubrod PS at enrollment | .76* | ||||
| 0 | 43 | 56.6 | 62 | 59.0 | |
| 1 | 33 | 43.4 | 43 | 41.0 | |
| Anemic at enrollment‡ | .08* | ||||
| No | 45 | 59.2 | 76 | 72.4 | |
| Yes | 31 | 40.8 | 29 | 27.6 | |
| Smoking history, pack-years§ | 58 | 96 | < .001† | ||
| Median, range | 38.5 | 16.5 | |||
| Range | 0-104 | 0-81 | |||
| Primary site at enrollment | .02* | ||||
| Oropharynx NOS | 9 | 11.8 | 14 | 13.3 | |
| Faucial arch | 1 | 1.3 | 0 | 0.0 | |
| Tonsillar fossa or tonsil | 19 | 25.0 | 39 | 37.1 | |
| Base of tongue | 36 | 47.4 | 52 | 49.5 | |
| Pharyngeal oropharynx | 8 | 10.5 | 0 | 0.0 | |
| Soft palate | 3 | 3.9 | 0 | 0.0 | |
| T stage at enrollment | .02† | ||||
| T2 | 19 | 25.0 | 43 | 41.0 | |
| T3 | 24 | 31.6 | 31 | 29.5 | |
| T4 | 33 | 43.4 | 31 | 29.5 | |
| N stage at enrollment | .99† | ||||
| N0 | 3 | 3.9 | 6 | 5.7 | |
| N1 | 10 | 13.2 | 7 | 6.7 | |
| N2a | 8 | 10.5 | 7 | 6.7 | |
| N2b | 20 | 26.3 | 45 | 42.9 | |
| N2c | 26 | 34.2 | 26 | 24.8 | |
| N3 | 9 | 11.8 | 14 | 13.3 | |
| AJCC stage at enrollment | .56* | ||||
| III | 6 | 7.9 | 6 | 5.7 | |
| IV | 70 | 92.1 | 99 | 94.3 | |
| On-protocol RT dose, Gy | .07† | ||||
| Median | 70 | 70 | |||
| Range | 68-73.8 | 0-75.9 | |||
| On-protocol cisplatin cycles | .06† | ||||
| 0 | 2 | 2.6 | 1 | 1.0 | |
| 1 | 9 | 11.8 | 4 | 3.8 | |
| 2 | 55 | 72.4 | 81 | 77.1 | |
| 3 | 10 | 13.2 | 19 | 18.1 | |
| First type of disease progression | .76* | ||||
| Local | 25 | 32.9 | 21 | 20.0 | |
| Regional | 14 | 18.4 | 32 | 30.5 | |
| Local and regional | 4 | 5.3 | 4 | 3.8 | |
| Local and distant | 3 | 3.9 | 3 | 2.9 | |
| Regional and distant | 1 | 1.3 | 2 | 1.9 | |
| Distant | 29 | 38.2 | 43 | 41.0 | |
| Salvage surgery within 6 weeks after progression | .87* | ||||
| No | 56 | 73.7 | 76 | 72.4 | |
| Yes | 20 | 26.3 | 29 | 27.6 | |
| Cause of death | 62 | 61 | .34* | ||
| This disease (local, regional, or distant) | 38 | 61.3 | 43 | 70.5 | |
| Second primary or other malignancy | 9 | 14.5 | 5 | 8.2 | |
| Other cause | 5 | 8.1 | 5 | 8.2 | |
| Unknown | 10 | 16.1 | 8 | 13.1 | |
Abbreviations: AJCC, American Joint Committee on Cancer, 5th edition (for RTOG 0129) or 6th Edition (for RTOG 0522); NOS, not otherwise specified; PS, performance status; RT, radiation therapy; RTOG, Radiation Therapy Oncology Group.
Fisher's exact test. All non-white races were combined. Primary site was tested as tonsil or base of tongue versus others. Progression type was tested as distant (± locoregional) versus locoregional only. Cause of death was tested as a result of this disease versus other categories.
Wilcoxon rank-sum test.
Anemia is defined as a hemoglobin level of 13.5 g per deciliter or less for men and 12.5 g per deciliter or less for women.
A pack-year is defined as the equivalent of smoking one pack of cigarettes per day for 1 year.
Patterns of Disease Progression
The median time to disease progression was similar for p16-positive and p16-negative patients (8.2 v 7.3 months; P = .67). The majority of p16-positive and p16-negative patients had disease progression within the first year after protocol therapy (65% v 63%) and in similar proportions each year thereafter (within the first 2 years, 82% v 86%; within the first 3 years, 86% v 93%).
The first sites of disease progression for the 181 patients were locoregional only (n = 100; 55.2%), distant metastases only (n = 72; 39.8%), or both (n = 9; 5.0%). The patterns of disease progression for p16-positive and p16-negative patients are compared in Table 1. Similar percentages of patients had distant metastases (with or without locoregional progression): 48 (45.7%) of 105 patients whose disease was p16 positive and 33 (43.4%) of 76 patients whose disease was p16 negative (P = .76; Table 1). The anatomic site distribution of distant metastases was also similar in the two groups. Of the 81 patients with distant metastases, the percentage of p16-positive and p16-negative patients who had lung (72.9% v 69.7%; P = .75), bone (14.6% v 15.2%), liver (8.3% v 15.2%), or other (16.7% v 12.1%) metastases was similar.
We also compared patterns of disease progression among patients with OPC categorized as having low, intermediate, or high risk of death based on their tumor p16 status, number of pack-years, and T and N stage, as defined in RTOG 0129.1 Of the OPC patients enrolled onto RTOG 0129 and RTOG 0522, 547 of 1,058 patients had available p16 tumor status and cigarette pack-years and were classified as having low (n = 263), intermediate (n = 166), or high risk (n = 118) of death. Disease progression was observed among 49 (18.6%) of 263 patients in the low-risk group, 54 (32.5%) of 166 patients in the intermediate-risk group, and 51 (43.2%) of 118 patients in the high-risk group. Patterns of disease progression are listed in Table 2. Among the patients with disease progression, the proportion of patients in the intermediate-risk group who had distant metastases (30 [55.6%] of 54 patients) was higher than in the low-risk group (38.8%) and high-risk (41.2%) group; however, this was not statistically significant (P = .18).
Table 2.
Patterns of Disease Progression for Low-, Intermediate-, and High- Risk Groups (per RTOG 0129)
| Pattern | Low Risk* |
Intermediate Risk† |
High Risk‡ |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| All patients | 263 | 48.1 | 166 | 30.3 | 118 | 21.6 |
| Disease progression | ||||||
| Yes | 49 | 18.6 | 54 | 32.5 | 51 | 43.2 |
| No | 214 | 81.4 | 112 | 67.5 | 67 | 56.8 |
| First type of disease progression | 49 | 54 | 51 | |||
| Local | 11 | 22.4 | 7 | 13.0 | 15 | 29.4 |
| Regional | 16 | 32.7 | 16 | 29.6 | 12 | 23.5 |
| Local and regional | 3 | 6.1 | 1 | 1.9 | 3 | 5.9 |
| Local and distant | 1 | 2.0 | 2 | 3.7 | 3 | 5.9 |
| Regional and distant | 1 | 2.0 | 0 | 0.0 | 1 | 2.0 |
| Distant | 17 | 34.7 | 28 | 51.9 | 17 | 33.3 |
| Lung metastasis | 19 | 30 | 21 | |||
| No | 7 | 36.8 | 6 | 20.0 | 7 | 33.3 |
| Yes | 12 | 63.2 | 24 | 80.0 | 14 | 66.7 |
Abbreviation: RTOG, Radiation Therapy Oncology Group.
Low risk: p16 positive and ≤ 10 pack-years, or p16 positive and > 10 pack-years and N0-N2a.
Intermediate-risk: p16 positive, > 10 pack-years, and N2b-N3, or p16 negative, ≤ 10 pack-years, and T2-T3.
High-risk: p16 negative, ≤ 10 pack-years, and T4, or p16 negative and > 10 pack-years.
Survival Analysis
The median follow-up time after first event of disease progression among surviving patients (n = 58) was 4.0 years (range, 0.04 to 8.97). At the time of analysis, 123 (68.0%) of 181 patients had died, including 61 (58.1%) of 105 p16-positive patients and 62 (81.6%) of 76 p16-negative patients. The cause of death was index cancer for 81 (65.9%) of 123 patients, second primary for 14 (11.4%) of 123 patients, or other/unknown cause for 28 (22.8%) of 123 patients, and these did not differ by p16 tumor status (P = .34).
In Kaplan-Meier analysis, p16-positive patients had significantly improved OS after disease progression when compared with p16-negative patients (hazard ratio [HR], 0.49; 95% CI, 0.34 to 0.70; P < .001; Fig 2). Estimated median OS after progression was 2.6 years (95% CI, 1.5 to 5.1) versus 0.8 years (95% CI, 0.6 to 0.9) for p16-positive versus p16-negative patients, respectively. Estimated 2-year OS after progression was 54.6% for patients with p16-positive tumors (95% CI, 44.9% to 64.4%) and 27.6% for patients with p16-negative tumors (95% CI, 17.3% to 37.9%).
Fig 2.
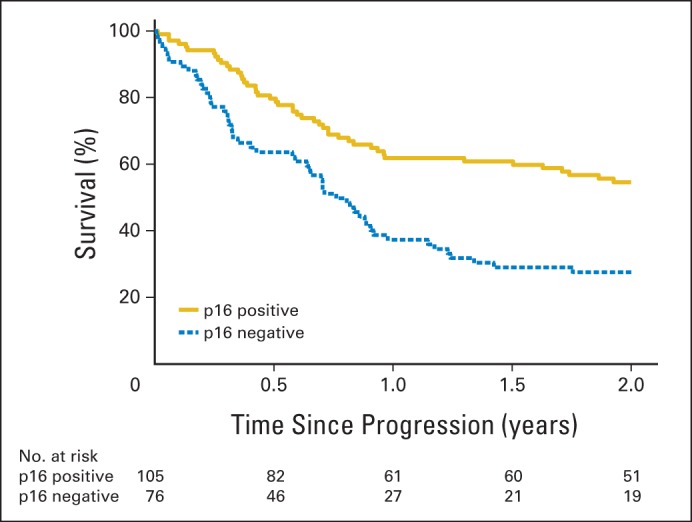
Kaplan-Meier estimates of overall survival after disease progression for patients with p16-positive and p16-negative oropharyngeal carcinoma (OPC). Patients with p16-positive OPC had significantly better overall survival after disease progression than patients with p16-negative OPC (P < .001). The 2-year rates of overall survival after disease progression were 54.6% for patients with p16-positive OPC (95% CI, 44.9 to 64.4) and 27.6% for patients with p16-negative OPC (95% CI, 17.3 to 37.9).
Additional factors associated with OS after disease progression in univariable analysis are listed in Table 3. Patient characteristics at protocol enrollment associated with OS after progression included age, performance status, anemia, and advanced tumor stage. Cumulative measures of cigarette smoking at enrollment were available for 154 (85.1%) of 181 patients. Among these patients, individuals with more than 20 pack-years of cigarette use had a significantly increased risk of death after disease progression, and the risk of death increased by 1% per pack-year (HR, 1.01; 95% CI, 1.0 to 1.02; P = .002).
Table 3.
Univariable Analysis of Overall Survival in Oropharyngeal Cancer Patients With Disease Progression
| Variable | HR | 95% CI | P |
|---|---|---|---|
| All patients (n = 181; 123 events) | |||
| p16 status (positive v negative) | 0.49 | 0.34 to 0.70 | < .001 |
| Age at enrollment (> 55 v ≤ 55) | 1.76 | 1.22 to 2.54 | .002 |
| Sex (male v female) | 1.22 | 0.72 to 2.09 | .46 |
| Race (non-white v white) | 1.22 | 0.75 to 2.00 | .42 |
| Zubrod PS at enrollment (1 v 0) | 1.52 | 1.06 to 2.18 | .02 |
| Anemic at enrollment (yes v no) | 1.47 | 1.02 to 2.13 | .04 |
| T stage at enrollment (T4 v T2-T3) | 2.02 | 1.41 to 2.91 | < .001 |
| N stage at enrollment (N2b-N3 v N0-N2a) | 1.27 | 0.82 to 1.98 | .29 |
| On protocol cisplatin cycles (0-1 v 2-3) | 2.16 | 1.25 to 3.74 | .006 |
| Progression type (distant v locoregional) | 2.03 | 1.41 to 2.90 | < .001 |
| Salvage surgery (yes v no) | 0.44 | 0.28 to 0.68 | < .001 |
| Patients with known pack-years (n = 154; 100 events) | |||
| Pack-years (continuous) | 1.01 | 1.00 to 1.02 | .002 |
| Pack-years (> 10 v ≤ 10) | 1.40 | 0.88 to 2.23 | .15 |
| Pack-years (> 20 v ≤ 20) | 2.01 | 1.32 to 3.07 | .001 |
Abbreviations: HR, hazard ratio from Cox proportional hazards model stratified by protocol; PS, performance status.
After protocol enrollment, fewer on-protocol cisplatin cycles significantly increased the risk of death after progression. At the time of disease progression, distant metastases compared with locoregional disease progression also significantly increased the risk of death (distant v locoregional: HR, 2.03; 95% CI, 1.41 to 2.90; P < .001).
After disease progression, 49 of 181 patients overall, and a similar proportion of p16-positive or p16-negative patients (27.6 v 26.3%; P = .85), underwent salvage surgery. The characteristics of patients who did and did not undergo salvage surgery were similar (Appendix Table A2), except for their mean age (56.8 v 54.2 years; P = .05). The majority (28 of 49 patients) underwent surgery more than 4 months after completing radiotherapy. The majority of the salvage surgeries performed was for local (19 [41.3%] of 46 surgeries) or regional (24 [52.2%] of 46 surgeries) disease progression, and these rates were not significantly different (P = .40). Median time to surgical salvage was similar for p16-positive patients (median, 136 days; range, 17 to 1,200 days) and p16-negative patients (median, 122.5 days; range, 58 to 349 days). Importantly, patients who underwent salvage surgery after disease progression had a significantly decreased risk of death after progression (HR, 0.44; 95% CI, 0.28 to 0.68; P < .001).
Factors independently associated with OS after disease progression in multivariable analyses included p16 tumor status, tumor stage, and cigarette pack-years at enrollment, as well as progression type (distant v locoregional) and salvage surgery (Table 4). When compared with patients with p16-negative tumors, patients with p16-positive tumors had an estimated 52% reduction in risk of death after adjustment for other factors (HR, 0.48; 95% CI, 0.31 to 0.74). When compared with patients who did not undergo surgical salvage, patients who had surgical salvage had an estimated 52% reduction in the risk of death after disease progression (HR, 0.48; 95% CI, 0.27 to 0.84). Sensitivity analyses considering surgical salvage at any time as a time-dependent covariate yielded similar results (HR, 0.54; 95% CI, 0.31 to 0.93). In contrast, patients with distant metastases had a two-fold increase in risk of death (HR, 1.99; 95% CI, 1.28 to 3.09) compared with patients who had locoregional progression. Sensitivity analyses that included imputations for patients with missing smoking status revealed similar results. Estimates of risk of death remained robust when enrollment age, race, and nodal stage were included in the model (Appendix Table A3).
Table 4.
Multivariable Analysis of Overall Survival in Oropharyngeal Cancer Patients With Disease Progression
| Covariate | Limited to Patients With Known Pack-Years (n = 154; 100 events) |
All Patients, With Imputed Pack-Years (n = 181; 123 events) |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| p16 status (positive v negative) | 0.48 | 0.31 to 0.74 | < .001 | 0.57 | 0.39 to 0.84 | .005 |
| T stage at enrollment (T4 v T2-T3) | 1.61 | 1.06 to 2.45 | .03 | 1.91 | 1.31 to 2.78 | < .001 |
| Progression type (distant v locoregional) | 1.99 | 1.28 to 3.09 | .002 | 1.70 | 1.13 to 2.54 | .01 |
| Salvage surgery (yes v no) | 0.48 | 0.27 to 0.84 | .01 | 0.56 | 0.34 to 0.92 | .02 |
| Pack-years (> 20 v ≤ 20) | 1.57 | 1.02 to 2.44 | .04 | 1.54 | 1.00 to 2.39 | .05 |
Abbreviation: HR, hazard ratio from Cox proportional hazards model stratified by protocol.
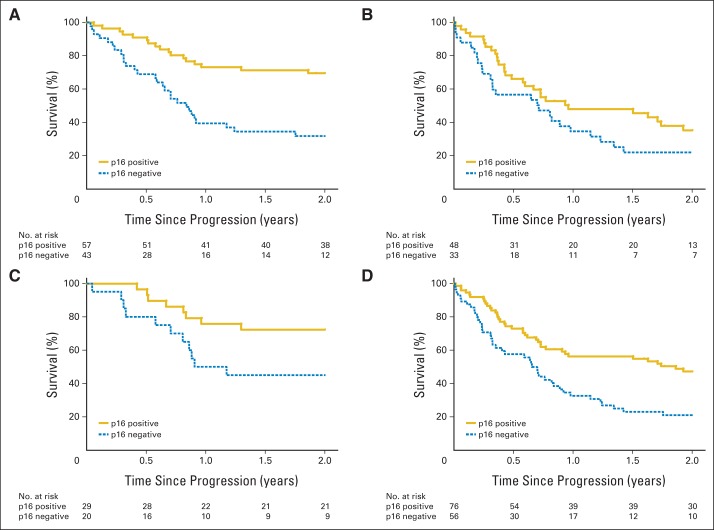
We evaluated potential interactions between p16 tumor status and both progression type and surgical salvage. Kaplan-Meier curves for OS stratified by progression type and surgical salvage are shown in Figure 3, stratified by p16 tumor status. The independent effect of p16 tumor status on survival appeared greater for patients with locoregional progression (HR, 0.34; 95% CI, 0.18 to 0.63) than for distant progression (HR, 0.66; 95% CI, 0.36 to 1.19), but this interaction did not reach statistical significance after adjustment for other factors (P = .12). The independent effect of p16 tumor status on survival also seemed greater for patients who underwent surgical salvage (HR, 0.26; 95% CI, 0.10 to 0.68) than for those who did not (HR, 0.55; 95% CI, 0.34 to 0.90), but this interaction did not reach statistical significance (P = .16).
Fig 3.
Kaplan-Meier estimates of overall survival after disease progression for patients with p16-positive and p16-negative oropharyngeal carcinoma (OPC) who had (A) locoregional progression, (B) distant metastases, (C) salvage surgery, and (D) no salvage surgery. Patients with p16-positive OPC had significantly better overall survival after disease progression than patients with p16-negative OPC in the subgroups that had locoregional failure (P < .001), distant metastases (P = .04), salvage surgery (P = .004), and no salvage surgery (P = .003).
DISCUSSION
Patients with p16-positive OPC are significantly less likely to experience cancer progression after chemoradiotherapy than patients with p16-negative OPC. In this article, we demonstrate that tumor p16 status is also strongly associated with OS among OPC patients after disease progression. Median survival after disease progression was almost 2 years longer for patients with p16-positive versus p16-negative OPC (median, 2.6 v 0.8 years). Importantly, surgical salvage significantly improved OS for both patient groups. Our data have several important implications for clinical trial design, salvage therapy, diagnostic evaluation, and patient counseling.
Clinical trials for patients with recurrent or metastatic OPC currently do not consider the impact of tumor p16 status on study outcomes. The marked difference in survival rates between p16-positive and p16-negative OPC observed in our study indicates that tumor p16 status must be a stratification factor for clinical trials for recurrent and metastatic OPC. Retrospective analyses indicate response rates to palliative chemotherapy may be higher for patients with p16-positive than p16-negative OPC.24 Indeed, the Eastern Cooperative Oncology Group observed higher response rates to platinum-based palliative chemotherapy and a trend toward improved survival for patients with p16-positive than p16-negative head and neck cancer.25 Because of nonuniform treatment after disease progression and a lack of data collection regarding use and type of palliative chemotherapy in RTOG 0129 and RTOG 0522, we were unable to address the influence of chemotherapy on observed survival differences. We considered whether surrogates of improved performance status (age, anemia, and cisplatin cycles) may have accounted for improved OS; however, these factors did not influence point estimates in multivariable analysis (data not shown). HPV-positive tumors may inherently be biologically more responsive to therapy because of differences in p53,26 p16,27 p21,27 and epidermal growth factor receptor expression.28,29
A better prognosis was observed for patients with OPC who underwent salvage surgery after disease progression compared with those who did not, regardless of p16 tumor status. To our knowledge, ours is the first study to report surgical salvage to be an independent predictor of survival for OPC within a prospective clinical trial. Our data are consistent with retrospective analyses that have observed improved survival rates after salvage surgery for locoregional persistent or recurrent disease.30–31 Historically, salvage surgery was associated with significant morbidity and was perhaps inappropriate in the context of a short expected median survival rate. However, the improved survival rates observed herein argues in favor of strong consideration of the use of surgical salvage after locoregional progression of OPC. Our data could not address the effect of distant metastases resection on survival rates, as only a few patients with distant metastases (five of 181) underwent salvage surgery. Nevertheless, clinical trials should prospectively collect data on salvage surgery to ensure that survival differences between treatment arms are not explained by differences in salvage rates.
We note that the demographic and clinical characteristics that distinguish p16-positive and p16-negative patients at diagnosis (such as age, race, smoking status, tumor stage, and subsite)1,3 are also present among the subset of patients who subsequently experience disease progression. This underscores our current inability to identify patients who will experience disease progression from those who will not. Ideally, predictive biomarkers will be identified to distinguish patients at risk for disease progression versus those at low risk, for whom deintensification protocols are appropriate. Disease progression occurred for approximately 19% of RTOG 0129 and RTOG 0522 patients with low-risk OPC, and the majority of them (61.2%) had locoregional disease progression. Data on the benefits of surgical salvage from our study will prove useful for patient counseling. In addition, our analysis of patterns of failure can inform clinical trial design for intermediate- and high-risk OPC patients. Although significant differences were not observed, trends toward increased risk of distant metastases in the intermediate-risk group were observed.
The data from RTOG 0129 and RTOG 0522 dispel the notion that patterns of failure and time to disease progression differed by tumor p16 status.4,8,10–11 Instead, median time to disease progression and anatomic site involvement were similar. Time to disease progression serves as a surrogate end point for OS in head and neck cancer32; however, further investigation will elucidate if this relationship differs by HPV status. The comparable time frame for disease progression and benefits of salvage surgery argue for similar post-treatment surveillance for p16-positive and p16-negative patients and close surveillance within the first 2 years of follow-up. The lungs were the most common site of distant metastases for both p16-positive and p16-negative OPC. Given this finding, p16 testing may be useful in differentiating lung metastases from second primary lung cancers.33
Our data highlight previously unappreciated prognostic differences after disease progression between HPV-positive and HPV-negative OPC and underscore the need to identify biomarkers for risk of progression.
Glossary Terms
- p16:
molecule that binds to cyclin-dependent kinase 4 and 6, thereby preventing their interaction with cyclin D. p16 (also known as p16INK4) behaves as a negative regulator of proliferation and arrests cells in the G0/G1 phase of the cell cycle.
Appendix
Table A1.
Missing Data Analysis: Patient and Tumor Characteristics
| Characteristic | p16 Tumor Status Unknown (n = 128) |
p16 Tumor Status Known (n = 181) |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Protocol | < .001* | ||||
| RTOG 0129 | 31 | 24.2 | 95 | 52.5 | |
| RTOG 0522 | 97 | 75.8 | 86 | 47.5 | |
| Age at enrollment, years | .55† | ||||
| Median | 57 | 56 | |||
| Range | 34-77 | 36-79 | |||
| Sex | .26* | ||||
| Male | 118 | 92.2 | 159 | 87.8 | |
| Female | 10 | 7.8 | 22 | 12.2 | |
| Race | .51* | ||||
| Asian | 1 | 0.8 | 2 | 1.1 | |
| Black or African American | 19 | 14.8 | 21 | 11.6 | |
| Native Hawaiian or other Pacific Islander | 1 | 0.8 | 0 | 0.0 | |
| White | 107 | 83.6 | 157 | 86.7 | |
| Unknown | 0 | 0.0 | 1 | 0.6 | |
| Zubrod PS at enrollment | .41* | ||||
| 0 | 81 | 63.3 | 105 | 58.0 | |
| 1 | 47 | 36.7 | 76 | 42.0 | |
| Anemic at enrollment‡ | 1.00* | ||||
| No | 86 | 67.2 | 121 | 66.9 | |
| Yes | 42 | 32.8 | 60 | 33.1 | |
| Smoking history, pack-years§ | 105 | 154 | .77† | ||
| Median | 28 | 23.75 | |||
| Range | 0-85 | 0-104 | |||
| Primary site at enrollment | .45* | ||||
| Oropharynx NOS | 8 | 6.3 | 23 | 12.7 | |
| Faucial arch | 0 | 0.0 | 1 | 0.6 | |
| Tonsillar fossa or tonsil | 55 | 43.0 | 58 | 32.0 | |
| Base of tongue | 53 | 41.4 | 88 | 48.6 | |
| Pharyngeal oropharynx | 5 | 3.9 | 8 | 4.4 | |
| Soft palate | 7 | 5.5 | 3 | 1.7 | |
| T stage at enrollment | .61† | ||||
| T2 | 51 | 39.8 | 62 | 34.3 | |
| T3 | 31 | 24.2 | 55 | 30.4 | |
| T4 | 46 | 35.9 | 64 | 35.4 | |
| N stage at enrollment | .54† | ||||
| N0 | 7 | 5.5 | 9 | 5.0 | |
| N1 | 11 | 8.6 | 17 | 9.4 | |
| N2a | 7 | 5.5 | 15 | 8.3 | |
| N2b | 37 | 28.9 | 65 | 35.9 | |
| N2c | 62 | 48.4 | 52 | 28.7 | |
| N3 | 4 | 3.1 | 23 | 12.7 | |
| AJCC stage at enrollment | .82* | ||||
| III | 10 | 7.8 | 12 | 6.6 | |
| IV | 118 | 92.2 | 169 | 93.4 | |
| On-protocol RT dose, Gy | .94† | ||||
| Median | 70 | 70 | |||
| Range | 4-74.4 | 0-75.9 | |||
| On-protocol cisplatin cycles | .33† | ||||
| 0 | 1 | 0.8 | 3 | 1.7 | |
| 1 | 7 | 5.5 | 13 | 7.2 | |
| 2 | 109 | 85.2 | 136 | 75.1 | |
| 3 | 11 | 8.6 | 29 | 16.0 | |
| First type of disease progression | .56* | ||||
| Local | 22 | 17.2 | 46 | 25.4 | |
| Regional | 37 | 28.9 | 46 | 25.4 | |
| Local and regional | 7 | 5.5 | 8 | 4.4 | |
| Local and distant | 7 | 5.5 | 6 | 3.3 | |
| Regional and distant | 3 | 2.3 | 3 | 1.7 | |
| Local, regional, and distant | 4 | 3.1 | 0 | 0.0 | |
| Distant | 48 | 37.5 | 72 | 39.8 | |
| Salvage surgery within 6 weeks after progression | .60* | ||||
| No | 97 | 75.8 | 132 | 72.9 | |
| Yes | 31 | 24.2 | 49 | 27.1 | |
Abbreviations: AJCC, American Joint Committee on Cancer, 5th Edition (RTOG 0129) or 6th Edition (RTOG 0522); NOS, not otherwise specified; PS, performance status; RT, radiation therapy; RTOG, Radiation Therapy Oncology Group.
Fisher's exact test. All non-white races were combined. Primary site was tested as tonsil or base of tongue versus others. Progression type was tested as distant (± locoregional) versus locoregional only.
Wilcoxon rank-sum test.
Anemia is defined as a hemoglobin level of 13.5 g per deciliter or less for men and 12.5 g per deciliter or less for women.
A pack-year is defined as the equivalent of smoking one pack of cigarettes per day for 1 year.
Table A2.
Patient and Tumor Characteristics for Patients Who Did and Did Not Have Salvage Surgery
| Characteristic | No Salvage Surgery (n = 132) |
Salvage Surgery (n = 49) |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Protocol | |||||
| RTOG 0129 | 69 | 52.3 | 26 | 53.1 | |
| RTOG 0522 | 63 | 47.7 | 23 | 46.9 | |
| Assigned treatment | |||||
| SFX and cisplatin | 41 | 31.1 | 10 | 20.4 | |
| AFX-C and cisplatin | 57 | 43.2 | 23 | 46.9 | |
| AFX-C, cisplatin, and cetuximab | 34 | 25.8 | 16 | 32.7 | |
| Age at enrollment, years | .05* | ||||
| Mean | 56.8 | 54.2 | |||
| SD | 7.81 | 8.38 | |||
| Median | 56.5 | 53 | |||
| Min-max | 39-79 | 36-71 | |||
| Q1-Q3 | 52-63 | 49-60 | |||
| Sex | .32† | ||||
| Male | 114 | 86.4 | 45 | 91.8 | |
| Female | 18 | 13.6 | 4 | 8.2 | |
| Race | .22† | ||||
| Asian | 1 | 0.8 | 1 | 2.0 | |
| Black or African American | 18 | 13.6 | 3 | 6.1 | |
| White | 112 | 84.8 | 45 | 91.8 | |
| Unknown | 1 | 0.8 | 0 | 0.0 | |
| Zubrod PS at enrollment | .23* | ||||
| 0 | 73 | 55.3 | 32 | 65.3 | |
| 1 | 59 | 44.7 | 17 | 34.7 | |
| Hemoglobin at enrollment, g/dL | .26* | ||||
| Mean | 13.8 | 14.1 | |||
| SD | 1.77 | 1.43 | |||
| Median | 14.1 | 14.2 | |||
| Min-max | 8.2-18.6 | 10-16.8 | |||
| Q1-Q3 | 12.7-14.95 | 13.4-15 | |||
| Anemic at enrollment | .25† | ||||
| No | 85 | 64.4 | 36 | 73.5 | |
| Yes | 47 | 35.6 | 13 | 26.5 | |
| Smoking history, pack-years‡ | 115 | 39 | .26* | ||
| Mean | 29.0 | 24.1 | |||
| SD | 24.81 | 24.52 | |||
| Median | 26.25 | 15 | |||
| Min-max | 0-96 | 0-104 | |||
| Q1-Q3 | 5-43.75 | 1.8-46.25 | |||
| Primary site at enrollment | .84† | ||||
| Oropharynx, NOS | 17 | 12.9 | 6 | 12.2 | |
| Faucial arch | 1 | 0.8 | 0 | 0.0 | |
| Tonsillar fossa or tonsil | 43 | 32.6 | 15 | 30.6 | |
| Base of tongue | 63 | 47.7 | 25 | 51.0 | |
| Pharyngeal oropharynx | 5 | 3.8 | 3 | 6.1 | |
| Soft palate | 3 | 2.3 | 0 | 0.0 | |
| p16 status | .85* | ||||
| Negative | 56 | 42.4 | 20 | 40.8 | |
| Positive | 76 | 57.6 | 29 | 59.2 | |
| T stage at enrollment | .37* | ||||
| T2 | 43 | 32.6 | 19 | 38.8 | |
| T3 | 40 | 30.3 | 15 | 30.6 | |
| T4 | 49 | 37.1 | 15 | 30.6 | |
| N stage at enrollment | .88* | ||||
| N0 | 6 | 4.5 | 3 | 6.1 | |
| N1 | 12 | 9.1 | 5 | 10.2 | |
| N2a | 11 | 8.3 | 4 | 8.2 | |
| N2b | 48 | 36.4 | 17 | 34.7 | |
| N2c | 39 | 29.5 | 13 | 26.5 | |
| N3 | 16 | 12.1 | 7 | 14.3 | |
| AJCC stage at enrollment | .41* | ||||
| III | 10 | 7.6 | 2 | 4.1 | |
| IV | 122 | 92.4 | 47 | 95.9 | |
| On-protocol RT dose, Gy | .88* | ||||
| Mean | 70.6 | 68.7 | |||
| SD | 1.25 | 10.28 | |||
| Median | 70 | 70 | |||
| Min-max | 68-75.9 | 0-72.76 | |||
| Q1-Q3 | 70-71.68 | 70-72 | |||
| On-protocol cisplatin cycles | .37* | ||||
| 0 | 2 | 1.5 | 1 | 2.0 | |
| 1 | 10 | 7.6 | 3 | 6.1 | |
| 2 | 96 | 72.7 | 40 | 81.6 | |
| 3 | 24 | 18.2 | 5 | 10.2 | |
Abbreviations: AJCC, American Joint Committee on Cancer, 5th edition (for RTOG 0129) or 6th Edition (for RTOG 0522); AFX-C, accelerated fractionation by concomitant boost radiotherapy; BOT, base of tongue; min, minimum; max, maximum; NOS, not otherwise specified; PS, performance status; Q1, first quartile; Q3, third quartile; RT, radiation therapy; RTOG, Radiation Therapy Oncology Group; SD, standard deviation; SFX, standard fractionation radiotherapy.
Wilcoxon rank-sum test.
Pearson χ2 test. All non-white races were combined. Primary site is tested as tonsil or BOT versus others.
A pack-year is defined as the equivalent of smoking one pack of cigarettes a day for 1 year.
Table A3.
Multivariable Analysis of Overall Survival in Oropharyngeal Cancer Patients With Disease Progression
| Covariate | Limited to Patients With Known Pack-Years (n = 154; 100 events) |
All Patients, With Imputed Pack-Years (n = 181; 123 events) |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| p16 status (positive v negative) | 0.48 | 0.31 to 0.74 | < .001 | 0.55 | 0.37 to 0.82 | .003 |
| T stage at enrollment (T4 v T2-T3) | 1.61 | 1.06 to 2.46 | .03 | 1.91 | 1.31 to 2.79 | < .001 |
| Progression type (distant v locoregional) | 1.96 | 1.24 to 3.10 | .004 | 1.61 | 1.06 to 2.43 | .02 |
| Salvage surgery (yes v no) | 0.48 | 0.27 to 0.84 | .01 | 0.56 | 0.34 to 0.92 | .02 |
| Pack-years (> 20 v ≤ 20) | 1.57 | 1.01 to 2.44 | .05 | 1.52 | 0.98 to 2.36 | .06 |
| Age at enrollment (> 50 v ≤ 50) | 0.97 | 0.56 to 1.68 | .92 | 1.07 | 0.66 to 1.74 | .77 |
| Race (non-white v white) | 1.01 | 0.56 to 1.84 | .97 | 0.95 | 0.56 to 1.64 | .87 |
| N stage at enrollment (N2b-N3 v N0-N2a) | 1.12 | 0.67 to 1.86 | .67 | 1.28 | 0.80 to 2.04 | .30 |
Abbreviation: HR, hazard ratio from Cox proportional hazards model stratified by protocol.
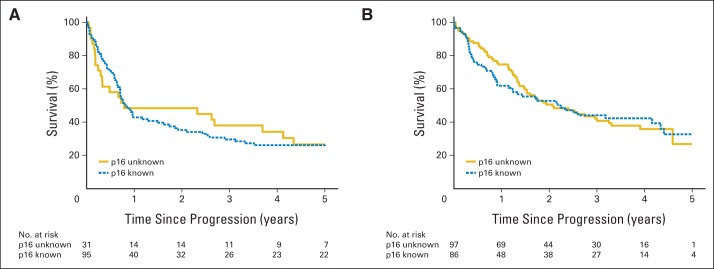
Fig A1.
Kaplan-Meier estimates of survival after disease progression for patients with known and unknown p16 tumor status in (A) Radiation Therapy Oncology Group (RTOG) 0129 and (B) RTOG 0522. There are no statistically significant differences in survival after disease progression between patients with known and unknown p16 tumor status in RTOG 0129 (log-rank test, P = .88) or RTOG 0522 (log-rank test, P = .85).
Footnotes
See accompanying editorial on page 3349; listen to the podcast by Dr Wirth at www.jco.org/podcasts
Processed as a Rapid Communication manuscript.
Supported by Radiation Therapy Oncology Group Grant No. U10 CA21661 and Community Clinical Oncology Program Grant No. U10 CA37422 from the National Cancer Institute and Bristol-Myers Squibb.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Maura Gillison, GlaxoSmithKline (U), Bristol-Myers Squibb (U) Stock Ownership: None Honoraria: Maura Gillison, Merck Serono Research Funding: None Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: Maura Gillison, Merck Serono
AUTHOR CONTRIBUTIONS
Conception and design: Carole Fakhry, Qiang Zhang, David Rosenthal, Quynh-Thu Le, Maura Gillison
Provision of study materials or patients: Phuc Felix Nguyen-Tan, Adam S. Garden, Vilija Avizonis, John Andrew Ridge, Maura Gillison
Collection and assembly of data: Phuc Felix Nguyen-Tan, Adel El-Naggar, Adam S. Garden, Quynh-Thu Le, Maura Gillison
Data analysis and interpretation: Carole Fakhry, Qiang Zhang, Adel El-Naggar, Denis Soulieres, Andy Trotti, Vilija Avizonis, John Andrew Ridge, Jonathan Harris, Quynh-Thu Le, Maura Gillison
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol. 2006;24:2606–2611. doi: 10.1200/JCO.2006.06.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 4.Huang SH, Perez-Ordonez B, Weinreb I, et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncol. 2013;49:79–85. doi: 10.1016/j.oraloncology.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 6.O'Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–550. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 7.Lin BM, Wang H, D'Souza G, et al. Long-term prognosis and risk factors among patients with HPV-associated oropharyngeal squamous cell carcinoma. Cancer. 2013;119:3462–3471. doi: 10.1002/cncr.28250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulut OC, Lindel K, Hauswald H, et al. Clinical and molecular characteristics of HNSCC patients with brain metastases: A retrospective study. Eur Arch Otorhinolaryngol. 2014;271:1715–1722. doi: 10.1007/s00405-013-2665-z. [DOI] [PubMed] [Google Scholar]
- 9.Ruzevick J, Olivi A, Westra WH. Metastatic squamous cell carcinoma to the brain: An unrecognized pattern of distant spread in patients with HPV-related head and neck cancer. J Neurooncol. 2013;112:449–454. doi: 10.1007/s11060-013-1075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller S, Khuri FR, Kono SA, et al. HPV positive squamous cell carcinoma of the oropharynx: Are we observing an unusual pattern of metastases? Head Neck Pathol. 2012;6:336–344. doi: 10.1007/s12105-012-0355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang SH, Perez-Ordonez B, Liu FF, et al. Atypical clinical behavior of p16-confirmed HPV-related oropharyngeal squamous cell carcinoma treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:276–283. doi: 10.1016/j.ijrobp.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ang KK, Zhang QE, Rosenthal DI, et al. A randomized phase III trial (RTOG 0522) of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III-IV head and neck squamous cell carcinomas (HNC) J Clin Oncol. 2011;29(suppl):360s. doi: 10.1200/JCO.2013.53.5633. abstr 5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.RTOG 0522: A randomized phase III trial of concurrent accelerated radiation and cisplatin versus concurrent accelerated radiation, cisplatin, and cetuximab [followed by surgery for selected patients] for Stage III and IV head and neck carcinomas. Clin Adv Hematol Oncol. 2007;5:79–81. [PubMed] [Google Scholar]
- 15.Flaming I, Cooper J, Henson D, et al. AJCC Cancer Staging Manual (ed 5) Philadephia, PA: Lippincott-Raven; 1997. [Google Scholar]
- 16.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 17.Begum S, Gillison ML, Ansari-Lari MA, et al. Detection of human papillomavirus in cervical lymph nodes: A highly efffective strategy for localizing site of tumor origin. Clin Cancer Res. 2003;9:6469–6475. [PubMed] [Google Scholar]
- 18.Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36:945–954. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grønhoj Larsen C, Gyldenløve M, Jensen DH, et al. Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: A systematic review. Br J Cancer. 2014;110:1587–1594. doi: 10.1038/bjc.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;5:163–170. [PubMed] [Google Scholar]
- 22.Cox D. Regression models and life tables. J Royal Statist Soc. 1972;34:187–229. [Google Scholar]
- 23.Rubin D. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 24.Vermorken JB, Stöhlmacher-Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): An open-label phase 3 randomised trial. Lancet Oncol. 2013;14:697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 25.Argiris A, Li S, Ghebremichael M, et al. Prognostic signficance of human papillomavirus in recurrent or metastatic head and neck squamous cell cancer: An analysis of Eastern Cooperative Group trials. Ann Oncol. doi: 10.1093/annonc/mdu167. [epub ahead of print on May 5, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimple RJ, Smith MA, Blitzer GC, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013;73:4791–4800. doi: 10.1158/0008-5472.CAN-13-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hafkamp HC, Mooren JJ, Claessen SM, et al. P21 Cip1/WAF1 expression is strongly associated with HPV-positive tonsillar carcinoma and a favorable prognosis. Mod Pathol. 2009;22:686–698. doi: 10.1038/modpathol.2009.23. [DOI] [PubMed] [Google Scholar]
- 28.Kong CS, Narasimhan B, Cao H, et al. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2009;74:553–561. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young RJ, Rischin D, Fisher R, et al. Relationship between epidermal growth factor receptor status, p16(INK4A), and outcome in head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2011;20:1230–1237. doi: 10.1158/1055-9965.EPI-10-1262. [DOI] [PubMed] [Google Scholar]
- 30.Zafereo ME, Hanasono MM, Rosenthal DI, et al. The role of salvage surgery in patients with recurrent squamous cell carcinoma of the oropharynx. Cancer. 2009;115:5723–5733. doi: 10.1002/cncr.24595. [DOI] [PubMed] [Google Scholar]
- 31.Kano S, Homma A, Hayashi R, et al. Salvage surgery for recurrent oropharyngeal cancer after chemoradiotherapy. Int J Clin Oncol. 2013;18:817–823. doi: 10.1007/s10147-012-0449-x. [DOI] [PubMed] [Google Scholar]
- 32.Michiels S, Le Maître A, Buyse M, et al. Surrogate endpoints for overall survival in locally advanced head and neck cancer: Meta-analyses of individual patient data. Lancet Oncol. 2009;10:341–350. doi: 10.1016/S1470-2045(09)70023-3. [DOI] [PubMed] [Google Scholar]
- 33.Bishop JA, Ogawa T, Chang X, et al. HPV analysis in distinguishing second primary tumors from lung metastases in patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2012;36:142–148. doi: 10.1097/PAS.0b013e3182395c7b. [DOI] [PMC free article] [PubMed] [Google Scholar]