Abstract
Purpose
Patients with esophageal carcinoma (EC) who are treated with definitive chemoradiotherapy (bimodality therapy [BMT]) experience frequent relapses. In a large cohort, we assessed the timing, frequency, and types of relapses during an aggressive surveillance program and the value of the salvage strategies.
Patients and Methods
Patients with EC (N = 276) who received BMT were analyzed. Patients who had surgery within 6 months of chemoradiotherapy were excluded to reduce bias. We focused on local relapse (LR) and distant metastases (DM) and the salvage treatment of patients with LR only. Standard statistical methods were applied.
Results
The median follow-up time was 54.3 months (95% CI, 48.4 to 62.4). First relapses included LR only in 23.2% (n = 64), DM with or without LR in 43.5% (n = 120), and no relapses in 33.3% (n = 92) of patients. Final relapses included no relapses in 33.3%, LR only in 14.5%, DM only in 15.9%, and DM plus LR in 36.2% of patients. Ninety-one percent of LRs occurred within 2 years and 98% occurred within 3 years of BMT. Twenty-three (36%) of 64 patients with LR only underwent salvage surgery, and their median overall survival was 58.6 months (95% CI, 28.8 to not reached) compared with those patients with LR only who were unable to undergo surgery (9.5 months; 95% CI, 7.8 to 13.3).
Conclusion
Unlike in patients undergoing trimodality therapy, for whom surveillance/salvage treatment plays a lesser role,1 in the BMT population, approximately 8% of all patients (or 36% of patients with LR only) with LRs occurring more than 6 months after chemoradiotherapy can undergo salvage treatment, and their survival is excellent. Our data support vigilant surveillance, at least in the first 24 months after chemotherapy, in these patients.
INTRODUCTION
Esophageal carcinoma (EC) remains a significant health problem around the world, with an estimated 482,300 new cases and 406,800 deaths each year.2 In North America, the most commonly prescribed therapy for localized EC is either chemoradiotherapy followed by surgery (trimodality therapy [TMT])3,4 or definitive chemoradiotherapy (bimodality therapy [BMT]),4,5 and the selection of TMT versus BMT is based on multiple factors that have been discussed previously.6,7 We previously reported the rate of local relapses (LRs) in a smaller cohort of patients,6,8 but the details of the timing and outcomes of salvage strategies in a larger group of patients have not been reported. Although the LR rate is low after TMT,1,9–11 it is higher without preoperative chemoradiotherapy12 and it is considerably higher after BMT.6,13 Another important question is whether surveillance of patients after BMT provides any benefit in those who develop an LR. We have aggressively surveyed our patients who underwent TMT and BMT. We reported that aggressive surveillance in patients who have undergone TMT is most likely not warranted1; however, the role of surveillance in patients who have received BMT remains unsettled.
In this report, we present one of the largest cohorts of patients who received BMT and provide their outcome after salvage treatment for LR. Our results seem to have implications for surveillance in terms of its duration and the types of investigations recommended.
PATIENTS AND METHODS
Patient Selection
We analyzed patients from our prospectively maintained database on EC in the Department of GI Medical Oncology at The University of Texas MD Anderson Cancer Center to find consecutive patients who, between 2002 and 2011, had histologically confirmed EC and successfully completed BMT. Patients who underwent planned surgery or salvage surgery (SS) within 6 months after chemoradiotherapy were excluded to avoid bias because that group could be construed as trimodality therapy patients for whom surgery had been delayed for one reason or another. All patients had baseline and postchemoradiation staging that included imaging studies and esophagogastroduodenoscopy (EGD) with biopsies. Endoscopic ultrasonography was performed in each patient at baseline. Imaging studies included chest and abdomen computed tomography (CT) and/or positron emission tomography (PET) with CT. Clinical staging was based on the sixth edition of the American Joint Committee Classification.14 The institutional review board approved this analysis.
Therapy
BMT consisted of radiation and concurrent chemotherapy. Before BMT, a fraction of patients received up to 8 weeks of induction chemotherapy (but we have reported that it does not seem to alter the outcome15). Radiation therapy was planned by four-dimensional simulation. All patients needed to be compliant with the dose-volume constraints of the MD Anderson Cancer Center Thoracic Radiation Oncology guidelines, which are similar to National Comprehensive Cancer Network 2013 Guidelines for Esophageal and Esophagogastric Junction Cancers.16 Gross target volume (gross tumor volume) was contoured on the basis of PET/CT or CT (when PET/CT was not obtained) images and the maximum-intensity phase image of the four-dimensional simulation. Beyond the gross tumor volume, 3-cm extensions of proximal and distal margins were used to determine the clinical target volume, and a 1-cm radial margin was used beyond the clinical target volume as the planning target volume. Patients were treated by intensity-modulated radiotherapy or proton beam treatment with a dose of 50.4 Gy in 28 fractions (1.8 Gy per fraction) given 5 days per week. Most patients received a fluoropyrimidine (intravenous or oral) and either a platinum compound or a taxane. Before proceeding with therapy, each patient was evaluated by appropriate disciplines and then discussed by the multidisciplinary team (which consisted of radiologists, gastroenterologists, thoracic surgeons, radiation oncologists, pathologists, nutritionists, geneticists [when appropriate], and medical oncologists). Patients who developed LR only were rediscussed in our multidisciplinary conference so that a consensus could be developed for a salvage strategy.
Surveillance After BMT
Each patient was observed according to the following surveillance strategy: all patients underwent EGD and multiple biopsies plus CT or PET/CT at their first visit (5 and 8 weeks after completion of BMT). Subsequent visits occurred every 3 months for the first year, every 6 months for 2 additional years, and then once per year for the next 2 years, for a total of 5 years of follow-up after BMT. Surveillance investigations included blood tests, EGD with biopsies (every other visit), and imaging studies including CT and PET/CT (every visit). LRs and DMs were recorded and tabulated. The survival follow-up was carried out through our institution's tumor registry, electronic medical records, and/or the Social Security database.
Statistical Analysis
Continuous variables were summarized by descriptive statistics such as means, standard deviations, medians, and ranges. Categorical variables were tabulated by frequency and percentage. For the 64 patients with LR only, analyses for timing of LR and overall survival (OS) from the point of LR were also performed. Timing of LR from completion of BMT was tabulated. The OS time was computed as the period from the date of diagnosis to the date of death or to the last follow-up date if patients were alive at the time of date collection. The Kaplan-Meier method was applied to estimate the probability of survival and median survival time. SAS software (version 9.3; SAS Institute, Cary, NC) and S Plus software (version 8.2; TIBCO Software, Palo Alto, CA) were used for analyses.
RESULTS
Patient Characteristics
The clinical characteristics of the 276 patients are listed in Table 1. Briefly, the median age was 67 years (range, 20 to 89 years). Most patients were men (87.7%), and most patients had adenocarcinoma (77.9%). At baseline staging, endoscopic ultrasonography and imaging studies demonstrated that the tumors were predominantly clinical stage II and III (29.3% and 54.7%, respectively).
Table 1.
Pretreatment Characteristics
| Covariate | Frequency |
|
|---|---|---|
| No. | % | |
| Age, years | ||
| Median | 67 | |
| Range | 20-89 | |
| Sex | ||
| Male | 242 | 87.7 |
| Female | 34 | 12.3 |
| Race | ||
| White | 239 | 86.6 |
| Other | 37 | 13.4 |
| Primary site | ||
| Esophagus | 61 | 22.1 |
| GEJ | 215 | 77.9 |
| Histology | ||
| Adenocarcinoma | 215 | 77.9 |
| Squamous cell carcinoma | 57 | 20.7 |
| Adenosquamous cell carcinoma | 4 | 1.4 |
| Histologic grade | ||
| Well/moderate | 132 | 47.8 |
| Poor | 143 | 51.8 |
| Unspecified | 1 | 0.4 |
| Baseline T stage | ||
| T1 | 10 | 3.6 |
| T2 | 23 | 8.3 |
| T3 | 229 | 83.0 |
| T4 | 11 | 4.0 |
| TX | 3 | 1.1 |
| Baseline N stage | ||
| N0 | 84 | 30.4 |
| N1 | 190 | 68.8 |
| NX | 2 | 0.7 |
| Baseline M stage | ||
| M0 | 241 | 87.3 |
| M1 | 35 | 12.7 |
| Baseline stage | ||
| Stage I | 9 | 3.3 |
| Stage II | 81 | 29.3 |
| Stage III | 151 | 54.7 |
| Stage IV | 35 | 12.7 |
Abbreviation: GEJ, gastroesophageal junction.
Treatment Characteristics
A total of 101 patients (36.6%) had induction chemotherapy before BMT. The median radiation dose was 50.4 Gy (range, 45 to 66 Gy). During radiation, 157 patients (56.9%) received a fluoropyrimidine (intravenous or oral) and a taxane, 72 patients (26.1%) received a fluoropyrimidine and a platinum compound, 40 patients (14.5%) received other drugs, and drug treatment was unspecified for seven patients (2.5%).
Failure Patterns
The median follow-up time was 54.3 months (95% CI, 48.4 to 62.4). At the first surveillance visit after BMT, 194 patients (70.3%) had achieved a clinical complete response (defined as no cancer in the EGD biopsies and physiologic PET/CT17), and 82 patients (29.7%) had persistent local cancer. Of the 194 patients with clinical complete response, 102 (53%) experienced a relapse. In the entire cohort, a total of 184 patients (66.7%) experienced a relapse. Of the 184 patients, 64 patients (23.2% of the entire cohort of 276 patients) were documented to have LR only as the first sign of treatment failure, and 120 patients (43.5% of 276 patients) had distant metastases. The details of initial relapse are shown in Figure 1A.
Fig 1.
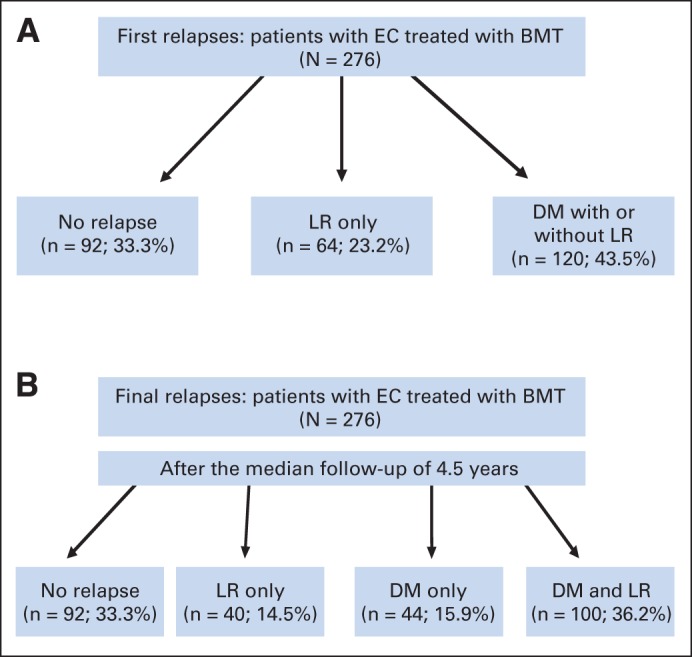
(A) Initial patterns of failure after bimodality therapy (BMT) in patients with esophageal and gastroesophageal junction cancer (EC). (B) Final failure patterns after completion of entire follow-up period after BMT. DM, distant metastasis; LR, local relapse.
The total failure rates after more than 4.5 years of median follow-up are shown in Figure 1B. Ninety-two patients (33.3%) never experienced a relapse, 40 patients (14.5%) experienced LR, 44 patients (15.9%) experienced DM only, and 100 patients (36.2%) were found to have DM plus LR. Overall, > 60% of patients had LR, DM, or both. Additional details regarding LR are shown in Table 2. Most (98%) of the LRs occurred within 3 years of completion of BMT.
Table 2.
Duration-Specific Rate of Locoregional Failure From Surgery (months)
| Type of Relapse | Persistent Disease at First Surveillance | ≤ 12 Months* | 13-24 Months | 25-36 Months | 37-48 Months | ≥ 49 Months | Total |
|---|---|---|---|---|---|---|---|
| Luminal only | |||||||
| No. | 15 | 27 | 10 | 4 | 0 | 1 | 57 |
| % of total cohort (N = 276) | 5.4 | 9.8 | 3.6 | 1.4 | 0.0 | 0.4 | 20.7 |
| % of first LR-only cohort after BMT (n = 64) | 23.4 | 42.2 | 15.6 | 6.3 | 0.0 | 1.6 | 89.1 |
| Regional | |||||||
| No. | 5 | 1 | 0 | 1 | 0 | 0 | 7 |
| % of total cohort (N = 276) | 1.8 | 0.4 | 0.0 | 0.4 | 0.0 | 0.0 | 2.5 |
| % of first LR-only cohort after BMT (n = 64) | 7.8 | 1.6 | 0.0 | 1.6 | 0.0 | 0.0 | 10.9 |
| Total LRs | |||||||
| No. | 20 | 28 | 10 | 5 | 0 | 1 | 64 |
| % of total cohort (N = 276) | 7.2 | 10.1 | 3.6 | 1.8 | 0.0 | 0.4 | 23.2 |
| % of first LR-only cohort after BMT (n = 64) | 31.3 | 43.8 | 15.6 | 7.8 | 0.0 | 1.6 | 100.0 |
NOTE. LR was grouped into the following subgroups: luminal only: documented intraluminal recurrence; regional: nodal relapse without luminal relapse; total LRs: combination of luminal only and regional relapses.
Abbreviations: BMT, bimodality therapy; LR, local relapse.
After first surveillance to 12 months after completion of BMT.
Among the 194 patients with a clinical complete response, 92 did not experience relapse. Forty-four patients had LR only (these patients were potential candidates for salvage treatment), 40 had DM only, and 18 had DM plus LR.
There was no difference in the clinical complete response rate (P = .359) or relapse rate (P = .257) with regard to whether patients received a platinum compound or a taxane in addition to a fluoropyrimidine with concurrent radiation therapy. In addition, there was no difference in the clinical complete response rate whether the histology was adenocarcinoma (70%) or squamous cell carcinoma (72%; P = .751). Similarly, the median survival of those who underwent SS was not different by histology (P = .957).
Salvage Strategies and Survival
The estimated median OS of the 64 patients with LR only (from relapse) was 13.3 months (95% CI, 9.5 to 32.7). At the time of this analysis, 43 of 64 patients (67.2%) have died. Twenty-three of the 64 patients were able to undergo SS for LR. Details regarding each of the 23 patients have been provided in Table 3. Of the 23 patients, 22 had LR after achieving a complete clinical response; one patient with persistent cancer was able to receive radiation therapy again but required surgery after a brief remission. Right transthoracic (Ivor-Lewis) esophagectomy was performed in 16 of 23 patients (70%), minimally invasive esophagectomy in five patients (22%), three–field technique esophagectomy in one patient (4%), and upper mediastinal lymph node dissection in one patient (4%). One patient underwent only upper mediastinal lymph node dissection without esophagectomy because we could not document esophageal (luminal) relapse. The estimated median OS of 23 patients was 58.6 months (95% CI, 28.8 to not available), and the estimated OS rates at 3 and 5 years were 61% (95% CI, 43% to 87%) and 45% (95% CI, 26% to 79%; Fig 2).
Table 3.
Characteristics and Outcomes of Patients Who Underwent Salvage Surgery (n = 23)
| Baseline |
Relapse |
Salvage Surgery |
Outcome |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Sex | Tumor Histology | Location of Tumor | T | N | M | Stage | Location | Time After BMT (months) | Type of Surgery | Extent of Resection | Relapse After Salvage Surgery | Survival Status | Survival Time After LRF (months) |
| 72 | Male | AC | GEJ | T3 | N1 | M0 | III | Luminal | 13-24 | Ivor-Lewis | R0 | Yes | Dead | 7 |
| 69 | Male | AC | GEJ | T3 | N1 | M0 | III | Luminal | < 12 | Ivor-Lewis | R0 | No | Dead | 58 |
| 68 | Male | AC | GEJ | T3 | N0 | M0 | IIA | Luminal | 13-24 | Ivor-Lewis | R0 | No | Alive | 82 |
| 62 | Male | SCC | GEJ | T3 | N0 | M0 | IIA | Luminal | < 12 | Minimally invasive | R0 | Yes | Alive | 79 |
| 56 | Male | AC | GEJ | T3 | N0 | M0 | IIA | Luminal | 13-24 | Ivor-Lewis | R0 | Yes | Alive | 71 |
| 64 | Male | AC | Esophagus | T3 | N1 | M0 | III | Luminal | < 12 | Ivor-Lewis | R0 | No | Alive | 68 |
| 77 | Male | AC | GEJ | T3 | N0 | M0 | IIA | Luminal | 13-24 | Ivor-Lewis | R0 | No | Alive | 54 |
| 69 | Male | AC | GEJ | T3 | N1 | M0 | III | Luminal | < 12 | Minimally invasive | R0 | Yes | Dead | 40 |
| 59 | Male | SCC | Esophagus | T3 | N1 | M0 | III | Regional | < 12 | Lymph node dissection | R0 | No | Alive | 60 |
| 49 | Male | AC | GEJ | T3 | N0 | M1 | IV | Luminal | 13-24 | Ivor-Lewis | R0 | No | Alive | 45 |
| 80 | Male | AC | GEJ | T3 | N0 | M0 | IIA | Luminal | < 12 | Ivor-Lewis | R2 | R2 resection | Dead | 3 |
| 64 | Male | AC | GEJ | T3 | N1 | M0 | III | Luminal | < 12 | Ivor-Lewis | R0 | Yes | Dead | 5 |
| 62 | Male | AC | GEJ | T2 | N0 | M0 | IIA | Luminal | < 12 | Ivor-Lewis | R0 | No | Dead | 32 |
| 57 | Male | AC | GEJ | T3 | N0 | M0 | IIA | Luminal | < 12 | Ivor-Lewis | R0 | Yes | Dead | 21 |
| 67 | Male | AC | GEJ | T3 | N0 | M0 | IIA | Luminal | 13-24 | Ivor-Lewis | R0 | No | Alive | 26 |
| 74 | Male | AC | GEJ | T3 | N0 | M0 | IIA | Luminal | 25-36 | Ivor-Lewis | R0 | Yes | Alive | 22 |
| 76 | Male | SCC | Esophagus | T3 | N1 | M0 | III | Luminal | < 12 | Minimally invasive | R0 | No | Dead | 4 |
| 57 | Male | AC | GEJ | T3 | N1 | M0 | III | Luminal | < 12 | Minimally invasive | R0 | Yes | Dead | 28 |
| 69 | Female | SCC | GEJ | T3 | N1 | M0 | III | Luminal | < 12 | Three-field technique | R1 | Yes | Dead | 14 |
| 78 | Male | AC | GEJ | T3 | N1 | M0 | III | Luminal | < 12 | Minimally invasive | R0 | No | Alive | 34 |
| 62 | Male | AC | GEJ | T3 | N1 | M0 | III | Luminal | < 12 | Ivor-Lewis | R0 | Yes | Alive | 31 |
| 76 | Male | SCC | Esophagus | T3 | N1 | M0 | III | Luminal | < 12 | Ivor-Lewis | R0 | No | Alive | 8 |
| 48 | Female | AC | GEJ | TX | N1 | M1 | IV | Regional | < 12 | Ivor-Lewis | R0 | No | Alive | 6 |
NOTE. One patient had upper mediastinal lymph node dissection without esophagectomy. All other patients (n = 22) had esophagectomy.
Abbreviations: AC, adenocarcinoma; BMT, bimodality therapy; GEJ, gastroesophageal junction; LRF, locoregional failure; SCC, squamous cell carcinoma.
Fig 2.
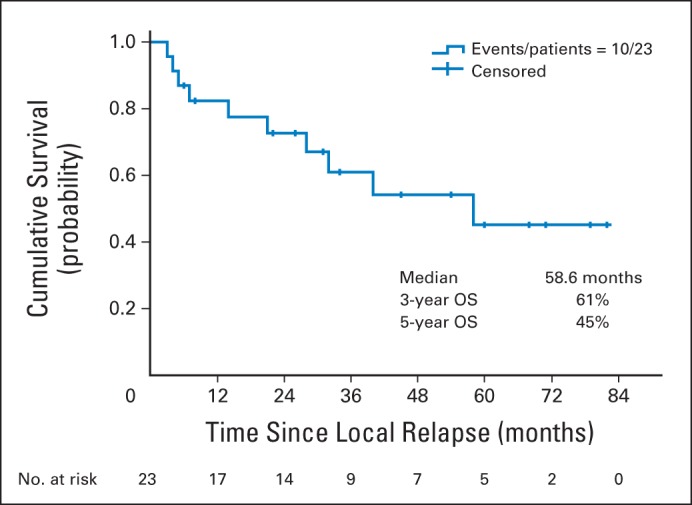
Kaplan-Meier survival curve from diagnosis of local relapse for 23 patients who underwent salvage surgery. OS, overall survival.
Among 41 patients who did not undergo surgery, eight received palliative chemotherapy, one received salvage chemoradiotherapy, one had an endoscopic mucosal resection, one had brachytherapy, one had endoscopic laser therapy, and 29 received best supportive care. Reasons for not undergoing surgery included considerable comorbidities (23 of 41 patients), technically unresectable cancer (invasion of adjacent structures; nine of 41 patients), DM before surgery (four of 41 patients), patient's choice (three of 41 patients), unconfirmed relapse just before surgery (one of 41 patients), and death (one of 41 patients). The OS for 41 patients without SS was 9.5 months (95% CI, 7.8 to 13.3).
Surgical Morbidity
Except for two patients, all had an R0 resection. The surgical complications (occurring in > 5% of patients) included major pulmonary events (17%), anastomotic leak (17%), and intensive care unit readmission (17%). There was no 30-day mortality; however, two patients (9%) died within 90 days of surgery.
DISCUSSION
Surveillance after local therapy (eg, TMT or BMT for patients with EC) is performed routinely in the Western world; however, there are considerable variations in the surveillance strategies because there has never been a prospective study examining various surveillance strategies (conservative v aggressive).1 The purpose of surveillance is to provide salvage therapy to patients who develop LR only. SS for LR only after BMT is recommended if surgery is feasible.16 We previously reported that the rate of LR is low (approximately 5%) after TMT for esophageal and gastroesophageal junction adenocarcinoma.1 However, the rate of persistent cancer or locally recurrent cancer after BMT can be as high as 50%.5,18,19 To our knowledge, the value of aggressive surveillance in terms of the success of implementation of salvage strategies has not been reported. In this report, we document that a sufficiently large fraction of patients (64 of 276 [23%]) had LR only, and of these, 36% (or 8% of 276) were able to undergo SS. More importantly, the median OS of patients who underwent salvage treatment was 58.6 months (95% CI, 28.8 to not available). This is in contrast with the TMT population: less than 2% of the TMT population who received salvage treatment survived more than 2 years.1
Our data compellingly argue for systematic surveillance of patients receiving BMT because a reasonable fraction of patients are able to undergo salvage treatment, and those who do receive salvage treatment seem to experience prolonged survival. However, we acknowledge that surveillance is costly, time consuming, and distressing to patients, their relatives, and their caregivers. We have not performed cost analysis of surveillance because costs vary geographically and thus cannot be generalized.
Our analysis has the following limitations: it is retrospective in nature; it involves a single-institution experience; the total denominator, although it is the largest reported, is still relatively small; and the data have emerged from a relatively aggressive surveillance strategy. However, our analysis has the following strengths: it provides the first evidence, to our knowledge, of the value of surveillance in terms of salvage treatment of patients; it demonstrates that the population of patients undergoing salvage treatment seems to enjoy a prolonged survival; it contrasts the value of surveillance/salvage in patients with EC who received TMT versus BMT; and our data also suggest that surveillance after 3 years (given that 98% of relapses occur by this time) may be of little benefit.
In conclusion, our data show that after BMT, patients with LR only can undergo SS and have a decent OS. Approximately 8% of the entire population seems to benefit from surveillance; therefore, vigilant surveillance in patients who have undergone BMT is recommended, at least during the first 24 months. We draw this conclusion with caution, given that cost analysis was not performed.
Footnotes
Supported by the Caporella, Dallas, Sultan, Park, Smith, Frazier, Oaks, Vanstekelenberg, Cantu, and Planjery Families, the Schecter Private Foundation, Rivercreek Foundation, Kevin Fund, Myer Fund, Dio Fund, Milrod Fund, and by multidisplinary grants provided by The University of Texas MD Anderson Cancer Center, Houston, TX. Supported in part by Grants No. RO1CA129906 and RO1CA 172741 from the National Cancer Institute (J.A.A.).
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Jaffer A. Ajani
Financial support: Jaffer A. Ajani
Administrative support: Jaffer A. Ajani
Provision of study materials or patients: Jaffer A. Ajani
Collection and assembly of data: Kazuki Sudo, Roopma Wadhwa, Hironori Shiozaki, Takashi Taketa, Mariela A. Blum, Jeffrey H. Lee, Manoop S. Bhutani, Brian Weston, William A. Ross, Ritsuko Komaki, David C. Rice, Stephen G. Swisher, Wayne L. Hofstetter, Dipen M. Maru, Heath D. Skinner, Jaffer A. Ajani
Data analysis and interpretation: Kazuki Sudo, Lianchun Xiao, Roopma Wadhwa, Elena Elimova, Jaffer A. Ajani
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Kazuki Sudo
No relationship to disclose
Lianchun Xiao
No relationship to disclose
Roopma Wadhwa
No relationship to disclose
Hironori Shiozaki
No relationship to disclose
Elena Elimova
No relationship to disclose
Takashi Taketa
No relationship to disclose
Mariela A. Blum
No relationship to disclose
Jeffrey H. Lee
No relationship to disclose
Manoop S. Bhutani
Research Funding: Boston Scientific
Travel, Accommodations, Expenses: Mauna Kea, Cook Medical
Brian Weston
No relationship to disclose
William A. Ross
Employment: Amgen (I)
Stock or Other Ownership: Johnson & Johnson, Abbott Laboratories, Amgen (I), Pfizer
Ritsuko Komaki
No relationship to disclose
David C. Rice
Consulting or Advisory Role: Olympus
Stephen G. Swisher
Consulting or Advisory Role: GlaxoSmithKline
Wayne L. Hofstetter
No relationship to disclose
Dipen M. Maru
Research Funding: Taiho Pharmaceutical
Heath D. Skinner
No relationship to disclose
Jaffer A. Ajani
No relationship to disclose
REFERENCES
- 1.Sudo K, Taketa T, Correa AM, et al. Locoregional failure rate after preoperative chemoradiation of esophageal adenocarcinoma and the outcomes of salvage strategies. J Clin Oncol. 2013;31:4306–4310. doi: 10.1200/JCO.2013.51.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 4.Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw. 2011;9:830–887. doi: 10.6004/jnccn.2011.0072. [DOI] [PubMed] [Google Scholar]
- 5.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01)—Radiation Therapy Oncology Group. JAMA. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 6.Welsh J, Settle SH, Amini A, et al. Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer. 2012;118:2632–2640. doi: 10.1002/cncr.26586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki A, Xiao L, Taketa T, et al. Results of the baseline positron emission tomography can customize therapy of localized esophageal adenocarcinoma patients who achieve a clinical complete response after chemoradiation. Ann Oncol. 2013;24:2854–2859. doi: 10.1093/annonc/mdt340. [DOI] [PubMed] [Google Scholar]
- 8.Amini A, Xiao L, Allen PK, et al. Celiac node failure patterns after definitive chemoradiation for esophageal cancer in the modern era. Int J Radiat Oncol Biol Phys. 2012;83:e231–e239. doi: 10.1016/j.ijrobp.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oppedijk V, van der Gaast A, van Lanschot JJ, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol. 2014;32:385–391. doi: 10.1200/JCO.2013.51.2186. [DOI] [PubMed] [Google Scholar]
- 10.Bekkar S, Gronnier C, Messager M, et al. The impact of preoperative radiochemotherapy on survival in advanced esophagogastric junction signet ring cell adenocarcinoma. Ann Thorac Surg. 2014;97:303–310. doi: 10.1016/j.athoracsur.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Mariette C, Robb WB, Piessen G. Reply to letter: “The role of surgery for patients with a complete clinical response after chemoradiation for esophageal cancer.”. Ann Surg. doi: 10.1097/SLA.0000000000000668. [epub ahead of print on March 25, 2014] [DOI] [PubMed] [Google Scholar]
- 12.Davies AR, Sandhu H, Pillai A, et al. Surgical resection strategy and the influence of radicality on outcomes in oesophageal cancer. Br J Surg. 2014;101:511–517. doi: 10.1002/bjs.9456. [DOI] [PubMed] [Google Scholar]
- 13.Amini A, Ajani J, Komaki R, et al. Factors associated with local-regional failure after definitive chemoradiation for locally advanced esophageal cancer. Ann Surg Oncol. 2013;21:306–314. doi: 10.1245/s10434-013-3303-0. [DOI] [PubMed] [Google Scholar]
- 14.Edge S, Byrd DR, Comptin CC, et al. AJCC Cancer Staging Manual (ed 6) New York, NY: Springer-Verlag; 2002. pp. 93–94. [Google Scholar]
- 15.Ajani JA, Xiao L, Roth JA, et al. A phase II randomized trial of induction chemotherapy versus no induction chemotherapy followed by preoperative chemoradiation in patients with esophageal cancer. Ann Oncol. 2013;24:2844–2849. doi: 10.1093/annonc/mdt339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network. Washington, PA: National Comprehensive Cancer Network; 2013. NCCN guidelines, version 2.2013. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 17.Cheedella NK, Suzuki A, Xiao L, et al. Association between clinical complete response and pathological complete response after preoperative chemoradiation in patients with gastroesophageal cancer: Analysis in a large cohort. Ann Oncol. 2013;24:1262–1266. doi: 10.1093/annonc/mds617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 19.Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593–1598. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]


