Abstract
The worldwide decline in coral cover has serious implications for the health of coral reefs. But what is the future of reef fish assemblages? Marine reserves can protect fish from exploitation, but do they protect fish biodiversity in degrading environments? The answer appears to be no, as indicated by our 8-year study in Papua New Guinea. A devastating decline in coral cover caused a parallel decline in fish biodiversity, both in marine reserves and in areas open to fishing. Over 75% of reef fish species declined in abundance, and 50% declined to less than half of their original numbers. The greater the dependence species have on living coral as juvenile recruitment sites, the greater the observed decline in abundance. Several rare coral-specialists became locally extinct. We suggest that fish biodiversity is threatened wherever permanent reef degradation occurs and warn that marine reserves will not always be sufficient to ensure their survival.
Many ecologists have expressed concern over the worldwide decline in coral cover due to global warming and associated coral bleaching, overfishing, and coastal pollution (1–5). Coral reefs support a high diversity of fishes that may ultimately depend on corals for their survival; however, the impact of long-term reef degradation on fish populations is unknown. Most attention to the protection of marine fish populations has focused on the benefits of controlling exploitation by establishing “no-take” marine reserves (6–8). However, comprehensive strategies for protecting marine biodiversity also require an understanding of how species respond to degradation of their habitats.
In the past, there has been a dichotomy of opinion over how closely fish communities are linked to their habitat, with some information indicating a high degree of variability that is independent of habitat change (9–14) and other data showing that coral-specialists clearly suffer when coral cover is reduced (13–17). Here we ask the following questions. If coral reefs continue along a path of degradation, what will be the fate of fish communities as a whole? Will marine reserves provide fish communities with any resilience to the effects of habitat loss?
Methods
In 1996, we observed the beginning of what progressed into a long-term decline in coral cover in four marine reserves in the Tamane Puli Conservation Area, Kimbe Bay, Papua New Guinea (150°06′E, 5°25′S). To predict the potential response of fish assemblages to declining coral in this area, we began by estimating the proportion of reef fish species that only fed on coral tissue or those that only lived in association with branching corals. We surveyed all species in 20 different families of fishes associated with coral reefs in the region (18). Those species dependent on live coral as food or living space were distinguished from the rest, based both on our own observations and published accounts of diet and habitat associations (19, 20).
The cover of branching scleractinian corals was estimated from annual surveys of eight reefs between 1996 and 2003 (including four marine reserves established in 1999 and four reefs open to fishing). Each reef was sampled by eight 50-m line transects (four in the reef crest and four in the upper reef slope habitat). Coral cover was estimated by quantifying the substratum under 100 random points along each transect.
In conjunction with coral surveys, we monitored the presence/absence and abundance of all species in four speciose reef fish families that could be reliably surveyed by visual transects (Acanthuridae, Chaetodontidae, Labridae, and Pomacentridae). Densities were estimated during annual surveys of the four reserve and four open reefs between 1997 and 2003. Scuba divers visually counted individual fish in eight replicate 50-m transects laid out in the reef crest and upper reef slope habitats (4 m wide for Acanthuridae, Chaetodontidae, and Labridae, and 1 m wide for Pomacentridae). Estimates of species richness were based on total species lists for each site at each time.
For 2 of the years (1999–2000) we carried out monthly surveys to record the substrata used by juveniles when they first settled into reef habitat. The settlement sites for all species in the four fish families were surveyed by using eight 50 × 2-m transects laid out in the reef crest and upper reef slope habitats. We counted all settlers, estimated their size, and recorded the microhabitats they occupied. Settlement sizes varied among species, and we used species-specific cut-off sizes to restrict our sampling to individuals <2 weeks old. The settlement sites of >102,000 juveniles were recorded over the 2-year period.
Results and Discussion
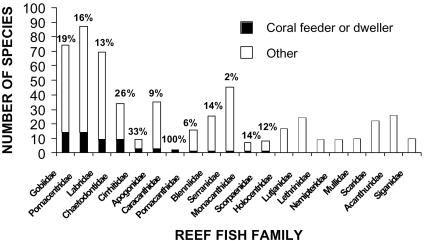
Our survey of the feeding modes and habitat use by species in 20 reef fish families indicated that ≈11% of 538 species had an obligate association with living corals (Fig. 1). This proportion appears to be typical for fishes of the Indo-Pacific region (19, 20). Only 12 of the 20 families contained any species that were specialized on corals, and in 5 of these 12 families, only 1 species fell into this category. Even for families such as Gobiidae, Pomacentridae, and Chaetodontidae, which include many coral-feeding or coral-dwelling species, these specialists were still a minority (<25%). Thus, we hypothesized that a relatively small proportion of species would be immediately threatened by the demise of corals.
Fig. 1.
Reef fish dependence on coral in Kimbe Bay. The number of coral reef fish species in 20 families that are dependent on live coral as food or living space (filled bars) is compared with all other species (open bars). Species are ranked in order of a decreasing number of species associated with corals. The percentage figures represent the proportion of species in each family that are associated with live coral.
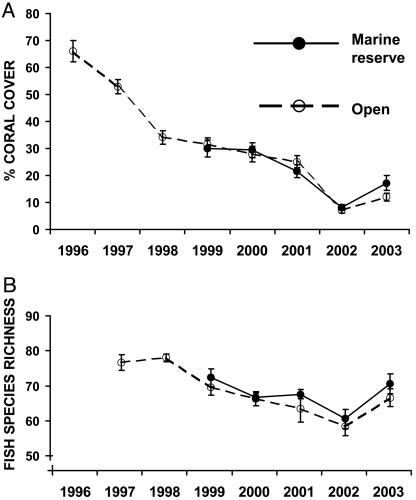
Our annual surveys of eight reefs between 1996 and 2003 (including four marine reserves established in 1999 and four areas open to fishing) documented a decline in coral cover from ≈66% in 1996 to a low of 7% in 2002 (Fig. 2A). This decline was associated with a corresponding increase in turfing algae. The decline in coral cover in marine reserves was very similar to that in open areas. It appeared to be due to a combination of coral bleaching (observed in 1997, 1998, 2000, and 2001), a gradual increase in sedimentation from terrestrial run-off, and outbreaks of crown-of-thorns starfish. By 2002, reef crest habitats on inshore reefs supported <2% cover of the branching acroporid corals that form the primary habitat and food of the majority of coral-specialist fishes.
Fig. 2.
(A) Declining mean cover of branching scleractinian corals between 1996 and 2003 in the Tamane Puli Conservation Area, Kimbe Bay. Coral cover was estimated from annual surveys of eight reefs (four marine reserves established in 1999 and four reefs open to fishing). Cover estimates were pooled for eight coastal reefs surveyed before 1999. Bars represent standard errors (n = 32). (B) Change in mean fish species richness for four reef fish families (Acanthuridae, Chaetodontidae, Labridae, and Pomacentridae) between 1997 and 2003 for the four marine reserves and four fished reefs. Species richness was based on total species lists for each site at each time. Bars represent standard errors (n = 4).
By 2002, diversity of fishes in the four focal families had declined by ≈22% compared to 1997 (Fig. 2B). This figure was close to the ≈15% decline predicted from patterns of diet and habitat use (Fig. 1) and paralleled the temporal change of coral cover (Fig. 2A). By 2003, the decline in diversity was reduced to ≈15%, the small recovery associated with the increase in coral cover in the last year. Importantly, the decline in biodiversity was not influenced by marine reserve status, with similar patterns observed in marine reserves and open areas. The proportional decline in local species richness was largest for the two families surveyed with the highest proportion of coral specialists (Chaetodontidae and Pomacentridae), each exhibiting a >25% decline in local species richness.
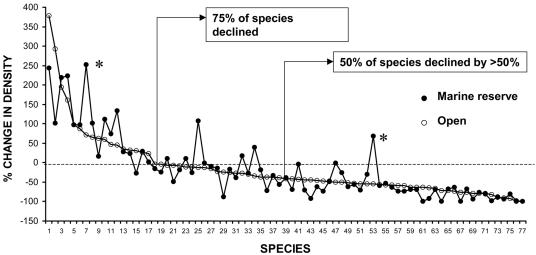
An ≈15% loss of species is likely to be ecologically significant for any community. However, the impact of coral decline on the structure of fish assemblages turned out to be far greater than either originally predicted or was evident from the focus on species richness. About 75% of the fish species surveyed declined in abundance from the beginning to the end of the survey period (Fig. 3). About half of all fish species declined by >50%. A smaller proportion of species (≈25%) increased in abundance, with numbers of some dead coral or rubble associated species rising dramatically. The magnitude of change in abundance for each species in areas open to fishing was strongly correlated with that for marine reserves (r = 0.83, P < 0.05). Only two surgeonfishes exploited in the traditional fishery (Ctenochaetus striatus and Acanthurus pyroferus) showed significant increases in marine reserves, a finding that can be attributed to protection (Fig. 3). Few other species surveyed are exploited, and the change in the abundance of most species can be attributed to habitat degradation.
Fig. 3.
Percentage change in fish abundance between 1997 and 2003 in marine reserves and on open reefs. Percentage change is calculated from the average of the first two surveys (1997–1998) and last two surveys (2002–2003) for all fish species in the families Acanthuridae, Chaetodontidae, Labridae, and Pomacentridae. The numbers on the x axis represent individual species (1–77) and are ranked from largest increase to largest decline for open reefs. Only species with a mean number per transect >1 are included, most showing a statistically significant change over time. Two surgeonfish species (marked by an asterisk) showed an increase in marine reserves relative to fished reefs.
The dramatic change in the abundance of almost all species indicates a phase-shift in reef fish community structure in response to habitat degradation and the increasing dominance of a small proportion of the original species pool. The catastrophic decline in the abundance of 50% of the species was not predicted from the initial snapshot of their ecology, because it affected far more than just coral-feeding or coral-dwelling fishes (Fig. 1).
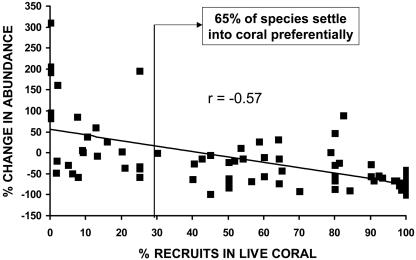
An analysis of fish settlement sites provided the most likely explanation for the community-wide change. Species varied on a continuum of those that only ever settled onto live coral substrata to those that never settled onto coral (Fig. 4). About 65% of fish species settled onto live coral in proportions significantly greater than expected because of the average coverage of live coral at these times. Furthermore, the magnitude of change in fish abundance was inversely correlated with the proportion of juveniles found settling on live coral (r = –0.57, P < 0.05). With a few exceptions, species that mainly settle into live coral declined, and those largely recruiting to noncoral substrata increased in abundance.
Fig. 4.
Relationship between the direction and magnitude of change in fish abundance between 1997 and 2003, and the proportion of all juveniles observed to be associated with live coral at settlement. Settlement data were collected in 1999 and 2000, when the average coral cover was 29.0 ± 0.7%.
Reef fish communities may be more contingent on their underlying habitat than has previously been considered. Our data suggests that this dependence arises through habitat-limited recruitment (16, 21), although adult mortality through declining food and shelter may also be important. The impact on species in reef fish families less reliant on coral may be correspondingly less extreme (e.g., Lethrinidae and Lutjanidae). However, this cannot be confirmed until we know more about the settlement site preferences in these groups. The impact on small specialized families (e.g., Gobiidae and Carancanthidae) may be even more devastating. Global extinction may be imminent for some coral-dwelling gobies with restricted geographic ranges (22). The entire caracanthid family is comprised of only two obligate coral-dwelling species (Fig. 1), both of which are now extremely rare at our study sites.
The magnitude of the decline in coral cover in Kimbe Bay is not atypical of other geographic locations where coral has also been largely replaced by turfing algae (1–5). The impacts of coral-algal phase-shifts on fish communities in other regions may have been similar. However, although short-term effects on coral-feeding fishes have been noted (23), the long-term effects on reef fish communities have not previously been described. Our results suggest that reefs without corals will no longer support diverse fish faunas but rather will be numerically dominated by a small subset of species preferring algal or rubble substrata.
Although there is considerable potential for recovery from local disturbance through larval dispersal, the spatial extent of habitat devastation appears to be expanding rather than contracting (4, 5). If this trend cannot be reversed by management actions, species with restricted dispersal or small geographic ranges will be threatened by extinction (24–26). Although there is a large body of evidence that indicates that marine reserves can be an effective management strategy for protecting marine biodiversity (6–8), there is a growing recognition that such areas cannot protect reefs from large-scale pollution or global warming (4, 27–30). Thus, although marine reserves are necessary to control the “top-down” impact of human predation, they must be combined with management strategies that fundamentally address “bottom-up” processes that appear to be a more likely path to extinction.
Acknowledgments
We thank the Nature Conservancy, the Australian Research Council, and Walindi Plantation Resort for financial support, the Mahonia Na Dari Research and Conservation Center at Kimbe for providing a research base, B. Green, U. Kaly, M. Logo, I. McCormick, S. Neale, and T. Sin for assistance in the field, and P. Munday and two anonymous reviewers for constructive advice. Special thanks to the traditional owners of the Tamare-Kilu reefs for allowing us to monitor their reefs.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hughes, T. P. (1994) Science 265, 1547–1551. [DOI] [PubMed] [Google Scholar]
- 2.Sebens, K. P. (1994) Am. Zool. 34, 115–133. [Google Scholar]
- 3.McClanahan, T. R. (2002) Environ. Conserv. 29, 460–483. [Google Scholar]
- 4.Hughes, T. P., Baird, A. H., Bellwood, D. R., Card, M., Connolly, S. R., Folke, C., Grosberg, R., Hoegh-Guldberg, O., Jackson, J. B. C., Kleypas, J., et al. (2003) Science 301, 929–933. [DOI] [PubMed] [Google Scholar]
- 5.Gardner, T. A., Côte, I. M., Gill, J. A., Grant, A. & Watkinson, A. R. (2003) Science 301, 958–960. [DOI] [PubMed] [Google Scholar]
- 6.Agardy, T. (1994) Trends Ecol. Evol. 9, 267–270. [DOI] [PubMed] [Google Scholar]
- 7.Halpern, B. S. & Warner, R. R. (2002) Ecol. Lett. 5, 361–366. [Google Scholar]
- 8.Lubchenco, J., Palumbi, S. R., Gaines, S. D. & Andelman, S. (2003) Ecol. Appl. 13, Suppl., S3–S7. [Google Scholar]
- 9.Doherty, P. J. & Fowler, A. J. (1994) Science 263, 935–939. [DOI] [PubMed] [Google Scholar]
- 10.Doherty, P. J. (2002) in Coral Reef Fishes: Dynamics and Diversity in a Complex Ecosystem, ed. Sale, P. F. (Academic, San Diego), pp. 327–355.
- 11.Williams, D. M. (1986) Mar. Ecol. Progr. Ser. 28, 157–164. [Google Scholar]
- 12.Sale, P. F. & Douglas, W. A. (1984) Ecology 65, 409–422. [Google Scholar]
- 13.Cheal, A. J., Coleman, G., Delean, S., Miller, I., Osborne, K. & Sweatman, H. A. (2002) Coral Reefs 21, 131–142. [Google Scholar]
- 14.Spalding, M. D. & Jarvis, G. E. (2002) Mar. Pollut. Bull. 44, 309–321. [DOI] [PubMed] [Google Scholar]
- 15.Munday, P. L., Jones, G. P. & Caley, M. J. (1997) Mar. Ecol. Progr. Ser. 152, 227–239. [Google Scholar]
- 16.Syms, C. & Jones, G. P. (2002) Ecology 81, 2714–2729. [Google Scholar]
- 17.Kokita, T. & Nakazono, A. (2001) Coral Reefs 20, 155–158. [Google Scholar]
- 18.Munday, P. L. & Allen, G. R. (2002) in The Status of Coral Reefs in Papua New Guinea, ed. Munday, P. L. (Australian Inst. of Marine Sci., Townsville), pp. 17–22.
- 19.Randall, J. E., Allen, G. R. & Steene, R. C. (1990) Fishes of the Great Barrier Reef and the Coral Sea (Crawford House, Bathurst, Australia).
- 20.Lieske, E. & Myers, R. (1994) Coral Reef Fishes: Indo-Pacific and Caribbean (HarperCollins, London).
- 21.Schmitt, R. J. & Holbrook, S. J. (2002) Ecology 81, 3479–3494. [Google Scholar]
- 22.Munday, P. L. (2004) Global Change Biol., in press.
- 23.Sano, M. (2004) Fish. Sci. 70, 41–46. [Google Scholar]
- 24.Jones, G. P., Caley, M. J. & Munday, P. L. (2002) in Coral Reef Fishes: Dynamics and Diversity in a Complex Ecosystem, ed. Sale, P. F. (Academic, San Diego), pp. 81–102.
- 25.Roberts, C. M. & Hawkins, J. P. (1999) Trends Ecol. Evol. 14, 241–246. [DOI] [PubMed] [Google Scholar]
- 26.Roberts, C. M., McClean, C. J., Veron, J. E. N., Hawkins, J. P., Allen, G. R., McAllister, D. E., Mittermeier, C. G., Schueler, F. W., Spalding, M., Wells, F., et al. (2002) Science 295, 1280–1284. [DOI] [PubMed] [Google Scholar]
- 27.Allison, G. W., Lubchenco, J. & Carr, M. H. (1998) Ecol. Appl. 8, Suppl., S79–S92. [Google Scholar]
- 28.Hixon, M. A., Boersma, P. D., Hunter, M. L., Jr., Micheli, F., Norse, E. A., Possingham, H. P. & Snelgrove, P. V. R. (2001) in Conservation Biology: Research Priorities for the Next Decade, eds. Soulé, M. & Orians, G. (Island, Covelo, CA), pp. 125–154.
- 29.Rogers, C. S. & Beets, J. (2001) Environ. Conserv. 28, 312–322. [Google Scholar]
- 30.Jameson, S. C., Tupper, M. H. & Ridley, J. M. (2002) Mar. Pollut. Bull. 44, 1177–1183. [DOI] [PubMed] [Google Scholar]