Abstract
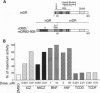
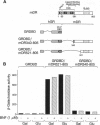
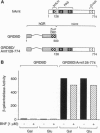
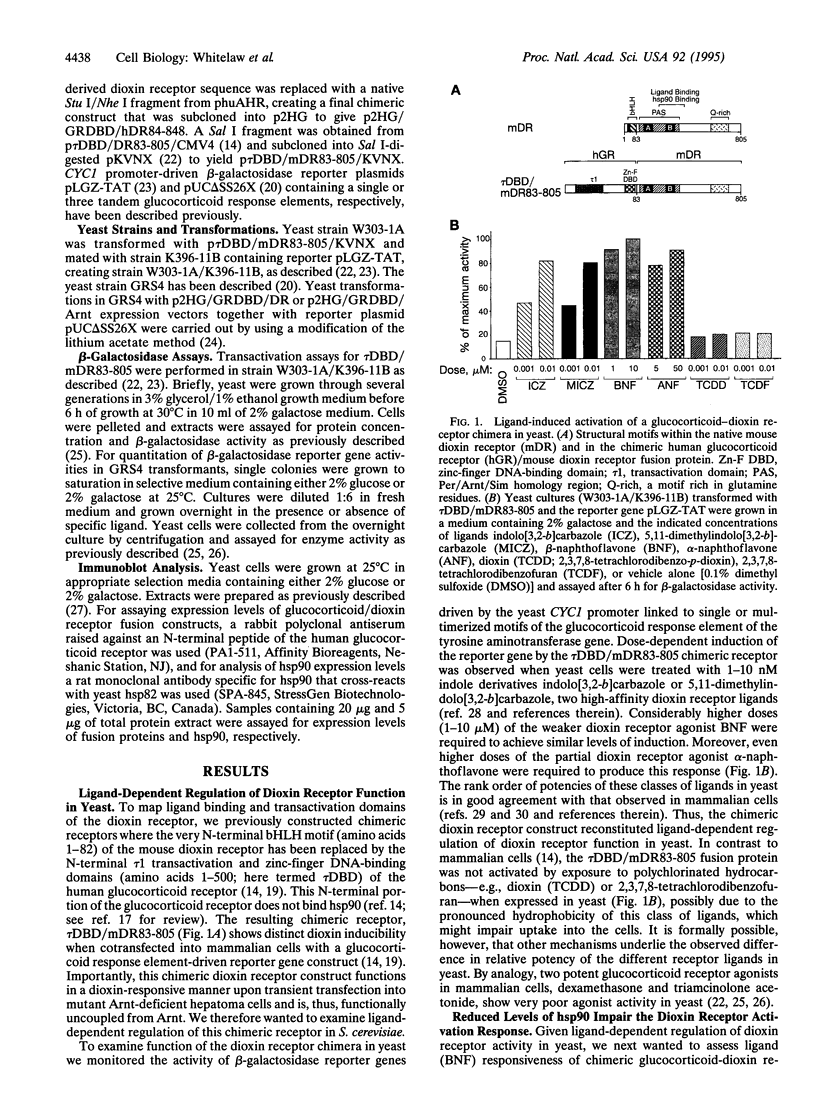
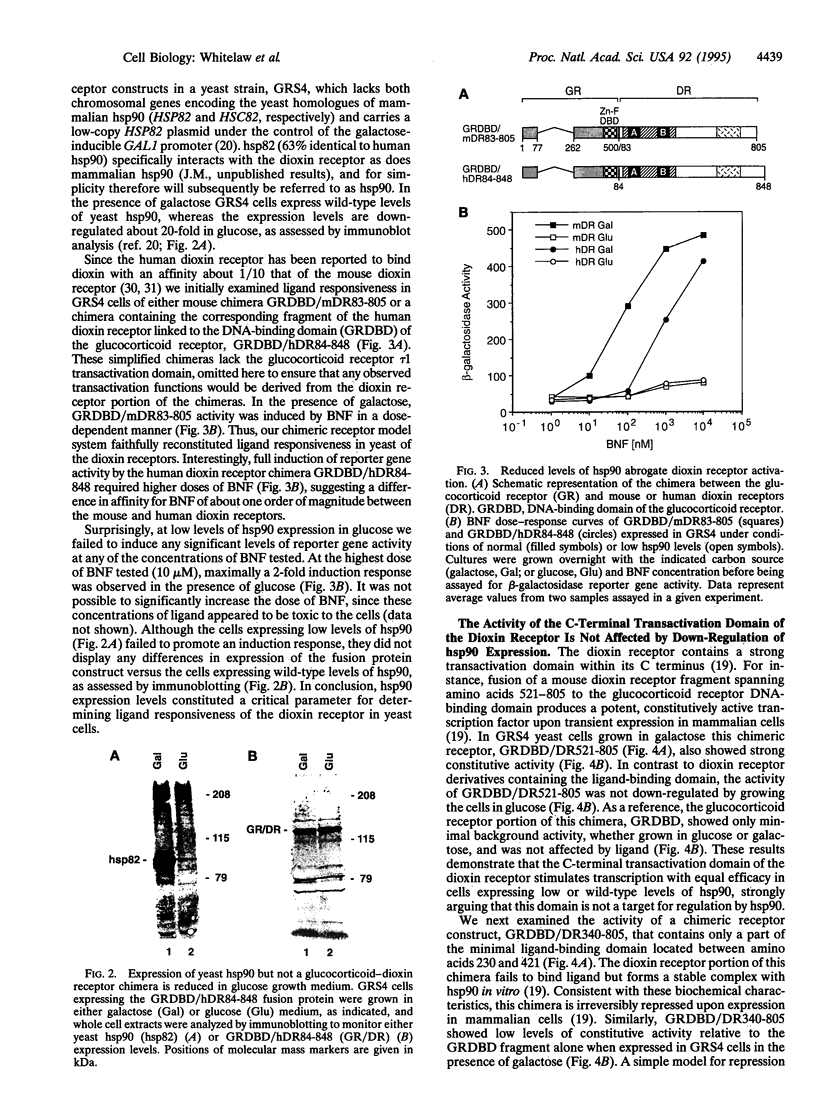
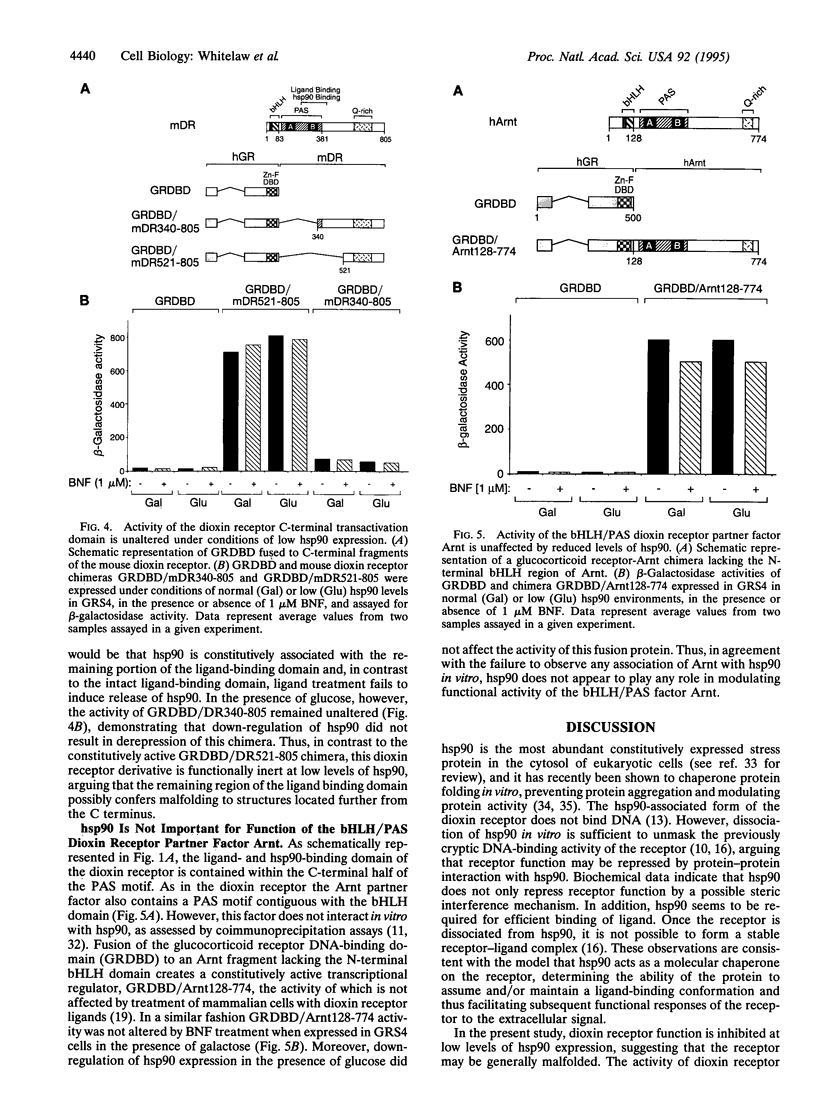
The dioxin (aryl hydrocarbon) receptor is a ligand-dependent basic helix-loop-helix (bHLH) factor that binds to xenobiotic response elements of target promoters upon heterodimerization with the bHLH partner factor Arnt. Here we have replaced the bHLH motif of the dioxin receptor with a heterologous DNA-binding domain to create fusion proteins that mediate ligand-dependent transcriptional enhancement in yeast (Saccharomyces cerevisiae). Previously, our experiments indicated that the ligand-free dioxin receptor is stably associated with the 90-kDa heat shock protein, hsp90. To investigate the role of hsp90 in dioxin signaling we have studied receptor function in a yeast strain where hsp90 expression can be down-regulated to about 5% relative to wild-type levels. At low levels of hsp90, ligand-dependent activation of the chimeric dioxin receptor construct was almost completely inhibited, whereas the activity of a similar chimeric construct containing the structurally related Arnt factor was not affected. Moreover, a chimeric dioxin receptor construct lacking the central ligand- and hsp90-binding region of the receptor showed constitutive transcriptional activity in yeast that was not impaired upon down-regulation of hsp90 expression levels. Thus, these data suggest that hsp90 is a critical determinant of conditional regulation of dioxin receptor function in vivo via the ligand-binding domain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonsson C., Whitelaw M. L., McGuire J., Gustafsson J. A., Poellinger L. Distinct roles of the molecular chaperone hsp90 in modulating dioxin receptor function via the basic helix-loop-helix and PAS domains. Mol Cell Biol. 1995 Feb;15(2):756–765. doi: 10.1128/mcb.15.2.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohen S. P., Yamamoto K. R. Isolation of Hsp90 mutants by screening for decreased steroid receptor function. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11424–11428. doi: 10.1073/pnas.90.23.11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver L. A., Jackiw V., Bradfield C. A. The 90-kDa heat shock protein is essential for Ah receptor signaling in a yeast expression system. J Biol Chem. 1994 Dec 2;269(48):30109–30112. [PubMed] [Google Scholar]
- Chen H. S., Perdew G. H. Subunit composition of the heteromeric cytosolic aryl hydrocarbon receptor complex. J Biol Chem. 1994 Nov 4;269(44):27554–27558. [PubMed] [Google Scholar]
- Denis M., Cuthill S., Wikström A. C., Poellinger L., Gustafsson J. A. Association of the dioxin receptor with the Mr 90,000 heat shock protein: a structural kinship with the glucocorticoid receptor. Biochem Biophys Res Commun. 1988 Sep 15;155(2):801–807. doi: 10.1016/s0006-291x(88)80566-7. [DOI] [PubMed] [Google Scholar]
- Dolwick K. M., Schmidt J. V., Carver L. A., Swanson H. I., Bradfield C. A. Cloning and expression of a human Ah receptor cDNA. Mol Pharmacol. 1993 Nov;44(5):911–917. [PubMed] [Google Scholar]
- Dolwick K. M., Swanson H. I., Bradfield C. A. In vitro analysis of Ah receptor domains involved in ligand-activated DNA recognition. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8566–8570. doi: 10.1073/pnas.90.18.8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M., Ohe N., Suzuki M., Mimura J., Sogawa K., Ikawa S., Fujii-Kuriyama Y. Dioxin binding activities of polymorphic forms of mouse and human arylhydrocarbon receptors. J Biol Chem. 1994 Nov 4;269(44):27337–27343. [PubMed] [Google Scholar]
- Garabedian M. J., Yamamoto K. R. Genetic dissection of the signaling domain of a mammalian steroid receptor in yeast. Mol Biol Cell. 1992 Nov;3(11):1245–1257. doi: 10.1091/mbc.3.11.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz D., St Jean A., Woods R. A., Schiestl R. H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992 Mar 25;20(6):1425–1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillner M., Bergman J., Cambillau C., Alexandersson M., Fernström B., Gustafsson J. A. Interactions of indolo[3,2-b]carbazoles and related polycyclic aromatic hydrocarbons with specific binding sites for 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat liver. Mol Pharmacol. 1993 Aug;44(2):336–345. [PubMed] [Google Scholar]
- Kadesch T. Consequences of heteromeric interactions among helix-loop-helix proteins. Cell Growth Differ. 1993 Jan;4(1):49–55. [PubMed] [Google Scholar]
- Kleman M. I., Poellinger L., Gustafsson J. A. Regulation of human dioxin receptor function by indolocarbazoles, receptor ligands of dietary origin. J Biol Chem. 1994 Feb 18;269(7):5137–5144. [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Matsushita N., Sogawa K., Ema M., Yoshida A., Fujii-Kuriyama Y. A factor binding to the xenobiotic responsive element (XRE) of P-4501A1 gene consists of at least two helix-loop-helix proteins, Ah receptor and Arnt. J Biol Chem. 1993 Oct 5;268(28):21002–21006. [PubMed] [Google Scholar]
- McGuire J., Whitelaw M. L., Pongratz I., Gustafsson J. A., Poellinger L. A cellular factor stimulates ligand-dependent release of hsp90 from the basic helix-loop-helix dioxin receptor. Mol Cell Biol. 1994 Apr;14(4):2438–2446. doi: 10.1128/mcb.14.4.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata Y., Yahara I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J Biol Chem. 1992 Apr 5;267(10):7042–7047. [PubMed] [Google Scholar]
- Perdew G. H. Association of the Ah receptor with the 90-kDa heat shock protein. J Biol Chem. 1988 Sep 25;263(27):13802–13805. [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990 Nov 8;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Poellinger L., Göttlicher M., Gustafsson J. A. The dioxin and peroxisome proliferator-activated receptors: nuclear receptors in search of endogenous ligands. Trends Pharmacol Sci. 1992 Jun;13(6):241–245. doi: 10.1016/0165-6147(92)90076-i. [DOI] [PubMed] [Google Scholar]
- Pollenz R. S., Sattler C. A., Poland A. The aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator protein show distinct subcellular localizations in Hepa 1c1c7 cells by immunofluorescence microscopy. Mol Pharmacol. 1994 Mar;45(3):428–438. [PubMed] [Google Scholar]
- Pongratz I., Mason G. G., Poellinger L. Dual roles of the 90-kDa heat shock protein hsp90 in modulating functional activities of the dioxin receptor. Evidence that the dioxin receptor functionally belongs to a subclass of nuclear receptors which require hsp90 both for ligand binding activity and repression of intrinsic DNA binding activity. J Biol Chem. 1992 Jul 5;267(19):13728–13734. [PubMed] [Google Scholar]
- Pratt W. B. The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem. 1993 Oct 15;268(29):21455–21458. [PubMed] [Google Scholar]
- Probst M. R., Reisz-Porszasz S., Agbunag R. V., Ong M. S., Hankinson O. Role of the aryl hydrocarbon receptor nuclear translocator protein in aryl hydrocarbon (dioxin) receptor action. Mol Pharmacol. 1993 Sep;44(3):511–518. [PubMed] [Google Scholar]
- Schena M., Yamamoto K. R. Mammalian glucocorticoid receptor derivatives enhance transcription in yeast. Science. 1988 Aug 19;241(4868):965–967. doi: 10.1126/science.3043665. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Toft D. O. Steroid receptors and their associated proteins. Mol Endocrinol. 1993 Jan;7(1):4–11. doi: 10.1210/mend.7.1.8446107. [DOI] [PubMed] [Google Scholar]
- Swanson H. I., Bradfield C. A. The AH-receptor: genetics, structure and function. Pharmacogenetics. 1993 Oct;3(5):213–230. doi: 10.1097/00008571-199310000-00001. [DOI] [PubMed] [Google Scholar]
- Takahashi J. S. Circadian clock genes are ticking. Science. 1992 Oct 9;258(5080):238–240. doi: 10.1126/science.1384127. [DOI] [PubMed] [Google Scholar]
- Whitelaw M. L., Gustafsson J. A., Poellinger L. Identification of transactivation and repression functions of the dioxin receptor and its basic helix-loop-helix/PAS partner factor Arnt: inducible versus constitutive modes of regulation. Mol Cell Biol. 1994 Dec;14(12):8343–8355. doi: 10.1128/mcb.14.12.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw M. L., Göttlicher M., Gustafsson J. A., Poellinger L. Definition of a novel ligand binding domain of a nuclear bHLH receptor: co-localization of ligand and hsp90 binding activities within the regulable inactivation domain of the dioxin receptor. EMBO J. 1993 Nov;12(11):4169–4179. doi: 10.1002/j.1460-2075.1993.tb06101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw M., Pongratz I., Wilhelmsson A., Gustafsson J. A., Poellinger L. Ligand-dependent recruitment of the Arnt coregulator determines DNA recognition by the dioxin receptor. Mol Cell Biol. 1993 Apr;13(4):2504–2514. doi: 10.1128/mcb.13.4.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech H., Buchner J., Zimmermann R., Jakob U. Hsp90 chaperones protein folding in vitro. Nature. 1992 Jul 9;358(6382):169–170. doi: 10.1038/358169a0. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson A., Cuthill S., Denis M., Wikström A. C., Gustafsson J. A., Poellinger L. The specific DNA binding activity of the dioxin receptor is modulated by the 90 kd heat shock protein. EMBO J. 1990 Jan;9(1):69–76. doi: 10.1002/j.1460-2075.1990.tb08081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsson A., Whitelaw M. L., Gustafsson J. A., Poellinger L. Agonistic and antagonistic effects of alpha-naphthoflavone on dioxin receptor function. Role of the basic region helix-loop-helix dioxin receptor partner factor Arnt. J Biol Chem. 1994 Jul 22;269(29):19028–19033. [PubMed] [Google Scholar]
- Wright A. P., Carlstedt-Duke J., Gustafsson J. A. Ligand-specific transactivation of gene expression by a derivative of the human glucocorticoid receptor expressed in yeast. J Biol Chem. 1990 Sep 5;265(25):14763–14769. [PubMed] [Google Scholar]
- Wright A. P., Gustafsson J. A. Glucocorticoid-specific gene activation by the intact human glucocorticoid receptor expressed in yeast. Glucocorticoid specificity depends on low level receptor expression. J Biol Chem. 1992 Jun 5;267(16):11191–11195. [PubMed] [Google Scholar]