Abstract
Proper patterns of genome-wide DNA methylation, mediated by DNA methyltransferases DNMT1, -3A and -3B, are essential for embryonic development and genomic stability in mammalian cells. The de novo DNA methyltransferase DNMT3B is of particular interest because it is frequently overexpressed in tumor cells and is mutated in immunodeficiency, centromere instability and facial anomalies (ICF) syndrome. In order to gain a better understanding of DNMT3B, in terms of the targeting of its methylation activity and its role in genome stability, we biochemically purified endogenous DNMT3B from HeLa cells. DNMT3B co-purifies and interacts, both in vivo and in vitro, with several components of the condensin complex (hCAP-C, hCAP-E and hCAP-G) and KIF4A. Condensin mediates genome-wide chromosome condensation at the onset of mitosis and is critical for proper segregation of sister chromatids. KIF4A is proposed to be a motor protein carrying DNA as cargo. DNMT3B also interacts with histone deacetylase 1 (HDAC1), the co-repressor SIN3A and the ATP-dependent chromatin remodeling enzyme hSNF2H. Further more, DNMT3B co-localizes with condensin and KIF4A on condensed chromosomes throughout mitosis. These studies therefore reveal the first direct link between the machineries regulating DNA methylation and mitotic chromosome condensation in mammalian cells.
INTRODUCTION
Chromatin structure, at both the local and whole-chromosome levels, is determined by many factors. One of the key regulators of chromatin structure is DNA methylation. Methylation of cytosine within the CpG dinucleotide in mammalian cells is an epigenetic modification of the DNA that has been shown to be important for diverse processes such as transcriptional regulation, embryonic development, genomic imprinting and genome stability (1–4). DNA methylation patterns are established and maintained by three DNA methyltransferases (DNMTs): DNMT1, -3A and -3B (5,6). DNMT1 has traditionally been regarded as a maintenance methyltransferase specialized for copying DNA methylation patterns following DNA replication, whereas DNMT3A and DNMT3B are believed to be involved primarily with de novo methylation, particularly during embryonic development (3).
DNA methylation is not randomly distributed throughout the mammalian genome. Rather, it tends to be concentrated in transposable elements (parasitic DNA), repeats and pericentromeric heterochromatin, and contributes to the transcriptionally repressed, highly condensed chromatin structure characteristic of these regions (7–9). DNA methylation is also believed to suppress mitotic recombination events involving highly repetitive regions (10–12). It remains unclear exactly how DNA methylation is ‘targeted’ to select regions of the genome; however, emerging evidence indicates that other epigenetic modifications, particularly acetylation and methylation of the histone H3 and H4 N-terminal tails, determine which regions of the genome will be methylated. The core histone tails are extensively modified by an ever-growing number of histone acetyltransferases and histone methylases (13–15). Establishment and maintenance of regional DNA methylation patterns is most likely accomplished by protein–protein interactions involving the DNA methyltransferases and one or more chromatin-associated factors. For example, DNMT1, -3A and -3B interact with histone deacetylases (16–19). In fact, most regions of the genome enriched in DNA methylation are also enriched for histone H3 lysine 9 methylation and deacetylated core histones (2,20). Although the exact targeting mechanism still needs to be deciphered, it is clear from studies on transformed cells just how important proper DNA methylation patterns are for normal cellular functioning. Hypermethylation of CpG island promoter regions of tumor suppressor genes, resulting in their silencing, and hypomethylation of repetitive DNA elements, resulting in increased genomic instability, are hallmarks of tumor cells (4,21).
While the condensed, transcriptionally silent, recombination-resistant state of heterochromatin brought about and/or maintained by DNA methylation is essential for genome stability, other factors clearly play an important role. Not only must the genome be protected from chromosomal rearrangements, there must also be a mechanism to ensure that daughter cells receive the complete array of genetic information following mitosis and guard against aneuploidy (22). Two protein complexes, condensin and cohesin, have evolved to help ensure the fidelity of this process (23–25). Cohesin is a multi-subunit protein complex, involved in sister chromatid cohesion, that contains the SMC (structural maintenance of chromosomes)-1/SMC3 heterodimer at its core (23,24). The condensin complex is involved in condensing chromosomes at the onset of mitosis, and is composed of the SMC2/SMC4 (hCAP-E/hCAP-C) heterodimer, as well as the non-SMC proteins hCAP-G, hCAP-H and hCAP-D2/CNAP1 (23,26,27). During mitosis, condensin appears to act as an intramolecular DNA crosslinker, which utilizes the energy of ATP hydrolysis to introduce supercoils into DNA and promote condensation (25). Immunodepletion of condensin from an in vitro Xenopus egg extract condensation assay causes sperm chromatin to decondense, supporting a role for the condensin complex in this process (26). Interestingly, inhibition of DNA methylation by treatment of cells with the DNA methyltransferase inhibitor 5-azacytidine also results in dramatic decondensation of certain regions of mitotic chromosomes (28). In addition, individuals with ICF (immunodeficiency, centromere instability and facial anomalies) syndrome, arising from mutations in the DNMT3B gene, exhibit marked demethylation and decondensation of pericentromeric heterochromatin, primarily on chromosomes 1, 9 and 16, indicating a functional relationship between DNA methylation and chromosome condensation during mitosis (29–32).
Chromosome condensation is also important during interphase, where it occurs at transcriptionally silent, heterochromatic regions such as centromeres (9). Local regions of condensed chromatin are likely to be involved in gene regulation on small and large scales. Both condensin (or condensin subunits) and the DNA methyltransferases have been shown to play roles in smaller scale chromatin structural changes relevant to gene expression during interphase. For example, the DNA methyltransferases can repress transcription in a largely HDAC-dependent manner (16–19) and can interact with other components of repressive heterochromatin, such as heterochromatin protein 1 (HP1) and the histone H3 lysine 9 methylase SUV39H1 (20,33). Condensin subunits (or their homologs) also appear to have gene-regulatory roles via chromatin structural changes during interphase (25), such as regulating sex chromosome dosage compensation in Caenorhabditis elegans (34), boundary element function at the Saccharomyces cerevisiae mating-type locus (35), and cooperating with Polycomb-group proteins to maintain gene repression in Drosophila (36).
In this report we sought to gain a better understanding of the factors regulating regional DNA methylation patterns, as well as to examine possible connections between DNA methylation and chromosome condensation, by identifying proteins that interact with DNMT3B. To accomplish this, we have biochemically purified DNMT3B from HeLa cells and identified the associated polypeptides by mass spectrometry. DNMT3B exists in a complex with the hCAP-C/hCAP-E heterodimer of condensin, chromokinesin homolog KIF4A, ATP-dependent chromatin remodeling enzyme hSNF2H, histone deacetylase HDAC1 and transcriptional co-repressor SIN3A. DNMT3B interacts with the hCAP-C/hCAP-E heterodimer and KIF4A, both in vivo and in vitro, and DNMT3B co-localizes with condensin, KIF4A and hSNF2H on condensed chromosomes throughout mitosis. Co-immunoprecipitation of DNMT3B with hCAP-G demonstrates that DNMT3B can also interact with the condensin complex in addition to the free hCAP-C/hCAP-E heterodimer. These studies therefore provide the first evidence for a direct link between DNA methylation and chromosome condensation by showing that a DNA methyltransferase interacts directly with proteins that can affect both local and genome-wide chromosomal condensation events, and indicate that DNMT3B may have a previously unrecognized function during the mitotic phase of the cell cycle.
MATERIALS AND METHODS
DNMT3B complex purification from HeLa cells
Nuclear extract from HeLa S3 cells (80 ml cell pellet, National Cell Culture Center) was prepared using a high salt (1.8 M KCl) extraction method as described previously (37). HeLa high salt (HS) nuclear protein was dialyzed against buffer C [20 mM HEPES (pH 7.9), 1.5 mM MgCl2, 0.3 mM EDTA, 2 mM EGTA, 25% glycerol, 1 mM dithiothreitol (DTT), 0.2 mM phenylmethylsulfonylfluoride (PMSF) and 1 µg/ml leupeptin, aprotinin, pepstatin A] to 100 mM NaCl. Nuclear extract was loaded onto a DEAE–Sepharose column, washed with buffer A [20 mM HEPES (pH 7.9), 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 1 mM DTT, 1 mM PMSF, 10 mM β-glycerophosphate and 1 µg/ml leupeptin, aprotinin, pepstatin A] containing 100 mM NaCl, and step eluted with buffer A containing 300 mM, 600 mM and 1 M NaCl. Peak DNMT3B fractions (identified by western blotting) were pooled, dialyzed against buffer A to 100 mM NaCl and applied to a Mono S column (Amersham Pharmacia). The column was washed with 100 mM NaCl in buffer A, and elution was performed with a 20 ml linear gradient from 100 mM to 1.0 M NaCl in buffer A. Peak DNMT3B fractions were pooled, dialyzed to 100 mM NaCl in buffer A, and then fractionated on a HiTrap heparin–agarose column (Amersham Pharmacia) with a gradient of 100 mM to 1 M NaCl in buffer A. All buffers in this and subsequent purification steps contained 0.05% Triton X-100. DNMT3B was purified further using step elutions from Blue 4 dye (Sigma) and histone–agarose (Amersham Pharmacia) resin columns. After elution from the histone–agarose column, DNMT3B was run on a Sephacryl 300 gel filtration column in buffer A containing 150 mM NaCl, followed by separation on a Mono Q ion exchange column by gradient elution (Amersham Pharmacia). All general chemicals were purchased from Sigma and protease inhibitors were purchased from Roche Applied Science. Sizing of DNMT3B complexes from nuclear extract was carried out on a Superose 6 gel filtration column (Amersham Pharmacia) using buffer A (150 mM NaCl).
Protein in-gel digestion and nano-HPLC/mass spectrometry analysis for protein identification
The protein bands of interest were excised, destained, in-gel digested and extracted as described previously (38). High performance liquid chromatography (HPLC) tandem mass spectrometry was carried out with a LCQ Deca XP with nanospray source (ThermoFinnigan) coupled online with a nano-HPLC system (Agilent 1100 capillary pump; Agilent Technologies) and an in-house packed capillary C18 column (5–10 cm in length, 75 µm inside diameter). Two microliters of tryptic peptide solution were injected each time and peptides were eluted from the HPLC column with a 15-min gradient of 25–80% buffer B (acetonitrile:water:acetic acid 90:9.9:0.1 v/v/v) at a flow rate of 0.7 µl/min. From the tip of the capillary column, the eluted peptides were sprayed directly into the mass spectrometer for mass spectrometry analysis (39). The LCQ Deca XP was operated in data-dependent mode that included a full mass spectrometry scan from mass range 400 to 1500 (mass:charge ratio) followed by three collision-induced dissociations of the peptide ions with the most intensity at a normalized collision energy level of 35%. Dynamic exclusion for the selected peptide ions was enabled to increase peptide coverage. The resulting spectra were searched for protein candidates in the National Center for Biotechnology Information non-redundant database with Mascot (Matrix Science).
Antibodies and western blots
DNMT3B was detected using an epitope affinity-purified rabbit polyclonal antiserum (No. 79/80) raised against a peptide located at DNMT3B amino acids 398–417 (Research Genetics). DNMT1 rabbit polyclonal antiserum was generated from a recombinant, bacterially expressed fragment of human DNMT1 (No. 1020, amino acids 914–1087). KIF4A was detected in western blots using rabbit polyclonal antiserum raised against a bacterially expressed fragment of KIF4A (amino acids 859–1232). Affinity-purified rabbit polyclonal hCAP-C and hCAP-E antibodies have been described previously (40). Rabbit polyclonal antibodies were raised against a recombinant polypeptide expressed in Escherichia coli from the middle region (amino acids 235–570) of hCAP-G. The hCAP-G antibody was affinity purified using the same peptide expressed as a glutathione S-transferase (GST) fusion protein, crosslinked to glutathione–Sepharose beads (Amersham Pharmacia). Other antibodies were purchased from commercial suppliers as follows: anti-SIN3A K-20 from Santa Cruz Biotechnology, anti-hCAP-C from Upstate Biotechnology, anti-HDAC1 from Affinity BioReagents, and anti-hSNF2H H-300 from Santa Cruz Biotechnology.
Immunoprecipitations
Immunoprecipitations were performed using the affinity-purified polyclonal anti-DNMT3B (No. 79/80) antibody with HeLa high salt nuclear extract. Irrelevant antibody immunoprecipitation controls were done with species-matched control antibodies purchased from Santa Cruz Biotechnology [rabbit anti-c-Fos K-25, full-length (FL) rabbit anti-GFP (green fluorescent protein) or normal immunoglobulin G (IgG)]. Immunoprecipitations for western blotting and DNA methyltransferase assays were performed using standard methods, in which the nuclear extract buffer was adjusted to 50 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 0.5% NP-40. In each case, 200 µl (800 µg) of nuclear extract was first pre-cleared with protein A/G plus agarose beads (Santa Cruz Biotechnology), incubated with 20 µl of antibody for 2 h at 4°C with rotation, followed by incubation with 20 µl protein A/G plus agarose for 2 h at 4°C with rotation. Washing was performed five times in 500 µl buffer [50 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 0.5% NP-40] at 4°C, for 5 min each on a rotator.
Enzymatic assays
DNA methyltransferase assays were performed as described previously (18) using poly d(I-C) (Roche Applied Science) and S-adenosyl-l-[3H]methyl)-methionine (Amersham Pharmacia) as the methyl donor. Histone deacetylase activity was determined as described previously (18). ATPase activity was determined as described previously in (41) using purified HeLa core histones as the substrate (42).
Plasmid construction
All expression vectors were generated by PCR using standard methods. PCRs were carried out with Pfu DNA polymerase according to the manufacturer’s instructions (Stratagene). The Dnmt3b1, hCAP-C, hCAP-E and KIF4A constructs were generated using their respective cDNA plasmids as templates. Dnmt3b1 primers were: (sense) 5′-GAT CTG AAT TCA TGA AGG GAG ACA GCA GAC ATC-3′ and (antisense) 5′-CAT GGG ATC CCT ATT CAC AGG CCA AGT AGT CC-3′ for FL Dnmt3b1; (sense) 5′-GAT CTG AAT TCA TGA AGG GAG ACA GCA GAC ATC-3′ and (antisense) 5′-GAT CGG ATC CCT AGA CTC TAA TGG GCC TCC TTT TGG C-3′ for Dnmt3b1 (1–583); and (sense) 5′-GAT CGA ATT CAT GCT GGA AGA ATT TGA GCC ACC C-3′ and (antisense) 5′-CAT GGG ATC CCT ATT CAC AGG CCA AGT AGT CC-3′ for Dnmt3b1 (560–860). PCR products were digested with EcoRI and BamHI and cloned into the relevant sites of pcDNA3.1 (Invitrogen) or pEGFP-C1 (Clontech) to create a fusion with GFP, or pGEX5X-1 to create a fusion with the GST protein for expression in E.coli. KIF4A was amplified with primers (sense) 5′-GAT CAA GCT TCA TGA AGG AAG AGG TGA AGG GAA TT-3′ and (antisense) 5′-GAT CGT CGA CTC AGT GGG CCT CTT CTT CGA TAG G-3′, hCAP-C with primers (sense) 5′-GAT CGA ATT CAT GCC CCG TAA AGG CAC CCA G-3′ and (antisense) 5′-GAT CGT CGA CTC AAC AAA GTC CCT TAG ATG CAA T-3′, and hCAP-E with primers (sense) 5′-GAT CGA ATT CAT GCA TAT TAA GTC AAT TAT TCT AG-3′ and (antisense) 5′-GAT CGT CGA CTT AAA CTT CCA CAT GTG CTC CTT TG-3′. The KIF4A PCR product was digested with HindIII and SalI, and the hCAPs were digested with EcoRI and SalI, then cloned into the same sites of pcDNA3.1(+) (Invitrogen) for use in in vitro transcription/translation reactions. All plasmids were confirmed by DNA sequencing.
Immunofluorescence microscopy
HeLa cells were grown in McCoy’s 5A media containing 10% fetal bovine serum (FBS) and 2 mM l-glutamine at 37°C. Transfections for immunofluorescence studies were performed by first plating 1–2 × 105 cells on 22-mm square glass coverslips in six-well plates for ∼24 h. Transfection was carried out using LT1 transfection reagent according to the manufacturer’s instructions (Mirus Corporation). Cells were processed for immunofluorescence microscopy after 24–48 h. HeLa cells were fixed with either 2% paraformaldehyde in phosphate-buffered saline (PBS) or with cold 100% methanol (–20°C), and permeabilized using 0.5% Triton X-100 in PBS. For mitotic cells, an additional digitonin treatment (0.005% in 1× PBS for 5 min) was performed before fixation. Fixed cells were incubated with primary antibody for 1 h, followed by the appropriate fluorescently labeled secondary antibody (1:300 dilution) for 1 h. Nuclei were stained using Hoechst 33342 (Sigma) and the coverslips mounted onto glass slides using Fluoromount-G mounting media (Southern Biotechnology). The following antibodies were used in this study: mouse anti-KIF4A (1:20 dilution), rabbit anti-hCAP-G (1:200), rabbit anti-hCAP-C (1:200), goat anti-hCAP-C T-18 (1:20; Santa Cruz Biotechnology), rabbit anti-HDAC1 (1:20; Affinity BioReagents) and rabbit anti-SIN3A K-20 (1:20). Secondary antibodies utilized include TRITC-conjugated anti-mouse and anti-rabbit antibody, and Texas Red-conjugated anti-goat antibody (1:300 dilutions; Southern Biotechnology). Images were collected on an IX70 inverted microscope equipped with a 100× 1.35 NA oil immersion objective (Olympus), and a charge-coupled device camera (Photometrics) configured at 0.07 µm pixels. Images were deconvolved with the SoftWoRx software package (Applied Precision) using Decon3d. The KIF4A antibody used for immunofluorescence was raised to a GST fusion protein (human KIF4A amino acids 996–1232) that was used as antigen to inject BALB/c mice to make the monoclonal KIF4AM2 antibody. For immunostaining, the KIF4AM2 culture supernatant was used at a dilution of 1:20. The SIN3A and HDAC1 antibodies did not work on mitotic chromosomes, most likely due to blocking of the epitope, as has been described previously for other proteins localizing to mitotic chromosomes (43).
Cell synchronization
HeLa cells were synchronized as described previously (44). Briefly, HeLa cells were plated on coverslips in a six-well plate and grown overnight. Cells were transfected (as described above) for 24 h, after which they were subjected to a single thymidine block. Cells were treated with 2.5 mM thymidine for 15 h and subsequently washed and allowed to recover in complete medium. After 8–9 h, cells were fixed with either 2% paraformaldehyde or 100% methanol (–20°C) and subjected to immunostaining as described above. This single thymidine block resulted in a significant enrichment of mitotic cells (∼40% of the cells plated were in M-phase).
GST pull-downs
All cDNAs for protein production were cloned into the GST fusion expression vector pGEX5X-1 as described above. Escherichia coli BL21 (DE3) Codon Plus-RP cells were utilized for protein production according to the manufacturer’s instructions (Stratagene). GST fusion proteins were prepared as described in (45). GST pull-downs were performed essentially as described in (18), using 5–10 µg of GST fusion protein and 3 µl of 35S-labeled in vitro transcribed/translated protein (made using the Promega TNT T7 Quick Coupled kit). Binding buffer is 60 mM Tris (pH 7.5), 200 mM NaCl, 0.67% NP-40 and 1.25 mM EDTA. After binding, unbound proteins were removed with six washes of the same buffer used for the binding reaction. Sample buffer was added to the beads and the specifically bound proteins were analyzed by SDS–PAGE followed by autoradiography.
Chromatin immunoprecipitation
The CCL256.1 lymphoblastoid cell line, purchased from the American Type Culture Collection, was used for chromatin immunoprecipitation (ChIP) analysis. Cells were maintained in RPMI-1640 medium with 10% FBS (Invitrogen). Cells were pelleted and washed with 1× PBS (pH 7.5). ChIP was performed as described previously with minor modifications (46,47). Cells were washed in RSB buffer [10 mM Tris (pH 7.5), 10 mM NaCl, 5 mM MgCl2], incubated on ice in RSB + 0.1% NP-40 for 10 min, and the nuclei were isolated by centrifugation. Nuclei were resuspended in RSB + 1% formaldehyde, incubated at room temperature for 10 min, followed by the addition of glycine to 125 mM final concentration. Nuclei were resuspended in SDS lysis buffer [1% SDS, 10 mM EDTA, 50 mM Tris (pH 8.0)] and sonicated. Debris was removed and soluble chromatin was diluted 10-fold in ChIP dilution buffer [0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris (pH 8.0), 167 mM NaCl]. ChIP reagents were purchased from Upstate Biotechnology and the assay was performed essentially according to the manufacturer’s protocol. The only exceptions were that 80 µl of salmon sperm DNA–protein agarose was used for pre-clearing and immunoprecipitation, and 20 µl of each antibody was used. Antibodies used include normal rabbit IgG (Santa Cruz Biotechnology) and the affinity-purified rabbit anti-DNMT3B (No. 79/80) antibody described above. ChIP-compatible antibodies were raised against synthetic peptides (two for each protein, co-injected) in rabbits for KIF4A (N-KEEVKGIPVRVALRC-C and N-CKHVATEYQENKA PGKK-C; Affinity BioReagents), hCAP-C (N-PRKGTQ PSTARRREEGC-C and N-CTKSVAVNPKEIASKG-C; Affinity BioReagents) and hSNF2H (N-CWIEPPKRERK ANYAVDAYF-C and N-CGTDRENVYEELRQSVRNAP-C; Bio-Synthesis, Inc.). Antibodies were tested and shown to immunoprecipitate the desired protein and to recognize a single band of the expected size in western blotting (not shown). After washing, complexes were eluted and cross-links reversed overnight at 65°C. Samples were treated with proteinase K, then were phenol/chloroform extracted and ethanol precipitated. PCR was performed in a 50 µl reaction under standard conditions with the following primers: satellite 2 repeat, (sense) 5′-ATG GAA ATG AAA GGG GTC ATC ATC T-3′ and (antisense) 5′-ATT CGA GTC CAT TCG ATG ATT CCA T-3′; the housekeeping gene GAPDH (sense) 5′-TCG GTG CGT GCC CAG TTG AAC C-3′ and (antisense) 5′-ATG CGG CTG ACT GTC GAA CAG GAG-3′; alu repeat (sense) 5′-GTA ATA GTG ATA ATT CTA TCC AAA GCA-3′ and (antisense) 5′-GAA TCT TGT TTT GAG GCA TAT AA-3′; and rDNA repeat (sense) 5′-CCT TCC ACG AGA GTG AGA AGC-3′ and (antisense) 5′-TCG ACC TCC CGA AAT CGT ACA-3′.
RESULTS
Purification of endogenous DNMT3B from HeLa cells reveals a novel group of DNMT3B-interacting proteins
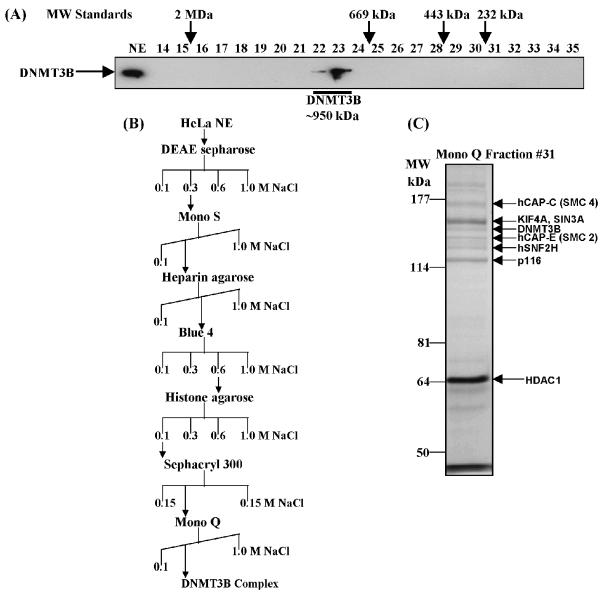
In order to understand better the functions of DNMT3B in terms of the regulation of its enzymatic activity and how it may be targeted to certain regions of the genome, we purified it from HeLa cell nuclear extract and identified and characterized the associated enzymatic activities and polypeptides. Before embarking on a large-scale purification of DNMT3B, we sought evidence that DNMT3B is indeed part of a larger protein complex. Figure 1A shows that endogenous DNMT3B from HeLa nuclear extract, when fractionated over a gel filtration sizing column, runs at a molecular weight of ∼950 kDa. Given that the DNMT3B monomer runs at ∼100 kDa (not shown), this finding supports the notion that DNMT3B is present in one or more multi-subunit protein complex in HeLa cells.
Figure 1.
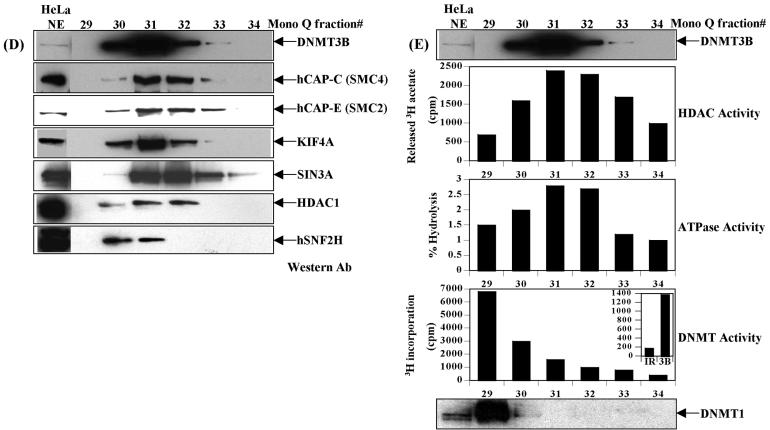
Purification of a novel DNMT3B complex from HeLa cells. (A) Endogenous DNMT3B in HeLa nuclear extract (NE) migrates at a molecular weight of ∼950 kDa on a Superose 6 gel filtration column. The position of the size standards and column fraction numbers are shown along the top. Western blotting was performed with an epitope affinity-purified rabbit polyclonal anti-DNMT3B antibody. (B) Purification scheme for the DNMT3B complex. (C) Silver staining of the peak fraction of DNMT3B (detected by western blotting) from the final Mono Q purification step. Protein bands were excised from the gel and identified by mass spectrometry and/or western blotting, as described in Materials and methods. (D) Western blot analysis of the Mono Q column fractions across the peak of DNMT3B. Antibodies used in the western analysis are shown at the right of the figure. Fraction 31 was used for the mass spectrometry identification of DNMT3B co-purifying proteins. (E) Enzymatic activities co-purifying with the HeLa DNMT3B complex. Column fractions across the DNMT3B peak were screened for HDAC activity, ATPase activity (ATP hydrolysis stimulated by the addition of purified core histones) and DNA methyltransferase activity [with poly d(I-C) as the DNA substrate]. The inset shows the DNA methyltransferase activity from an immunoprecipitation with anti-DNMT3B antibody (‘3B’) demonstrating that the DNMT3B complex possesses DNA methyltransferase activity. ‘IR’ is an irrelevant antibody control (normal rabbit IgG). In the bottom panel, column fractions were probed for DNMT1 by western blotting.
We next employed the biochemical fractionation strategy shown in Figure 1B to purify a DNMT3B complex, beginning with 80 l of HeLa S3 cells. Once the DNMT3B complex was biochemically pure, co-purifying polypeptides were excised from the gel and identified by mass spectrometry. With the exception of one polypeptide (p116), all co-purifying bands were identified (Fig. 1C). Co-purifying polypeptides included hCAP-C (SMC4), KIF4A, SIN3A, hCAP-E (SMC2), hSNF2H and HDAC1. hCAP-C and hCAP-E have been shown to exist as a free heterodimer as well as forming the core of a protein complex known as condensin (48). In the condensin complex, hCAP-C/hCAP-E interact with three other proteins: hCAP-G, hCAP-H and hCAP-D2/CNAP1 (23,26,27). Condensin is involved in the chromosome-wide condensation events that take place at the onset of mitosis (24,26,49). hSNF2H is an ATP-dependent chromatin remodeling factor present in a number of protein complexes, such as NURF, ACF and CHRAC (50). KIF4A is a poorly characterized kinesin-like motor protein of the chromokinesin subfamily. It is believed to carry DNA as its cargo and be involved in mitosis (51). SIN3A is a transcriptional co-repressor that is often found in protein complexes containing histone deacetylases (52). The band identified as HDAC1 is significantly darker than the other bands in the silver-stained gel due to the presence of a contaminating protein running at the same molecular weight, but not co-purifying with DNMT3B (data not shown). Given the novelty of our finding that DNMT3B co-purifies with components of the mitotic chromosome condensation machinery, the remainder of the present paper will focus on understanding and characterizing these interactions further. Additional characterization of the interactions between DNMT3B and hSNF2H and HDAC1 are described elsewhere (53).
In order to confirm the protein identifications made by mass spectrometry, we obtained antibodies to each of the proteins shown in Figure 1C and used them in western blotting of the column fractions surrounding the peak of DNMT3B. In all cases we were able to demonstrate that the proteins identified by mass spectrometry co-purified with DNMT3B (Fig. 1D). Consistent with the presence of HDAC1 in the DNMT3B-containing fractions, we found that histone deacetylase activity co-purified precisely with the peak of DNMT3B (Fig. 1E, upper graph). DNMT3B also co-purified with ATPase activity, stimulated by the addition of purified core histones to the reaction (Fig. 1E, middle graph). This result is consistent with the co-purification of DNMT3B with three proteins (hSNF2H, hCAP-C and hCAP-E) that are known to bind and hydrolyze ATP (25,50). Finally, we tested the DNMT3B-containing fractions for DNA methyltransferase activity. Low-level DNMT activity was detectable in fraction 31, the peak DNMT3B fraction by western blotting; however, the majority of activity did not co-purify with DNMT3B (Fig. 1E, lower graph). Interestingly, the major peak of DNMT activity in these fractions was due to an adjacent but clearly separate DNMT1-containing complex, shown using an antibody against DNMT1 (Fig. 1E, bottom panel). The characterization of this complex will be the subject of future studies. Immunoprecipitation with an anti-DNMT3B antibody (Fig. 1E, lower graph inset), however, demonstrates that endogenous DNMT3B is associated with DNA methyltransferase activity. These data support the notion that the DNMT3B complex we have purified possesses DNA methyltransferase activity.
DNMT3B co-immunoprecipitates with components of the chromosome condensation machinery
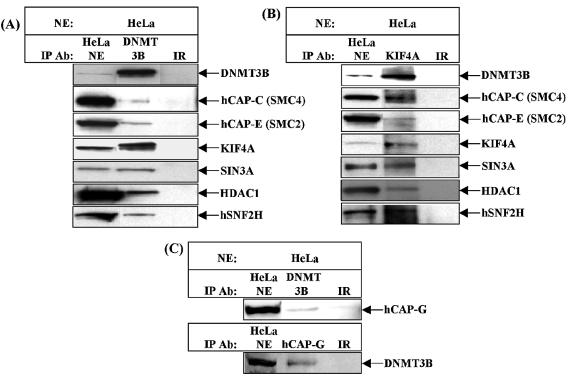
In order to confirm the interactions identified in the biochemical fractionation, we employed co-immunoprecipitation of endogenous proteins from HeLa nuclear extract. Epitope affinity-purified anti-DNMT3B antibody was capable of immunoprecipitating hCAP-C, hCAP-E, KIF4A, SIN3A, HDAC1 and hSNF2H (Fig. 2A). In a reciprocal experiment, an anti-KIF4A antibody efficiently immunoprecipitated DNMT3B, hCAP-C, hCAP-E, SIN3A, HDAC1 and hSNF2H from HeLa nuclear extract (Fig. 2B). The Xenopus homolog of KIF4A, Xklp1, has been reported to be a component of mitotic chromosomes and is essential for mitosis in Xenopus (43). To our knowledge, this is the first demonstration that KIF4A interacts with components of the human condensin complex.
Figure 2.
Confirmation of the DNMT3B protein interactions identified in the biochemical purification by co-immunoprecipitation. (A) Anti-DNMT3B antibody was used as the immunoprecipitating antibody to identify DNMT3B-interacting proteins from HeLa nuclear extract. Immunoprecipitation reactions were then probed with the antibodies shown at the right of the panel by western blotting. (B) Use of a rabbit polyclonal antibody to KIF4A in an immunoprecipitation from HeLa nuclear extract confirms all interactions in (A). (C) The hCAP-C/hCAP-E heterodimer can exist free or in the condensin complex. The condensin complex also contains hCAP-D2/CNAP1, hCAP-H and hCAP-G. hCAP-G can be reciprocally co-immunoprecipitated with DNMT3B, indicating that DNMT3B also interacts with the condensin complex in HeLa cells. In each case, the input (HeLa NE) and an irrelevant antibody control (species-matched normal IgG) were run on the same gels.
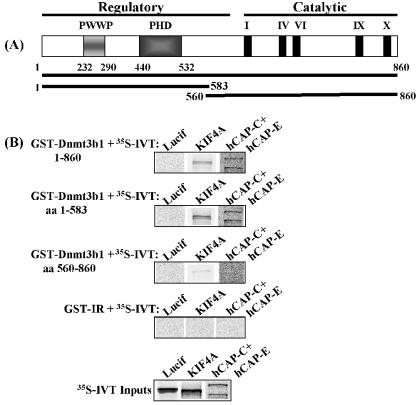
The hCAP-C/hCAP-E heterodimer has been shown to exist as free heterodimer or as the ‘core’ component of the condensin complex (48). Although we did not detect other components of the standard condensin complex (non-SMC proteins) in our DNMT3B-containing fractions, we sought to determine whether DNMT3B interacts only with free hCAP-C/hCAP-E heterodimer or with the condensin complex in HeLa cells. To accomplish this, we utilized an antibody against the hCAP-G subunit of condensin as a marker of the capacity of DNMT3B to interact with condensin. It has been shown that the components of the condensin complex interact with each other throughout the cell cycle regardless of their nuclear/cytoplasmic localization, and that antibodies against hCAP-G efficiently immunoprecipitate the entire condensin complex [(48,54) and J. A. Schmiesing and K. Yokomori, unpublished data]. Endogenous DNMT3B co-immunoprecipitated hCAP-G, and in a reciprocal experiment, an hCAP-G antibody co-immunoprecipitated DNMT3B (Fig. 2C). Therefore DNMT3B is clearly capable of interacting with the condensin complex in addition to the hCAP-C/hCAP-E heterodimer. Loss of a subset of the proteins associated with DNMT3B during the purification may be the reason we did not identify other non-SMC condensin components in the biochemical purification. Alternatively, the DNMT3B–condensin interactions we have identified may represent a novel condensin-like complex. Finally, we utilized a GST pull-down assay to determine if the N-terminal regulatory or the C-terminal catalytic domain of DNMT3B (Fig. 3A) mediates the interaction with the hCAPs and KIF4A. Figure 3B demonstrates that full-length versions of DNMT3B (as a GST fusion protein), in vitro-transcribed and co-translated 35S-labeled hCAP-C/hCAP-E and KIF4A readily interact in vitro, and also reveals that the N-terminal regulatory domain of DNMT3B is responsible for these interactions.
Figure 3.
DNMT3B binds to the hCAP-C/hCAP-E heterodimer and KIF4A in vitro in a GST pull-down assay. (A) Schematic structure of DNMT3B showing the extent of the two deletion mutants used in the interaction domain mapping study. (B) The GST fusion proteins indicated at the upper left of each panel were incubated with in vitro-transcribed and -translated 35S-labeled proteins (IVT), then washed extensively. An irrelevant GST fusion (‘GST-IR’) served as a control for non-specific binding to the IVT proteins, while IVT–luciferase served as a control for non-specific binding to GST–DNMT3B proteins. The IVT inputs used in the GST pull-down are shown in the bottom panel.
Co-localization of DNMT3B and its associated proteins in interphase HeLa cells
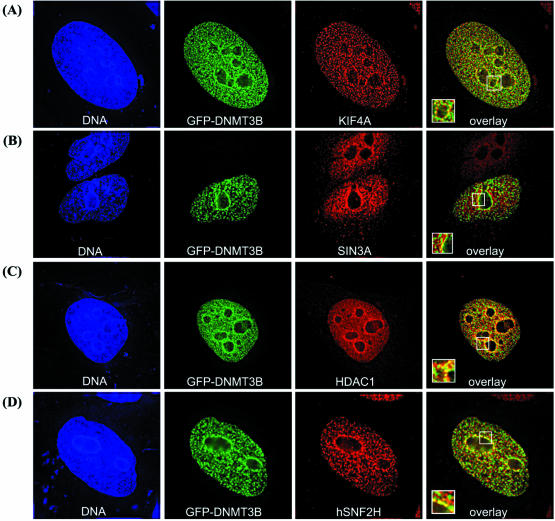
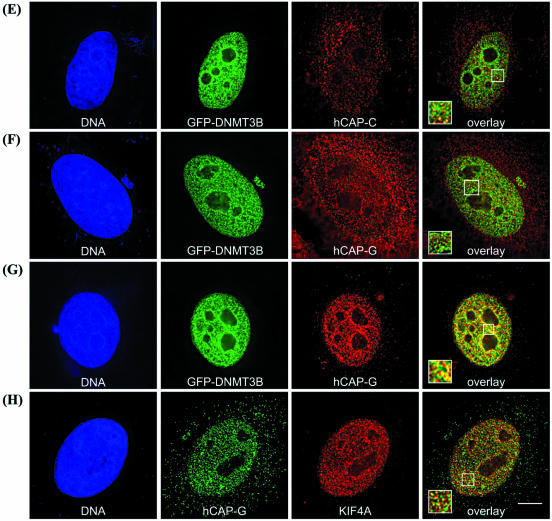
To gain additional support for the interactions identified biochemically, we first examined the cellular distribution of DNMT3B-interacting proteins (KIF4A, SIN3A, HDAC1, hSNF2H, hCAP-C and hCAP-G) in interphase cells using immunofluorescence microscopy. DNMT3B was visualized by fusion with GFP. The localization of GFP–DNMT3B was essentially indistinguishable from endogenous DNMT3B, as detected using affinity-purified rabbit polyclonal antibody (data not shown), indicating that the GFP tag does not interfere with normal DNMT3B distribution. DNMT3B co-localizes extensively with KIF4A in interphase cells, in both DAPI-dense areas indicative of heterochromatin (surrounding the nucleolus for example) and the nucleoplasm (Fig. 4A). A significant fraction of DNMT3B also co-localizes with SIN3A, HDAC1 and hSNF2H, particularly in heterochromatin domains (Fig. 4B–D). Much of the condensin complex resides in the cytoplasm during interphase, although studies have also clearly demonstrated that some condensin remains in the nucleus during interphase (27,54). Consistent with these studies, we find that a major portion of hCAP-C (Fig. 4E), and hCAP-G (Fig. 4F) localizes to the cytoplasm. Nuclear staining is also readily visible in these cells, and a fraction of the nuclear hCAP-C and hCAP-G clearly co-localizes with DNMT3B (Fig. 4E and F). In order to ‘enhance’ the nuclear hCAP-G staining, cells were treated with digitonin before immunostaining, which removes most of the cytoplasmic proteins but does not disrupt the nuclear envelope. This treatment allowed for greatly enhanced detection of the fluorescent signal for hCAP-G in the nucleus, and also revealed that nuclear hCAP-G (condensin) co-localized with DNMT3B in interphase nuclei (Fig. 4G). Similar findings were obtained with hCAP-C (data not shown). KIF4A and hCAP-G were also highly co-localized in interphase nuclei (Fig. 4H).
Figure 4.
Immunofluorescence localization of DNMT3B-associated proteins in interphase HeLa cells. DNMT3B (as a GFP fusion) co-localizes extensively with KIF4A (A). GFP–DNMT3B co-localization with SIN3A (B), HDAC1 (C), hSNF2H (D), hCAP-C (E) and hCAP-G (F) in interphase cells. Note that much of the hCAP-C and hCAP-G localizes to the cytoplasm during interphase, but a fraction of the nuclear hCAP-C/hCAP-G clearly co-localizes with DNMT3B. In order to enhance the nuclear condensin immunofluorescence signal (monitored by hCAP-G staining), HeLa cells were treated with digitonin to remove cytoplasmic proteins. This treatment allowed for better visualization of nuclear hCAP-G, and revealed that hCAP-G and DNMT3B co-localize extensively (G). Co-localization of endogenous hCAP-G (green) and KIF4A (red) in interphase nuclei (H). The left panel of each row is the Hoechst 33342 DNA stain (blue), and the middle two panels show the localization of GFP–DNMT3B (green) and the indicated proteins (red). In (H), hCAP-G is shown in green and KIF4A is in red. The two images are merged in the right-most panel, where yellow color represents co-localization. Boxed areas are enlarged and shown in the lower-left corner of each overlay panel. The scale bar corresponds to 5 µm.
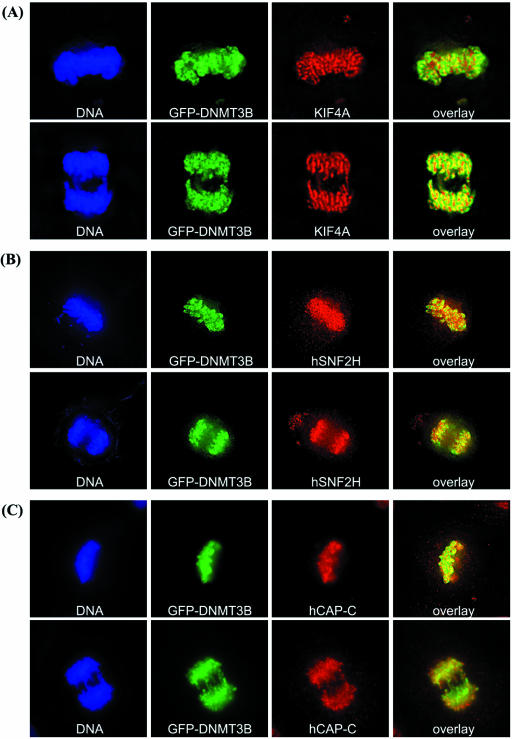
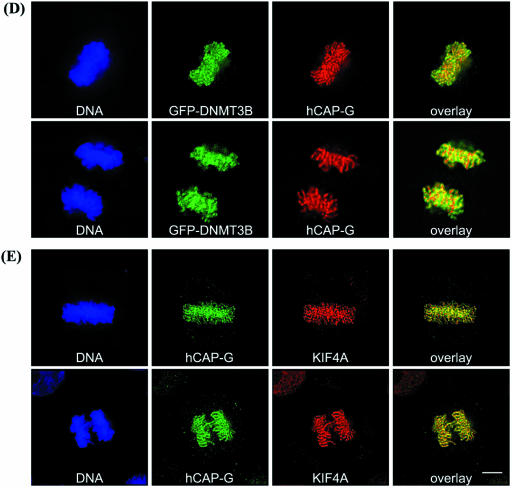
DNMT3B co-localizes with the condensin complex, KIF4A and hSNF2H on condensed chromosomes throughout mitosis
Given the clearly defined role of condensin during mitosis, we next studied the localization of DNMT3B, KIF4A, hSNF2H and condensin during this phase of the cell cycle. HeLa cells were transfected with GFP–DNMT3B, then synchronized using a thymidine block to enrich for cells in mitosis (44). Using this method, we found that DNMT3B localized exclusively to condensed chromosomes during all phases of mitosis [Fig. 5, representative cells in metaphase (upper panels) and anaphase (lower panels) are shown]. Antibodies specific for KIF4A, hSNF2H, hCAP-C and hCAP-G were then utilized to determine the localization of endogenous proteins. Interestingly, KIF4A (Fig. 5A), hSNF2H (Fig. 5B), hCAP-C (Fig. 5C) and hCAP-G (Fig. 5D) co-localized with DNMT3B on condensed mitotic chromosomes. Therefore the immunofluorescence co-localization data are entirely consistent with the biochemical purification and co-immunoprecipitation data, and support our findings that DNMT3B interacts with components of the mitotic chromosome condensation machinery. KIF4A and hCAP-G also co-localized very well in HeLa cells (Fig. 5E). We were unable to perform immunofluorescence localization for SIN3A and HDAC1 in mitotic cells because the epitope for these antibodies appeared to become blocked on condensed chromosomes. It has recently been shown, however, that both SIN3A and HDAC1 are localized to condensed chromosomes during mitosis (55), further supporting our findings.
Figure 5.
DNMT3B localizes to condensed chromosomes throughout mitosis, and co-localizes with condensin, KIF4A and hSNF2H. HeLa cells were transfected with GFP–DNMT3B, synchronized using a thymidine block, then released. When cells were entering mitosis, they were fixed and stained for endogenous KIF4A (A), hSNF2H (B), hCAP-C (C), hCAP-G (D), and KIF4A and hCAP-G (E). Representative cells from metaphase (upper panels) and anaphase (lower panels) are shown. The left panel is the DNA stain (blue), the middle two panels show the localization of GFP–DNMT3B (green) and the indicated protein (red), and the right panel is an overlay of the green and red channels. In (E), hCAP-G staining is in green and KIF4A staining is in red.
Binding of DNMT3B complex components to repetitive DNA in vivo
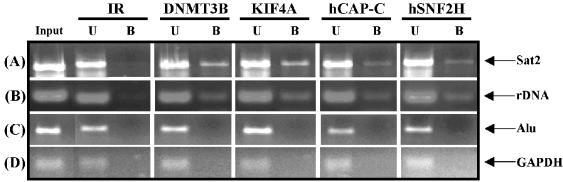
To examine whether DNMT3B and its associated proteins bind to sequences that are known to be methylated by DNMT3B, we utilized ChIP. Proteins associated with chromatin were fixed with formaldehyde, then precipitated with antibodies specific to DNMT3B, KIF4A, hCAP-C and hSNF2H. The resulting specifically bound DNA fragments were then analyzed by semi-quantitative PCR with primers specific for satellite 2 (sat2) and the rDNA repeats, two regions whose methylation and transcriptional repression, respectively, are known to be regulated by DNMT3B (30–32,56). We could readily detect binding of DNMT3B, KIF4A, hCAP-C and hSNF2H to both sat2 and rDNA (Fig. 6A and B). We also used primers amplifying an alu repeat, a high copy-number repetitive element whose methylation status appears to be regulated by both DNMT3A and DNMT3B (57); however, we did not detect binding of any of the four proteins tested. As a negative control, we used primers specific for the GAPDH housekeeping gene. As expected, neither DNMT3B nor its interaction partners were detected. These results therefore further support our interaction data and demonstrate that the DNMT3B complex we have identified binds to known DNMT3B target sequences in vivo.
Figure 6.
DNMT3B and its associated proteins bind to similar repetitive sequences in vivo. ChIP assays were carried out on formaldehyde-fixed chromatin isolated from the CCL256.1 lymphoblastoid cell line. Following the washes, cross-links were reversed and the purified DNA was analyzed by PCR with primers specific for (A) satellite 2 repeat, (B) rDNA repeat, (C) alu repeat and (D) the GAPDH housekeeping gene. For each antibody, PCRs were set up for the input, unbound ‘U’ and specifically bound ‘B’ fractions. Antibodies used in the ChIP are shown along the top of the panel. Control ChIP reactions were performed with species-matched normal IgG. Aliquots of PCRs were run on a 3% agarose gel and visualized with ethidium bromide.
DISCUSSION
In the present paper, we have used biochemical fractionation methods to purify endogenous DNMT3B and its associated proteins from HeLa nuclear extract. Similar to DNMT1 and DNMT3A, we find that DNMT3B also interacts with HDAC1, indicating that the interaction between these two classes of epigenetic modification systems is highly conserved. Our results also link together the DNA methylation and ATP-dependent chromatin remodeling machineries by demonstrating that DNMT3B interacts with hSNF2H. Interestingly, we also identified components of the mitotic chromosome condensation machinery as DNMT3B-interacting factors. The core component of the condensin complex (hCAP-C/hCAP-E) and KIF4A, which has been shown to associate with mitotic chromosomes and be essential for mitosis in Xenopus (43), interact with DNMT3B both in vivo and in vitro. Previous studies have revealed that the Xenopus homolog of hSNF2H (ISWI) is also a component of mitotic chromosomes in Xenopus (58), and hSNF2H interacts with the related cohesin complex, which is involved in sister chromatin cohesion during mitosis (59). To our knowledge, this is the first demonstration that hSNF2H localizes to mitotic chromosomes by immunofluorescence. Finally, we show that the co-repressor SIN3A interacts with DNMT3B. Given that the most well characterized role for condensin is its involvement in condensing chromosomes at the onset of mitosis, we examined the localization of DNMT3B during mitosis (49,60). DNMT3B co-localized well with condensin and KIF4 on condensed chromosomes throughout mitosis, providing further support for the interactions identified biochemically. DNMT3B and its interacting proteins identified here also bind to known DNMT3B ‘target’ sequences in vivo. These studies therefore provide the first direct link between a DNA methyltransferase and the machinery involved in chromatin structure maintenance, and suggest a novel role for DNMT3B during the mitotic phase of the cell cycle.
A number of indirect links between DNA methylation/DNA methyltransferases and the regulation of chromatin structure on both small and large scales has been reported. ICF syndrome provides an interesting example. ICF patients display severe hypomethylation of pericentromeric satellite repeats on chromosomes 1 and 16, and to a lesser extent 9. These same regions also display marked decondensation during mitosis, resulting in chromosome breakage within the pericentromeric regions, as well as rearrangements resulting in whole-arm deletions or gains and the formation of multi-radial chromosomes (29). The multi-radials may be formed by unresolved Holliday junctions, and the crosslinks may lead to breaks and missegregation at anaphase when the spindle fibers begin to pull on the crosslinked chromatids (29). Treatment of cells with the DNA methylation inhibitor 5-aza-2′-deoxycytidine, which covalently traps DNA methyltransferases on DNA during the methyltransfer reaction, also induces chromosomal decondensation at the same regions affected in ICF syndrome, as well as other regions (28). Interestingly, mutations in condensin subunits not only negatively affect the degree of chromosome condensation during mitosis, but have also been shown to result in severe chromosome segregation defects, such as the formation of chromatin bridges during anaphase, indicating that condensin is also important in sister chromatid resolution (61,62). Most studies of the chromosomal defects in ICF syndrome have focused on the loss of DNA methylation from pericentromeric regions as the prime cause of the defects. Our findings, demonstrating a direct link between the DNA methyltransferase mutated in ICF syndrome (DNMT3B) and the mitotic chromosome condensation and segregation machinery, open up other possible mechanisms to explain some of the ICF syndrome-specific chromosomal defects. Mutations in DNMT3B may alter its ability to interact with condensin and KIF4A. If DNMT3B is required for proper localization or function of condensin and KIF4A in pericentromeric regions, then this may help to explain the formation of multi-radial chromosomes in this disease. Since we found that DNMT3B is co-localized with condensin and KIF4A over all chromosomes, the chromosome 1 and 16 pericentromeric regions may be specifically targeted in ICF cells because they are more susceptible to minor perturbations in condensin than other regions of the genome, perhaps due to the large size of the repetitive regions in these chromosomes. Monitoring the integrity of DNMT3B, condensin and KIF4A in ICF syndrome cells and tumor cells will be the subject of future studies.
Several additional interesting connections between DNA methyltransferases and chromatin condensation have been reported. For example, several DNA methyltransferases, including DNMT3B, have been shown to interact with components of the histone modification machinery, such as histone deacetylases (HDAC1), histone methylases (SUV39H1) and the methylated histone H3 lysine 9 binding proteins (HP1 proteins) (17,20,33). Studies in yeast demonstrated that pericentromeric histone H3 lysine 9 methylation and HP1 binding are critical for proper assembly of the cohesin complex (63,64). It is reasonable to assume that similar chromatin modifications may be needed for condensin assembly as well. Indeed, it is believed that mitotic chromosome condensation initiates from within pericentromeric heterochromatin (25,27). Finally, it was recently reported that knockout of a protein known to interact with the murine homolog of Sin3a (also a DNMT3B-interacting protein), called mSds3, results in disruption of pericentromeric heterochromatin, delocalization of HP1 proteins, aneuploidy and the formation of multi-radial chromosomes reminiscent of those seen in human ICF syndrome patients (65). The examples in this and the preceding paragraph serve to emphasize the many indirect connections between DNA methyltransferases and chromosome condensation, and many of these observations may, in part, be explained by our novel findings that the de novo DNA methyltransferase DNMT3B interacts directly with the cellular machinery involved in chromosome condensation.
Although we did not detect all components of the ‘standard’ condensin complex in our DNMT3B-containing fractions, we have presented clear evidence that DNMT3B interacts and co-localizes with condensin. We believe that this discrepancy is due to loss of subunits from the protein complex during the biochemical fractionation, since DNMT3B co-immunoprecipitates with multiple components of the condensin complex in nuclear extracts and co-localizes well with condensin on mitotic chromosomes. It remains possible, however, that we have identified a novel condensin-like complex that may localize similarly to the standard condensin complex. There may be a population of DNMT3B interacting with hCAP-C/hCAP-E and KIF4A that is not part of the conventional condensin complex. Condensin-like complexes with alternative subunit compositions have recently been described (60,66). Clarification of the exact nature of the interactions between DNMT3B and condensin subunits will be the subject of future work.
What might be the function of the DNMT3B–condensin interaction? As was mentioned earlier, DNMT3B, as a DNA binding protein, may be required for proper targeting of condensin and/or KIF4A to certain regions of the genome, such as pericentromeric heterochromatin. Our ChIP studies revealed that DNMT3B and its associated proteins do indeed bind to such regions. Since DNMT3B co-localized with KIF4A during interphase, whereas most of the condensin was located in the cytoplasm, it is possible that nuclear chromatin-bound KIF4A and DNMT3B may serve as a platform for the assembly of condensin at the onset of mitosis. Given that DNMT3B co-localized with condensin–KIF4A over all chromosomes during mitosis, we propose another function for these interactions. Condensin–KIF4A binding to chromatin may recruit DNMT3B to carry out de novo and/or maintenance methylation. It has been reported that the maintenance methylation activity of DNMT1 is inefficient and requires the presence of DNMT3A and DNMT3B (57,67). In fact, DNMT1 and DNMT3B interact (68), and DNMT1, like DNMT3B, is recruited to mitotic chromosomes (55). Therefore, we suggest that DNMT1 may not be able to fully complete maintenance methylation during S-phase and that low-level maintenance methylation may continue to occur in G2, M or even the next G1, as long as it is completed before the next S-phase. There is evidence for DNA methylation occurring outside S-phase, particularly in G2, which has been termed ‘delayed’ methylation (69,70). DNA methylation during mitosis has also been reported (71). Low-level maintenance or ‘proofreading’ activity after S-phase may be required to methylate ‘difficult’ regions of the genome, such as constitutive, highly repetitive heterochromatin. DNMT3B may accomplish this by direct interaction with DNMT1 or independently of DNMT1 outside S-phase in conjunction with condensin. It remains to be determined which DNMT, or combination of DNMTs, gives rise to the ‘delayed’ methylation outside of S-phase since the de novo DNMTs were not known at the time of these earlier studies. The identification of a novel direct connection between DNA methylation and chromatin structure at a genome-wide level provides an important starting point that may help to explain how DNA methylation regulates chromatin structure, how cells target DNA methylation to particular regions of the genome, and also provide a new pathway for aberrations in the DNA methylation system to contribute to the genomic instability of tumor cells.
Acknowledgments
ACKNOWLEDGEMENTS
We thank En Li for providing the DNMT3B cDNA. This work was supported by intramural funds from the National Cancer Institute, National Institutes of Health (NIH). K.D.R. is a National Cancer Institute Scholar. T.M.G. was supported by the Pharmacology Research Associate Training Fellowship Program (National Institute of General Medical Sciences, NIH). K.Y. is a Scholar of the Leukemia and Lymphoma Society and is supported by NIH grant GM59150. Y.Z. is supported by the Robert A. Welch Foundation (I-1550) and NIH grant CA85146.
REFERENCES
- 1.Bestor T.H. and Tycko,B. (1996) Creation of genomic methylation patterns. Nature Genet., 12, 363–367. [DOI] [PubMed] [Google Scholar]
- 2.Bird A. (2002) DNA methylation patterns and epigenetic memory. Genes Dev., 16, 6–21. [DOI] [PubMed] [Google Scholar]
- 3.Li E. (2002) Chromatin modification and epigenetic reprogramming in mammalian development. Nature Rev. Genet., 3, 662–673. [DOI] [PubMed] [Google Scholar]
- 4.Baylin S.B., Esteller,M., Rountree,M.R., Bachman,K.E., Schuebel,K. and Herman,J.G. (2001) Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum. Mol. Genet., 10, 687–692. [DOI] [PubMed] [Google Scholar]
- 5.Bestor T.H. (2000) The DNA methyltransferases. Hum. Mol. Genet., 9, 2395–2402. [DOI] [PubMed] [Google Scholar]
- 6.Robertson K.D. (2002) DNA methylation and chromatin—unraveling the tangled web. Oncogene, 21, 5361–5379. [DOI] [PubMed] [Google Scholar]
- 7.Miller O.J., Schnedl,W., Allen,J. and Erlanger,B.F. (1974) 5-methylcytosine localised in mammalian constitutive heterochromatin. Nature, 251, 636–637. [DOI] [PubMed] [Google Scholar]
- 8.Yoder J.A., Walsh,C.P. and Bestor,T.H. (1997) Cytosine methylation and the ecology of intragenomic parasites. Trends Genet., 13, 335–340. [DOI] [PubMed] [Google Scholar]
- 9.Henikoff S. (2000) Heterochromatin function in complex genomes. Biochim. Biophys Acta, 1470, O1–O8. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh C.-L. and Lieber,M.R. (1992) CpG methylated minichromosomes become inaccessible for V(D)J recombination after undergoing replication. EMBO J., 11, 3115–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen R.Z., Pettersson,U., Beard,C., Jackson-Grusby,L. and Jaenisch,R. (1998) DNA hypomethylation leads to elevated mutation rates. Nature, 395, 89–93. [DOI] [PubMed] [Google Scholar]
- 12.Eden A., Gaudet,F., Waghmare,A. and Jaenisch,R. (2003) Chromosomal instability and tumors promoted by DNA hypomethylation. Science, 300, 455. [DOI] [PubMed] [Google Scholar]
- 13.Jenuwein T. and Allis,C.D. (2001) Translating the histone code. Science, 293, 1074–1080. [DOI] [PubMed] [Google Scholar]
- 14.Berger S.L. (2001) An embarrassment of niches: the many covalent modifications of histones in transcriptional regulation. Oncogene, 20, 3007–3013. [DOI] [PubMed] [Google Scholar]
- 15.Schneider R., Bannister,A.J. and Kouzarides,T. (2002) Unsafe SETs: histone lysine methyltransferases and cancer. Trends Biochem. Sci., 27, 396–402. [DOI] [PubMed] [Google Scholar]
- 16.Fuks F., Bergers,W.A., Brehm,A., Hughes-Davies,L. and Kouzarides,T. (2000) DNA methyltransferase Dnmt1 associates with histone deacetylase activity. Nature Genet., 24, 88–91. [DOI] [PubMed] [Google Scholar]
- 17.Fuks F., Burgers,W.A., Godin,N., Kasai,M. and Kouzarides,T. (2001) Dnmt3a binds deacetylases and is recruited by a sequence-specific repressor to silence transcription. EMBO J., 20, 2536–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson K.D., Ait-Si-Ali,S., Yokochi,T., Wade,P.A., Jones,P.L. and Wolffe,A.P. (2000) DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nature Genet., 25, 338–342. [DOI] [PubMed] [Google Scholar]
- 19.Bachman K.E., Rountree,M.R. and Baylin,S.B. (2001) Dnmt3a and Dnmt3b are transcriptional repressors that exhibit unique localization properties to heterochromatin. J. Biol. Chem., 276, 32282–32287. [DOI] [PubMed] [Google Scholar]
- 20.Lehnertz B., Ueda,Y., Derijck,A.A.H.A., Braunschweig,U., Perez-Burgos,L., Kubicek,S., Chen,T., Li,E., Jenuwein,T. and Peters,A.H.F.M. (2003) Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr. Biol., 13, 1192–1200. [DOI] [PubMed] [Google Scholar]
- 21.Jones P.A. and Baylin,S.B. (2002) The fundamental role of epigenetic events in cancer. Nature Rev. Genet., 3, 415–428. [DOI] [PubMed] [Google Scholar]
- 22.Nasmyth K. (2002) Segregating sister genomes: the molecular biology of chromosome separation. Science, 297, 559–565. [DOI] [PubMed] [Google Scholar]
- 23.Hirano T. (2000) Chromosome cohesion, condensation and separation. Annu. Rev. Biochem., 69, 115–144. [DOI] [PubMed] [Google Scholar]
- 24.Ball A.R. and Yokomori,K. (2001) The structural maintenance of chromosomes (SMC) family of proteins in mammals. Chromosome Res., 9, 85–96. [DOI] [PubMed] [Google Scholar]
- 25.Hagstrom K.A. and Meyer,B.J. (2003) Condensin and cohesin: more than chromosome compactor and glue. Nature Rev. Genet., 4, 520–534. [DOI] [PubMed] [Google Scholar]
- 26.Hirano T., Kobayashi,R. and Hirano,M. (1997) Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein. Cell, 88, 511–521. [DOI] [PubMed] [Google Scholar]
- 27.Schmiesing J.A., Gregson,H.C., Zhou,S. and Yokomori,K. (2000) A human condensin complex containing hCAP-C-hCAP-E and CNAP1, a homolog of Xenopus XCAP-D2, colocalizes with phosphorylated histone H3 during the early mitotic chromosome condensation. Mol. Cell. Biol., 20, 6996–7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haaf T. and Schmid,M. (2000) Experimental condensation inhibition in constitutive and facultative heterochromatin of mammalian chromosomes. Cytogenet. Cell Genet., 91, 113–123. [DOI] [PubMed] [Google Scholar]
- 29.Tuck-Muller C.M., Narayan,A., Tsien,F., Smeets,D.F.C.M., Sawyer,J., Fiala,E.S., Sohn,O.S. and Ehrlich,M. (2000) DNA hypomethylation and unusual chromosome instability in cell lines from ICF patients. Cytogenet. Cell Genet., 89, 121–128. [DOI] [PubMed] [Google Scholar]
- 30.Hansen R.S., Wijmenga,C., Luo,P., Stanek,A.M., Canfield,T.K., Weemaes,C.M.R. and Gartler,S.M. (1999) The DNMT3B DNA methyltransferase gene is mutated in the ICF immunodeficiency syndrome. Proc. Natl Acad. Sci. USA, 96, 14412–14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okano M., Bell,D.W., Haber,D.A. and Li,E. (1999) DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell, 99, 247–257. [DOI] [PubMed] [Google Scholar]
- 32.Xu G.-L., Bestor,T.H., Bourc’his,D., Hsieh,C.-L., Tommerup,N., Bugge,M., Hulten,M., Qu, Russo,J.J. and Viegas-Pequignot,E. (1999) Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature, 402, 187–191. [DOI] [PubMed] [Google Scholar]
- 33.Fuks F., Hurd,P.J., Deplus,R. and Kouzarides,T. (2003) The DNA methyltransferases associate with HP1 and SUV39H1 histone methyltransferase. Nucleic Acids Res., 31, 2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chuang R.-T., Albertson,D.G. and Meyer,B.J. (1994) DPY-27: A chromosome condensation protein homolog that regulates C. elegans dosage compensation through association with the X chromosome. Cell, 79, 459–474. [DOI] [PubMed] [Google Scholar]
- 35.Bhalla N., Biggins,S. and Murray,A.W. (2002) Mutation of YCS4, a budding yeast condensin subunit, affects mitotic and nonmitotic chromosome behavior. Mol. Biol. Cell, 13, 632–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lupo R., Breiling,A., Bianchi,M.E. and Orlando,V. (2001) Drosophila chromosome condensation proteins topoisomerase II and barren colocalize with polycomb and maintain Fab-7 PRE silencing. Mol. Cell, 7, 127–136. [DOI] [PubMed] [Google Scholar]
- 37.Ruf I.K. and Rawlins,D.R. (1995) Identification and characterization of ZIIBC, a complex formed by cellular factors and the ZII site of the Epstein–Barr virus BZLF1 promoter. J. Virol., 69, 7648–7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y., Zhang,W., White,M.A. and Zhao,Y. (2003) Capillary high-performance liquid chromatography/mass spectrometric analysis of proteins from affinity-purified plasma membranes. Anal. Chem., 75, 3751–3757. [DOI] [PubMed] [Google Scholar]
- 39.Gatlin C.L., Kleemann,G.R., Hays,L.G., Link,A.J. and Yates,J.R. (1998) Protein identification at the low femtomole level from silver-stained gels using a new fritless electrospray interface for liquid chromatography-microspray and nanospray mass spectrometry. Anal. Biochem., 263, 93–101. [DOI] [PubMed] [Google Scholar]
- 40.Schmiesing J.A., Ball,A.R., Gregson,H.C., Alderton,J.M., Zhou,S. and Yokomori,K. (1998) Identification of two distinct human SMC protein complexes involved in mitotic chromosome dynamics. Proc. Natl Acad. Sci. USA, 95, 12906–12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wade P.A., Jones,P.L., Vermaak,D. and Wolffe,A.P. (1998) A multiple subunit Mi-2 histone deacetylase from Xenopus laevis cofractionates with an associated Snf2 superfamily ATPase. Curr. Biol., 8, 843–846. [DOI] [PubMed] [Google Scholar]
- 42.Ausubel F.M., Brent,R., Kingston,R.E., Moore,D.D., Seidman,J.G., Smith,J.A. and Struhl,K. (1995) Current Protocols in Molecular Biology. John Wiley and Sons, Inc., New York. [Google Scholar]
- 43.Vernos I., Raats,J., Hirano,T., Heasman,J., Karsenti,E. and Wylie,C. (1995) Xklp1, a chromosomal Xenopus kinesin-like protein essential for spindle organization and chromosome positioning. Cell, 81, 117–127. [DOI] [PubMed] [Google Scholar]
- 44.Gregson H.C., Schmiesing,J.A., Kim,J.-S., Kobayashi,T., Zhou,S. and Yokomori,K. (2001) A potential role for human cohesin in mitotic spindle aster assembly. J. Biol. Chem., 276, 47575–47582. [DOI] [PubMed] [Google Scholar]
- 45.Ling Y., Sankpal,U.T., Robertson,A.K., McNally,J.G., Karpova,T. and Robertson,K.D. (2004) Modification of de novo DNA methyltransferase 3a (Dnmt3a) by SUMO-1 modulates its interaction with histone deacetylases (HDACs) and its capacity to repress transcription. Nucleic Acids Res., 32, 598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magdinier F. and Wolffe,A.P. (2001) Selective association of the methyl-CpG binding protein MBD2 with the silent p14/p16 locus in human neoplasia. Proc. Natl Acad. Sci. USA, 98, 4990–4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Osta A. and Wolffe,A.P. (2001) Analysis of chromatin-immunopurified MeCP2-associated fragments. Biochem. Biophys. Res. Commun., 289, 733–737. [DOI] [PubMed] [Google Scholar]
- 48.Kimura K., Cuvier,O. and Hirano,T. (2001) Chromosome condensation by a human condensin complex in Xenopus egg extracts. J. Biol. Chem., 276, 5417–5420. [DOI] [PubMed] [Google Scholar]
- 49.Hudson D.F., Vagnarelli,P., Gassmann,R. and Earnshaw,W.C. (2003) Condensin is required for nonhistone protein assembly and structural integrity of vertebrate mitotic chromosomes. Dev. Cell, 5, 323–336. [DOI] [PubMed] [Google Scholar]
- 50.Varga-Weisz P. (2001) ATP-dependent chromatin remodeling factors: nucleosome shufflers with many missions. Oncogene, 20, 3076–3085. [DOI] [PubMed] [Google Scholar]
- 51.Lee Y.M., Lee,S., Lee,E., Shin,H., Hahn,H., Choi,W. and Kim,W. (2001) Human kinesin superfamily member 4 is dominantly localized in the nuclear matrix and is associated with chromosomes during mitosis. Biochem. J., 360, 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knoepfler P.S. and Eisenman,R.N. (1999) Sin meets NuRD and other tails of repression. Cell, 99, 447–450. [DOI] [PubMed] [Google Scholar]
- 53.Geiman T.M., Sankpal,U.T., Robertson,A.K., Zhao,Y., Zhao,Y. and Robertson,K.D. (2004) DNMT3B interacts with hSNF2H chromatin remodeling enzyme, HDACs 1 and 2, and components of the histone methylation system. Biochem. Biophys. Res. Commun., 318, 544–555. [DOI] [PubMed] [Google Scholar]
- 54.Takemoto A., Kimura,K., Yokoyama,S. and Hanaoka,F. (2003) Cell cycle-dependent phosphorylation, nuclear localization and activation of human condensin. J. Biol. Chem., 279, 4551–4559. [DOI] [PubMed] [Google Scholar]
- 55.Craig J.M., Earle,E., Canham,P., Wong,L.H., Anderson,M. and Choo,K.H.A. (2003) Analysis of mammalian proteins involved in chromatin modification reveals new metaphase centromeric proteins and distinct chromosomal distribution patterns. Hum. Mol. Genet., 12, 3109–3121. [DOI] [PubMed] [Google Scholar]
- 56.Santoro R., Li,J. and Grummt,I. (2002) The nucleolar remodeling complex NoRC mediates heterochromatin formation and silencing of ribosomal gene transcription. Nature Genet., 32, 393–396. [DOI] [PubMed] [Google Scholar]
- 57.Chen T., Ueda,Y., Dodge,J.E., Wang,Z. and Li,E. (2003) Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3b. Mol. Cell. Biol., 23, 5594–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacCallum D.E., Losada,A., Kobayashi,R. and Hirano,T. (2002) ISWI remodeling complexes in xenopus egg extracts: identification as major chromosomal components that are regulated by INCENP-aurora B. Mol. Biol. Cell, 13, 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hakimi M.-A., Bochar,D.A., Schmiesing,J.A., Dong,Y., Barak,O.G., Speicher,D.W., Yokomori,K. and Shiekhattar,R. (2002) A chromatin remodelling complex that loads cohesin onto human chromosomes. Nature, 418, 994–998. [DOI] [PubMed] [Google Scholar]
- 60.Ono T., Losado,A., Hirano,M., Myers,M.P., Neuwald,A.F. and Hirano,T. (2003) Differential contributions of condensin I and condensin II to mitotic chromosome architecture in vertebrate cells. Cell, 115, 109–121. [DOI] [PubMed] [Google Scholar]
- 61.Bhat M.A., Philip,A.V., Glover,D.M. and Bellen,H.J. (1996) Chromatid segregation at anaphase requires the barren product, a novel chromosome-associated protein that interacts with topoisomerase II. Cell, 87, 1103–1114. [DOI] [PubMed] [Google Scholar]
- 62.Steffensen S., Coelho,P.A., Cobbe,N., Vass,S., Costa,M., Hassan,B., Prokopenko,S.N., Bellen,H., Heck,M.M.S. and Sunkel,C.E. (2001) A role for Drosophila SMC4 in the resolution of sister chromatids in mitosis. Curr. Biol., 11, 295–307. [DOI] [PubMed] [Google Scholar]
- 63.Bernard P., Maure,J.-F., Partridge,J.F., Genier,S., Javerzat,J.-P. and Allshire,R.C. (2001) Requirement of heterochromatin for cohesion at centromeres. Science, 294, 2539–2542. [DOI] [PubMed] [Google Scholar]
- 64.Nonaka N., Kitajima,T., Yokobayashi,S., Xiao,G., Yamamoto,M., Grewal,S.I.S. and Watanabe,Y. (2001) Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nature Cell Biol., 4, 89–93. [DOI] [PubMed] [Google Scholar]
- 65.David G., Turner,G.M., Yao,Y., Protopopov,A. and DePinho,R.A. (2003) mSin3-associated protein, mSds3, is essential for percentric heterochromatin formation and chromosome segregation in mamalian cells. Genes Dev., 17, 2396–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yeong F.M., Hombauer,H., Wendt,K.S., Hirota,T., Mudrak,I., Mechtler,K., Loregger,T., Marchler-Bauer,A., Tanaka,K., Peters,J.-M. et al. (2003) Identification of a subunit of a novel kleisin-β/SMC complex as a potential substrate of protein phosphatase 2A. Curr. Biol., 13, 2058–2064. [DOI] [PubMed] [Google Scholar]
- 67.Rhee I., Bachman,K.E., Park,B.H., Jair,K.-W., Yen,R.-W.C., Schuebel,K.E., Cui,H., Feinberg,A.P., Lengauer,C., Kinzler,K.W. et al. (2002) DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature, 416, 552–556. [DOI] [PubMed] [Google Scholar]
- 68.Kim G.-D., Ni,J., Kelesoglu,N., Roberts,R.J. and Pradhan,S. (2002) Co-operation and communication between the human maintenance and de novo DNA (cytosine-5) methyltransferases. EMBO J., 21, 4183–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woodcock D.M., Adams,J.K. and Cooper,I.A. (1982) Characteristics of enzymatic DNA methylation in cultured cells of human and hampster origin and the effect of DNA replication inhibition. Biochim. Biophys Acta, 696, 15–22. [DOI] [PubMed] [Google Scholar]
- 70.Davis T., Kirk,D., Rinaldi,A., Burdon,R.H. and Adams,R.L.P. (1985) Delayed methylation and the matrix bound DNA methylase. Biochem. Biophys. Res. Commun., 126, 678–684. [DOI] [PubMed] [Google Scholar]
- 71.Bugler B., Bertaux,O. and Valencia,R. (1980) Nucleic acids methylation of synchronized BHK 21 HS 5 fibroblasts during the mitotic phase. J. Cell. Physiol., 103, 149–157. [DOI] [PubMed] [Google Scholar]