Abstract
This study was designed to evaluate whether subjects with amyloid beta (Aβ) pathology, detected using florbetapir positron emission tomorgraphy (PET), demonstrated greater cognitive decline than subjects without Aβ pathology. Sixty-nine cognitively normal (CN) controls, 52 with recently diagnosed mild cognitive impairment (MCI) and 31 with probable Alzheimer's disease (AD) dementia were included in the study. PET images obtained in these subjects were visually rated as positive (Aβ+) or negative (Aβ−), blind to diagnosis. Fourteen percent (10/69) of CN, 37% (19/52) of MCI and 68% (21/31) of AD were Aβ+. The primary outcome was change in ADAS-Cog score in MCI subjects after 36 months; however, additional outcomes included change on measures of cognition, function and diagnostic status. Aβ+ MCI subjects demonstrated greater worsening compared with Aβ− subjects on the ADAS-Cog over 36 months (5.66±1.47 vs −0.71±1.09, P=0.0014) as well as on the mini-mental state exam (MMSE), digit symbol substitution (DSS) test, and a verbal fluency test (P<0.05). Similar to MCI subjects, Aβ+ CN subjects showed greater decline on the ADAS-Cog, digit-symbol-substitution test and verbal fluency (P<0.05), whereas Aβ+ AD patients showed greater declines in verbal fluency and the MMSE (P<0.05). Aβ+ subjects in all diagnostic groups also showed greater decline on the CDR-SB (P<0.04), a global clinical assessment. Aβ+ subjects did not show significantly greater declines on the ADCS-ADL or Wechsler Memory Scale. Overall, these findings suggest that in CN, MCI and AD subjects, florbetapir PET Aβ+ subjects show greater cognitive and global deterioration over a 3-year follow-up than Aβ− subjects do.
Keywords: alzheimer's disease, amyloid, cognitive decline, florbetapir, MCI, PET
Introduction
The prognostic evaluation of people at risk for AD, such as normal elderly or those with mild cognitive impairment (MCI) is challenging due to considerable variability in progression rates and underlying pathologic heterogeneity. A reliable biomarker that could accurately identify subjects at greatest risk for progressive cognitive decline could enhance the clinical evaluation of at-risk subjects and accelerate the testing of preventive strategies.1,2,3
Accumulation of amyloid-β (Aβ) fibrils in the form of amyloid plaques is a neuropathological requirement for definitive diagnosis of dementia due to Alzheimer's disease (AD).4 Insights gained from pathologic and biomarker studies suggest that Aβ changes in the brain begin years, and possibly decades, before cognitive symptoms emerge. Two recent cross-sectional studies of carriers at-risk for familial AD estimated that Aβ changes may occur 15 years prior to expected symptom onset.5,6 Likewise, biomarker studies of older asymptomatic and MCI subjects have reported an increased rate of AD pathologic changes. Such findings have led to the concept of preclinical7 and MCI stages of AD;2 however, the predictive value of available biomarkers at both the individual patient level and group level is not yet fully elucidated.
Among the various biomarkers in development to assess Aβ, position emission tomorgraphy (PET) tracers offer the potential of directly imaging changes in cortical Aβ. 11C-labeled Pittsburgh compound B (PiB) was the first PET tracer to image cortical Aβ plaques8,9, and prior PiB studies have shown that PiB-positive normal and MCI subjects are more likely to show faster cognitive deterioration than PiB-negative subjects.10, 11, 12, 13, 14, 15, 16, 17, 18, 19 The short half-life of 11C (20 min) limits its viability for routine clinical use. Florbetapir F 18 is a PET ligand with high affinity and specificity to Aβ,20,21 and a multicenter clinical histopathologic study has shown a significant correlation between visual ratings of florbetapir PET in living subjects and autopsy measured Aβ pathology.22 It was recently approved by FDA to detect neuritic plaques in the evaluation of patients with progressive cognitive decline. Other F 18 amyloid PET tracers are also being developed.23,24 A limitation of current F 18 amyloid PET tracers has been the relative lack of longitudinal data. The current study was designed to test whether florbetapir PET can predict subsequent cognitive decline in older at-risk subjects.
Materials and Methods
This prospective, observational study (AV45-A11 (NCT00857506)) was sponsored by Avid Radiopharmaceuticals (a subsidiary of Eli Lilly & Co.) and conducted at 21 US clinical sites. It was a longitudinal extension of a cross-sectional Phase 2 florbetapir PET study (AV45-A05; NCT00702143). Baseline cross-sectional results25,26 and 18-month interim findings27 have been reported separately.
Subjects
Participants in the longitudinal study included 69 cognitively normal (CN) healthy controls, 52 MCI and 31 clinically diagnosed AD dementia patients. All previously received a florbetapir PET scan in the cross-sectional study. AD patients met NINCDS-ADRDA28 criteria for probable AD with mini-mental state examination (MMSE) scores between 10 and 24. MCI subjects were recently diagnosed (either at the screening visit or within the past year) on the basis of a global clinical dementia rating (CDR) score of 0.5 with an MMSE>24. All had memory complaint or cognitive impairment corroborated by an informant, but no episodic memory threshold was imposed. CN subjects were assessed clinically as cognitively normal, with a Global CDR of 0.0 and an MMSE of 29–30. CN subjects were all ≥50 years of age, recruited approximately equal distribution across age deciles (50–59, 60–69, 70–79 and ≥80 years of age). At the screening visit, subjects underwent a medical history, clinical interview, physical and neurologic examinations and laboratory evaluations. MRI was obtained at screening or within 6 months prior to enrollment to rule out significant central nervious system lesions. Subjects who had other neuropsychiatric diseases, contraindications to PET, received anti-amyloid investigational drugs, or were unable to complete psychometric testing were excluded.
Baseline assessments
Baseline measures included a clinical diagnostic interview and a cognitive/functional battery comprised of the Alzheimer's Disease Assessment Scale (ADAS-cog; 11-item version), MMSE, CDR Global (CDR Global) and Sum of Boxes (CDR-SB), Alzheimer's Disease Cooperative Study Activities of Daily Living Scale (ADCS-ADL), Digit-Symbol Substitution (DSS), Category Verbal Fluency (animals and vegetables) and Wechsler Logical Memory (immediate and delayed recall).
Florbetapir PET Scan
Site PET scanners were qualified with a Hoffman brain phantom. PET amyloid imaging was performed as part of study AV45-A05.25 Fifty minutes after intravenous injection of 10 mCi (370 MBq) of florbetapir F 18, a 10-min emission scan (acquired in 2 × 5 min frames) was obtained. PET scanners included Discovery LS PET/CT (GE, Fairfield, CT, USA), Advance PET (GE), ECAT HR+ (Siemens, Washington DC, USA) and Biograph PET/CT (Siemens) models. Image reconstruction utilized an iterative algorithm (4 iterations, 16 subsets) and a post reconstruction Gaussian filter of 5 mm.
Three nuclear medicine physicians, blinded to clinical data, independently rated the PET images at an imaging core lab (ICON Medical Imaging, Warrington, PA, USA). A binary qualitative scale (amyloid positive: Aβ+ or amyloid negative: Aβ−) was implemented in this study according to the pattern of tracer uptake observed in cortical gray matter areas. The PET rating methods, visual rater training and reliability have been described previously.22,25,27 In brief, scans were rated as amyloid negative if tracer retention was seen predominantly in white matter, with no appreciable or low levels of tracer retention in cortical gray matter. Scans were rated as amyloid positive when tracer showed a gray matter pattern of distribution with accumulation along the midline and surface of the cortex.
Follow-up assessments
Eligibility to participate in the follow-up protocol was contingent upon completing a PET scan in the Phase II study AV45-A05. A brief phone screen and status update with the subject/informant was conducted every 6 months. A diagnostic interview and a cognitive and functional test battery were administered in the clinic 18 and 36 months after the PET scan. Subjects were classified as CN, MCI, AD or non-AD dementia based on these evaluations. Clinical diagnoses were generated without knowledge of the florbetapir scan results.
Standard protocol approvals, registrations and patient consents
This study (NCT00857506) was approved by the Institutional Review Boards at all participating sites and all subjects or their appropriate representatives provided informed consent. The study sponsor was involved in all aspects of the study. The first author had full access to the statistical analyses and planned study report.
Statistical analysis
The primary analysis used analysis of covariance (ANCOVA) to compare the magnitude of change from baseline on the ADAS-Cog between Aβ+ and Aβ− subjects in the MCI population at 36 months. A last observation carried forward (LOCF) methodology was implemented to impute the missing values during the followup. Subjects who had at least one post-baseline visit were included in the analysis, and the ANCOVA models were adjusted for baseline test score and age. As a sensitivity analysis, with observed data only, a mixed-effect repeated measure (MMRM) model was implemented that included fixed effects for baseline amyloid beta status (Aβ+ or Aβ−), visit, amyloid-by-visit interaction, baseline score and age to compare the least square mean (LSM) change from baseline between Aβ+ and Aβ− at month 36. An unstructured covariance structure was used to model the within-subject correlation. We also generated illustrative line graphs using mixed model analyses that included fixed effects for amyloid status, age and month of followup, to estimate slopes of cognitive test score change per month. Key secondary analyses compared the percentage of Aβ+ and Aβ− subjects in the MCI population who experienced a 4-point change in the ADAS-Cog, or had a change in diagnosis from MCI to AD dementia using Fisher's exact test. Other secondary analyses included the change from baseline ADAS-Cog and CDR-SB in CN and AD patients. Analyses of other psychometric and functional assessments, conversions in diagnosis, time to first AD medication (donepezil, rivastigmine, galantamine and memantine), were also done for all relevant patient groups.
All statistical tests were conducted with a two-sided type I error rate of 0.05, unless otherwise noted. Differences between diagnostic groups and Aβ status on baseline characteristics continuous variables were assessed with two-sample t-tests; categorical variables were assessed with χ2 tests. The Mantel–Haenszel statistic was used to test for trend differences between Aβ+ and Aβ− MCI subjects converting to either CN or AD. The Pearson χ2 test was used to test for differences in AD medication use between Aβ+ and Aβ− groups. Fisher's exact test was used when a frequency table had a cell count less than or equal to 5. Analyses were conducted with SAS Windows (Version 9 or later). P-values for the secondary and exploratory analyses are unadjusted for multiple comparisons.
Results
Subject disposition
A total of 152 of 184 subjects from the cross-sectional study (AV45-A05) enrolled in the follow-up study (AV45-11) due to an approximate 8-month delay between the start of the two studies (Supplementary Figure 1). Of these 152 subjects (69 CN; 52 MCI; 31 AD), 97% of CN, 88% of MCI and 87% of AD completed 18 months of followup, while 74% of CN, 71% of MCI and 52% of AD completed 36 months of followup. The most common reasons for termination were withdrawal of consent (N=38) and loss to follow up (N=8). The proportion of study completers did not differ by visual ratings of amyloid status in the AD, MCI or CN groups (P=0.52, P=0.21, P=0.33, respectively).
Baseline Florbetapir PET Amyloid Positivity by Diagnosis
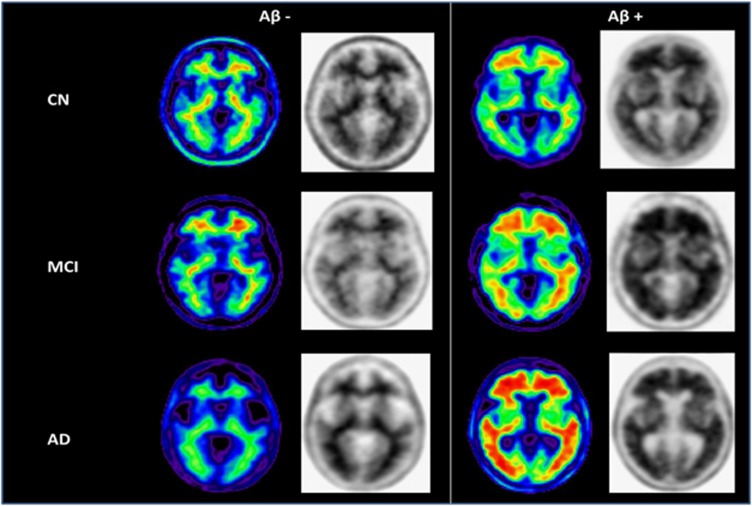
Figure 1 depicts illustrative amyloid positive (Aβ+) and negative (Aβ−) PET scans. As reported previously25, 37% (19/52) of MCI, 14% (10/69) of CN and 68% (21/31) of AD dementia subjects were rated as PET Aβ+ (P<0.0001) at study entry.
Figure 1.
Example images of Aβ− and Aβ+ subjects clinically classified as CN, MCI and AD. Normalized SUVR images (color) and gray scale images (used for visual interpretation of Aβ− vs Aβ+ status) from representative subjects. Note the absence of gray matter uptake and the difference in average cortical SUVR in the Aβ− vs Aβ+ classified scans. The color images are shown for illustrative purposes.
Baseline demographic characteristics and cognitive performance
At baseline (Table 1), Aβ+ subjects classified by visual ratings tended to be older and have worse cognitive performance at baseline than Aβ− subjects on some measures. Because of these potential differences, we adjusted for baseline score and age in longitudinal statistical models evaluating rate of change by amyloid status.
Table 1. Baseline characteristics in Aβ+ and Aβ− subjects classified by visual ratings on florbetapir F 18 PET.
|
Cognitively normal |
Mild cognitive impairment |
AD dementia |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Aß+ (N =10) | Aß− (N=57) | P | Aß+ (N=17) | Aß− (N=30) | P | Aß+ (N=19) | Aß− (N=9) | P | |
| Age in years | 77.30 (8.04) | 68.70 (11.32) | 0.0250 | 74.47 (7.72) | 70.40 (10.72) | 0.1762 | 77.63 (7.29) | 73.33 (12.98) | 0.3750 |
| Gender: Female N (%) | 4 (40%) | 36 (63.2%) | 0.1685 | 9 (52.9%) | 16 (53.3%) | 0.9793 | 9 (47.4%) | 2 (22.2%) | 0.2032 |
| APOE4+ N (%) | 3 (30%) | 13 (23.2%) | 0.6446 | 11 (73.3%) | 4 (13.3%) | <0.0001 | 11 (73.3%) | 2 (22.2%) | 0.0150 |
| Education in years | 15.90 (0.74) | 15.21 (2.39) | 0.0860 | 14.47 (2.18) | 15.27 (2.42) | 0.2681 | 14.18 (2.08) | 14.22 (3.38) | 0.9709 |
| SUVR | 1.34 (0.18) | 1.00 (0.09) | 0.0001 | 1.50 (0.15) | 1.00 (0.08) | <0.0001 | 1.57 (0.17) | 1.05 (0.11) | <0.0001 |
| MMSE | 29.50 (0.53) | 29.65 (0.48) | 0.3761 | 27.29 (2.14) | 27.53 (1.63) | 0.6691 | 21.53 (3.96) | 22.33 (1.73) | 0.4604 |
| ADAS-COG | 5.60 (2.50) | 4.51 (2.42) | 0.1953 | 10.88 (4.85) | 8.53 (4.45) | 0.0993 | 20.74 (8.87) | 13.33 (3.32) | 0.0037 |
| CDR-SB | 0.00 (0.00) | 0.02 (0.09) | 0.1591 | 1.59 (0.87) | 1.38 (0.91) | 0.4541 | 5.68 (2.34) | 5.39 (2.53) | 0.7637 |
| Activities of daily living | 75.80 (2.44) | 77.40 (1.46) | 0.0711 | 72.29 (7.79) | 75.27 (3.70) | 0.1541 | 64.05 (11.63) | 62.67 (10.79) | 0.7658 |
| Digital symbol substitution | 41.90 (7.71) | 49.95 (9.46) | 0.0135 | 35.41 (9.83) | 39.97 (11.15) | 0.1677 | 20.05 (12.15) | 28.89 (13.20) | 0.0920 |
| Verbal fluency animals | 18.30 (4.45) | 20.00 (4.24) | 0.2500 | 16.41 (4.40) | 16.07 (4.52) | 0.8007 | 11.11 (4.58) | 12.25 (4.33) | 0.5527 |
| Verbal fluency vegetables | 13.10 (3.21) | 14.02 (3.51) | 0.4437 | 11.59 (3.81) | 11.63 (3.74) | 0.9687 | 6.79 (2.80) | 7.38 (3.07) | 0.6335 |
| WMS delayed recall | 10.40 (4.38) | 12.86 (3.52) | 0.0535 | 7.29 (4.71) | 9.23 (4.54) | 0.1719 | 1.11 (1.79) | 3.67 (4.82) | 0.1568 |
| WMS immediate recall | 11.90 (4.20) | 14.11 (2.77) | 0.0363 | 9.35 (4.47) | 11.10 (3.34) | 0.1348 | 3.16 (2.85) | 7.89 (4.78) | 0.0029 |
PET cortical global SUVR (standard uptake values relative to cerebellum) was quantified as reported previously.27 Please see Materials and Methods section for statistical details and abbreviations of test names.
Florbetapir PET and rate of change from baseline to 36 months
Table 2 provides the observed changes on each measure at 36 months for Aβ+ and Aβ− classified subjects analysed by ANCOVA (LOCF) and by MMRM, adjusting for baseline age and cognitive function scores. The significance of the results using either analytical method was similar. At month 36, the LS mean change from baseline in ADAS-Cog in the MCI group (the primary outcome variable) was 5.66 (worsening) for MCI subjects who had Aβ+ scans, compared with −0.71 (improvement) for MCI subjects who had Aβ− scans (P=0.0014). For CN subjects, the mean change from baseline at month 36 for subjects who had Aβ+ scans was 3.24 (worsening), compared with −0.09 (improvement) for subjects who had Aβ− scans (P=0.0013). For subjects with clinically diagnosed AD dementia, the mean change from baseline at month 36 for subjects who had Aβ+ scans was 8.88, compared with 3.81 for subjects who had Aβ− scans (P>0.05). For both the MCI and CN groups, the percentage of subjects with a clinically significant 4-point worsening in ADAS-Cog was significantly greater for subjects who had Aβ+ scans than for subjects who had Aβ− scans (Aβ+ MCI 8/17, 47%, Aβ− MCI 3/30 10%, P<0.01; Aβ+ CN 4/10, 40%, Aβ− CN 3/57 5%, P<0.01).
Table 2. Change from baseline to 36 months by florbetapir PET amyloid status.
| Change over 36 months (LOCF) | Change over 36 months (MMRM) | ||||||
|---|---|---|---|---|---|---|---|
| |
|
Aß+ |
Aß− |
P |
Aß+ |
Aß− |
P |
| CN | ADAS score | 3.24 (0.90) | −0.09 (0.37) | 0.0013 | 3.65 (0.96) | −0.18 (0.43) | 0.0007 |
| CDR sum of box | 0.76 (0.15) | 0.10 (0.06) | 0.0002 | 0.82 (0.16) | 0.10 (0.07) | <0.0001 | |
| Mini mental state examination | −0.74 (0.33) | −0.40 (0.13) | 0.3412 | −0.74 (0.30) | −0.28 (0.13) | 0.1598 | |
| Activities of daily living | −0.63 (0.73) | −0.19 (0.28) | 0.5878 | −0.76 (0.77) | −0.28 (0.34) | 0.5742 | |
| Digital symbol substitution | −6.52 (2.91) | 0.21 (1.17) | 0.0383 | −7.05 (2.65) | 0.78 (1.15) | 0.0099 | |
| Verbal fluency animal | −2.78 (1.57) | −0.62 (0.64) | 0.2114 | −3.54 (1.55) | −0.66 (0.70) | 0.0986 | |
| Verbal fluency vegetable | −2.09 (1.02) | 0.16 (0.42) | 0.0481 | −2.23 (0.97) | 0.16 (0.44) | 0.0311 | |
| Wechsler logical memory scale | −0.43 (1.13) | 0.97 (0.45) | 0.2613 | −0.44 (1.12) | 1.12 (0.48) | 0.2135 | |
| WMS immediate recall | −0.93 (1.06) | 0.93 (0.42) | 0.1127 | −0.82 (1.10) | 1.07 (0.48) | 0.1252 | |
| MCI | ADAS score | 5.66 (1.47) | −0.71 (1.09) | 0.0014 | 6.62 (1.71) | −0.84 (1.20) | 0.0009 |
| CDR sum of box | 1.99 (0.53) | 0.39 (0.40) | 0.0223 | 2.33 (0.59) | 0.54 (0.42) | 0.0170 | |
| Mini mental state examination | −2.88 (0.81) | −0.30 (0.60) | 0.0148 | −2.78 (0.96) | −0.32 (0.66) | 0.0421 | |
| Activities of daily living | −4.93 (2.20) | −2.84 (1.63) | 0.4624 | −7.03 (2.76) | −4.35 (1.93) | 0.4333 | |
| Digital symbol substitution | −10.94 (2.16) | 0.13 (1.61) | 0.0002 | −9.34 (2.87) | −0.45 (1.85) | 0.0143 | |
| Verbal fluency animal | −3.18 (1.10) | −0.53 (0.82) | 0.0630 | −3.48 (1.34) | −0.59 (0.93) | 0.0864 | |
| Verbal fluency vegetable | −2.28 (0.82) | 0.76 (0.61) | 0.0051 | −2.12 (0.91) | 0.61 (0.64) | 0.0195 | |
| Wechsler logical memory scale | −1.46 (1.12) | 0.49 (0.84) | 0.1781 | −1.22 (1.30) | 0.57 (0.92) | 0.2729 | |
| WMS immediate recall | −1.87 (0.99) | 0.50 (0.74) | 0.0674 | −1.89 (1.32) | 0.66 (1.00) | 0.0914 | |
| AD dementia | ADAS score | 8.88 (2.88) | 3.81 (4.43) | 0.3763 | 14.76 (4.71) | 4.11 (5.65) | 0.1705 |
| CDR sum of box | 4.05 (0.80) | 0.12 (1.17) | 0.0116 | 5.46 (1.09) | 0.49 (1.51) | 0.0165 | |
| Mini mental state examination | −3.92 (1.24) | 1.17 (1.83) | 0.0327 | −5.96 (1.97) | 0.76 (2.63) | 0.0680 | |
| Activities of daily living | −20.79 (4.52) | −5.67 (6.63) | 0.0746 | −28.16 (6.52) | −7.62 (8.83) | 0.0846 | |
| Digital symbol substitution | −5.99 (2.24) | −0.01 (3.35) | 0.1651 | −6.48 (4.29) | 0.44 (5.51) | 0.3543 | |
| Verbal fluency animal | −4.77 (0.81) | 0.08 (1.27) | 0.0041 | −6.26 (1.21) | −0.04 (1.58) | 0.0067 | |
| Verbal fluency vegetable | −3.05 (0.70) | 0.62 (1.10) | 0.0105 | −4.52 (0.95) | 0.99 (1.21) | 0.0029 | |
| Wechsler logical memory scale | −0.18 (0.67) | 1.49 (1.01) | 0.1949 | 0.35 (0.90) | 1.13 (1.33) | 0.6413 | |
| WMS immediate recall | −0.89 (0.82) | −0.23 (1.26) | 0.6862 | −1.49 (0.88) | −0.05 (1.20) | 0.3910 | |
Abbreviations: LOCF, last observation carried forward; MMRM, mixed model repeated measure. P-values<0.05 are noted in bold. Please see Materials and Methods for details of models.
Exploratory analyses of other cognitive outcomes (Table 2): In ANCOVA analyses adjusting for age and baseline score, Aβ+ MCI subjects also showed a significantly greater deterioration than Aβ− rated subjects on the CDR-SB, MMSE, DSS and verbal fluency (vegetables). Aβ+ CN subjects similarly had greater decline on the CDR-SB, DSS and verbal fluency for vegetables. Among clinically diagnosed AD dementia subjects, Aβ+ classification predicted greater decline on the CDR-SB, MMSE, tests of verbal fluency and a trend on the ADCS-ADL. Significantly greater declines by amyloid status on the ADCS-ADL and Wechsler Memory Scale were not observed across any of the diagnostic groups.
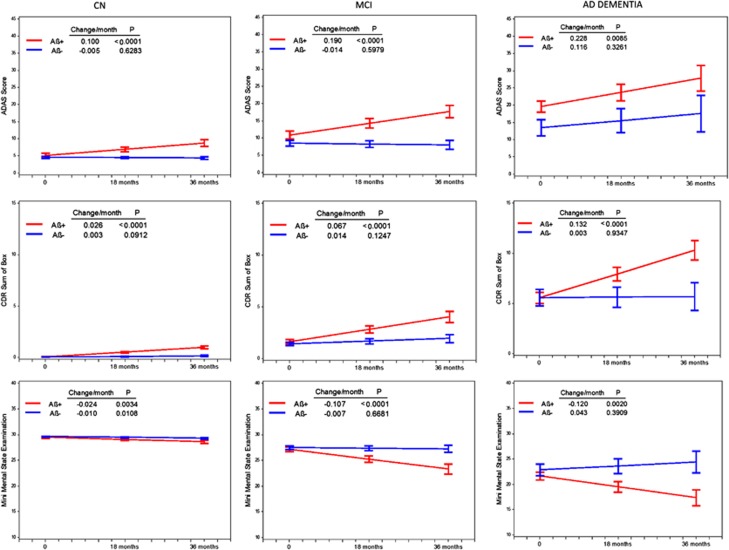
Figure 2 illustrates the slopes calculated for the ADAS-COG, CDR-SB and MMSE by clinical diagnosis in Aβ+ and Aβ− classified subgroups. In contrast to Aβ− subjects in whom significant declines were generally not observed, significant declines in slopes were observed for all Aβ+ classified subgroups regardless of clinical diagnosis.
Figure 2.
Baseline test score and change in score per month (estimated slopes) for the ADAS-Cog, CDR-SB and MMSE in Aβ+ and Aβ− subjects classified as CN, MCI and AD. Baseline scores and slopes estimated from MMRM model adjusted for baseline age. See text for details.
Florbetapir PET ratings and change in diagnosis
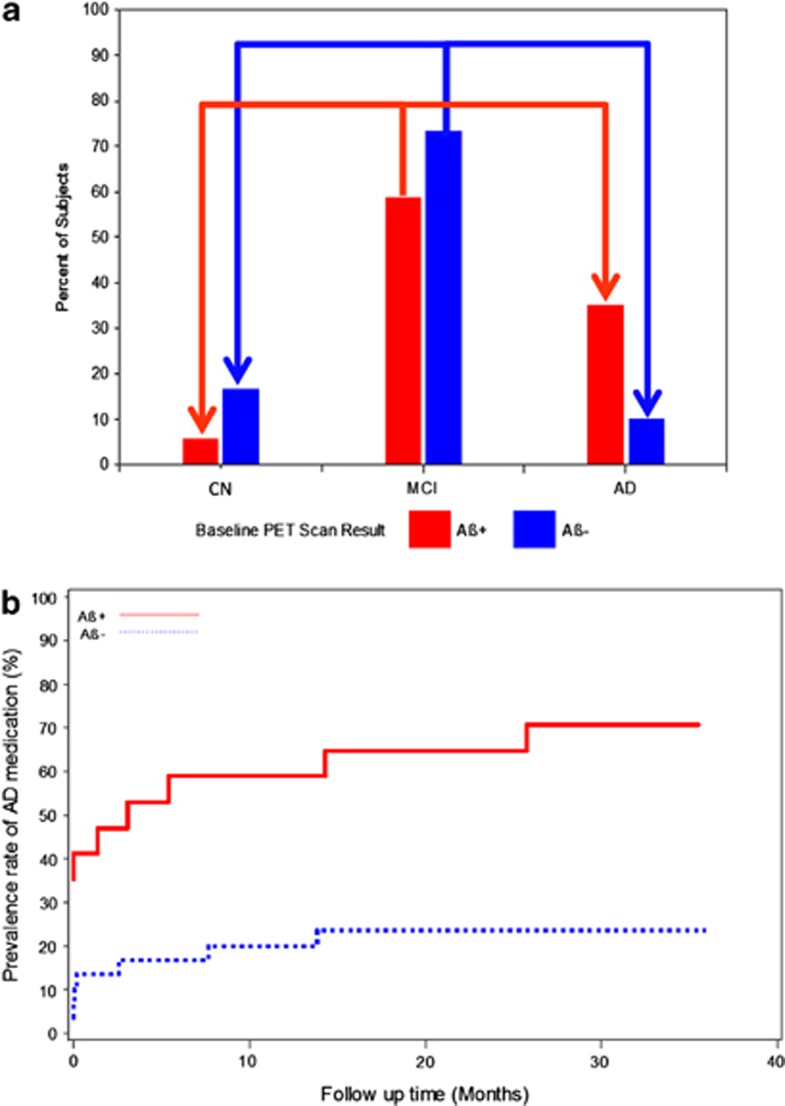
The proportion of MCI subjects progressing to AD dementia or reverting to CN over the 36-month study can be seen in Figure 3a. Overall, more MCI Aβ+ subjects converted to dementia and fewer converted to CN status than Aβ− subjects (P=0.036). MCI subjects rated Aβ+ had an ~3.5-fold higher conversion rate to AD dementia (6/17 Aβ+ MCI subjects (35.3%) vs 3/30 rated Aβ− (10.0%); P=0.054). Fewer Aβ+ (1/17 (5.9%)] vs Aβ− (5/30, (16.7%)) MCI subjects reverted to CN status, although this difference was not statistically significant. Among the 30 MCI Aβ− subjects, 27 failed to progress to dementia and failed to show clinically significant worsening (a 4-point decline on the ADAS-Cog) over 36 months resulting in a negative predictive value of florbetapir PET for both outcomes of 90% (95% CI: 74.4%-96.5%). In MCI subjects, the positive predictive value was 47% with respect to a clinically significant ADAS-Cog decline by 4 points and 35.3% for conversion to AD dementia. The positive and negative predictive values based on SUVR were similar to those for the visual reads.
Figure 3.
(a) Percentage of Aβ+ and Aβ− MCI subjects progressing to dementia or reverting to cognitive normal (CN) status over 36 months. The bars depict the 36-month endpoint diagnostic status of subjects originally classified as MCI at baseline. The red and blue bars titled CN depict the percentage of MCI subjects who reverted to CN by amyloid status. The red and blue bars titled MCI depict the percentage of MCI subjects who continued to be classified as MCI at endpoint (that is, cognitive change was not sufficient to trigger conversion). The red and blue bars titled AD depict the percentage of MCI subjects who progressed to dementia by amyloid status. Conversion to dementia from MCI was almost three times higher in Aβ+ subjects while reversion to CN was almost three times lower than Aβ− subjects. The arrows depict conversion and reversion. Please see text for details. (b) Concomitant and initiation of AD medication use in MCI subjects over 36 months by baseline florbetapir PET status. The proportion of MCI subjects who started the study taking AD medications was greater among Aβ+ vs Aβ− subjects (35.3% vs 3.3% P=0.006). By study end, 70.6% of Aβ+ MCI subjects were taking AD medications vs 23.3% Aβ− MCI (P=0.002) because of the greater percentage who initiated AD medications in Aβ+MCI vs Aβ−MCI (54.5% vs 20.7%). Please see text for details.
Florbetapir PET Ratings and Cumulative AD medication use over time
The proportion of MCI subjects taking AD medications at the start of the study was greater among Aβ+ than Aβ− subjects (6/17 Aβ+ (35.3%) vs 1/30 Aβ− (3.3%); P=0.0062) (Figure 3b). By study end, 12/17 Aβ+ MCI subjects (70.6%) were taking AD medications vs 7/30 rated Aβ− (23.3%) (P=0.0022). Among MCI subjects not taking AD medications at baseline, Aβ+ subjects had a greater likelihood of starting AD medications during the study (6/11 Aβ+ subjects (54.5%) vs 6/29 Aβ− (20.7%); P=0.056).
Discussion
These results, from the first multicenter, 36-month follow-up study of florbetapir F 18 amyloid imaging, confirm and extend results from our prior 18-month interim report27 and prior prognostic studies of 11C-PiB10, 11, 12, 13, 14, 15, 16, 17, 18,29,30 and CSF Aβ42. Subjects with Aβ+ florbetapir PET scans displayed greater cognitive and global deterioration than Aβ− subjects over the course of 36 months, regardless of diagnostic status. Conversely, the minimal decline in the Aβ− group is an important finding that has impact on clinical trials at all stages of AD when these subjects are included.
The diagnostic classification of MCI indicates an increased risk for AD but is not a definitive diagnosis. An Aβ biomarker may increase the probability that the MCI syndrome is due to AD.2 In this study, compared with Aβ− subjects, Aβ+ MCI subjects showed greater mean worsening on the ADAS-Cog, MMSE, CDR-SB and tests of executive function (DSS, fluency for vegetables). Aβ+ MCI subjects were also more likely to experience a 4-point decline on the ADAS-Cog, a previously used benchmark for evaluating clinically meaningful change.31, 32, 33 The greater deterioration on the CDR-SB and greater rate of AD medication prescriptions in Aβ+ MCI suggest that the declines observed in these subjects are relevant for clinicians and to the design of clinical trials. CN Aβ+ subjects worsened significantly more than CN Aβ− subjects on the ADAS-Cog, CDR-SB, DSS and verbal fluency test (vegetables). These findings confirm prior PiB study results in normal subjects10,18 extend them over a broader range of tests relevant to clinical trials and practice, and provide optimism that it may be possible to identify preclinical AD in CN, and test preventive interventions in these subjects. Among clinically diagnosed AD dementia subjects, Aβ+ classification predicted greater decline on the CDR-SB, MMSE, tests of verbal fluency and a trend on the ADCS-ADL. Cognitive decline in Aβ+ patients was typically least in the CN cohort and greatest in the AD cohort and (Figure 2) consistent with slower cognitive decline at earlier stages of the disease. In contrast, the slope of cognitive decline was relatively flat for Aβ− subjects, regardless of diagnostic classification.
Despite the consistent changes in psychometric test scores, the difference in percentage of MCI subjects converting to AD fell just short of significance (P=0.054). The latter may be due, in part, to the small sample size of the MCI cohort (a post hoc power analysis demonstrated only 56% power for this endpoint) and the limited 3-year duration of followup. The present findings suggest that amyloid PET may have bidirectional predictive value in MCI for progressing to AD dementia or reverting to CN. The 3.5-fold lower conversion rate from MCI to AD and the threefold higher reversion rate to normal status in Aβ− compared with Aβ+ MCI suggest that Aβ− MCI has a diverse etiology and is less likely to indicate a progressive neurodegenerative disease.
Rates of conversion from PiB Aβ+ MCI to AD dementia have varied in prior studies from 29–82% depending on entry criteria and duration of follow-up.11,12,14,16,27 The relatively low conversion rate to AD dementia in our study (35% over 36 months) should be interpreted in the context of some key issues; as noted previously, 27 MCI subjects in this study were recently diagnosed and with respect to age, APOE4 status, ADAS-Cog and memory performance (Wechsler memory scale), they were more similar to early MCI than to late MCI subjects in prior studies such as ADNI (See Supplementary Table 1).34, 35, 36, 37 No threshold on delayed recall performance was required for inclusion in order to more closely simulate the diagnostic process typically used in clinical practice 2 and to not bias the amyloid status results toward only those MCI subjects who were more rapidly progressing or closest to conversion to AD dementia. These factors may also explain the lower rate of amyloid positivity observed in our MCI subjects (37%) compared with late MCI subjects in ADNI1(~62%), and the greater similarity to early MCI subjects from ADNI-GO/2 (~43%).38 A significant proportion of Aβ+ subjects were already on cognitive medications at baseline, and there was a disproportionately higher rate of cholinesterase therapy initiation among Aβ+ subjects (71% vs 23% among Aβ− MCI subjects), which might have reduced conversion rates. Although the majority of Aβ+ MCI subjects did not decline to the point that they were considered ‘converted', they did decline sufficiently to warrant pharmacological intervention. Dropouts between 18 and 36 months may have also resulted in a lower estimated conversion rate (and our power for detecting differences); however, the dropout rate in our study is consistent with similar length industry sponsored trials. Finally, prior studies have noted a prolonged gestation period between amyloid deposition and development of dementia suggesting that longer follow-up periods may be necessary to ascertain the ultimate conversion rate in Aβ+ MCI subjects, which would be consistent with the higher observed positive predictive value (47%) observed for 4-point decline on the ADAS-Cog.
Some limitations should be considered when evaluating the present results. The primary objective succeeded in demonstrating greater decline on the ADAS-Cog in Aβ+ MCI patients; however, we did not adjust for multiple comparisons in the secondary and exploratory analyses; these analyses should therefore be interpreted in that context, despite general consistency across the ANCOVA, MMRM and conversion analyses. The Aβ+ subjects tended to be older than Aβ− subjects, so we included age as an adjustment factor in our analyses. We attempted to explore the effect of APOE ɛ4 in combination with amyloid status on cognitive and functional decline. However, as we and others have previously reported,27,39 these two factors often provide overlapping information, leading to statistical models that fail to converge or require elimination of one factor from the model; in our exploratory analyses, typically only one of these variables was retained in the model indicating that when amyloid status was included in the model APOE ɛ4 generally did not provide additional prognostic information. The majority interpretation of three readers may differ from that provided by an individual reader in the clinical setting. Finally, we did not collect CSF, FDG-PET or MRI volumetric data and could not test the comparative or combined utility of florbetapir with other biomarkers.
Our results suggest that amyloid PET has promise for detecting risk of subsequent cognitive decline in patients with MCI and CN older adults, and support the negative predictive value of amyloid PET40,41 in identifying patients unlikely to show clinical deterioration over several years of followup. Future longitudinal PET and cognitive data should further clarify the prognostic role of amyloid PET in the clinical setting, its ability to improve confidence in the recently proposed diagnoses of dementia42 and MCI2 due to AD, and for subject enrichment of therapeutic trials in the preclinical stages of AD.7
Acknowledgments
The authors would like to acknowledge the contributions of principal investigators, study staff and families who participated in the AV45-A11 study group. The authors would also like to acknowledge the substantial contributions of Professors Christopher Clark and R Edward Coleman (both deceased) in the design and conduct of the study.
PMD has received research grants through Duke University from NIA, NIMH, NINDS, NHLBI, University of California (ADCS), Northern California Research Institute (ADNI), Avid/Lilly, Elan, Bristol-Myers, Ono, Sanofi, Novartis, Medivation and Neuronetrix in the recent past. He has received advisory or speaking fees from the University of California, National University of Singapore, University of Cambridge, Royal College of Psychiatrists, Radiologic Society of North America, Alzheimer's Association, Alzheimer's Foundation of America, Postgraduate Press, Avid/Lilly, Medivation, Bristol Myers, Accera, Piramal, Grifols, Baxter, Nutricia, Great Falls Living, Sonexa, Schering, TauRx, Baxter, Elan, Genomind, Shire, Neuroptix, Bayer, Neuronetrix, Otsuka, AstraZeneca, Envivo, Targacept, Abbvie, Neurocog Trials, Lundbeck and Edwards Hospital. He received publishing royalties from a book. He owns stock in Sonexa, Clarimedix, Maxwell Health and Adverse Events Inc, whose products are not discussed in this manuscript. RAS has served as a site investigator for Avid, BMS, Elan, Janssen, Pfizer and Wyeth and as a consultant to Bayer, BMS, Elan, Eisai, Janssen, Pfizer, Roche and Wyeth and as an unpaid consultant to Avid. She has received speaking honoraria from Prizer, Janssen, Eli Lilly and Bayer. KJ was a co-investigator in the trial and has consulted for GE Healthcare, Bayer-Schering, Pfizer, Elan/Janssen and Seimens. KJ has received research support from Avid/Lilly, Bristol-Myers-Squib, Janssen (JanssenAI) and Pfizer. EMR has served as a scientific advisor to Sygnis, AstraZeneca, Bayer, Eisai, Elan, Eli Lilly, GlaxoSmithKline, Intellect, Link Medicine, Novartis, Siemens and Takeda. He has had research contracts with NIA the Arizona Department of Health Services, AstraZeneca and Avid. TZW has served as a scientific advisor for Lilly and a site co-investigator on Avid studies. MNS has served in a consulting or advisory capacity for Lilly, Amerisciences, Takeda, Eisai, Pfizer, GSK and has received royalties from Wiley and Amerisciences. He has received contracts and grants from Celgene, Ceregene, Bayer, Baxter, BMS, Lilly, Pfizer, Wyeth, Janssen, Elan, Avid, Genentech and Eisai. CHS has served on speaker bureaus for Novartis, Forest, Accera and as a consultant to Lilly. ASF has served as a consultant to Lilly and Avid, and received grant funding from Avid. AC, ADJ, ML, MAM, DMS and MJP are employees of Avid, a division of Eli Lilly, and except for ML, formerly held Avid stock or options. MG has served as a consultant to Acumen, Adamas, ALSP, Avid, Astra-Zeneca, Biogen Idec, Elan, Helicon, Intellect Neurosciences, Janssen Alzheimer Immunotherapy, J&J, Lilly, Medimmune, Neurophage, Neurogenetic Pharmaceuticals, Phloronol and Teva and on advisory boards for Helicon, Nutricia North America and Bristol Myers Squibb. MG owns stock in Prothena, and formerly held Avid stock options.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Contributor Information
AV45-A11 Study Group:
Ranjan Duara, Marwan Sabbagh, Geoffrey Lawrence Ahern, Richard F Holub, Mildred V Farmer, Beth Emmie Safirstein, Gustavo Alva, Crystal F Longmire, George Jewell, Keith A Johnson, Ron Korn, Eric M Reiman, Jeanette K Wendt, Dean Wong, P Murali Doraiswamy, R Edward Coleman, Michael Devous, Danna Jennings, Michael W Weiner, Cynthia A Murphy, Karel D Kovnat, Jeff D Williamson, and Carl H Sadowsky
Supplementary Material
References
- Aisen PS, Andrieu S, Sampaio C, Carrillo M, Khachaturian ZS, Dubois B, et al. Report of the task force on designing clinical trials in early (predementia) AD. Neurology. Jan. 2011;76:280–286. doi: 10.1212/WNL.0b013e318207b1b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MW, Aisen PS, Jack CR, Jr., Jagust WJ, Trojanowski JQ, Shaw L, et al. The Alzheimer's disease neuroimaging initiative: progress report and future plans. Alzheimers Dement. 2010;6:202–211 e207. doi: 10.1016/j.jalz.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18 ((4 Suppl:S1–S2. [PubMed] [Google Scholar]
- Fleisher AS, Chen K, Quiroz YT, Jakimovich LJ, Gomez MG, Langois CM, et al. Florbetapir PET analysis of amyloid-beta deposition in the presenilin 1 E280A autosomal dominant Alzheimer's disease kindred: a cross-sectional study. Lancet Neurol. 2012;11:1057–1065. doi: 10.1016/S1474-4422(12)70227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Rinne JO, Kadir A, Langstrom B. The use of PET in Alzheimer disease. Nat Rev Neurol. 2010;6:78–87. doi: 10.1038/nrneurol.2009.217. [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66:1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okello A, Koivunen J, Edison P, Archer HA, Turkheimer FE, Någren K, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009;73:754–760. doi: 10.1212/WNL.0b013e3181b23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Wiste HJ, Vemuri P, Weigand SD, Senjem ML, Zeng G, et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain. 2010;133:3336–3348. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivunen J, Scheinin N, Virta JR, Weigand SD, Senjem ML, Zeng G, et al. Amyloid PET imaging in patients with mild cognitive impairment: a 2-year follow-up study. Neurology. 2011;76:1085–1090. doi: 10.1212/WNL.0b013e318212015e. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Pike KE, Darby D, et al. Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer's disease. Neuropsychologia. 2008;46:1688–1697. doi: 10.1016/j.neuropsychologia.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65:557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Sojkova J, Zhou Y, Zhou Y, An Y, Ye W, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74:807–815. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YY, Ellis KA, Pietrzak RH, Ames D, Darby D, Harrington K, et al. Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology. 2012;79:1645–1652. doi: 10.1212/WNL.0b013e31826e9ae6. [DOI] [PubMed] [Google Scholar]
- Pontecorvo MJ, Mintun MA. PET amyloid imaging as a tool for early diagnosis and identifying patients at risk for progression to Alzheimer's disease. Alzheimers Res Ther. 2011;3:11. doi: 10.1186/alzrt70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SR, Golding G, Zhuang Z, Zhang W, Lim N, Hefti F, et al. Preclinical properties of 18F-AV-45: a PET agent for Abeta plaques in the brain. J Nucl Med. 2009;50:1887–1894. doi: 10.2967/jnumed.109.065284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel H, Gertz HJ, Dresel S, et al. Cerebral amyloid-beta PET with florbetaben (18F) in patients with Alzheimer's disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol. 2011;10:424–435. doi: 10.1016/S1474-4422(11)70077-1. [DOI] [PubMed] [Google Scholar]
- Rowe CC, Ackerman U, Browne W, Mulligan R, Pike KL, O'Keefe G, et al. Imaging of amyloid beta in Alzheimer's disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 2008;7:129–135. doi: 10.1016/S1474-4422(08)70001-2. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Sperling RA, Gidicsin CM, Carmasin JS, Maye JE, Coleman RE, et al. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer's disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 2013;9 ((5 Suppl:S72–S83. doi: 10.1016/j.jalz.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Johnson KA, Doraiswamy PM, Reiman EM, Fleisher AS, Sabbagh MN, et al. Amyloid deposition detected with florbetapir F 18 ((18)F-AV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiol Aging. 2013;34:822–831. doi: 10.1016/j.neurobiolaging.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doraiswamy PM, Sperling RA, Coleman RE, Johnson KA, Reiman EM, Davis MD, et al. Amyloid-beta assessed by florbetapir F 18 PET and 18-month cognitive decline: a multicenter study. Neurology. 2012;79:1636–1644. doi: 10.1212/WNL.0b013e3182661f74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Chen K, Liu X, Roontiva A, Thiyyagura P, Ayutyanont N, et al. Using positron emission tomography and florbetapir F18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch Neurol. 2011;68:1404–1411. doi: 10.1001/archneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A. 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Donepezil Study Group. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C. A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology. 2000;54:2269–2276. doi: 10.1212/wnl.54.12.2269. [DOI] [PubMed] [Google Scholar]
- Rosler M, Anand R, Cicin-Sain A, Gauthier S, Agid Y, Dal-Bianco P, et al. Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomised controlled trial. BMJ. 1999;318:633–638. doi: 10.1136/bmj.318.7184.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisen PS, Petersen RC, Donohue MC, Gamst A, Raman R, Thomas RG, et al. Clinical core of the Alzheimer's disease neuroimaging initiative: progress and plans. Alzheimers Dement. 2010;6:239–246. doi: 10.1016/j.jalz.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan VK, Risacher SL, Nho K, Kim S, Swaminathan S, Shen L, et al. APOE and BCHE as modulators of cerebral amyloid deposition: a florbetapir PET genome-wide association study Mol Psychiatryadvance online publication, 19 February 2013; doi: 10.1038/mp.2013.19 [DOI] [PMC free article] [PubMed]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. The Alzheimer's disease neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2012;8 ((1 Suppl:S1–68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Rowley J, Mohades S, Leuzy A, Dauar MT, Shin M, et al. Dissociation between brain amyloid deposition and metabolism in early mild cognitive impairment. PLoS ONE. 2012;7:e47905. doi: 10.1371/journal.pone.0047905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg A, Carter SF, Rinne J, Drzezga A, Brooks DJ, Vandenberghe R, et al. A European multicentre PET study of fibrillar amyloid in Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2013;40:104–114. doi: 10.1007/s00259-012-2237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–678. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.