Abstract
Ketamine, an N-methyl-D-aspartate receptor (NMDAR) channel blocker, has been found to induce rapid and robust antidepressant-like effects in rodent models and in treatment-refractory depressed patients. However, the marked acute psychological side effects of ketamine complicate the interpretation of both preclinical and clinical data. Moreover, the lack of controlled data demonstrating the ability of ketamine to sustain the antidepressant response with repeated administration leaves the potential clinical utility of this class of drugs in question. Using quantitative electroencephalography (qEEG) to objectively align doses of a low-trapping NMDA channel blocker, AZD6765 (lanicemine), to that of ketamine, we demonstrate the potential for NMDA channel blockers to produce antidepressant efficacy without psychotomimetic and dissociative side effects. Furthermore, using placebo-controlled data, we show that the antidepressant response to NMDA channel blockers can be maintained with repeated and intermittent drug administration. Together, these data provide a path for the development of novel glutamatergic-based therapeutics for treatment-refractory mood disorders.
Keywords: antidepressant efficacy, lanicemine, NMDA channel blocker
Introduction
Major depressive disorder (MDD) is a highly prevalent, debilitating and life-threatening psychiatric disorder affecting an estimated 350 million people.1 Despite a large number of existing antidepressant drugs, developed largely within the context of a monoamine hypothesis of mood disorders, recent, large-scale, community-based studies have made us increasingly aware of the limitations on current treatment strategies.2 In particular, these strategies are associated with both a significantly delayed onset of therapeutic action and a large percentage of treatment-resistant patients. Acknowledgment of the current unmet medical need provides an impetus for the development of alternative treatments based on a deeper understanding of the pathophysiology of depression and related disorders.
Converging evidence implicates the glutamate neurotransmitter system in the pathophysiology of mood and other stress-related disorders3, 4 making it a target for development of novel antidepressant agents.5 Supporting this approach, small placebo-controlled and open-label trials provide evidence that ketamine, an N-methyl-D-aspartate receptor (NMDAR) antagonist, possesses rapid-acting antidepressant effects in a subset of individuals who had not previously responded to classical monoaminergic-based medications.6 Nevertheless, while the results of these studies are compelling, their interpretation is limited by the incomplete ability to blind study subjects and investigators to study drug assignment because of ketamine's well-documented acute physiological and psychological side effects.7 Furthermore, as MDD is a chronic disorder, the clinical usefulness of ketamine as an antidepressant remains suspect due to the lack of studies showing enduring benefit of repeated administration and anticipated difficulties in translating the treatment from the laboratory to clinic setting.
The antidepressant effects of ketamine are increasingly believed to result from changes in cortical excitability, likely caused by cortical disinhibition,8, 9 related to a reduction in the activity of inhibitory interneurons.10 Acute changes in cortical excitability and glutamate release are proposed to initiate a sequence of biochemical and structural changes within cortical networks, leading to a therapeutic response capable of outlasting actual drug exposure.11, 12 Similarly, the decreased activation of inhibitory interneurons and increased activation of pyramidal cells in the prefrontal cortex are postulated to be associated with the psychotomimetic properties of ketamine and other NMDAR antagonists.10, 13 This raises the question: can the acute psychotomimetic effects be disentangled from the antidepressant benefits of this class of compounds?
We conducted preclinical and clinical investigations with a low-trapping, NMDA channel blocker, lanicemine (also known as AZD6765 or AR-R 15896AR).14, 15 Low-trapping NMDA channel blockers are posited to have greater therapeutic windows relative to classic NMDAR antagonists (for example, ketamine),14 and we hypothesized that lanicemine would produce antidepressant effects at doses without limiting dissociative side effects. Using gamma-band electroencephalography (EEG), a putative marker of cortical disinhibition, to ensure appropriate brain penetration and dose alignment between ketamine and lanicemine, we tested the ability of lanicemine to produce antidepressant efficacy with minimal psychological and physiological side effects. Moreover, to examine the true potential clinical utility of this novel drug class, we determined whether the antidepressant effect of lanicemine could be safely extended by repeated administration over 3 weeks.
Materials and methods
Effects of ketamine and lanicemine on EEG in rodent models
Male Sprague-Dawley rats (n=6–9) were implanted with frontal and temporal skull screw electrodes for continuous EEG recording and trained to perform a single-tone operant discrimination task for food reward. EEG was recorded and behavioral performance was evaluated for a 30-min period before dosing and for three 30-min periods following dosing with intraperitoneal lanicemine (3, 10 or 30 mg kg−1), ketamine (1, 3, 10 or 30 mg kg−1) or vehicle control. EEG data acquired by Neuralynx (Bozeman, MO, USA) were imported to NeuroExplorer Ver. 3.183 software suite (Plexon, Dallas, TX, USA). Consecutive 10-s epochs of EEG data from each channel were subjected to a fast Fourier transform, from which EEG power density was computed from 1 to 50 Hz.
For analysis of drug effects, power spectral density data were compared for the 20-min period before dosing and for 1.5 h after dosing using 1- to 5-min analysis bins. Relative changes were normalized for post- to pre-dosing periods in each of five frequency bands (delta, theta, alpha, beta and gamma).
All animal experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institute of Animal Care and Use Committee (IACUC) of AstraZeneca (Wilmington, DE, USA).
Lanicemine studies in human
All studies in man were approved by the institutional review boards at each site and were conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and the International Conference on Harmonization guideline E6: Good Clinical Practice. All participants provided written, informed consent before study entry and had the right to withdraw from the study at any time.
EEG, physiological and dissociative effects of lanicemine relative to ketamine in human (phase I, D2285M00008/NCT01130909)
A phase I, randomized, double-blind, four-way, crossover study in healthy subjects was performed at a single center in France between May 2010 and January 2011 (D2285M00008/NCT01130909). Males aged 30–45 years, with body mass index 18–30 kg m−2 and non-smoking status for at least 4 weeks, without clinically relevant acute or chronic disease, received lanicemine 75 mg, lanicemine 150 mg, ketamine 0.5 mg kg−1 or placebo as single intravenous (i.v.) administrations. Washout was ⩾7 days between study periods.
Quantitative gamma-band EEG (qEEG) was assessed predose and 0.25, 1, 1.25, 3 and 8 h after starting a 60-min infusion of each study drug. Calculations were performed on 28 scalp electrodes placed according to the international 10–20 system. EEG acquisition was performed using the AS40 Comet headstage and amplifier (Grass Technologies, West Warwick, RI, USA). Analysis of composite brain maps utilized proprietary Standard Decision Tree methodology to compare active treatment vs placebo and to identify the direction of drug effect using an electrode-by-electrode procedure. Gamma-band data in the range from 32.5 to 48 Hz were presented as absolute change and relative change vs other frequency bands. Additional parameters derived from the EEG spectrum below 13 Hz were the alpha slow-wave index, used as a monitor of vigilance level,16 and theta-cordance (4–7.5 Hz band), which combines information from both absolute and relative power.17
Safety evaluations included safety and tolerability assessments, adverse events, pupil size and electronystagmography, and subjective dissociative effects measured by the 27-item Clinician Administered Dissociative States Scale (CADSS). Opticokinetic parameters were measured 25 min after start of infusion using Metrovision MON 2008H (Pérenchies, France) and CADSS was assessed at prespecified times up to 8 h after start of infusion.
qEEG cordance was analyzed using repeated measures analysis of covariance on absolute values, with treatment/dose, period, time point and treatment/dose by time point and sequence as fixed effects, with subjects nested within sequence as a random effect and baseline values as a covariate. For CADSS, a mixed model analyzed change from baseline, with treatment/dose, period, time point and treatment/dose by time point interaction as fixed effects, with subjects nested within sequence as a random effect and baseline values as a covariate.
Single-dose (100 mg) exploratory safety and efficacy trial of lanicemine in patients with treatment-resistant MDD (phase IIA, D6702C00001/NCT00491686)
The phase IIA, double-blind, randomized study (D6702C00001/NCT00491686; study 1) was performed at five centers in the United States between July 2007 and November 2007. It consisted of a screening period (⩽30 days), one inpatient treatment period, and one follow-up visit 7–10 days after treatment. Outpatients (men and women) aged 21–65 years with DSM-IV-TR-diagnosed MDD, confirmed by the MINI (Mini International Neuropsychiatric Interview), a history of poor response to ⩾2 antidepressants, and baseline Hamilton Rating Scale for Depression (HAM-D-17) score ⩾20 were eligible. Exclusion criteria included: current episode of depression ⩽12 weeks or ⩾5 years; history of DSM-IV Axis I disorder other than MDD or substantial Axis II disorder; use of mood stabilizers, other antipsychotic or psychoactive drugs within 7 days of day 1 or fluoxetine or monoamine oxidase inhibitors within 14 days of day 1 of the treatment period; and evidence of other clinically relevant disease.
Lanicemine 100 mg or placebo (0.9% saline) was administered as single i.v. infusions (30 ml volume over 60 min). The primary efficacy evaluation was change in Montgomery-Åsberg Depression Rating Scale (MADRS) total score from baseline to 24 h post infusion. Secondary variables included: change in MADRS total score at other scheduled time points; Bond-Lader Visual Analogue Scale; Brief Psychiatric Rating Scale; and CogState (CogState, Melbourne, Australia). Safety evaluations included: adverse events, vital signs, physical examination, clinical laboratory evaluations and electrocardiograms.
Change from baseline in MADRS total score was compared between treatment groups with last observation carried forward (LOCF) in the intent-to-treat (ITT) population, using an analysis of covariance model with baseline MADRS as a covariate and treatment as a fixed effect. Descriptive statistics were used for secondary efficacy and safety data. To detect a signal for efficacy variables in this exploratory study, the prespecified statistical tests were two-sided at alpha of 20%. No adjustments were made for multiplicity.
Adjunctive, multiple-infusion efficacy trial of lanicemine in patients with moderate-to-severe MDD and a history of poor response to antidepressants (phase IIB, D6702C00009/NCT00781742)
The phase IIB, double-blind, randomized, outpatient study (D6702C00009/NCT00781742; study 9) was performed at 30 centers in the United States between October 2008 and March 2010. It consisted of a screening period (⩽30 days), a 3-day placebo run-in (when patients received one single-blind placebo infusion (0.9% saline)), and a 3-week treatment period, followed by a 5-week treatment-free follow-up.
Outpatients (men and women) aged 18–65 years with DSM-IV-TR-diagnosed MDD, confirmed by the MINI, and a history of poor response were included. Poor response was defined as treatment failure on two or more antidepressants after exposure at adequate doses or maximum tolerated doses for ⩾4 weeks. Informed by data on inadequate response to different classes of antidepressants in STAR*D,18 inadequate response to different classes of antidepressants was not a requirement. Initially, despite treatment with at least one antidepressant, a baseline HAM-D-17 score of ⩾26, a Clinical Global Impression of Severity (CGI-S) score of ⩾5 and a Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR-16) score of ⩾21 were required. In addition to the first antidepressant, a second antidepressant was permitted as well. Acceptable combinations of two antidepressants were SSRI/SNRI+bupropion and SSRI/SNRI+mirtazapine. Concomitant buspirone, triiodothyronine and lithium were permitted. Also permitted were concomitant benzodiazepines and hypnotics. To improve recruitment, the inclusion criteria were reduced to include patients with baseline HAM-D-17 ⩾20, CGI-S ⩾4 and QIDS-SR-16 ⩾16. Of 152 patients randomized, 72 patients were enrolled under the original protocol and 80 patients were enrolled under the amended protocol. Exclusion criteria included: current episode of depression ⩽12 weeks or ⩾5 years; lifetime history of DSM-IV Axis I disorder other than MDD with the exception of generalized anxiety disorder, comorbid panic disorder and simple phobias; HAM-D-17 item 3 score ⩾2; use of mood stabilizers, other antipsychotic drugs or tricyclic antidepressants within 7 days of day 1 or monoamine oxidase inhibitors within 14 days of day 1 of the treatment period; and evidence of other clinically relevant disease.
Patients were randomized in a 1:1:1 ratio to lanicemine 100 mg, lanicemine 150 mg or placebo (three i.v. infusions per week) as adjunct to ongoing psychotropics that included at least one antidepressant. The predefined primary efficacy variable was change from randomization to week 3 in MADRS total score. Secondary variables included: MADRS score change at other scheduled assessments; remission (that is, MADRS score ⩽10); response (that is, ⩾50% reduction from baseline in MADRS score); Hamilton Rating Scale for Anxiety (HAM-A; anxiety); HAM-D-17 and QIDS-SR-16 (depressive symptoms); CGI-S and Clinical Global Impression of Improvement (CGI-I; global improvement); and Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q; quality of life). Efficacy evaluations were performed at weekly intervals from baseline (randomization) to week 8. Changes in QIDS-SR-16 score at day 1 and MADRS score at day 3 were also measured to assess onset of effect.
Safety evaluations included: adverse events during treatment and follow-up, vital signs, weight and body mass index changes, physical examination, clinical laboratory evaluations and dissociative state assessed by the CADSS. Adverse events, vital signs and weight and body mass index changes were assessed at planned visits to week 8. Clinical laboratory evaluations were performed at weeks 1–4 and 8. CADSS was assessed at weeks 1–3.
Change from baseline in MADRS total score and continuous secondary efficacy variables were compared between the two lanicemine groups and placebo at week 3 with LOCF in the ITT analysis set, using an analysis of covariance model with baseline MADRS total score as a covariate, with treatment, MDD disease severity and comorbid generalized anxiety disorder status as fixed effects, and pooled center as a random effect. A logistic regression model including treatment and baseline in the model was used for categorical secondary efficacy variables.
All statistical comparisons were based on a two-sided significance level of alpha=0.05. For the primary analysis, Dunnett's procedure was used to adjust for multiplicity (comparisons between each lanicemine dose and placebo). For secondary analyses, no multiplicity adjustments to P-values were made. The ITT analysis set included all randomized patients who received at least one dose of study drug and who had a randomization (baseline) MADRS total score assessment and at least one MADRS score postrandomization. The safety analysis set included all randomized patients who received at least one dose of study drug.
Results
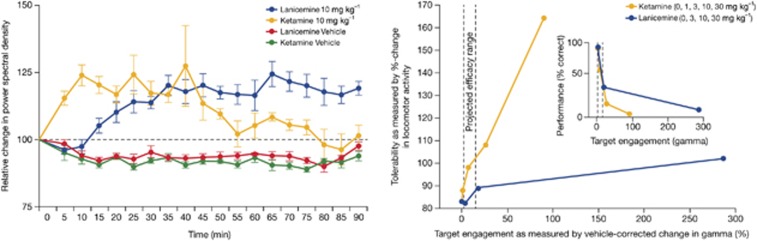
Previous studies with lanicemine and ketamine demonstrated that both compounds bind with low-to-moderate affinity to sites within the NMDA channel pore, exhibit strong voltage dependence, and have similar lack of NR2A vs NR2B subunit selectivity (Table 1).14, 19, 20, 21, 22 However, at steady-state concentrations, ketamine had a greater propensity to be trapped within the NMDA channel pore following the removal and reapplication of glutamate (trapping: 86% with ketamine vs 54% with lanicemine). Low trapping theoretically preserves use-dependent channel block under conditions of normal, pyramidal cell-driven, synaptic transmission.14 Thus, while NMDARs are ubiquitously expressed within the central nervous system, the low-trapping property of lanicemine may bias channel block to those elements of the brain, such as cortical interneurons, with high levels of tonic activity. Since selective reduction in NMDAR activity on cortical interneurons has been shown to increase spontaneous, high-frequency (gamma-band ∼40 Hz) EEG,23, 24 gamma-band EEG may serve as a useful biomarker for NMDA channel blockers in general and lanicemine in particular. To test this hypothesis, cortical EEG recordings were obtained from rats trained to perform an auditory detection task for food reward. Both ketamine and lanicemine produced pronounced dose-dependent elevations in spontaneous gamma-band EEG (Figure 1, left), but only gamma changes for ketamine were tightly coupled to increases in locomotor activity (Figure 1, right)—suggesting that lanicemine not only engages brain circuits involved in the generation of gamma-EEG, but also influences these networks independent of the broader systems-level disruptions typical of ketamine.
Table 1. Comparative NMDA channel binding and trapping profiles of lanicemine and ketamine in in-vitro studies.
| Assay | Ketamine | Lanicemine |
|---|---|---|
| Binding (Ki)21 | 0.15 μM22 | 0.56–2.1 μM14a |
| IC50 (Xenopus oocyte)20 | 2.8 μMa | 6.4 μMa |
| IC50 (CHO cell)19 | 0.57 μMa | 4–7 μMa |
| NR2A/NR2B IC50 ratio (Xenopus oocyte)a | 2.6a | 1.4a |
| Voltage dependence of block for NR1A/2B22 | 7–30 folda | 5–7 folda |
| Trapping14 | 86%14 | 54%14 |
Abbreviations: CHO, Chinese hamster ovary; IC50, half maximal inhibitory concentration; Ki, receptor binding affinity; NMDA, N-methyl-D-aspartate; NR2A/NR2B, NMDA receptor subunits.
For methodology for the determination of IC50 values, please see Supplementary Methods.
AstraZeneca data on file.
Figure 1.
Electroencephalography (EEG) effects of lanicemine relative to ketamine in rodent model. Left: Time course of gamma-band EEG following administration of lanicemine and ketamine (doses: 10 mg kg−1) and respective vehicles. Right: Tolerability of lanicemine vs ketamine, measured by hyper-locomotor activity, at comparable levels of target engagement.
EEG, physiological and dissociative effects of lanicemine relative to ketamine in human
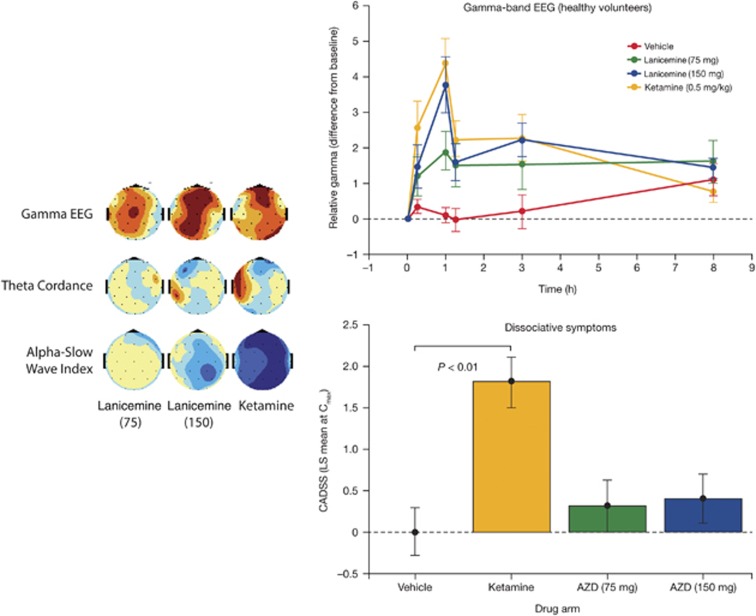
To determine whether the differentiated EEG/side-effect profile seen preclinically with ketamine and lanicemine translates to humans, we conducted the qEEG crossover study in healthy volunteers. Out of 23 subjects randomized, 14 subjects were treated with lanicemine 75 mg, 19 with lanicemine 150 mg, 17 with ketamine and 15 with placebo. The study was stopped earlier than planned following two serious adverse events (syncope due to orthostatic hypotension) occurring during ketamine infusion. Significant increases in gamma-band EEG were observed at the stop of infusion for both ketamine and lanicemine, and baseline-corrected gamma-EEG following 150 mg lanicemine was statistically indistinguishable from ketamine (0.5 mg kg−1) (Figure 2, left and top right). In addition, both ketamine and the 150-mg dose of lanicemine produced significant reductions in prefrontal theta-cordance, a derived EEG biomarker putatively linked to early antidepressant treatment response (Figure 2, left).25, 26 There were no serious adverse events associated with lanicemine.
Figure 2.
Electroencephalography (EEG) effects of lanicemine relative to ketamine in man. Left: Distribution over skull surface of absolute gamma EEG magnitude, theta-cordance and alpha slow-wave index at 1 h of perfusion. Color mapping depicts levels of statistical significance between study drug (lanicemine 75 or 150 mg; ketamine 0.5 mg kg−1) and vehicle at a given electrode location (blue: decrease; red: increase in P-value). Top right: Comparison of lanicemine (75 or 150 mg), ketamine (0.5 mg kg−1), and vehicle for mean (s.e.) change in relative gamma-EEG magnitude over time (eyes closed). Bottom right: Comparison of lanicemine (75 or 150 mg), ketamine (0.5 mg kg−1), and vehicle for mean (s.e.) change in Clinician Administered Dissociative States Scale (CADSS) total score from baseline. CADSS total score was increased significantly with ketamine vs vehicle at 1 h (P<0.01) and across all times (P<0.05). Lanicemine (each dose) did not significantly increase CADSS total score vs vehicle at any time.
Importantly, whereas ketamine infusion was associated with significant dissociative effects compared with placebo as determined by the CADSS, lanicemine did not produce significant dissociative symptoms (Figure 2, bottom right). Adverse events potentially related to dissociative-type events (including feeling abnormal, disinhibition, illusion and dissociation) were reported in 7% (n=1) of the lanicemine 75 mg, 11% (n=2) of the lanicemine 150 mg and 24% (n=4) of the ketamine group. Visual hallucination (a psychotomimetic effect) was reported in 5% (n=1) of the lanicemine 150 mg group and in no patients in other groups. Possibly aligned to the group differences in CADSS scores, a strong effect of ketamine, relative to lanicemine, was apparent on a measure of low-frequency EEG activity—the alpha-slow wave index—putatively aligned to sedative brain states (Figure 2, left).27 Mean±s.d. supine systolic blood pressure at the end of the 60-min infusion was increased by 4.9±9.5 mm Hg in the ketamine, 3.9±11.4 mm Hg in the lanicemine 150 mg, 3.4±7.3 mm Hg in the lanicemine 75 mg, and −0.3±8.2 mm Hg in the placebo group compared with preinfusion values.
Together, these data support the utility of gamma-band EEG as a translational biomarker for NMDA channel blockers, and provide evidence for differentiation of lanicemine from ketamine across multiple end points, including psychotomimetic liability at comparable levels of gamma-EEG.
Single-dose (100 mg) exploratory safety and efficacy trial of lanicemine in patients with treatment-resistant MDD
Translational and previous preclinical data28 suggested a psychotomimetic-free therapeutic window for lanicemine in humans at doses of 75–150 mg. We therefore conducted a pilot study to determine whether a dose in this range (100 mg) might be relatively well tolerated and yet still provide an antidepressant signal. In a phase IIA monotherapy study (study 1), 34 treatment-resistant patients (mean HAM-D-17 score ∼25; Supplementary Table 1) were randomized to a single infusion of lanicemine 100 mg i.v. (n=16 (7, male; 9, female)) or placebo (n=18 (7, male; 11, female)).
Lanicemine 100 mg was generally well tolerated, with the most common adverse event being dizziness (Supplementary Table 2). Lanicemine produced no clinically meaningful effects on psychotomimetic symptoms measured by the Brief Psychiatric Rating Scale (mean±s.e. at 1 h: 22.8±1.1 for lanicemine vs 23.9±1.2 for placebo; at 4 h: 23.1±1.2 vs 24.4±1.5), dissociative symptoms measured by the CADSS (least squares mean (LSM)±s.e. change from baseline at 1 h: 0.6 (0.59) for lanicemine vs −0.8 (0.55) for placebo), or cognitive functions measured by CogState (Supplementary Figures 1 and 2).
There were no serious adverse events reported during treatment. At the 24-h time point, we failed to observe a statistically significant difference in the change in MADRS scores between lanicemine vs placebo. However, this comparison was confounded by a strong placebo effect (for example, 14.2 MADRS score change) at this time point, rendering the meaningfulness of the non-statistically significant, numerically greater change for lanicemine vs placebo (2.44, P=0.472) difficult to interpret. However, we observed statistically significant differences (according to prespecified criteria for this exploratory study) for lanicemine vs placebo in MADRS scores at 1 and 72 h after the infusion (P=0.183 and 0.089, respectively) (Supplementary Figure 3). An antidepressant-like effect was also indicated by changes in sad/happy Bond-Lader Visual Analogue Scale at 4 h (P=0.137). The trend for the antidepressant effect of lanicemine peaked at 72 h (MADRS score change vs placebo of −5.7 (P=0.089)) and had dissipated vs placebo by 10–13 days after the single i.v. infusion, while remaining on average 10 points below baseline measures.
Adjunctive, multiple-infusion efficacy trial of lanicemine in patients with moderate-to-severe MDD and a history of poor response to antidepressants
On the basis of the exploratory data from study 1, a second phase II study (study 9) was designed to determine whether repeated administration of lanicemine over 3 weeks at an interval of three infusions per week would consolidate and extend the therapeutic benefits observed from a single administration. In study 9, we examined the effect of augmenting patients' existing antidepressant therapies with repeated lanicemine administration on symptom improvement in outpatients with moderate-to-severe MDD with a history of poor response to multiple antidepressants. Infusions were stopped after 3 weeks to explore the durability of the antidepressant effect over a subsequent 5-week observation period in which drug was not administered.
Male and female outpatients (Supplementary Table 3) received lanicemine 100 mg (n=15, male; n=36, female) or 150 mg (n=20, male; n=31, female) or placebo (n=15, male; n=35, female), three times per week on non-consecutive days for 3 weeks (Supplementary Figure 4). To help mitigate the large placebo effect seen in study 1, this second study had a single-blind i.v. saline run-in infusion. In addition, patients were allowed to remain on their background antidepressant medications (Supplementary Table 4) and be treated in an outpatient setting with the anticipation that this would minimize changes to their baseline levels of depression. The doses of adjunctive antidepressants and benzodiazepines were not changed after enrollment, including the 3-week treatment period and 5-week follow-up period. All 152 randomized patients received their assigned study treatment.
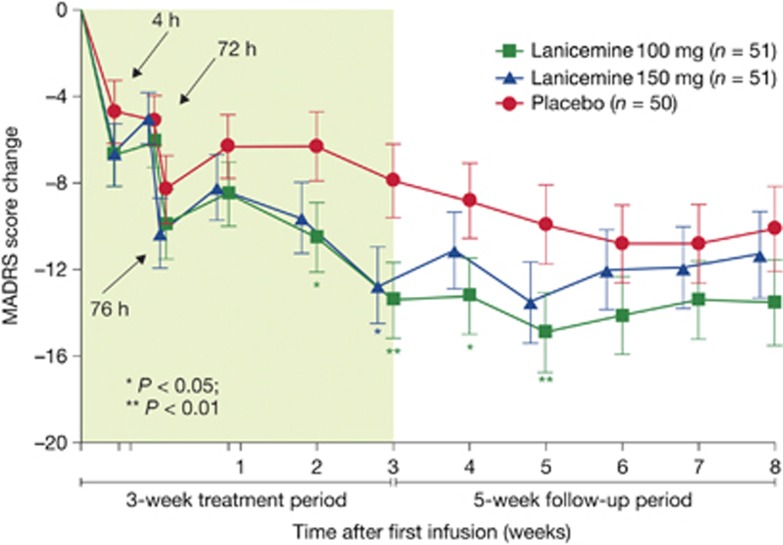
Patients treated with lanicemine (100 or 150 mg) exhibited a significantly greater change from baseline at week 3 in MADRS total score (the primary efficacy variable) than placebo-treated patients (Figure 3). The LSM difference between lanicemine 100 mg and placebo and between lanicemine 150 mg and placebo was −5.5 (95% confidence interval (CI)=−9.1 to −1.9, P=0.006) and −4.8 (95% CI=−8.5 to −1.2, P=0.019), respectively.
Figure 3.
Montgomery Åsberg Depression rating Scale (MADRS) score change at prespecified time points during the 3-week treatment and 5-week follow-up period in lanicemine 100 mg, lanicemine 150 mg and placebo groups (intent-to-treat (ITT), last observation carried forward (LOCF)) (phase IIB study, study 9).
There was a numerical difference for the change in total MADRS score in favor of lanicemine 100 mg over placebo at all visits (Figure 3). Significant onset of efficacy measured by mean MADRS score was demonstrated as early as 2 weeks for 100 mg (LSM difference from placebo=−4.2, 95% CI −7.50 to −0.99; two-sided P=0.011), and this mean difference persisted to week 5 (LSM difference=−4.9, 95% CI −8.64 to −1.24; two-sided P=0.009)––that is, 2 weeks after the last infusion.
Most secondary end points supported the finding of significant improvement in MADRS score at 3 weeks. Both lanicemine doses induced a significant benefit in the CGI-I scale and significant improvements in anxiety symptoms as measured by HAM-A score, compared with placebo (Table 2). The 100 mg, but not 150 mg, lanicemine group also showed significant effects on the HAM-D and the patient-reported QIDS-SR-16 and Q-LES-Q. The study was not powered to detect a difference between the active dosing groups; however, the 100-mg dose showed numerically better results than the 150- mg dose on all these measures (Table 2). Finally, the type of background antidepressant (SSRI vs SNRI) did not appear to affect the response to lanicemine.
Table 2. Secondary efficacy variables at 3 weeks in lanicemine 100 mg, lanicemine 150 mg and placebo groups (ITT, LOCF) (phase IIB study, study 9).
| Efficacy variable | Lanicemine (100 mg) (n=51) | Lanicemine (150 mg) (n=51) | Placebo (n=50) |
|---|---|---|---|
| Response,a n (%) | 19 (37) | 15 (29) | 8 (16) |
| (OR vs placebo) | OR=3.34 | OR=2.12 | |
| P-value | P=0.014 | P=0.137 | |
| Remission,b n (%) | 10 (20) | 11 (22) | 5 (10) |
| (OR vs placebo) | OR=2.20 | OR=2.36 | |
| P-value | P=0.186 | P=0.144 | |
| CGI-I, n (%), category ⩽2 | 32 (65) | 24 (47) | 13 (26) |
| (OR vs placebo) | OR=5.41 | OR=2.54 | |
| P-value | P=<0.001 | P=0.030 | |
| CGI-S score vs baseline | –1.5 | −1.5 | −0.8 |
| (Δ vs placebo) | Δ=−0.7 | Δ=−0.6 | |
| P value | P=0.006 | P=0.009 | |
| QIDS-SR-16 score vs baseline | −8.7 | −6.8 | −6.3 |
| (Δ vs placebo) | Δ=−2.5 | Δ=−0.6 | |
| P-value | P=0.016 | P=0.575 | |
| HAM-D-17 score vs baseline | −11.9 | −10.7 | −8.0 |
| (Δ vs placebo) | Δ=−3.8 | Δ=−2.6 | |
| P-value | P=0.010 | P=0.079 | |
| HAM-A score vs baseline | −7.8 | −7.2 | −4.2 |
| (Δ vs placebo) | Δ=−3.5 | Δ=−3.0 | |
| P-value | P=0.002 | P=0.009 | |
| Q-LES-Q-SF score vs baseline | 8.1 | 6.8 | 4.2 |
| (Δ vs placebo) | Δ=3.9 | Δ=2.6 | |
| P-value | P=0.026 | P=0.137 |
Abbreviations: OR, odds ratio; CGI-I, Clinical Global Impression of Improvement; CGI-S, Clinical Global Impression of Severity; HAM-D, Hamilton Rating Scale for Depression; HAM-A, Hamilton Rating Scale for Anxiety; ITT, intent-to-treat; LOCF, last observation carried forward; MADRS, Montgomery Åsberg Depression Rating Scale; QIDS-SR-16, Quick Inventory of Depressive Symptomatology-Self-Report; Q-LES-Q-SF, Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form.
P values: vs placebo.
Response defined as ⩾50% reduction from baseline in MADRS total score at week 3.
Remission defined as MADRS total score ⩽10 at week 3.
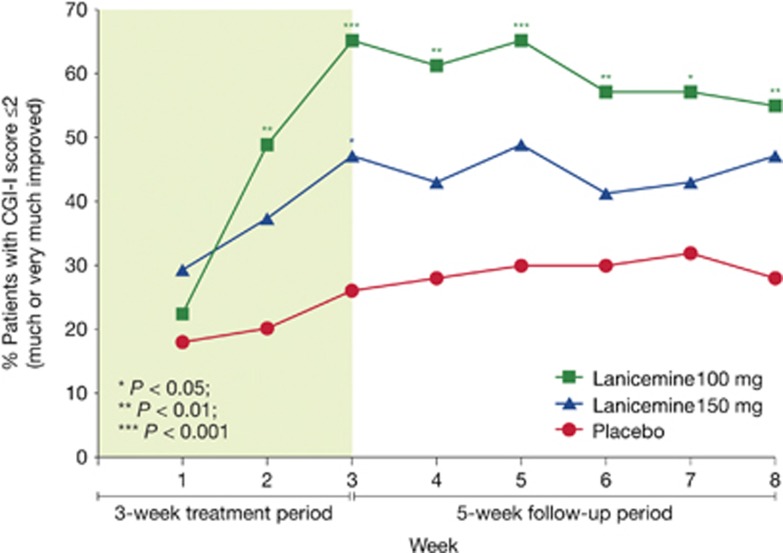
Treatment response was also assessed utilizing the proportion of patients achieving a CGI-I score of 1 (very much improved) or 2 (much improved). In prospectively planned analyses, statistically significant separation from placebo was seen at week 3 for both 100 and 150 mg lanicemine (Figure 4). In post hoc analyses based on the odds ratio of response vs placebo, statistical separation from placebo was seen at every time point after stopping infusions of 100 mg lanicemine. This suggests persistence of efficacy for up to 5 weeks after stopping infusions employing a widely utilized scale of overall improvement.
Figure 4.
Clinical Global Impression of Improvement (CGI-I) response rate (%) at prespecified time points during the 3-week treatment and 5-week follow-up period in lanicemine 100 mg, lanicemine 150 mg and placebo groups. Response is defined as CGI-I score⩽2 (much or very much improved) (phase IIB study, study 9).
Both doses of lanicemine were generally well tolerated, with the most common adverse event being dizziness around the time of infusion, with 12, 49, and 37%, in the placebo, lanicemine 100 and 150 mg groups, respectively, experiencing transient dizziness (Table 3). Potentially dissociative-related adverse events (including mental impairment, depersonalization, dissociation and illusion) occurred during the treatment period in 0, 6 and 10% of the respective groups. Similar to other studies, transient increases in supine blood pressure that were considered not clinically meaningful were observed around the time of infusion, with lanicemine 150 mg having a greater effect than 100 mg. Mean (s.d.) supine systolic blood pressure at the end of treatment increased by 4.8±16.1 and 2.0±12.2 mm Hg in the lanicemine 150 and 100 mg groups, respectively, vs −0.6±12.1 with placebo. Increases in supine systolic blood pressure ⩾20 mm Hg during the treatment period occurred in 37% (n=19) of the lanicemine 100 mg day−1, 55% (n=28) of the lanicemine 150 mg day−1, and 26% (n=13) of the placebo group. As in study 1, lanicemine produced no clinically meaningful difference compared with placebo on dissociative symptoms as measured by CADSS, with mean±s.d. changes vs baseline at week 3 of −2.6±7.1 in the lanicemine 100 mg day−1, −0.8±3.8 in the lanicemine 150 mg day−1 and −1.8±4.9 in the placebo group. There were no serious adverse events reported during treatment.
Table 3. Most common adverse events in the treatment period by decreasing incidence in the 150-mg group (based on ⩾5% in either lanicemine group) (safety analysis set) (phase IIB study, study 9).
| Preferred term | Lanicemine (100 mg) (n=51) | Lanicemine (150 mg) (n=51) | Placebo (n=50) |
|---|---|---|---|
| Dizziness, n (%) | 25 (49) | 19 (37) | 6 (12) |
| Nausea, n (%) | 5 (10) | 10 (19) | 8 (16) |
| Somnolence, n (%) | 8 (16) | 4 (8) | 2 (4) |
| Blood pressure increased, n (%) | 3 (6) | 4 (8) | 0 (0) |
| Dry mouth, n (%) | 1 (2) | 4 (8) | 2 (4) |
| Hypoesthesia, n (%) | 1 (2) | 4 (8) | 1 (2) |
| Headache, n (%) | 8 (16) | 3 (6) | 4 (8) |
| Constipation, n (%) | 1 (2) | 3 (6) | 0 (0) |
| Nasopharyngitis, n (%) | 1 (2) | 3 (6) | 0 (0) |
| Diarrhea, n (%) | 4 (8) | 2 (4) | 5 (10) |
| Urinary tract infection, n (%) | 3 (6) | 2 (4) | 4 (8) |
| Upper abdominal pain, n (%) | 4 (8) | 1 (2) | 1 (2) |
| Fatigue, n (%) | 4 (8) | 1 (2) | 0 (0) |
| Sedation, n (%) | 3 (6) | 1 (2) | 1 (2) |
Discussion
There is a rapidly growing interest in the development of glutamatergic drugs, especially NMDAR antagonists, for the treatment of severe mood disorders.5 Here, we report robust and sustained antidepressant effects for a low-trapping NMDA channel blocker, lanicemine (100 mg), at doses without the limiting prominent dissociative side effects observed with ketamine. Completed studies with lanicemine now encompass the largest pool of depressed patients (n>120) exposed to an NMDA channel blocker to date, and represent a stringent test of the hypothesis that NMDAR antagonists can deliver antidepressant efficacy independent of psychotomimetic side effects.
The ability to produce antidepressant efficacy without limiting psychotomimetic and dissociative side effects is not only critical from a patient safety perspective, but also important for clarifying the direct pharmacological link between efficacy and NMDA channel blockade. Depression trials are notoriously susceptible to both placebo effects and investigator unblinding. While studies with ketamine6 and the NR2B-selective NMDAR antagonist, CP101,606,29 provided initial evidence of therapeutic potential, the prominent dissociative effects confounded data interpretation. The observation that lanicemine produced antidepressant efficacy without significant psychotomimetic effects suggests that difficulties in maintaining study blind are unlikely in and of themselves to account, completely, for the antidepressant effects of NMDAR antagonists. This contention is further supported by a single-dose lanicemine study in which subjects and investigators were no better than chance at guessing whether a patient had been exposed to drug or placebo.30
In contemplating the differentiated psychotomimetic and dissociative profile of lanicemine relative to ketamine, one must consider that the lack of prominent cognitive, perceptual and dissociative effects could be a consequence of lanicemine being dosed relatively lower down on the dose–response curve than ketamine. We employed gamma-band EEG in an attempt to functionally align ketamine and lanicemine doses. In our clinical studies, lanicemine produced similar effects on gamma-band EEG measures to the doses of ketamine previously associated with antidepressant activity, thus suggesting that functional dose alignment was achieved, at least in relationship to cortical excitability. However, while comparative acute efficacy effects between ketamine and lanicemine are not directly addressed by our studies, the magnitude of acute efficacy for lanicemine vs placebo appears to be lower than in previously published studies with ketamine.30 This may be related to differences in relative dosing of the drugs, although the lack of evidence suggesting that the higher (150 mg) dose of lanicemine was more effective than the 100-mg dose would not support this hypothesis. It is also possible that the differences in acute response to the two drugs could be related to the differences in study designs (existing placebo-controlled ketamine studies being crossover in design) and the incomplete blinding of the ketamine trials. This could also result from variability in the effect size seen across different studies, which is not uncommon in the antidepressant literature.
In any case, the observation that antidepressant efficacy without marked psychotomimetic side effects can occur with an NMDAR antagonist at doses that produce significant elevations in gamma-band EEG provides important insight into mechanisms of action. The correlation between spontaneous gamma-EEG and behavioral abnormalities in animals (and to a lesser extent in humans) for non-selective NMDA channel blockers led to the hypothesis that gamma-EEG is a biomarker of acute psychosis. However, recent data undermine this hypothesis. First, the NR2B-selective compound, CP101,606, produces minimal elevations in gamma-EEG preclinically,24 but strong clinical dissociation. Second, we show that drug exposure increases gamma-EEG at a faster rate than concomitant increases in either locomotion (preclinical) or psychotomimetic side effects. While a strict mapping between spontaneous gamma-EEG and psychosis is unlikely, the role of subtle alterations in regionally or task-specific gamma production cannot be excluded.
Perhaps, the most clinically relevant information resulting from these studies is the demonstration of a sustained antidepressant effect with repeated dosing of lanicemine. The existing placebo-controlled data with ketamine focus solely on the acute response to a single infusion, showing that the antidepressant response effect rapidly wanes in the majority of patients over the subsequent week. A previous open-label study in which 24 subjects received a series of up to six infusions of ketamine (0.5 mg kg−1) three times weekly over a 12-day period suggested the antidepressant response to NMDAR antagonists could be extended with repeated dosing.31 However, until now there was no evidence of a sustained antidepressant effect in a randomized trial. We clearly demonstrate not only that the antidepressant effect is sustained with repeated dosing of an NMDA channel blocker, but also that the duration of response following discontinuation of the repeated dosing course lasts for several weeks. These findings address one of the major concerns associated with ultimate clinical implementation of this novel class of medication for the treatment of mood disorders.
While providing useful data concerning the potential therapeutic utility of lanicemine for the treatment of depression, there are important limitations to consider. First, it is not possible to determine whether there is a true difference in the clinical response to the two doses of lanicemine studied. Although the 150-mg dose of lanicemine appeared to produce less robust effects than 100 mg, suggesting a potential inverted U-shape dose–response curve, it is important to note that study 9 was not powered to make a direct comparison of the different doses of lanicemine and it is not possible to determine the dose–response relationship based on this study. However, there is emerging preclinical data related to ketamine12 and GLYX-1332 that may indicate a possible U-shaped dose response. This effect may be related to the dose–response relationship between NMDAR antagonism and glutamate release,33, 34 and reports suggesting that activation of AMPA receptors is a necessary component in generating the antidepressant-like response to the drug.12, 35 Additional studies will be necessary to confirm the dose–response relationship.
Second, while study 1 and published data by Zarate et al.36 provide evidence supporting a single-dose antidepressant effect for lanicemine, a similar single-dose trend was not as robustly observed in study 9. Given that study 9 (in contrast to study 1) was an adjunctive study and most reports to date with ketamine have also been monotherapy studies, an open question remains regarding the extent to which concomitant medications (that is, benzodiazepine administration) may alter the time course of antidepressant treatment effects for NMDA channel blockers. Preliminary reports on ketamine when used adjunctively with antidepressants also suggest a delayed onset of efficacy.37 Finally, while study 9 provides limited data regarding the efficacy and safety of repeated intermittent administrations, it will be critical to better characterize and understand the long-term safety and efficacy profile of NMDA channel blockers including lanicemine. These questions and others are currently being addressed in ongoing studies (for example, Study 31: ClinicalTrials.gov Identifier: NCT01482221).
Summary
Lanicemine, a low-trapping NMDA channel blocker, demonstrated antidepressant effects in patient studies, with fewer dissociative and psychotomimetic symptoms than ketamine at dose exposures that caused similar changes in cortical activation. In clinical studies, lanicemine produced robust and significant efficacy without clinically appreciable dissociative and psychotomimetic adverse effects. These data are consistent with the pharmacological separation of efficacy from psychotomimetic side effects observed in preclinical and phase I studies. Importantly, in a 3-week, placebo-controlled phase IIB study of patients with moderate-to-severe MDD, repeated administration of lanicemine (100 or 150 mg per infusion) at 3-day intervals provided sustained antidepressant efficacy, without psychotomimetic effects. The results of these studies demonstrate that an NMDA channel blocker can achieve antidepressant responses in the absence of prominent psychotomimetic effects and are sustained with repeated dosing. The putative antidepressant characteristics of lanicemine are being explored in ongoing clinical trials.
Acknowledgments
These studies were funded by AstraZeneca Pharmaceuticals. We thank Tom Hudzik (for conducting and analyzing preclinical experiments), David Gurley (for conducting in vitro experiments) and Dean Snyder (for conducting and analyzing EEG studies), who were AstraZeneca employees at the time the research was conducted. Bill Wolvey from PAREXEL provided editorial support funded by AstraZeneca Pharmaceuticals. Clinical trials registry numbers: phase 1, healthy volunteer NCT01130909/phase IIA, study 1, NCT00491686/phase IIB, study 9, NCT00781742.
Gerard Sanacora has received consulting fees from Abbott, AstraZeneca, Bristol-Myers Squibb, Evotec, Eli Lilly & Co, Hoffman La-Roche, Johnson & Johnson, Novartis and Noven Pharmaceuticals; and grant support from AstraZeneca, Bristol-Myers Squibb, Hoffman La-Roche, Merck & Co and Sepracor. In addition, he is a co-inventor on a filed patent application by Yale University (PCTWO06108055A1). Mark A Smith was an AstraZeneca employee at the time this work was conducted and is currently at Shire Pharmaceuticals. He owns stock in AstraZeneca. Sanjeev Pathak is a full-time AstraZeneca employee and he holds stock in AstraZeneca. Hong-Lin Su is a full-time AstraZeneca employee and he holds stock in AstraZeneca. Peter H Boeijinga was a FORENAP employee at the time this work was conducted. Dennis J McCarthy is a full-time AstraZeneca employee and he holds stock in AstraZeneca. Michael Quirk is a full-time AstraZeneca employee and he holds stock in AstraZeneca.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Author contributions
GS was the Principal Investigator for study 9. Dr Sanacora contributed to the analysis and interpretation of the studies and was the lead author on manuscript preparation and writing. MAS contributed to the design, conduct, analysis and interpretation of the clinical studies with lanicemine. SP led the conceptualization, design, execution, data collection and interpretation of study 9. H-LS helped to design the clinical trials and conduct the data analyses (studies 1 and 9), and was also involved in interpreting the data. PHB supervised the human volunteer EEG data collection and processing, and was responsible for interpretation/reporting. DJM established the antidepressant effect of lanicemine in preclinical models, proposed the clinical testing of lanicemine in depressed patients, selected the dose for testing in humans, and as a team member helped design, execute and interpret the clinical studies. MQ was the preclinical and translational (qEEG) science lead overseeing design, execution, analysis and data interpretation, and was co-lead author on manuscript preparation and writing.
Supplementary Material
References
- World Health Organization The Global Burden of Disease: 2004 Update WHO Press: Geneva, Switzerland; Available athttp://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/index.html. Accessed 14 September2012 [Google Scholar]
- Insel TR, Wang PS. The STAR*D trial: revealing the need for better treatments. Psychiatr Serv. 2009;60:1466–1467. doi: 10.1176/ps.2009.60.11.1466. [DOI] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aan Het RM, Zarate CA, Jr., Charney DS, Mathew SJ. Ketamine for depression: where do we go from here. Biol Psychiatry. 2012;72:537–547. doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr., Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Quirk MC, Sosulski DL, Feierstein CE, Uchida N, Mainen ZF. A defined network of fast-spiking interneurons in orbitofrontal cortex: responses to behavioral contingencies and ketamine administration. Front Syst Neurosci. 2009;3:13. doi: 10.3389/neuro.06.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. The GABA-glutamate connection in schizophrenia: which is the proximate cause. Biochem Pharmacol. 2004;68:1507–1514. doi: 10.1016/j.bcp.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Mealing GA, Lanthorn TH, Murray CL, Small DL, Morley P. Differences in degree of trapping of low-affinity uncompetitive N-methyl-D-aspartic acid receptor antagonists with similar kinetics of block. J Pharmacol Exp Ther. 1999;288:204–210. [PubMed] [Google Scholar]
- Mealing GA, Lanthorn TH, Small DL, Murray RJ, Mattes KC, Comas TM, et al. Structural modifications to an N-methyl-D-aspartate receptor antagonist result in large differences in trapping block. J Pharmacol Exp Ther. 2001;297:906–914. [PubMed] [Google Scholar]
- Matejcek M. Cortical correlates of vigilance regulation and their use in evaluating the effects of treatment. Adv Biochem Psychopharmacol. 1980;23:339–348. [PubMed] [Google Scholar]
- Jobert M, Wilson FJ, Ruigt GS, Brunovsky M, Prichep LS, Drinkenburg WH. Guidelines for the recording and evaluation of pharmaco-EEG data in man: the International Pharmaco-EEG Society (IPEG) Neuropsychobiology. 2012;66:201–220. doi: 10.1159/000343478. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Warden D, Wisniewski SR, Fava M, Trivedi MH, Gaynes BN, et al. STAR*D: revising conventional wisdom. CNS Drugs. 2009;23:627–647. doi: 10.2165/00023210-200923080-00001. [DOI] [PubMed] [Google Scholar]
- Balestra M, Bernstein P, Ernst GE, Frietze W, McCauley JP, Nugiel D, et al. (inventors). AstraZeneca Pharmaceuticals LP (assignee). Ethanamine Compounds and Methods of Using the Same. Patent US20120277272 A12012
- Dravid SM, Erreger K, Yuan H, Nicholson K, Le P, Lyuboslavsky P, et al. Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J Physiol. 2007;581:107–128. doi: 10.1113/jphysiol.2006.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster AC, Wong EH. The novel anticonvulsant MK-801 binds to the activated state of the N-methyl-D-aspartate receptor in rat brain. Br J Pharmacol. 1987;91:403–409. doi: 10.1111/j.1476-5381.1987.tb10295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilling KE, Jatzke C, Hechenberger M, Parsons CG. Potency, voltage-dependency, agonist concentration-dependency, blocking kinetics and partial untrapping of the uncompetitive N-methyl-D-aspartate (NMDA) channel blocker memantine at human NMDA (GluN1/GluN2A) receptors. Neuropharmacology. 2009;56:866–875. doi: 10.1016/j.neuropharm.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Hakami T, Jones NC, Tolmacheva EA, Gaudias J, Chaumont J, Salzberg M, et al. NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PLoS ONE. 2009;4:e6755. doi: 10.1371/journal.pone.0006755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B. Differential role of NR2A and NR2B subunits in N-methyl-D-aspartate receptor antagonist-induced aberrant cortical gamma oscillations. Biol Psychiatry. 2012;71:987–995. doi: 10.1016/j.biopsych.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook IA, Leuchter AF, Morgan M, Witte E, Stubbeman WF, Abrams M, et al. Early changes in prefrontal activity characterize clinical responders to antidepressants. Neuropsychopharmacology. 2002;27:120–131. doi: 10.1016/S0893-133X(02)00294-4. [DOI] [PubMed] [Google Scholar]
- Horacek J, Brunovsky M, Novak T, Tislerova B, Palenicek T, Bubenikova-Valesova V, et al. Subanesthetic dose of ketamine decreases prefrontal theta cordance in healthy volunteers: implications for antidepressant effect. Psychol Med. 2010;40:1443–1451. doi: 10.1017/S0033291709991619. [DOI] [PubMed] [Google Scholar]
- Boeijinga PH, Parot P, Soufflet L, Landron F, Danel T, Gendre I, et al. Pharmacodynamic effects of acamprosate on markers of cerebral function in alcohol-dependent subjects administered as pretreatment and during alcohol abstinence. Neuropsychobiology. 2004;50:71–77. doi: 10.1159/000077944. [DOI] [PubMed] [Google Scholar]
- Palmer GC, Miller JA, Cregan EF, Gendron P, Peeling J. Low-affinity NMDA receptor antagonists. The neuroprotective potential of ARL 15896AR. Ann NY Acad Sci. 1997;825:220–231. doi: 10.1111/j.1749-6632.1997.tb48432.x. [DOI] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr., Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, et al. Replication of ketamine's antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, Aan Het RM, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2012;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38:729–742. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury GM, Behar KL, Cho W, Thomas MA, Rothman DL, Sanacora G. 1H-[13C]-nuclear magnetic resonance spectroscopy measures of ketamine's effect on amino acid neurotransmitter metabolism. Biol Psychiatry. 2012;71:1022–1025. doi: 10.1016/j.biopsych.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr., Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, et al. A randomized trial of a low-trapping nonselective N-Methyl-D-Aspartate channel blocker in major depression. Biol Psychiatry. 2012;74:257–264. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusin C, Soskin DP, Kara PJ, Kelley D, Trina C, Paolo C, et al. Open-label, flexible-dose repeated intravenous ketamine infusions as adjunct in outpatients with treatment resistant major depression with suicidal ideation New Research Approaches for Mental Health Interactions28–31 May2013. Abstract 43.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.