Abstract
Understanding the cellular uptake and intracellular trafficking of dendrimer–DNA complexes is an important prerequisite for improving the transfection efficiency of non-viral vector-mediated gene delivery. Dendrimers are synthetic polymers used for gene transfer. Although these cationic molecules show promise as versatile DNA carriers, very little is known about the mechanism of gene delivery. This paper investigates how the uptake occurs, using an endothelial cell line as model, and evaluates whether the internalization of dendriplexes takes place randomly on the cell surface or at preferential sites such as membrane rafts. Following extraction of plasma membrane cholesterol, the transfection efficiency of the gene delivered by dendrimers was drastically decreased. Replenishment of membrane cholesterol restored the gene expression. The binding and especially internalization of dendriplexes was strongly reduced by cholesterol depletion before transfection. However, cholesterol removal after transfection did not inhibit expression of the delivered gene. Fluorescent dendriplexes co-localize with the ganglioside GM1 present into membrane rafts in both an immunoprecipitation assay and confocal microscopy studies. These data strongly suggest that membrane cholesterol and raft integrity are physiologically relevant for the cellular uptake of dendrimer–DNA complexes. Hence these findings provide evidence that membrane rafts are important for the internalization of non-viral vectors in gene therapy.
INTRODUCTION
Dendrimers represent one of several non-viral systems used for delivering nucleic acids into cells. They are synthetic hyperbranched polymers which are highly soluble in aqueous solutions (1). Dendrimers with positively charged terminal groups can bind DNA forming complexes (2), termed dendriplexes (3) by analogy with similar complexes formed by liposomes and DNA called lipoplexes. The DNA within dendriplexes is protected from cellular and restriction nucleases (4). Although they are less efficient than viral vectors, they have potential for use in gene therapy and other therapeutic applications due to their safety and lack of immunogenicity.
An essential prerequisite for achieving improved transfection efficiencies is an understanding of the whole delivery process. This study investigates cellular uptake of dendriplexes, and specifically whether the entry of the complexes into cells takes place randomly or at preferential sites in the plasma membrane.
Eukaryotic cell membranes contain glycerolipids, sphingolipids, cholesterol and proteins. The physical properties of biological membranes are linked to their lipid composition. The differences between lipids, such as their melting temperature and the nature of their chains, lead to the formation of glycero- or sphingo-domains in which phospholipids have either rapid lateral and rotational diffusion or limited mobility. Cholesterol, suggested to play a role in endocytosis (5), tends to partition with sphingolipids to occupy the free space between acyl chains, therefore promoting the formation of liquid ordered domains (6–9). Microdomains of cholesterol and sphingolipids in the exoplasmic leaflet of the plasma membrane are termed ‘membrane rafts’ (10–12) and are characterized by their resistance to solubilization with non-ionic detergent (13–21). Microdomain formation has been reported in planar supported lipid layers and in giant unilamellar vesicles (22). Both nanometer-sized (23–26) and micron-sized (27) domains have been observed.
While questions remain about the nature and size of rafts in natural membranes (14,28), there is accumulating evidence regarding their functional role in cells. Several reports have shown that cholesterol and membrane rafts are involved in membrane trafficking, signalling, protein and lipid sorting, bacterial infection, binding and internalization of viruses (10,11,29–32). These functional dynamic structures on the membranes also play a role in cellular uptake and endocytosis of other non-viral vectors, such as liposomes (33).
This work shows that depletion of plasma membrane cholesterol profoundly affects the gene delivery mediated by dendriplexes and that membrane rafts are involved in the cellular uptake of dendriplexes. These microdomains are crucial for endocytosis of molecules, although probably not in a strictly receptor-mediated or specific protein-mediated manner.
MATERIALS AND METHODS
Cell culture and transfection
EA.hy 926 cells (34,35), a human endothelial hybridoma (a kind gift from Dr C. J. S. Edgell, University of North Carolina, USA) and Chinese hamster ovary (CHO) cells were maintained in RPMI 1640 medium (Invitrogen, Paisley, UK). Human embryonic kidney (HEK) 293 cells were grown in Dulbecco’s modified Eagle’s medium (Invitrogen). Tissue culture media were supplemented with 10% heat-inactivated fetal calf serum (FCS) (Globepharm, Cranleigh, UK), 2 mM glutamine (Invitrogen), 100 µg/ml streptomycin (Invitrogen) and 100 U/ml penicillin (Invitrogen), and the cells were grown at 37°C in a humidified atmosphere with 5% CO2.
Cell transfections were carried out using plasmid DNA encoding for either enhanced green fluorescent protein (pEGFP-C1) (Clontech, Palo Alto, USA) or β-galactosidase (pCMVβ-gal) (Clontech). Plasmids were grown in Escherichia coli DH5α strain and purified using endotoxin-free plasmid purification kits (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Plasmid concentration and purity were assessed by spectrophotometry. Cells, seeded on either 12- or 24-well plates overnight, were 80–90% confluent at the time of transfection. Dendriplexes were formed by incubating for 30 min at room temperature plasmid DNA and Superfect® (Qiagen), a commercially available dendrimer, at 1:3 (w/w) ratio in Optimem medium (Invitrogen). Preformed complexes were added to cells for 3 h at 37°C in a humidified atmosphere with 5% CO2 and then removed, replacing the culture medium. After 48 h at 37°C the cells were analysed for protein expression unless otherwise specified. Enhanced green fluorescent protein (EGFP) was detected by flow cytometry (FACScalibur, 488 nm argon ion and 635 nm red diode lasers, Becton Dickinson, Oxford, UK). All experiments were carried out using a mock control. The percentage of positive cells was calculated (36) by setting the background population as 98% negative when analysing control cells that had undergone mock transfection. At least 5 × 103 (usually 104) cells were acquired for each condition.
Enzymatic activity was detected using X-Gal (5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside) staining or the amount of β-galactosidase protein was quantified with respect to total protein using o-nitrophenyl galactosidase (ONPG) colometric assay (37). All transfection experiments were carried out in triplicate; however, for pEGFP transfections, three wells were pooled together and the results were considered as an average value of triplicates.
Cholesterol depletion
Methyl-β-cyclodextrin (MβCD), water-soluble cholesterol (balanced with MβCD), cholesterol (5-cholesten-3β-ol), nystatin and filipin III were purchased from Sigma (Gillingham, Dorset, UK). MβCD was dissolved in phosphate buffer saline (PBS) without Ca2+ and Mg2+ (Invitrogen), kept on ice and freshly used. Nystatin stock solution was prepared in endotoxin-free water (Sigma) while filipin III was dissolved in methanol; aliquots were immediately frozen in liquid nitrogen to reduce the possible oxidation process. The compounds were used at final concentrations of 10 mM MβCD (31,38), 25 µg/ml nystatin (29) and 5 µg/ml filipin III (29). In post-transfection depletion studies the cholesterol removal with MβCD was carried out 24 h after transfection.
Cells were grown in 24-well plates until they reached 80–90% confluence. The culture medium was then replaced with PBS containing either no additive or MβCD, nystatin or filipin III. Plates were incubated at 37°C for 30 min in a waterbath. Following incubation, the PBS was replaced in all wells which were then incubated for a further 30 min at 37°C in humidified atmosphere with 5% CO2. The cells were then transfected using dendriplexes as indicated above.
In some experiments the cells were depleted with MβCD 24 h after transfection, using the same protocol.
To investigate the activity of the drug in the presence of cholesterol, cells were treated with 10 mM MβCD in the presence of either 0.25 or 0.5 mg/ml water soluble cholesterol using the same conditions as described above. The cells were then transfected as described above.
To determine the effect of replacing the cholesterol, cells were treated with 10 mM MβCD as indicated above. They were then incubated for 30 min at 37°C with 0.4 mg/ml of either water-soluble cholesterol or cholesterol (5-cholesten-3β-ol), first dissolved in ethanol and then diluted in PBS. The cells were then transfected as described.
Cellular binding of dendriplexes
The interaction of dendriplexes with cells was evaluated using fluorescent complexes. Dendrimer conjugation with fluorescein isothiocyanate (FITC) isomer I (Sigma) was performed as described (39) with minor modifications. Briefly, Superfect® was diluted 1:3 (v/v) in 150 mM carbonate buffer pH 9.3. FITC (1 mg/ml in dimethylsulfoxide) was added 1:20 (v/v) into the solution containing dendrimers with slow vortexing. After 30 min incubation the reaction was terminated by addition of 100 mM NaH2PO4. The labelled dendrimers were purified using a PD10 column, pre-equilibrated with PBS (pH 7.4) and concentrated with a Centricon YM-10 (Amicon, Millipore, Billerica, USA) at 3000 g at 4°C. The concentration of conjugated dendrimers was measured by spectrophotometry.
Dendriplexes formed using FITC-labelled dendrimers were added to both MβCD-treated and untreated cells on ice. The cells were then incubated at either 4 or 37°C. At various times, unbound complexes were removed by washing with PBS and then cells were harvested. The cells were then analysed by flow cytometry and the median fluorescence intensity of the population was determined. This represents the amount of dendriplexes that have bound to the cell surface (including those that will have subsequently internalized).
Internalization study
In order to follow the internalization of the vectors, dendriplexes made using FITC-labelled dendrimers were added to the cells and incubated for 15 min at 4°C. The unbound dendriplexes were then washed off and cells were warmed up to 37°C (t0). At various times the cells were harvested and then treated for 4 min with 5 mM NaOH to remove dendriplexes still bound to the cell surface. After gentle washing the cells were then analysed by flow cytometry. The internalization of the complexes was expressed as the ratio of the mean fluorescence intensity of cells that had been treated with NaOH (MFI+NaOH) to that of those that had not been treated (MFI–NaOH) after subtraction of background fluorescence (MFIbkg):
![]()
Confocal microscopy
Cells grown on coverslips (borosilicate, Ø 16 mm, No. 1; VWR, Poole, Dorset, UK) were treated with MβCD or untreated and then transfected with dendriplexes as described above. The complexes were formed using Cy3-labelled dendrimers and Cy5-labelled DNA. Cy3 was purchased from Amersham Biosciences (Uppsala, Sweden). Dendrimers were labelled using the same protocol as described above for FITC conjugation. Cy5-DNA was obtained using the Cy5 ULS labelling kit (Amersham Biosciences) and fluorescent DNA was purified by elution in Microspin G-50 columns (Amersham Biosciences) according to the manufacturer’s instructions. At the stated times post-transfection, cells on the coverslip were washed in PBS and fixed in 4% paraformaldehyde for 15 min at 4°C. Cells were then washed twice in PBS and once in water, and mounted in Mowiol-DAPI mounting medium (40). Imaging data were collected using an inverted Zeiss LSM 510 Meta confocal laser scanning microscope (Zeiss, Jena, Germany) and processed using Adobe Photoshop. Cells were identified with a 60× oil immersion objective. The system was switched to frame mode for confocal image acquisition.
For co-localization studies EA.hy 926 cells were grown on Ø 0.22 mm glass-well dishes (Willcowells, Amsterdam, The Netherlands), either treated with MβCD or untreated and then transfected with either Cy3-dendrimers alone or complexes formed by Cy3-conjugated dendrimers and Cy5-labelled DNA. Immediately after addition of either dendrimers or dendriplexes, 0.02 µg/µl FITC-conjugated cholera toxin, B subunit (ChTxB) (Sigma) was added and the cells were observed under the confocal laser scanning microscope for up to 15 min.
Cell lysis and immunoprecipitation
First, 5 × 107 EA.hy 926 cells were MβCD treated or untreated as previously described. The cells were then placed at 4°C and treated for 2 h with dendriplexes formed using FITC-dendrimers. After washing to remove the unbound dendriplexes, cells were detached using 150 mM NaCl, 1 mM EDTA and 40 mM Tris pH 7.5. The resulting cell pellets were washed once in PBS and then resuspended in 500 µl of cold PBS and incubated with 100 µl of MACS Magnetic Cell Sorting anti-FITC microbeads (Miltenyi Biotec Ltd, Bisley, UK) for 15 min at 4°C. For raft detection, cells were washed twice and then lysed on ice for 20 min in 1 ml of MNE buffer (150 mM NaCl, 2 mM EDTA, 25 mM MES pH 6.5) containing 1% Triton X-100 and protease and phosphatase inhibitors (41). MACS High Gradient Magnetic separation columns type LS or MS (Miltenyi Biotec Ltd) were used for magnetic immunoprecipitation. The column was placed in the magnetic field of a MACS separator and equilibrated with 2 ml of PBS followed by ≥200 µl of MNE buffer. Then 500 µl of cell lysate was applied onto the column. The unbound fraction was collected. The column was washed with 500 µl of MNE buffer. After removing the column from the magnetic field, a further 500 µl of MNE buffer was added and the fraction bound to the column collected. Samples (40 µl) of the lysate were spotted onto Protan® Ø 0.45 µm nitrocellulose transfer membrane (Schleicher & Schuell Biosciences GmbH, Dassel, Germany). Dot blots were dried under vacuum using Hybri-blot® Manifold (BRL, Bethesda Research Laboratories, USA). The membrane was rinsed several times with PBS and treated with 10% skimmed milk (Marvel, Premier Beverages, Stafford, UK) for 30 min at room temperature. After washing with PBS–0.05% Tween-20 (Sigma), dot blots were probed with horseradish peroxidase (HRP) conjugated ChTxB (Sigma) at 5 µg/ml for 2.5 h at room temperature to detect the glycosphingolipid GM1. The presence of FITC-dendriplexes was revealed using rabbit anti-FITC isomer I polyclonal antibody (DAKO, Ely, UK) at 46 µg/ml for 1.5 h. After washing, 26 µg/ml HRP-conjugated swine anti-rabbit antibody (DAKO) was added for 1 h at room temperature. HRP activity was detected by 3,3′,5,5′-tetramethylbenzidine (TMB) blotting substrate (Sigma).
RESULTS
Effects of cholesterol depletion on transfection mediated by dendrimers
To examine the role of cholesterol in gene delivery mediated by dendrimers, the plasma membrane cholesterol was depleted. Three different agents were used to deplete cholesterol, each acting by a different mechanism. MβCD, a water-soluble cyclic oligomer of glucopyranoside, acts strictly on the cell surface, selectively extracting cholesterol without being incorporated into plasma membranes (42–45). Filipin III and nystatin are antibiotics that incorporate into lipid membranes and chelate cholesterol (46–51).
Initial experiments were carried out using the EA.hy 926 endothelial hybridoma, as the main interest in the laboratory is gene transfer to the endothelium. Cells were transfected with plasmid DNA, which carried reporter genes encoding for either EGFP (Fig. 1A) or β-galactosidase protein (Fig. 1B and C). Gene expression was evaluated by flow cytometry and either X-Gal staining or colorimetric assay. When cells were treated with 10 mM MβCD (31,38), 5 µg/ml filipin III or 25 µg/ml nystatin (29) 1 h prior to transfection, a strong decrease was seen in the transfection efficiency for all three agents (though it was not as dramatic in the case of nystatin) in terms of both cells expressing the marker genes (Fig. 1A and B) and the total amount of β-galactosidase protein produced (Fig. 1C).
Figure 1.
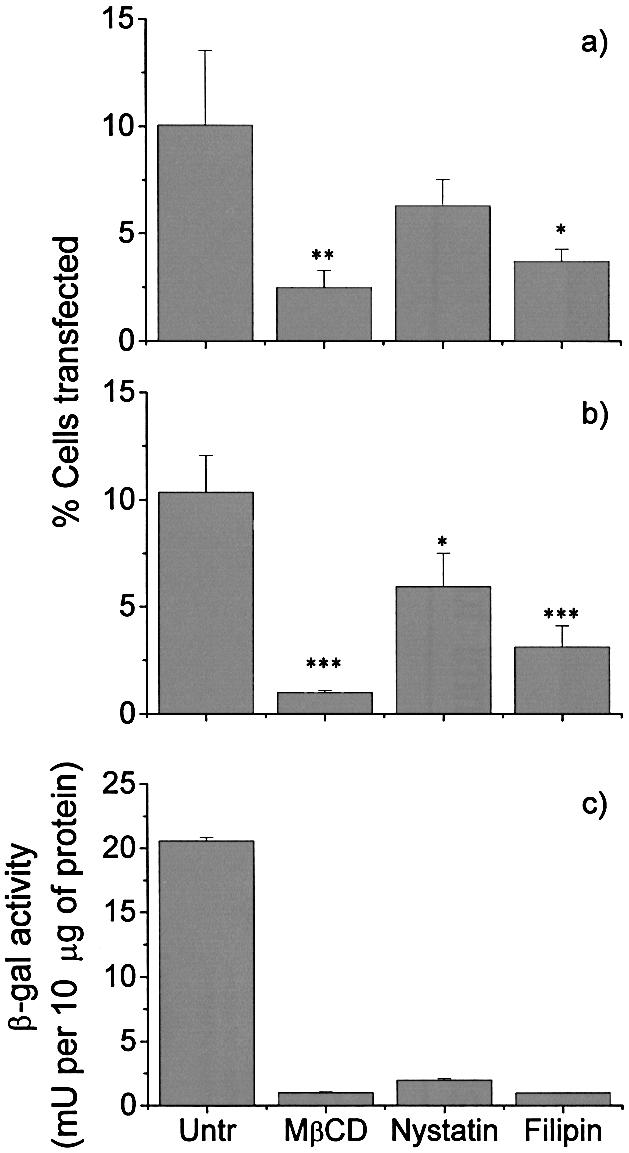
Cholesterol depletion reduces transfection efficiency. Cells were transfected with plasmids encoding either (A) EGFP or (B and C) β-galactosidase. Transfections were carried out following cholesterol chelation with MβCD, nystatin or filipin III. Transfection efficiencies were evaluated by (A) flow cytometry, (B) X-Gal staining or (C) ONPG colometric assay. All the experiments were carried out in triplicate. Error bars represent the standard error of the mean: (A) n = 4; (B) n = 2. Statistical significance (unpaired t test) is represented as follows: ***, p < 0.05; **, p < 0.1; *, p < 0.2 (A and B). The error bars of a representative experiment in (C), expressing the amount of β-galactosidase protein produced, correspond to the standard deviation of the mean.
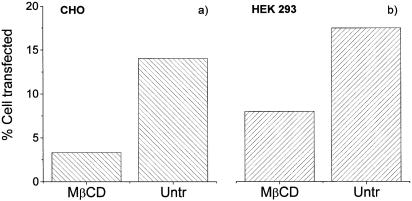
In order to confirm that these results are not unique to EA.hy 926 cells, and also to test cells that are easier to transfect, the experiments were repeated with CHO and HEK293 cells. As is shown in Figure 2, the same pattern of inhibition by MβCD was seen. Similar data were also obtained when filipin or nystatin was used to treat CHO cells (data not shown). The transfection efficiency seen with these cells (15–20%) is, in our hands, around the maximum observed for any cell type using this reporter system.
Figure 2.
Transfection efficiencies of cholesterol depletion on CHO and HEK 293 cells. A representative experiment shows the effect of cholesterol depletion with MβCD on the transfection efficiency on (A) CHO and (B) HEK 293, as previously shown in Figure 1 for EA.hy 926 cells. The cells were transfected using EGFP reporter gene and triplicate wells were pooled together prior to flow cytometry.
MβCD treatment and/or simultaneous addition of soluble cholesterol
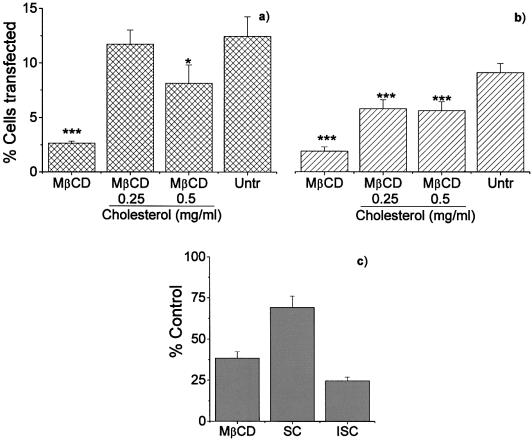
MβCD binds to cholesterol in its internal cavity, and co-administration of MβCD and cholesterol results in an equilibrium in which cholesterol is simultaneously depleted and replenished. In order to confirm that the effect of MβCD was due to cholesterol depletion, rather than some other action of the drug, we co-incubated EA.hy 926 cells with MβCD and 0.25 or 0.5 mg/ml cholesterol. As shown in Figure 3A and B, the addition of cholesterol largely reversed the inhibition of transfection caused by MβCD, indicating that it was the cholesterol depletion activity of MβCD that was responsible for the reduction in transfection. Similar data were also seen in CHO and HEK 293 cells (data not shown).
Figure 3.
The effect of reconstitution of cholesterol on gene delivery. (A) The transfection efficiency using pEGFP (n = 6); (B) transfections carried out using pCMVβ-gal (n = 4). Cells were treated with MβCD alone (10 mM), MβCD with soluble cholesterol at either 0.25 or 0.5 mg/ml or left untreated (untr). Following treatment, the cells were transfected with dendriplexes and the transfection efficiency determined as described in Figure 1. Error bars represent the standard error of the mean. Statistically significant differences (unpaired t test) are shown as follows: ***, p < 0.05; *, p < 0.2. Following MβCD-depletion, the cholesterol pool was restored (C) by adding 0.4 mg/ml of either cholesterol (water-soluble, SC) or 5-cholesten-3β-ol (water insoluble, ISC). This latter source was not available for restoration. The results are presented as the mean percentage of replenishment compared with untreated cells (control) ± the standard error of the mean of three independent experiments.
Cholesterol replenishment re-establishes the efficiencies mediated by dendriplexes
The role of cholesterol in the cellular uptake of dendriplexes was confirmed by first depleting plasma membrane cholesterol with MβCD and then incubating cells with 0.4 mg/ml cholesterol for 30 min at 37°C. Two different sources of cholesterol were provided: first, water-soluble cholesterol, which is in a form suitable for replenishment, and, secondly, cholesterol initially dissolved in ethanol and then further diluted in PBS as a source not available for membrane reconstitution. Figure 3C shows that cholesterol replenishment immediately following MβCD deprivation could restore the expression the delivered gene by up to 70%.
Effect of cholesterol depletion after transfection
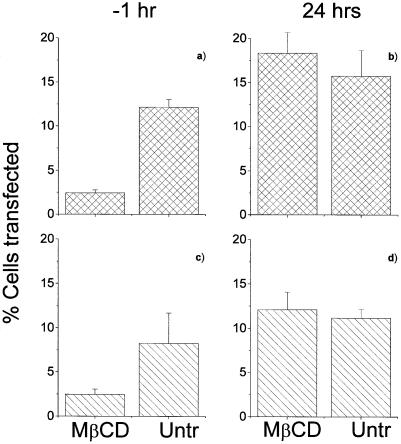
We went on to exclude the possibility that the reduction in transfection was due to MβCD affecting gene expression or protein production. Cells were treated with MβCD either 1 h before or 24 h after transfection with pEGFP or pCMV-βgal. As shown in Figure 4, while treatment with MβCD before transfection reduced expression of the genes, treatment at 24 h had no effect. Similar data were seen at 40 h (not shown). These data indicate that MβCD depletion of cholesterol had no effect on gene expression following transfection.
Figure 4.
Post-transfection MβCD-depletion and gene expression. The figure shows the effect of MβCD treatment of cells 1 h prior to transfection and 24 h after transfection with dendriplexes formed using either (A and B) pEGFP or (C and D) pCMV-βgal. At 48 h the transfection efficiency was evaluated as previously reported. The result shows a representative experiment where error bars represent the standard deviation of the mean.
Binding of dendriplexes to the cell
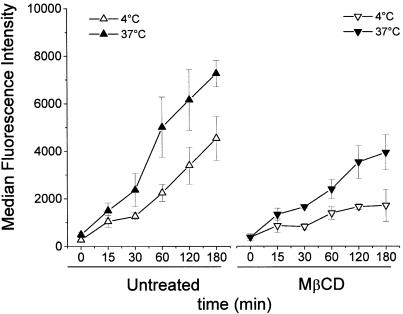
To investigate whether the decrease in gene expression was determined by an impairment in binding to the cell surface, the complexes were formed using dendrimers labelled with fluorescein. The kinetics of cellular binding of dendriplexes (which, at least at 37°C, will include subsequent internalization) was compared at two different temperatures: 4°C (at which endocytosis is reduced or blocked) and 37°C (at which internalization occurs). In untreated cells (Fig. 5, left panel), the binding at 37°C was higher than at 4°C. In MβCD-treated cells, however, the binding of dendriplexes was markedly decreased at both 4 and 37°C (Fig. 5, right panel), indicating that cholesterol depletion affects the binding of complexes. At 37°C the viability of MβCD-treated cells and untreated cells was similar (>92%), as determined by FACS analysis using propidium iodide and annexin V staining. However, at 4°C there was greater necrosis of MβCD-treated cells (35%), when compared with untreated cells.
Figure 5.
Kinetics of cellular uptake of dendrimers in the presence or absence of MβCD and cell viability at different temperatures. Cells were untreated (left panel) or treated with MβCD (right panel) and then incubated at 37°C (closed symbols) or 4°C (open symbols) with FITC-labelled dendriplexes. The cells, after washing off the unbound complexes, were analysed by flow cytometry at various times to determine the amount of dendriplexes both bound to the cell surface and internalized. The results are plotted as mean ± SEM of four and three independent experiments in untreated cells and MβCD-treated cells, respectively.
Internalization of dendriplexes
Internalization kinetics of the complexes was monitored in cells exposed to the fluorescent dendriplexes for 15 min at 4°C, before being warmed up to 37°C after washing away the unbound dendriplexes. A period of 15 min was chosen to allow similar amounts of dendriplex to adhere to the surface of untreated and MβCD-depleted cells (Fig. 5). Cell-surface-bound dendriplexes were removed at different times using 5 mM NaOH, thus allowing detection of dendriplexes that had internalized.
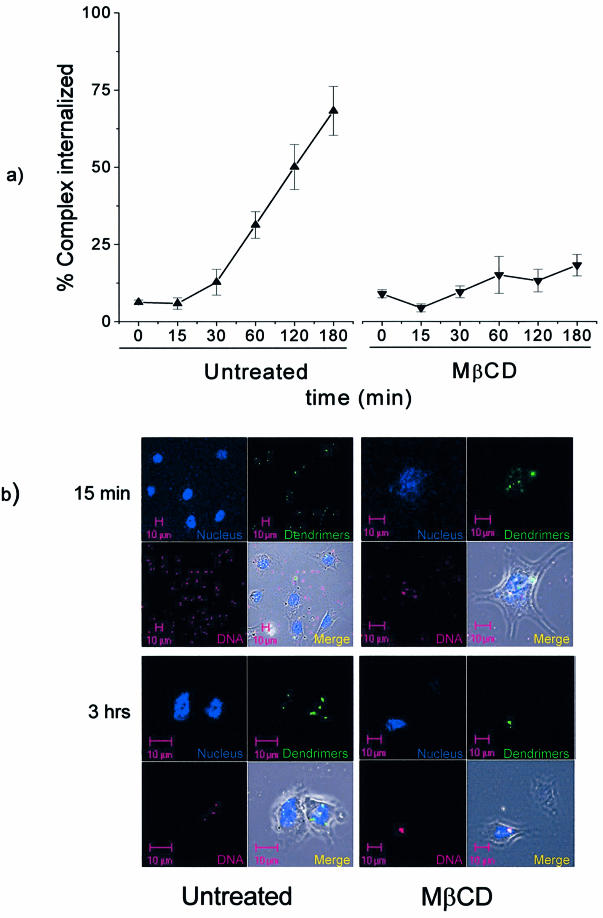
In untreated cells the endocytosis of dendriplexes occurred at a constant rate, following a 30 min lag period after increasing the temperature to 37°C (Fig. 6A, left panel), with 70% of cell-bound dendrimers becoming internalized by 3 h. In contrast, only 20% of dendrimers were internalized in MβCD-treated cells (Fig. 6A, right panel).
Figure 6.
Internalization of fluorescein-labelled dendriplexes. (A) Cells were incubated with complexes formed using FITC-conjugated dendrimers at 4°C for 15 min to prevent internalization. The samples were then warmed to 37°C and, at various times, the amount of internalized dendriplexes was determined after removing cell-surface-bound material with 5 mM NaOH. The results represent the mean ± SEM of three independent experiments of both untreated and cholesterol-depleted cells. (B) Dendriplexes formed with Cy3-dendrimers (green) and Cy5-DNA (red) were incubated with untreated or MβCD-treated EA.hy 926 cells for 15 min or 3 h as indicated. The cells were fixed and stained with DAPI (nuclear stain, blue) and then examined by confocal microscopy.
The internalization of dendriplexes was also followed in MβCD-treated and control cells using confocal microscopy. As shown in Figure 6B, where DNA labelled with Cy5 and dendrimers labelled with Cy3 were detected at 15 min and 3 h, considerably more complex has internalized in control cells than in MβCD-treated cells.
Co-localization of glycosphingolipid GM1 and dendrimers
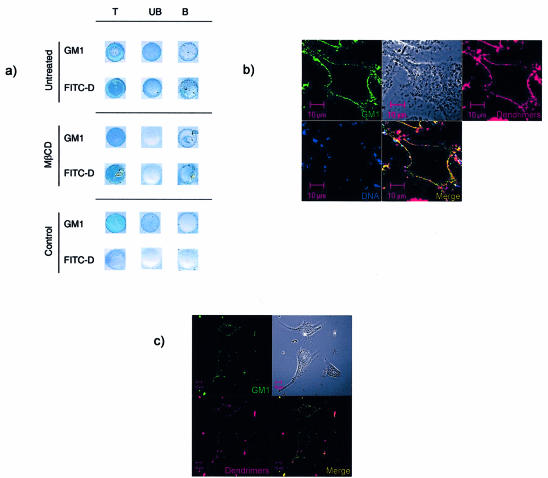
The interactions between dendriplexes and membrane rafts were assessed using immunoprecipitation with dendriplexes formed with FITC-labelled dendrimers. Following transfection at 4°C, cells were incubated with anti-FITC beads, then lysed and the beads separated. The bound and unbound fractions were collected. The collected fractions were assessed by dot blot with HRP-ChTxB (to detect raft-associated glycosphingolipids GM1) and anti-FITC antibodies (to detect the dendrimers).
As shown in Figure 7A, dots stained with ChTxB show the presence of ganglioside GM1, indicating that this glycosphingolipid is present, as would be expected, in the total cell lysates of all cells. In addition, ChTxB stained the anti-FITC immunoprecipitated fractions of cells that were incubated with dendriplexes, but not the equivalent fraction of control cells that had not been exposed to FITC-dendrimers. This indicates that GM1 was associated with the FITC-dendrimers during the immunoprecipitation stage. Furthermore, these results also show that rafts can be isolated by immunoprecipitation. In dot blots stained with anti-FITC antibody, FITC-dendrimers were detected in total lysates and the immunoprecipitated fractions of cells (either untreated or treated with MβCD), but not in control cells that had not been incubated with dendrimers. A weak positive stain was seen in the unbound fraction of the lysates, possibly due to some of the FITC-dendrimers having internalized and so not being available to the anti-FITC beads, which were used on intact cells.
Figure 7.
Co-localization of dendriplexes and ganglioside GM1. (A) EA.hy 926 cells were either untreated or treated with MβCD followed by transfection with dendriplexes formed using FITC-dendrimers. Cells not incubated with dendriplexes were used as a control. Cells were then probed with anti-FITC beads and lysed. This lysate was run through a column retaining the beads. Total lysate (T), the unbound material (UB) and the bound fraction (B) were then spotted onto the nitrocellulose. Dot blots were probed with either HRP-conjugated ChTxB, for detecting GM1 ganglioside, or anti-FITC antibody to localize dendriplexes. Data show that GM1 as well as dendrimers were both detected in the total lysates and the fractions bound to the columns of both untreated and MβCD treated cells. A weak signal for both GM1 and FITC-dendrimers was also detected in the unbound fraction of untreated cell lysate. In control untransfected cell lysates, only dots of total and unbound fraction, but not the bound fraction, were positive for GM1, whereas no FITC-dendriplexes were detected. (B) Confocal microscopy was performed on living cells that had been incubated with dendriplexes formed using Cy3-dendrimers (red) and Cy5 DNA (blue), together with FITC-ChTxB (green) which binds to the raft marker ganglioside GM1. (C) In addition, cells were imaged that had been incubated with Cy3 dendrimers alone (no DNA) together with FITC-ChTxB.
To determine further the nature of the interaction between dendriplexes and membrane rafts, confocal microscopy studies on living cells were performed. As shown in Figure 7B, dendriplexes labelled with Cy3 (dendrimer) and Cy5 (DNA) co-localized with ChTxB, indicating that they co-localized with membrane rafts. Dendriplexes are larger than membrane rafts, so we repeated the microscopy (Fig. 7C) using cells incubated with dendrimers alone (no DNA). Again, co-localization of dendrimers with ChTxB was seen, indicating that dendrimers preferentially bind to rafts.
DISCUSSION
Cationic dendrimers are being developed for application as gene therapy vectors, as well as for other forms of drug delivery. Their branched structure, terminating in charged groups, results in binding of DNA. The overall positive charge of the dendrimer–DNA complex (dendriplex) is thought to mediate electrostatic interactions with the cell surface, though there is also a potential to modify these groups to incorporate molecules suitable for targeting.
At present there is little understanding of how dendrimers mediate gene delivery to cells. Our data clearly indicate that membrane cholesterol has a significant function, at least in the cells shown here, as gene delivery is greatly reduced when cholesterol is removed from the cells and replacement of cholesterol restores the ability of the cell to be transfected. In a similar manner, cholesterol depletion has been shown to reduce the ability of liposomes to deliver genes to cells (33). Transfection has been shown to be enhanced by free cholesterol (52) or cationic cholesterol derivatives (53,54). Cholesterol has been reported to play an important role in the ability of intracellular bacteria (29,31) and viruses (55) to enter cells, as well as for protein fusions containing the TAT protein transduction domain (56).
The effect of cholesterol on gene delivery could be at the level of binding of dendriplexes to the cell surface, in their internalization (probably in endosomes) or in endosomal escape. The use of chelating agents acting with different mechanisms provides evidence that cholesterol is essential for the cellular uptake of dendriplexes. We have shown that gene expression was not affected by cholesterol depletion after transfection, indicating that the results are not due to a reduction in transcription and/or translation processes.
Our data indicate that the binding of dendriplexes to cells is reduced by cholesterol depletion. Cholesterol may have a direct role in the binding of dendriplexes, for example by interaction of the polar head at position C3 of the molecule with the charged amino groups of the dendrimer. Alternatively, dendriplexes may preferentially interact with membrane rafts, which are dynamic structures involved in signalling and endocytosis. Depletion of cholesterol has been shown to disrupt rafts, which are cholesterol enriched. We have reported that dendriplexes that are on the surface of cells co-precipitate with the glycosphingolipid GM1. Furthermore co-localization of the complexes with the raft marker ChTxB supports the hypothesis that dendrimers associate with rafts. It is not known what structures of the membrane microdomains the dendriplexes are interacting with, though one candidate would be the annular anionic phospholipids surrounding rafts (57).
However, while binding to cell surface is reduced in the absence of cholesterol, the most striking result is that the internalization of dendriplexes is virtually abolished in cholesterol-depleted cells. One possible explanation is that internalization of dendriplexes is dependent on lipid rafts. Internalization of viruses and bacteria has previously been shown to be mediated by membrane rafts (29–31,58,59). Cholesterol may not only play a role in maintaining the structure of the rafts but may also be involved in a signalling cascade that initiates endocytosis.
Once within the cell, the dendriplex (or at least the DNA component) has to escape into the cytosol before trafficking to the nucleus. The data presented in this paper do not address the issue as to whether cholesterol has a role in this process. It has been proposed that dendrimers may act as a ‘proton sponge’, leading to osmotic lysis of endosomes/lysosomes and so resulting in escape of the dendrimers (2). An additional model is that electrostatic interactions between the surface of dendrimers and charged groups on the lipid bilayer cause ‘bending’ of the membrane and resulting disruption/leakage of the vesicle (60). The presence of cholesterol in the endosomes could influence the endosomal escape by altering either the lipid–dendrimer interaction or the strength, rigidity or permeability of the membrane. In many systems, addition of chloroquine has been shown to enhance transfection by interfering with the acidification of the endosomes/lysosomes and so reducing degradation of the DNA. However, in the case of dendrimer-mediated gene delivery, chloroquine has a variable effect on the efficiency of gene transfer; one report shows no enhancement and another shows a variable effect depending on the cell line used (1,61). This is presumably because the pH of the endosomes/lysosomes is important in the escape of the DNA into the cytosol, and so addition of choloroquine has opposing effects on gene delivery (reducing both the degradation of DNA and the efficiency of escape into the cytosol). We have therefore not carried out experiments in which we have used chloroquine to determine its effect on gene delivery following cholesterol depletion.
In summary, we have shown that cholesterol profoundly affects the ability of dendrimers to mediate gene transfer to endothelial and other cells. While cholesterol is involved in the binding of dendrimers to the cell surface, and may have a role in endosomal escape, our data suggest that the most important effect is on internalization of the dendriplexes. This is most likely because of the importance of lipid rafts in endocytosis, though the mechanisms by which they direct dendrimer gene delivery have yet to be elucidated. Further characterization of the internalization and delivery pathways of dendrimers, which may be different in different cell types, will be important for the design and use of the next generation of dendrimer-based gene therapy vectors. In addition, as dendrimers are also used for drug delivery, such insights are likely to have a wider application.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Hermann Gruber for helpful discussion about labelling procedure, Giovanna Lombardi for giving us the methyl-β-cyclodextrin protocol and Cliburn Chan for critical review of the manuscript. This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) Grant 28/GTH12562. A.J.T.G. is a BBSRC Research Leave Fellow.
REFERENCES
- 1.Kukowska-Latallo J.F., Bielinska,A.U., Johnson,J., Spindler,R., Tomalia,D.A. and Baker,J.R.,Jr (1996) Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. Proc. Natl Acad. Sci. USA, 93, 4897–4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang M.X., Redemann,C.T. and Szoka,F.C.,Jr (1996) In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug. Chem., 7, 703–714. [DOI] [PubMed] [Google Scholar]
- 3.Ramaswamy C., Sakthivel,T., Wilderspin,A.F. and Florence,A.T. (2003) Dendriplexes and their characterisation. Int. J. Pharm., 254, 17–21. [DOI] [PubMed] [Google Scholar]
- 4.Bielinska A.U., Kukowska-Latallo,J.F. and Baker,J.R.,Jr (1997) The interaction of plasmid DNA with polyamidoamine dendrimers: mechanism of complex formation and analysis of alterations induced in nuclease sensitivity and transcriptional activity of the complexed DNA. Biochim. Biophys. Acta, 1353, 180–190. [DOI] [PubMed] [Google Scholar]
- 5.Heiniger H.J., Kandutsch,A.A. and Chen,H.W. (1976) Depletion of L-cell sterol depresses endocytosis. Nature, 263, 515–517. [DOI] [PubMed] [Google Scholar]
- 6.Sankaram M.B. and Thompson,T.E. (1990) Interaction of cholesterol with various glycerophospholipids and sphingomyelin. Biochemistry, 29, 10670–10675. [DOI] [PubMed] [Google Scholar]
- 7.Silvius J.R. (2003) Role of cholesterol in lipid raft formation: lessons from lipid model systems. Biochim. Biophys. Acta, 1610, 174–183. [DOI] [PubMed] [Google Scholar]
- 8.Silvius J.R., del Giudice,D. and Lafleur,M. (1996) Cholesterol at different bilayer concentrations can promote or antagonize lateral segregation of phospholipids of differing acyl chain length. Biochemistry, 35, 15198–15208. [DOI] [PubMed] [Google Scholar]
- 9.Xu X., Bittman,R., Duportail,G., Heissler,D., Vilcheze,C. and London,E. (2001) Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal and disease-associated sterols and comparison of sphingomyelin, cerebrosides and ceramide. J. Biol. Chem., 276, 33540–33546. [DOI] [PubMed] [Google Scholar]
- 10.Brown D.A. and London,E. (1998) Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol., 14, 111–136. [DOI] [PubMed] [Google Scholar]
- 11.Simons K. and Ikonen,E. (1997) Functional rafts in cell membranes. Nature, 387, 569–572. [DOI] [PubMed] [Google Scholar]
- 12.Simons K. and van Meer,G. (1988) Lipid sorting in epithelial cells. Biochemistry, 27, 6197–6202. [DOI] [PubMed] [Google Scholar]
- 13.Harder T. and Simons,K. (1997) Caveolae, DIGs and the dynamics of sphingolipid-cholesterol microdomains. Curr. Opin. Cell Biol., 9, 534–542. [DOI] [PubMed] [Google Scholar]
- 14.London E. (2002) Insights into lipid raft structure and formation from experiments in model membranes, Curr. Opin. Struct. Biol., 12, 480–486. [DOI] [PubMed] [Google Scholar]
- 15.London E. and Brown,D.A. (2000) Insolubility of lipids in triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim. Biophys. Acta, 1508, 182–195. [DOI] [PubMed] [Google Scholar]
- 16.Melkonian K.A., Chu,T., Tortorella,L.B. and Brown,D.A. (1995) Characterization of proteins in detergent-resistant membrane complexes from Madin–Darby canine kidney epithelial cells. Biochemistry, 34, 16161–16170. [DOI] [PubMed] [Google Scholar]
- 17.Ostermeyer A.G., Beckrich,B.T., Ivarson,K.A., Grove,K.E. and Brown,D.A. (1999) Glycosphingolipids are not essential for formation of detergent-resistant membrane rafts in melanoma cells. methyl-beta-cyclodextrin does not affect cell surface transport of a GPI-anchored protein. J. Biol. Chem., 274, 34459–34466. [DOI] [PubMed] [Google Scholar]
- 18.Roper K., Corbeil,D. and Huttner,W.B. (2000) Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat. Cell Biol., 2, 582–592. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder R., London,E. and Brown,D. (1994) Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc. Natl Acad. Sci. USA, 91, 12130–12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stulnig T.M., Berger,M., Sigmund,T., Raederstorff,D., Stockinger,H. and Waldhausl,W. (1998) Polyunsaturated fatty acids inhibit T cell signal transduction by modification of detergent-insoluble membrane domains. J. Cell Biol., 143, 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuosto L., Parolini,I., Schroder,S., Sargiacomo,M., Lanzavecchia,A. and Viola,A. (2001) Organization of plasma membrane functional rafts upon T cell activation. Eur. J. Immunol., 31, 345–349. [DOI] [PubMed] [Google Scholar]
- 22.Dietrich C., Bagatolli,L.A., Volovyk,Z.N., Thompson,N.L., Levi,M., Jacobson,K. and Gratton,E. (2001) Lipid rafts reconstituted in model membranes. Biophys. J., 80, 1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harder T., Scheiffele,P., Verkade,P. and Simons,K. (1998) Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol., 141, 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pralle A., Keller,P., Florin,E.L., Simons,K. and Horber,J.K. (2000) Sphingolipid–cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol., 148, 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schutz G.J., Kada,G., Pastushenko,V.P. and Schindler,H. (2000) Properties of lipid microdomains in a muscle cell membrane visualized by single molecule microscopy. EMBO J., 19, 892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vereb G., Matko,J., Vamosi,G., Ibrahim,S.M., Magyar,E., Varga,S., Szollosi,J., Jenei,A., Gaspar,R., Jr, Waldmann,T.A. et al. (2000) Cholesterol-dependent clustering of IL-2Ralpha and its colocalization with HLA and CD48 on T lymphoma cells suggest their functional association with lipid rafts. Proc. Natl Acad. Sci. USA, 97, 6013–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson T.G. and McConnell,H.M. (2001) Condensed complexes and the calorimetry of cholesterol-phospholipid bilayers. Biophys. J., 81, 2774–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edidin M. (2001) Shrinking patches and slippery rafts: scales of domains in the plasma membrane. Trends Cell Biol., 11, 492–496. [DOI] [PubMed] [Google Scholar]
- 29.Norkin L.C., Wolfrom,S.A. and Stuart,E.S. (2001) Association of caveolin with Chlamydia trachomatis inclusions at early and late stages of infection. Exp. Cell Res., 266, 229–238. [DOI] [PubMed] [Google Scholar]
- 30.Pelkmans L., Kartenbeck,J. and Helenius,A. (2001) Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol., 3, 473–483. [DOI] [PubMed] [Google Scholar]
- 31.Peyron P., Bordier,C., N’Diaye,E.N. and Maridonneau-Parini,I. (2000) Nonopsonic phagocytosis of Mycobacterium kansasii by human neutrophils depends on cholesterol and is mediated by CR3 associated with glycosylphosphatidylinositol-anchored proteins. J. Immunol., 165, 5186–5191. [DOI] [PubMed] [Google Scholar]
- 32.Varma R. and Mayor,S. (1998) GPI-anchored proteins are organized in submicron domains at the cell surface. Nature, 394, 798–801. [DOI] [PubMed] [Google Scholar]
- 33.Zuhorn I.S., Kalicharan,R. and Hoekstra,D. (2002) Lipoplex-mediated transfection of mammalian cells occurs through the cholesterol-dependent clathrin-mediated pathway of endocytosis. J. Biol. Chem., 277, 18021–18028. [DOI] [PubMed] [Google Scholar]
- 34.Edgell C.J., McDonald,C.C. and Graham,J.B. (1983) Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl Acad. Sci. USA, 80, 3734–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thornhill M.H., Li,J. and Haskard,D.O. (1993) Leucocyte endothelial cell adhesion: a study comparing human umbilical vein endothelial cells and the endothelial cell line EA-hy-926. Scand. J. Immunol., 38, 279–286. [DOI] [PubMed] [Google Scholar]
- 36.Lampariello F. (1994) Evaluation of the number of positive cells from flow cytometric immunoassays by mathematical modeling of cellular autofluorescence. Cytometry, 15, 294–301. [DOI] [PubMed] [Google Scholar]
- 37.Arancibia-Carcamo C.V., Oral,H.B., Haskard,D.O., Larkin,D.F. and George,A.J. (1998) Lipoadenofection-mediated gene delivery to the corneal endothelium: prospects for modulating graft rejection. Transplantation, 65, 62–67. [DOI] [PubMed] [Google Scholar]
- 38.Subtil A., Gaidarov,I., Kobylarz,K., Lampson,M.A., Keen,J.H. and McGraw,T.E. (1999) Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl Acad. Sci. USA, 96, 6775–6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gruber H.J., Hahn,C.D., Kada,G., Riener,C.K., Harms,G.S., Ahrer,W., Dax,T.G. and Knaus,H.G. (2000) Anomalous fluorescence enhancement of Cy3 and cy3.5 versus anomalous fluorescence loss of Cy5 and Cy7 upon covalent linking to IgG and noncovalent binding to avidin. Bioconjug. Chem., 11, 696–704. [DOI] [PubMed] [Google Scholar]
- 40.Sanderson C.M., Parkinson,J.E., Hollinshead,M. and Smith,G.L. (1996) Overexpression of the vaccinia virus A38L integral membrane protein promotes Ca2+ influx into infected cells. J. Virol., 70, 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kabouridis P.S., Magee,A.I. and Ley,S.C. (1997) S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J., 16, 4983–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilangumaran S. and Hoessli,D.C. (1998) Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem. J., 335, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein U., Gimpl,G. and Fahrenholz,F. (1995) Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry, 34, 13784–13793. [DOI] [PubMed] [Google Scholar]
- 44.Ohtani Y., Irie,T., Uekama,K., Fukunaga,K. and Pitha,J. (1989) Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur. J. Biochem., 186, 17–22. [DOI] [PubMed] [Google Scholar]
- 45.Yancey P.G., Rodrigueza,W.V., Kilsdonk,E.P., Stoudt,G.W., Johnson,W.J., Phillips,M.C. and Rothblat,G.H. (1996) Cellular cholesterol efflux mediated by cyclodextrins. Demonstration of kinetic pools and mechanism of efflux. J. Biol. Chem., 271, 16026–16034. [DOI] [PubMed] [Google Scholar]
- 46.Gent M.P. and Prestegard,J.H. (1976) Interaction of the polyene antibiotics with lipid bilayer vesicles containing cholesterol. Biochim. Biophys. Acta, 426, 17–30. [DOI] [PubMed] [Google Scholar]
- 47.Milhaud J. (1992) Permeabilizing action of filipin III on model membranes through a filipin–phospholipid binding. Biochim. Biophys. Acta, 1105, 307–318. [DOI] [PubMed] [Google Scholar]
- 48.Montesano R., Vassalli,P., Perrelet,A. and Orci,L. (1980) Distribution of filipin–cholesterol complexes at sites of exocytosis—a freeze–fracture study of degranulating mast cells. Cell Biol. Int. Rep., 4, 975–984. [DOI] [PubMed] [Google Scholar]
- 49.Orlandi P.A. and Fishman,P.H. (1998) Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol., 141, 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patterson J., Holland,J. and Bieber,L.L. (1979) Studies on the competition of polyene antibiotics for sterols. J. Antibiot. (Tokyo), 32, 646–653. [DOI] [PubMed] [Google Scholar]
- 51.Schnitzer J.E., Oh,P., Pinney,E. and Allard,J. (1994) Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis and capillary permeability of select macromolecules. J. Cell Biol., 127, 1217–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Worgall S., Worgall,T.S., Kostarelos,K., Singh,R., Leopold,P.L., Hackett,N.R. and Crystal,R.G. (2000) Free cholesterol enhances adenoviral vector gene transfer and expression in CAR-deficient cells. Mol. Ther., 1, 39–48. [DOI] [PubMed] [Google Scholar]
- 53.Ghosh Y.K., Visweswariah,S.S. and Bhattacharya,S. (2000) Nature of linkage between the cationic headgroup and cholesteryl skeleton controls gene transfection efficiency. FEBS Lett., 473, 341–344. [DOI] [PubMed] [Google Scholar]
- 54.Ghosh Y.K., Visweswariah,S.S. and Bhattacharya,S. (2002) Advantage of the ether linkage between the positive charge and the cholesteryl skeleton in cholesterol-based amphiphiles as vectors for gene delivery. Bioconjug. Chem., 13, 378–384. [DOI] [PubMed] [Google Scholar]
- 55.Guyader M., Kiyokawa,E., Abrami,L., Turelli,P. and Trono,D. (2002) Role for human immunodeficiency virus type 1 membrane cholesterol in viral internalization. J. Virol., 76, 10356–10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wadia J.S., Stan,R.V. and Dowdy,S.F. (2004) Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med., 10, 310–315. [DOI] [PubMed] [Google Scholar]
- 57.Kirsch C., Eckert,G.P. and Mueller,W.E. (2003) Statin effects on cholesterol micro-domains in brain plasma membranes. Biochem. Pharmacol., 65, 843–856. [DOI] [PubMed] [Google Scholar]
- 58.Lafont F., Tran Van Nhieu,G., Hanada,K., Sansonetti,P. and van der Goot,F.G. (2002) Initial steps of Shigella infection depend on the cholesterol/sphingolipid raft-mediated CD44-IpaB interaction. EMBO J., 21, 4449–4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Norkin L.C., Anderson,H.A., Wolfrom,S.A. and Oppenheim,A. (2002) Caveolar endocytosis of simian virus 40 is followed by brefeldin A-sensitive transport to the endoplasmic reticulum, where the virus disassembles. J. Virol., 76, 5156–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Z.Y. and Smith,B.D. (2000) High-generation polycationic dendrimers are unusually effective at disrupting anionic vesicles: membrane bending model. Bioconjug. Chem., 11, 805–814. [DOI] [PubMed] [Google Scholar]
- 61.Haensler J. and Szoka,F.C.,Jr (1993) Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug. Chem., 4, 372–379. [DOI] [PubMed] [Google Scholar]