Abstract
Study Objectives:
The aims of this study were to (1) investigate the nature of cognitive impairment in individuals with insomnia, (2) document their clinical significance, (3) examine their correlates, and (4) explore differences among individuals with insomnia with and without cognitive complaints.
Design:
Participants underwent 3 consecutive nights of polysomnography. On the morning following the third night, they completed a battery of questionnaires and neuropsychological tests.
Participants:
The sample included 25 adults with primary insomnia (mean age: 44.4 ± 11.5 y, 56% women) and 16 controls (mean age: 42.8 ± 12.9 y, 50% women) matched for sex, age, and education.
Intervention:
N/A.
Measurement and Results:
Participants completed neuropsychological tests covering attention, memory, working memory, and executive functions, as well as questionnaires assessing the subjective perception of performance, depression, anxiety, fatigue, sleepiness, and hyperarousal. There were significant group differences for the attention and episodic memory domains. Clinically significant deficits were more frequent in the insomnia group. Within the insomnia group, individuals with cognitive complaints exhibited significantly poorer performance on a larger number of neuropsychological variables. All impaired aspects of performance were significantly associated with either subjective or objective sleep continuity, and some were also independently related to sleep microstructure (i.e., relative power for alpha frequencies) or selected psychological variables (i.e., beliefs or arousal).
Conclusions:
These findings suggest clinically significant alterations in attention and episodic memory in individuals with insomnia. Objective deficits were more pronounced and involved more aspects of performance in a subgroup of individuals with cognitive complaints. These deficits appear associated with sleep continuity, and may also be related to sleep microstructure and dysfunctional beliefs.
Citation:
Fortier-Brochu É, Morin CM. Cognitive impairment in individuals with insomnia: clinical significance and correlates. SLEEP 2014;37(11):1787-1798.
Keywords: attention, cognitive performance, insomnia, memory
INTRODUCTION
Insomnia is a widespread condition: between 30% and 50% of adults experience occasional sleep difficulties, and 6% to 13% meet the diagnostic criteria for an insomnia disorder.1–3 The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, (DSM-5)4 defines insomnia as a predominant complaint of dissatisfaction with sleep quantity or quality, along with difficulty initiating or maintaining sleep, which causes clinically significant distress or impairment in important areas of functioning (i.e., social, occupational, behavioral, etc.). Although primarily defined as a sleep disorder, repercussions of insomnia extend beyond the sleep period to affect the individuals' daytime lives. While an accurate understanding of the nature and mechanisms of these daytime symptoms is necessary to improve interventions, the daytime counterpart of insomnia has received relatively little scientific attention compared to its nighttime manifestations.
A number of studies have documented increased levels of fatigue, anxiety, or mood disturbances in individuals with insomnia in comparison with normal sleepers, although these symptoms usually remain in the subclinical range. Complaints of cognitive impairment are also often encountered in clinical practice and have been documented in a few studies,5–7 but scientific investigation has not succeeded in providing an unequivocal account of the objective impairments underlying these complaints. A number of studies have compared the performance of individuals with insomnia and normal sleepers on neuropsychological tests.7–9 Results of these studies have generated confusing findings: only one in four comparisons produced statistically significant differences between individuals with insomnia and normal sleepers, and the specific differences that are found are inconsistent across studies. Accordingly, most available reviews have concluded that there was no clear evidence of cognitive impairment in individuals with insomnia.7–9 However, the conclusions of many of these studies are limited by the relatively small and, in some cases, poorly characterized samples, the potential effect of confounding variables (e.g., age, education, comorbidity, hypnotic use, etc.) and the use of cognitive tasks of questionable sensitivity for detecting milder impairments (e.g., line judging or tracing, speed during a card-sorting task, immediate recognition tasks, cancellation tasks, etc.). In this context, definite conclusions regarding cognitive performance in individuals with insomnia still appear premature. Incidentally, a meta-analysis of available studies identified reliable differences of mild to moderate magnitude between individuals with insomnia and normal sleepers in a few cognitive domains, including working memory, episodic memory, and problem solving.10 These findings are paralleled by recent functional magnetic resonance imaging studies, which suggest that individuals with insomnia exhibit decreased activation of the frontoparietal cortex during a working memory task,11 and decreased recruitment of the head of the left caudate nucleus during a problem-solving task,12 even when performance on these tasks is unaltered. A similar hypoactivation of prefrontal regions was also found during a verbal fluency task.13 Still, additional well-controlled studies are required to further document the nature and magnitude of differences in cognitive performance among individuals with insomnia and controls.
One hypothesis that could account for inconsistent results across studies is the existence of individual differences regarding the cognitive effect of insomnia. Studies have shown trait-like individual differences in the vulnerability to the cognitive effects of sleep deprivation.14 Different patterns of associations between sleep and fatigue have also been documented among individuals with insomnia.15 Interestingly, the few available epidemiological accounts of cognitive complaints among individuals with insomnia suggest that as many as 50% do not report impaired memory or concentration.5 Recent studies also suggest different patterns of cognitive performance among individuals with insomnia presenting different symptom profiles (e.g., with or without objective short sleep duration,16 with or without hyperarousal17). Considering individuals with insomnia as a homogeneous sample with regard to daytime symptoms or cognitive performance may consequently be misleading and contribute to mask existing deficits for a subgroup of individuals.
The inconsistent evidence for statistically significant differences between individuals with insomnia and normal sleepers, together with the small to moderate magnitude of these differences, also raises questions regarding their clinical significance or practical impact in everyday functioning. One commonly used strategy to document clinical significance is to compare performance to normative values in order to identify individuals whose performances significantly depart from what would be expected. Among existing studies, those that used tests for which normative data were available have usually reported that averaged standardized scores for insomnia groups remained within the normal range.18 However, this does not exclude the possibility that some participants within the group could exhibit abnormal performance on selected variables. A meaningful repercussion of otherwise small differences in strategic aspects of cognitive performance could give rise to cognitive complaints in a subgroup of individuals, and contribute to the apparent discrepancy between objective and subjective findings.
If cognitive impairment is clinically significant in individuals with insomnia, or in a subgroup of individuals with insomnia, then treatment should also aim at improving this area of functioning. This would require an improved understanding of the underlying mechanisms of these impairments. Numerous factors could affect performance in individuals with insomnia. The detrimental effect of insufficient sleep on performance in basic attention tasks is well established, although controversies remain regarding its effect on higher level cognitive functions such as memory and executive functions.19 Alterations in sleep microstructure, which are suspected in individuals with insomnia,20 may also influence cognitive performance. Recent findings suggest that experimental manipulation of sleep micro-structure (i.e., reduced slow frequencies (< 1 Hz) and increased alpha frequencies (8-12 Hz)) hampers memory performance in healthy elderly individuals without sleep problems.21 In addition to potential contributions of altered sleep, factors such as increased depressive symptoms, fatigue, anxiety, arousal, and sleepiness have also been associated with decreased cognitive performance in healthy individuals,22–26 and could likewise be at play in individuals with insomnia. Among the studies investigating correlates of cognitive performance in individuals with insomnia, a few suggest associations between cognitive performance and sleep continuity27,28 or sleep microstructure,29 but investigations of relations between cognitive symptoms and other daytime symptoms remain scarce, and further investigation appears necessary.
In summary, individuals with insomnia present cognitive complaints that are not very well understood. Previous findings suggest mild to moderate deficits in a limited number of areas of cognitive functioning, but individual differences may exist among the heterogeneous group of insomnia sufferers. The clinical significance of these impairments and their correlates remain poorly documented. A better understanding of these symptoms and their mechanisms is likely to lead to improved knowledge of the effects of insomnia on the brain, and to improved intervention strategies more closely suited to the wide range of symptoms reported by individuals with insomnia.
Study Aims
The aims of this study were (1) to further investigate the nature of cognitive impairment in individuals with insomnia; (2) to gather some information regarding the clinical significance of these impairments; (3) to examine the association between cognitive impairments, different measures of sleep, and other daytime symptoms; and (4) to explore cognitive impairments in individuals with insomnia with and without cognitive complaints.
METHODS
This study was approved by the Comité d'éthique de la recherche de l'Institut universitaire en santé mentale de Québec.
Participants
The participants included 25 adults with insomnia (INS) and 16 age-, sex- and education-matched individuals without sleep problems (CTL). Participants were between 25 and 59 years old. Participants with insomnia (INS) met the following combined DSM-IV and International Classification of Diseases-10 criteria for insomnia: (1) difficulty initiating or maintaining sleep, with sleep onset latency (SOL) or wake time after sleep onset (WASO) superior to 30 minutes, or early morning awakening after sleeping less than 6.5 hours, and a sleep efficiency (SE) below 85%; (2) insomnia symptoms occurring at least 3 nights per week for at least 6 months and (3) significant distress or altered functioning in social, occupational, or other significant domains. Of note, these criteria imply that participants also met the new DSM-5 criteria for an insomnia disorder4. Participants without sleep problems (CTL) were (1) satisfied with their sleep; (2) did not meet diagnostic criteria for insomnia; and (3) did not use medication to facilitate their sleep. Exclusion criteria for both groups were (1) presence of a medical or neurological condition likely to interfere with sleep or cognitive functioning; (2) use of a medication altering sleep or cognitive functioning; (3) presence of a current major depressive episode or more than two prior major depressive episodes, generalized anxiety disorder, bipolar disorder or history of past episode of manic episode, or lifetime history of psychotic symptoms; (4) substance abuse (including alcohol) in the previous year; (5) suspicion of a sleep disorder other than insomnia; and (6) night or shift work, or irregular sleep schedule (i.e., habitual bedtime later than 01:00). Participants with insomnia who were using medication to facilitate their sleep on an occasional basis (i.e., a maximum of 2 nights per week) were included after ceasing medication for at least 2 consecutive weeks.
Procedure
Participants were recruited through larger ongoing studies at the Centre d'étude des troubles du sommeil in Université Laval and through advertisements in local media. After an initial screening telephone interview, they underwent a clinical assessment including the Structured Clinical Interview for DSM-IV,30 the Duke Structured Interview for Sleep Disorders,31 the Modified Mini-Mental State Examination (MMSE),32 and a medical history questionnaire. They also completed sleep diaries during 2 consecutive weeks. Participants then slept in the laboratory for 3 consecutive nights. On the morning following the third night, they completed a series of questionnaires and a battery of neuropsychological tests. The testing session began between 30 and 60 minutes after participants got out of bed, and lasted about 2.5 hours.
Measures
Measures of sleep
Polysomnographic recordings: Polysomnographic (PSG) recordings were conducted over 3 consecutive nights. Bedtimes and arising times were set to match participants' usual sleep schedule at home as closely as possible. Recordings were conducted using a standard montage and scored by qualified technicians according to standard criteria.33 On the first night, respiratory parameters and leg movement were also recorded in order to screen for sleep apnea and periodic leg movements. This first night was considered a screening and adaptation night, and data were not used in subsequent analyses. Dependent variables included measures of sleep continuity (i.e., SOL, WASO, number of awakening (NWAK), total sleep time (TST), time spent in bed (TIB) and percent sleep efficiency (%SE)) as well as measures of sleep architecture (percentage of time spent in stages N1, N2, N3, and R).
Spectral analysis of the electroencephalogram: Spectral analysis was conducted on the electroencephalogram (EEG) recordings of the F4-A1 lead for every epoch of non-rapid eye movement (NREM) sleep (stages N2 and N3) on night 3. EEG recordings were submitted to an automatic procedure for detection of artefacts based on Brunner's criteria for muscle artefacts detection34. A visual inspection was also conducted by a qualified technologist who was blind to participants' group. Portions of EEG including artefacts such as eye movements, muscle contractions, or heart beats were excluded, as were portions of EEG including miniarousals or microarousals. Spectral analysis was conducted using the Harmonie Sensa software from Stellate Systems.TM The resolution was set at 512 Hz. Power spectra were computed for 4-sec epochs with a 50% overlap. A Hanning window was used for pretreatment of the signal. The duration of recordings was 30 sec (i.e., 15 overlapping 4-sec epochs). Spectra were divided in the following seven frequency bands: slow waves (0.00-1.00 Hz), delta (1.00-4.00 Hz), theta (4.00-8.00 Hz), alpha (8.00-12.00 Hz), sigma slow (12.00-14.00 Hz), sigma fast (14.00-16.00 Hz), and beta (16.00-30.00 Hz). Analyses were computed on log-transformed relative power values.
Sleep diary: Before coming into the laboratory for PSG recordings, participants completed sleep diaries at home every morning for 14 consecutive nights. Dependent variables from the sleep diaries included SOL, NWAK, and WASO, duration of the terminal awakening (TWAK), TIB, TST, and %SE, as well as a subjective rating of sleep quality (SQ) on a five-point Likert scale (1 = poorest sleep quality, 5 = best sleep quality).
Insomnia Severity Index: The Insomnia Severity Index (ISI)35 assesses severity of initial insomnia, maintenance insomnia, and terminal insomnia, as well as satisfaction with sleep, interference of insomnia with daytime functioning, noticeability of functional difficulties associated with insomnia, and level of distress caused by insomnia. Each of the items is scored on a five-point Likert scale (0 = not at all, 4 = extremely). The dependent variable for this scale was the total score on these seven items, with higher scores suggesting more severe insomnia. An additional item investigating the perceived effect of insomnia on concentration and memory was also included and was used to categorize individuals with insomnia presenting cognitive complaints (i.e., those with a rating of 3 or 4) from those not presenting cognitive complaints (i.e., those with a score ranging from 0 to 2).
Measures of cognitive performance
Tests of cognitive performance were chosen to cover the cognitive domains for which impairments are suspected in individuals with insomnia, namely attention, working memory, episodic memory, and executive functioning.10,36 These tests have also been selected because they either were sensitive to detect cognitive impairment in individuals with insomnia in previous studies, or have demonstrated sufficient sensitivity to detect relatively subtle cognitive impairment in studies carried with other clinical populations.
Digit Span: The Digit Span subtest from the Wechsler Memory Scale-III37 is divided into two parts. In Digit Span Forward, series of digits of increasing length are verbally presented to participants, and their task is to repeat these digits in the exact order in which they were presented. In Digit Span Backward, participants must repeat the series of digits in the reverse order than that in which they have been presented. Dependent variables were the z-scores for the longest series of digits correctly recalled for each condition. This task is considered a measure of retention and manipulation of verbal information in working memory.
Paced Auditory Serial Addition Task: The Paced Auditory Serial Addition Task (PASAT)38 requires that participants add 60 pairs of digits presented in the auditory modality. Digits are presented in a continuous sequence from an audio digital recording. Participant must add the last digit heard to the digit immediately preceding it, and state their answers verbally. The task is carried at four different paces, with intervals of 2.4, 2.0, 1.6, and 1.2 seconds between digits. The dependent variable used is the z-score for the total number of correct answers over all four trials. The PASAT is presumed to assess speed of information processing, divided attention, and manipulation of information in working memory.
Tower Test from the D-KEFS: Materials for the Tower Test from the D-KEFS39 consist of a wooden board with three pegs and five discs of different colors and sizes. Participants must move the discs on the pegs in order to achieve a given model in the smallest number of moves possible. In doing so, participants must not move more than one disc at a time, and avoid placing a larger disc on a smaller one. The number of moves required to reach the solution varies from 1 to 26. Dependent variables for this task include an execution score computed as a function of the number of moves for each problem, as well as the ratio of rule violations per item completed. This test is presumed to assess planning abilities in a spatial modality, rule learning, inhibition of impulsive and perseverative responses and capacity to establish and maintain a set of instructions.
Verbal fluency: The verbal fluency task from the D-KEFS39 requires participants to name as many words corresponding to specific criteria as possible in a 60-second period. In the alphabetical condition, participants are asked to name words beginning with a given letter (three trials: F,A,S). In the category condition, participants must name words belonging to a given semantic category (two trials: animals, boy names). This task involves strategic access to information stored in semantic memory. Dependent variables selected for this task include the number of words generated in both conditions, as well as the ratios of set-loss (i.e., words that do not correspond to the required criteria for a given trial) and repetition errors (i.e., words repeated within a given trial) as a function of the total number of words generated. This task involves strategic access to information stored in semantic memory.
Continuous Performance Test-II: The Conners Continuous Performance Test-II (CPT-II)40 is a computerized task that assesses the ability to inhibit the response to a stimulus. Letters are successively presented on the computer screen and participants must press on the space bar as fast as possible, except when the letter is an “X”, when the participant must do nothing. The entire test lasts 14 minutes. Dependent variables derived from the CPT-II are reaction time, number omission errors (i.e., not pressing on the bar when the stimulus is not an X), commission errors (i.e., pressing on the bar when the stimulus is an X), and perseveration errors (i.e., hits with a reaction time below 100 msec), the detectability index (i.e., an index of the ability to detect targets from nontargets) and the hit rate block change (i.e., change in reaction time with time-on-task). This task assesses sustained attention as well as executive control of attention.
California Verbal Learning Task: In the California Verbal Learning Task (CLVT-II), a list of 16 words (list A) belonging to four broad categories is read five times to participants, and each trial is followed by a free immediate recall.41 An interference list (list B) is then read one time, followed by a free immediate recall. The participant must then recall list A: a free recall is completed, followed by a cued recall using the names of the four categories of items in the list. After a 20-min delay, participants must again proceed to a free recall of list A, followed by a cued recall. A recognition trial follows. Dependent variables selected include the total number of words recalled on trials 1 and 5, the total number of words recalled on delayed free recall, as well as errors of intrusion (i.e., recall of words that did not belong to the learning list) and repetition (i.e., words repeated within a given recall trial). This task provides an assessment of encoding, retrieval and storage in episodic verbal memory.
Measures of perceived cognitive performance
Multifactorial Memory Questionnaire: The Multifactorial Memory Questionnaire (MMQ)42,43 includes 57 items that are divided into three scales. The Contentment subscale involves 18 items dealing with the subjective feelings related to memory (satisfaction, irritation, embarrassment, etc.), with higher scores reflecting greater contentment with memory. The Ability subscale includes 20 items assessing the frequency of a number of memory failures in day-to-day life, with higher scores indicating better memory ability. The Strategy subscale involves 19 items assessing the frequency of use of different compensatory strategies, with higher scores indicating a more frequent use of memory strategies. Each item is rated on a five-point Likert scale. Dependent variables were the scores for each scale.
Cognitive Failures Questionnaire: The Cognitive Failures Questionnaire (CFQ)44 assesses the frequency of 25 cognitive failures during the past 6 months on a five-point Likert scale. The dependent variable for this scale is the total score.
Sleep diary cognitive variables: Additional items were added to sleep diaries to assess daytime cognitive function. Every evening, participants had to rate the perceived frequency of difficulty with attention/concentration, memory and planning/ organization (1 = never, 5 = very often). Given very high correlations between those three items (0.73 < r < 0.89, P < 0.001), the dependent variable for this measure was the sum of all three items.
Measures of other daytime symptoms
Dysfunctional Beliefs and Attitudes about Sleep: The Dysfunctional Beliefs and Attitudes about Sleep (DBAS) is a 30-item questionnaire that assesses different types of unhelpful cognitions associated with sleep or insomnia (i.e., beliefs, attitudes, expectations, attributions, etc.). A higher score indicates a higher level of unhelpful cognitions.
Beck Depression Inventory-II: The Beck Depression Inventory-II (BDI-II)45 was used to assess the severity of depressive symptoms. A higher score indicates a higher severity of depressive symptoms.
State Trait Anxiety Inventory: The State Trait Anxiety Inventory (STAI)46 assesses two forms of anxiety, namely anxiety in a specific situation (state anxiety) and the tendency to experience anxiety in general (trait anxiety). Each scale includes 20 items rated on a four-point Likert Scale. Higher scores indicate higher levels of anxiety.
Multidimensional Fatigue Inventory: The Multidimensional Fatigue Inventory (MFI)47,48 is a 20-item questionnaire that assesses the main manifestations of fatigue on a five-point Likert-type scale. It distinguishes the following five dimensions of fatigue: general fatigue, physical fatigue, mental fatigue, decreased motivation, and decreased activities. Higher scores suggest greater fatigue.
Arousal Predisposition Scale: The Arousal Predisposition Scale (APS)49 is a 12-item questionnaire intended to assess the reactivity in response to different types of environmental situations. It is considered a measure of the hyperarousal trait that is hypothesized to characterize individuals with insomnia. Responses are provided on a five-point Likert scale. Higher scores reflect a greater predisposition to arousal.
Epworth Sleepiness Scale: The Epworth Sleepiness Scale (ESS)50 is a seven-item questionnaire intended to measure the likelihood of someone falling asleep in different situations. Items are rated on a four-point Likert scale. A higher score indicates a higher level of sleepiness.
Statistical Analyses
Data were examined using standard procedure to establish normality and investigate outliers and missing data.51 Missing data were not imputed. The alpha level was set at 5% for all analyses. Analyses were conducted using IBM SPSS 20.
Comparison of INS and CTL groups
Group comparisons on demographic, clinical, sleep, power spectral analyses, and daytime symptoms variables were conducted using chi-square tests (for categorical variables) and independent samples t-tests (for continuous variables). For each cognitive test, dependent variables were converted to normalized z-scores using the published population-based normative data, with higher z-scores indicating better performance. A global performance score was computed by averaging z-scores on the 20 selected dependent variables, and between-group comparison on global performance was examined using an independent samples t-test. To identify specific differences in cognitive performance, variables were grouped in four cognitive domains: attention (CPT-II hit rate, CPT-II hit rate block change, CPT-II omissions, CPT-II commissions, CPT-II perseverations and CPT-II detectability), working memory (digit span forward, digit span backward, PASAT total score, and CVLT-II trial I), episodic memory (CVLT-II trial 5, CVLT-II delayed free recall, CVLT-II repetitions, CVLT-II intrusions) and executive functions (Tower execution score and rule violations, Verbal fluency alphabetic and category scores as well as percentage of set-loss and repetition errors). A multivariate analysis of variance (MANOVA) was performed for each cognitive domain. When appropriate, univariate tests were computed to identify which variables were responsible for the significant differences in the multivariate analysis. For each comparison, effect sizes were estimated using Cohen's d. In order to assess the clinical significance of findings, chi-square tests were computed to compare groups on the frequency of clinically impaired performance, defined as a performance inferior to one standard deviation below the normative mean (i.e., z scores < -1).
Comparison of INS with cognitive complaints and CTL
INS were divided in subgroups according to their score on the concentration/memory item of the ISI, which identified a subgroup of individuals with insomnia and cognitive complaints (INS-C, n = 13), and a subgroup with insomnia but without cognitive complaints (INS-NC, n = 12). All participants in the CTL group had scores below 3 on the concentration/memory item of the ISI, and were accordingly considered as not presenting cognitive complaints. Between-group comparisons between INS-C, INS-NC, and CTL were then computed using chi-square tests (for categorical variables) and univariate analyses of variance (ANOVAs) for clinical, sleep, daytime symptoms, and cognitive performance variables. Post hoc comparisons were computed when the omnibus tests suggested significant differences between groups.
Correlates of cognitive impairments
Bivariate correlations were computed between cognitive performance variables for which differences between INS and CTL groups were statistically significant (i.e., CPT-II detectability, CPT-II perseverations, CVLT-II intrusions), sleep variables (derived from sleep diaries, PSG, and spectral analyses) and daytime symptoms variables (BDI-II, STAI, APS, ESS, and MFI scores). A preliminary examination of correlation matrices was conducted to identify variables potentially associated with cognitive impairments (i.e., correlations for which P ≤ 0.10). These variables were then entered in linear regression analyses (one analysis for each variable) and unique predictors were identified using a backward elimination procedure. Given the well documented tradeoff between speed and accuracy on the CPT-II,40 hit rate was also entered as a predictor for regression analyses on CPT-II variables. Group differences in predictors were verified by testing interactions terms.
RESULTS
Sample Description
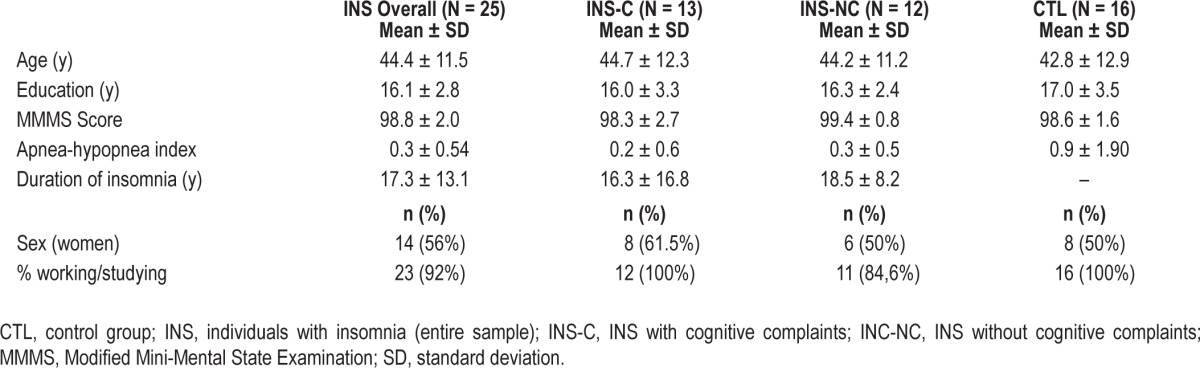
Means, standard deviations, and between-group comparisons for demographic and clinical variables are presented in Table 1. There was no significant difference between groups for age (t(39) = 0.431, P = 0.669), education (t(39) = -0.884, P = 0.382), or sex distribution (χ2 (1, N = 41) = 0.141, P = 0.707), which validated the matching procedure. There were no significant differences between groups regarding the score on the Modified Mini-Mental Status Examination (t(39) = 0.462, P = 0.647), the occupational status (χ2 (1, N = 41) = 1.346, P = 0.246), or the apnea/hypopnea index (t(39) = -1.683, P = 0.102).
Table 1.
Demographic and clinical characteristics of participants by group.
Comparison of INS and CTL Groups
Sleep
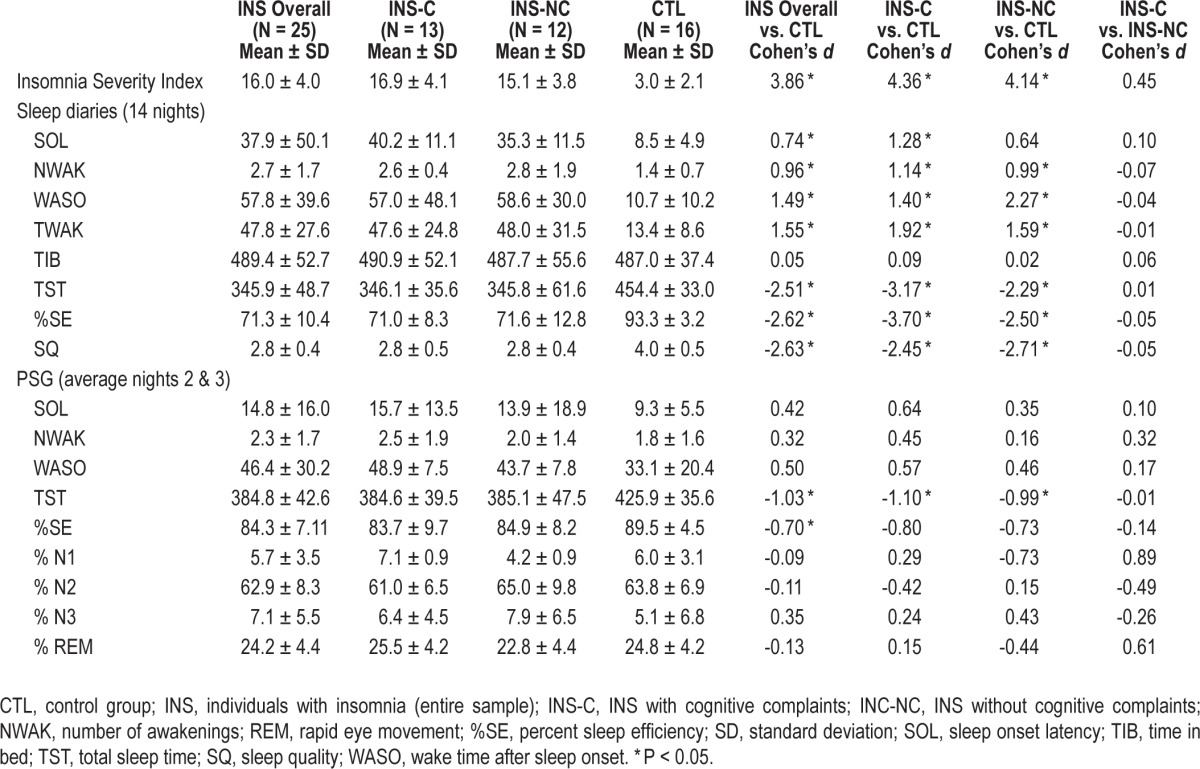
Means, standard deviations, and between-group comparisons for sleep variables are presented in Table 2. INS had a significantly higher score on the ISI compared to CTL (t(39) = 12.033, P < 0.001), which validates group formation. For sleep diary variables (averaged over 14 nights), comparisons indicated that INS had a significantly longer SOL (t(39) = 2.329, P = 0.025), WASO (t(39) = 4.629, P < 0.001), and TWAK (t(39) = 4.881, P < 0.001), more frequent NWAK (t(39) = 2.980), P = 0.005), shorter TST (t(39) = -7.810, P < 0.001), lower %SE (t(39) = -8.160, P < 0.001), and poorer SQ (t(39) = -8.219, P < 0.001). Average TIB did not differ between groups (t(39) = 0.158, P = 0.875). For PSG variables, a significant difference was found between INS and CTL for TST (t(39) = -3.200, P = 0.003) and SE (t(39) = -2.194, P = 0.034). There was no significant difference for sleep architecture variables, nor for log-transformed relative spectral power data: slow waves (t(36) = -0.542, P = 0.591), delta (t(36) = 0.588, P = 0.581), theta (t(36) = 0.156, P = 0.877), alpha (t(36) = 0.276, P = 0.784), slow sigma (t(36) = -0.077, P = 0.939), fast sigma (t(36) = -0.169, P = 0.866), and beta (t(36) = -1.704, P = 0.097).
Table 2.
Between-group comparisons on sleep variables.
Daytime symptoms
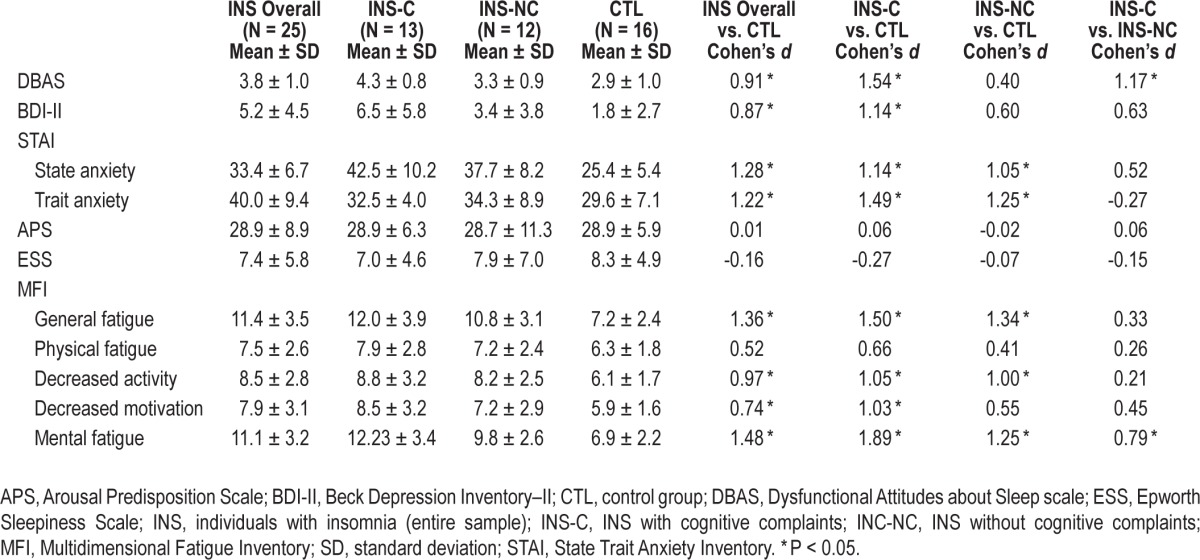
Means, standard deviations, and between-group comparisons for daytime symptoms variables are presented in Table 3. INS had higher scores on the DBAS scale (t(38) = 2.812, P = 0.008), the BDI (t(39) = 2.565, P = 0.014), both STAI state (t(39) = 3.983, P < 0.001) and trait (t(39) = 3.774, P = 0.001) scales, and most scales from the MFI (i.e., general fatigue (t(39) = 4.241, P < 0.001), decreased activities (t(39) = 3.024, P = 0.004), decreased motivation (t(39) = 2.316, P = 0.026), and mental fatigue (t(39) = 4.603, P < 0.001). Groups did not differ on scores on the APS (t(39) = 0.034, P = 0.973), the ESS (t(39) = -0.501, P = 0.619), and the physical fatigue scale of the MFI (t(39) = 1.634, P = 0.110.
Table 3.
Between-group comparisons on daytime variables.
Cognitive performance
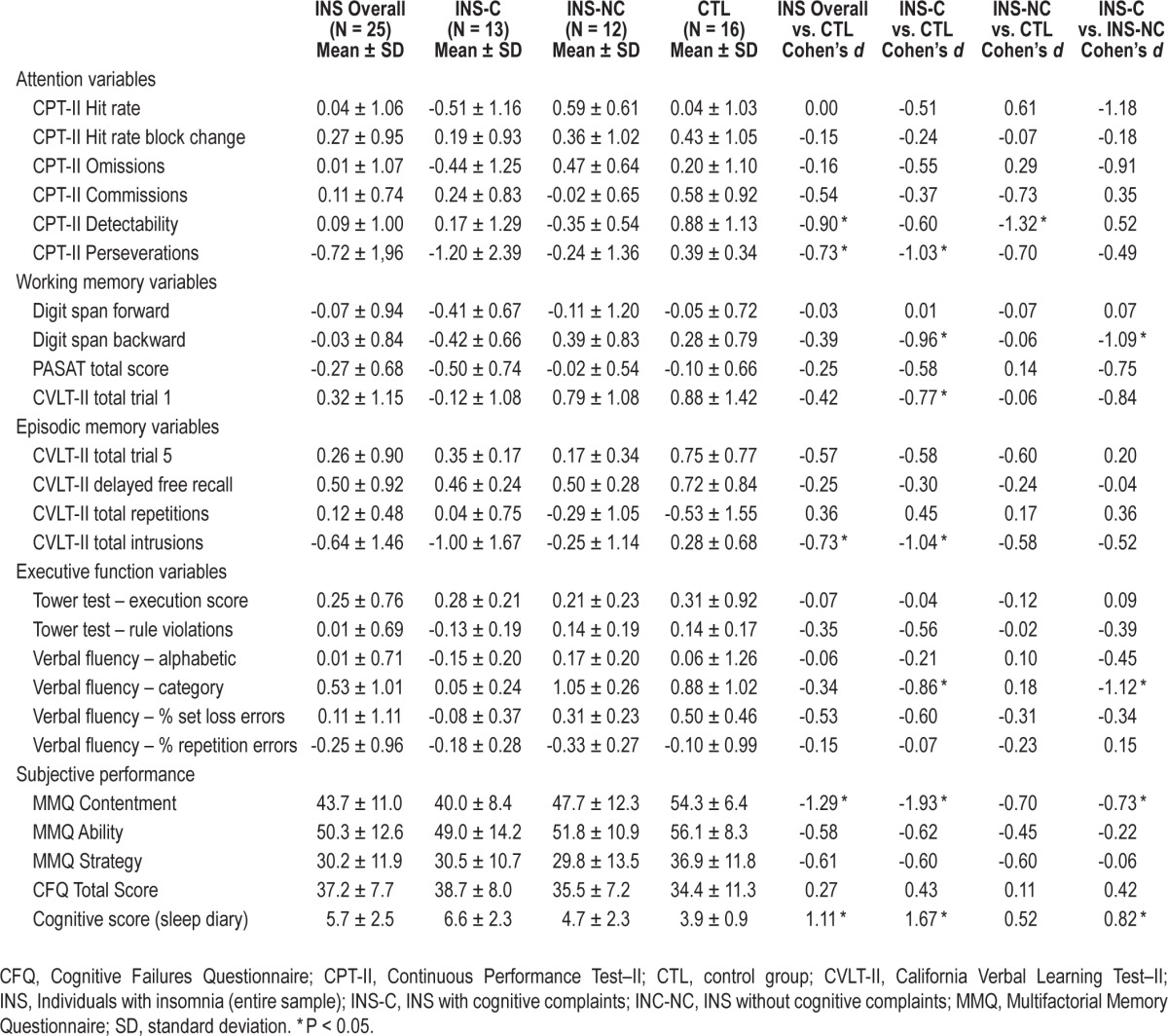
An independent samples t-test indicated poorer global performance in individuals with insomnia (mean z-score: -0.07 ± 0.43) compared with normal sleepers (mean z-score: 0.25 ± 0.36; t(39) = -2.519, P = 0.016, Cohen's d = -0.81). Detailed means and standard deviations for cognitive variables are presented in Table 4. Significant differences were found between INS and CTL groups for the attention (F(1, 35) = 2.611, P = 0.039) and episodic memory domains (F(1, 39) = 2.830, P = 0.039), and for subjective assessment of cognitive functioning (F(1, 39) = 5.186, P = 0.001). There were no significant differences between groups for the working memory (F(4, 36) = 0.75, P = 0.57) and executive domains (F(6, 34) = 0.57, P = 0.75). For the attention domain, univariate analyses suggested poorer detectability (P = 0.011) and greater number of perseverative errors (P = 0.039) in the INS group compared to CTL. For the episodic memory domain, univariate tests suggested increased intrusion errors (P = 0.024) in INS compared to CTL. Effect sizes for significant differences in cognitive performance were moderate to large (0.73 ≤ d ≤ 0.92). Among subjective measures of performance, INS had significantly higher frequency of reported cognitive difficulties on the sleep diaries (P = 0.006) and lower scores on the Contentment scale of the MMQ (P = 0.001). Differences did not reach statistical significance for the score on the Cognitive Failures Questionnaire (P = 0.358) nor for scores on the Ability (P = 0.115) and Strategy scales of the MMQ (P = 0.073).
Table 4.
Between-group comparisons on cognitive performance variables.
Clinical significance
Chi-square tests on the frequency of impaired performance for the selected variables suggest that participants from the INS group were more likely than CTL to have impaired performance on CVLT-II intrusions (INS, n = 9; CTL, n = 1, χ2 (1, N = 41) = 4.682, P = 0.03) and CPT-II perseveration errors (INS, n = 6; CTL, n = 0, χ2 (1, N = 35) = 5,431, P = 0.02). Groups did not differ on the frequency of impaired performance for CPT-II detectability.
Comparison of INS-C, INS-NC and CTL Groups
Demographic variables
There was no significant difference between groups for age (F(2, 38) = 0.098, P = 0.907), education (F(2, 38) = 0.401, P = 0.672), apnea-hypopnea index (F(2, 36) = 1.378, P = 0.266), MMSE score (F(2, 38) = 1.235, P = 0.302), sex distribution (χ2 (2, N = 41) = 0.475, P = 0.788), or occupation (χ2 (2, N = 41) = 4.529, P = 0.104). INS-NC and INS-C did not significantly differ on insomnia duration (t(23) = 0.409, P = 0.686).
Sleep
Means, standard deviations, and between-group comparisons for sleep variables are presented in Table 2. There were significant differences between groups on the ISI total score (F(2, 38) = 74.6, P < 0.001), with both INS-C and INS-NC groups having higher scores than CTL. Regarding sleep diary variables, significant differences were found between groups for NWAK (F(2, 38) = 4.357, P = 0.02), WASO (F(2, 38) = 10.452, P < 0.001), TWAK (F(2, 38) = 11.277, P < 0.001), SQ (F(2, 38) = 32.932, P < 0.001), TST (F(2, 38) = 29.719, P < 0.001), and %SE (F(2, 38) = 32.469, P < 0.001). Post hoc tests suggested decreased sleep continuity on these variables in both INS subgroups compared to CTL, but no difference was found between the two INS groups. Differences for SOL were marginally significant (F(2, 38) = 2.697, P = 0.080). There was no significant difference between groups for TIB (F(2, 38) = 0.026, P = 0.974). For PSG variables, there were no significant differences between the three groups: SOL (F(2, 38) = 0.354, P = 0.704), NWAK (F(2, 38) = 0.674, P = 0.515), TST (F(2, 38) = 2.336, P = 0.11), WASO (F(2, 38) = 0.805, P = 0.454), %SE (F(2, 38) = 1.203, P = 0.312), %N2 (F(2, 38) = 0.553, P = 0.580), %N3 (F(2, 38) = 0.842, P = 0.439), or %R (F(2, 38) = 0.113, P = 0.894), although differences approached significance for %N1 (F(2, 38) = 3.076, P = 0.058). There were no significant differences for log-transformed relative spectral power values: slow waves (F(2, 35) = 0.193, P = 0.825), delta (F(2, 35) = 1.164, P = 0.324), theta (F(2, 35) = 2.108, P = 0.137), alpha (F(2, 35) = 0.613, P = 0.547), slow sigma (F(2, 35) = 0.006, P = 0.994), fast sigma (F(2, 35) = 0.014, P = 0.986) and beta (F(2, 35) = 2.194, P = 0.127).
Daytime symptoms
Means, standard deviations, and between-group comparisons for cognitive functioning variables are presented in Table 3. Significant between-group differences were found for DBAS (F(2, 38) = 7.016, P = 0.001), BDI-II (F(2, 38) = 5.237, P = 0.01), STAI trait anxiety (F(2, 38) = 8.273, P = 0.001), and STAI state anxiety (F(2, 38) = 8.115, P = 0.001), IMF general fatigue (F(2, 38) = 9.392, P = 0.000), decreased motivation (F(2, 38) = 3.606, P = 0.037), decreased activities (F(2, 38) = 4.675, P = 0.015), and mental fatigue (F(2, 38) = 14.053, P < 0.001). INS-C and INS-NC had significantly higher scores than CTL on STAI trait scale, IMF general fatigue, decreased activities, and mental fatigue. INS-C also had higher scores than CTL on DBAS, BDI-II, and IMF decreased motivation. INS-C had higher scores than INS-NC on DBAS and IMF mental fatigue. There were no group differences for APS (F(2, 38) = 1.028, P = 0.984), ESS (F(2, 38) = 6.335, P = 0.812), and IMF physical fatigue (F(2, 38) = 1.587, P = 0.218).
Cognitive performance
The ANOVA suggested group differences for overall z score (F(2, 38) = 6.052, P = 0.005). Post hoc comparisons suggested that INS-C (mean z-score: -0.24 ± 0.48) had a significantly lower overall z-score compared to INS-NC (mean z-score: 0.11 ± 0.30, P = 0.027) and CTL (mean z-score: 0.25 ± 0.36, P = 0.001). Detailed means and standard deviations for the cognitive variables are presented in Table 5. Within the attention domain, ANOVAs suggested significant differences for CPT-II detectability (F(2, 32) = 4.275, P = 0.023) and CPT-II perseverations (F(2, 32) = 3.442, P = 0.044). For the working memory domain, ANOVAs suggested group differences for Digit span backward (F(2, 38) = 2.569, P = 0.019). For episodic memory variables, differences were found for CVLT-II intrusion errors (F(2, 38) = 4.093, P = 0.025). For the executive functions domain, differences were found for Verbal fluency in the category condition (F(2, 38) = 4.142, P = 0.024). Differences in subjective ratings of performance were found on the sleep diary cognitive score (F(2, 38) = 7.966, P = 0.001), and the MMQ contentment scale (F(2, 38) = 8.885, P = 0.001.
Table 5.
Independent correlates of cognitive deficits.
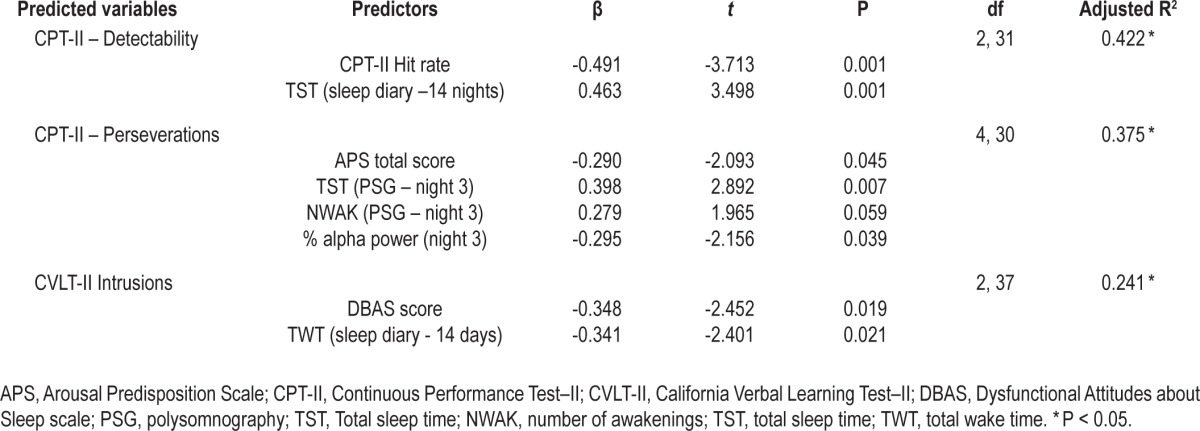
Correlates of Cognitive Impairments
Stepdown regression analyses were conducted on normalized scores for CPT-II detectability and perseveration errors and CVLT-II intrusion errors. Results are reported in Table 5. Habitual sleep duration on sleep diaries was positively associated with CPT-II detectability. Habitual TWT was negatively associated with CVLT-II intrusion errors. PSG-defined sleep duration on night 3 and relative power for alpha frequencies were associated with CPT-II perseveration errors. DBAS scores were significantly related to CVLT-II intrusions errors, whereas APS scores were significantly associated with CPT-II perseveration errors. STAI, MFI, and ESS scores were not correlated with cognitive impairments (P > 0.10) and were consequently not entered in regression analyses. Exploratory correlations between BDI-II scores and performance variables were not significant for CPT-II perseverations and detectability (P > 0.10). A significant association was found between the BDI-II score and CVLT-II intrusions (r = 0.349, P = 0.025). The BDI-II score was consequently entered in the regression analysis for CVLTII intrusions, but was not retained as a significant predictor in the final model. There were no significant interactions between group and predictor variables for any of the dependent variables examined in the regression procedure.
DISCUSSION
This study examined the nature and clinical significance of cognitive impairment associated with insomnia. Individuals with insomnia had poorer overall performance compared to participants of the control group, and committed more errors in attention and episodic memory tasks. Mild clinically significant cognitive impairments were more frequent among individuals with insomnia. Those who specifically complained about the effect of insomnia on their cognitive performance appeared more impaired than those who did not have cognitive complaints. Most cognitive impairments were associated with either objective or subjective sleep continuity variables, whereas some variables were also associated with relative power alpha frequencies, predisposition to hyperarousal, and beliefs about sleep.
Findings of impaired performance in selective cognitive domains in individuals with insomnia are in agreement with prior accounts in the literature. The magnitude of significant differences between individuals with insomnia and controls on attention and memory variables was in the moderate to large range. Previous results have been inconsistent with regard to attention processes in individuals with insomnia, and varied depending on the nature of the task and dependent variable considered,10 with more complex tasks usually being considered more sensitive to the effects of insomnia.52,53 In the current study, individuals with insomnia exhibited decreased detectability and increased perseveration errors on the CPT-II, but similar reaction time, time-on-task effect, and rate of omission errors compared to normal sleepers. These findings suggest that although they respond on average as quickly as normal sleepers, individuals with insomnia are unable to maintain a comparable level of accuracy. Increased perseveration errors (i.e., hits with a reaction time inferior to 100 msec) are most commonly interpreted as anticipatory responses reflecting increased impulsivity but they can also result from inattention or decreased motivation.40 Contrary to perseveration errors committed in the context of other cognitive tasks (e.g., Wisconsin Card Sorting Test), these anticipatory responses are not considered to result from set-shifting impairment, although both types of perseveration errors can be related to decreased inhibition.40,54 Previous studies have also generated conflicting findings regarding memory in individuals with insomnia,18,55–61 although a meta-analysis recently suggested reliable differences of moderate magnitude for memory variables across studies.10 In the current study, individuals with insomnia recalled the same amount of information as controls, but generated more intrusion errors. This could reflect decreased response inhibition during recall,41 which would be consistent with a recent report suggesting an increased vulnerability to interference in episodic memory in individuals with insomnia.62 Overall, deficits in memory and attention performance both appear consistent with a mild dysfunction of cognitive inhibitory processes. Inhibitory processes are considered to be highly dependent on the integrity of the prefrontal cortex, for which previous studies suggest decreased activation during task completion in individuals with insomnia.11,13
A secondary aim of this study was to gather information regarding the clinical significance of differences in performance between normal sleepers and individuals with insomnia. Clinical significance can be documented indirectly through indexes such as comparisons with normative data, subjective ratings, and social impact measures.63 In this study, averaged scores for individuals with insomnia remained in the normal range when compared with normative means, but insomnia was associated with an increased frequency of clinically significant mild deficits in attention and episodic memory domains. These findings suggest that, although they are of small magnitude, differences between groups involve impairments that would be considered clinically meaningful by current practice standards for a subgroup of participants with insomnia. As a group, individuals with insomnia were also more dissatisfied with their memory and reported more day-to-day cognitive difficulties compared to controls, which could be interpreted as evidence that cognitive difficulties were sufficient to be noticeable to participants in their everyday lives. Group differences in subjective ratings of performance in conjunction with absence of significant group differences on cognitive tests have previously led to the hypothesis that individuals with insomnia tend to overestimate their daytime cognitive deficits.18,57,64 However, the relationship of neuropsychological test performance to everyday functioning is considered complex, as it is mediated by a large number of variables (e.g., availability and use of compensatory strategies, nature of daytime roles and occupations, mood, etc). Mild deficits on strategic aspects of cognitive functioning may be sufficient to impair day-to-day functioning in complex activities. This hypothesis is further supported by previous population-based studies suggesting that insomnia is associated with different adverse outcomes that could be related to cognitive impairments, such as decreased productivity and increased costly accidents and errors in the workplace.65,66
Results further show that individuals with insomnia who reported more severe cognitive complaints perform significantly worse overall than both controls and individuals with insomnia without cognitive complaints. Exploratory analyses suggest that specific cognitive profiles differ between the two insomnia subgroups. More specifically, individuals without cognitive complaints had a faster reaction time but poorer accuracy on the CPT-II compared to controls, whereas those with cognitive complaints did not differ on reaction time, but exhibited significantly more perseveration errors on the CPT-II, recalled a smaller number of items on Digit span backward and CVLT-II trial 1, and generated more intrusions on the CVLT-II and fewer words on categorical verbal fluency. These findings tend to support the hypothesis that individual differences exist regarding both cognitive complaints and performance in individuals with insomnia. Consistent with the findings of the overall group comparisons, recent studies have also failed to find significant differences between individuals with insomnia and controls on measures of working memory,11,67 verbal fluency,13,64 or tower tasks.12 These results appeared inconsistent with those of a previous meta-analysis, which suggested decreased performance in individuals with insomnia on working memory and problem-solving tasks.10 Findings of more extended deficits among a subgroup of individuals with insomnia and cognitive complaints suggest that considering insomnia as a homogeneous condition may contribute to mask performance impairments in a subset of individuals. Heterogeneous cognitive profiles among individuals with insomnia may in turn account, at least in part, for contradictory findings in the literature. From the clinical standpoint, findings also indicate that increased cognitive complaints may not solely result from an overestimation of deficits, but may actually be associated with more pronounced and extended cognitive impairment.
All three variables for which impaired performance was found were associated with some aspects of sleep disturbance. Interestingly, none of these variables was associated with anxiety, depressed mood, fatigue, or sleepiness. Increased CPT-II perseverations, which can be conceptualized as a measure of impulsivity, were associated with shorter sleep duration and increased relative alpha power on the night prior to cognitive testing, as well as increased hyperarousal predisposition. This is consistent with recent evidence suggesting increased errors in an attention task in individuals with insomnia who also show evidence of hyperarousal.17 Decreased accuracy on the CPT-II and the CVLT-II were both associated with decreased habitual sleep continuity (i.e., sleep duration and total wake time, respectively), as assessed by sleep diaries completed at home over 14 consecutive nights. Associations between different aspects of cognitive performance and sleep continuity have been reported in previous studies.28,68 Increased intrusions during recall were also associated with more unhelpful beliefs about sleep. Theoretical models for insomnia predict that increased beliefs and vigilance toward the expected consequences of poor sleep can lead to real impairments in performance.69,70 Although this could account for the relationship between memory performance and beliefs about sleep, it is also possible that individuals with insomnia who experience more difficulties with their memory become more prone to endorse beliefs regarding the negative effect of their poor sleep than those with milder memory impairments. Different patterns of correlations for different cognitive variables may also imply that distinct mechanisms are at play in altering different aspects of performance.
This study had a number of limitations, the most important of which is a relatively small sample size. For some variables, group differences of moderate magnitude were not sufficient to reach statistical significance, and it seems plausible that a larger sample would have allowed identification of additional areas of cognitive impairment. In order to decrease the influence of potential confounding factors, the sample included only adults without comorbid medical or psychological disorders, who did not use psychotropic medication on a regular basis. This may limit generalization to other groups of individuals with insomnia (e.g., elders, individuals with comorbid insomnia, etc.). It seems likely that the nature, clinical significance, and correlates of cognitive impairments would be different among other clinical groups with insomnia. Generalizability could also be limited by the relatively high level of education in our sample. In fact, a recent study71 performed on elders suggested that sleep onset or maintenance difficulties were associated with decreased cognitive performance only in individuals with a lower education (or smaller cognitive reserve).
Nonetheless, findings from this study suggest that at least a subgroup of individuals with insomnia experience significant cognitive impairments that are clinically significant and warrant clinical attention. Future studies may achieve better characterization of cognitive deficits associated with insomnia by selecting individuals who specifically complain about their cognitive functioning.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by a research fellowship awarded to Émilie Fortier-Brochu, MPs, by the Canadian Institutes of Health Research. Dr Charles Morin served as a consultant on advisory boards for Merck, Novartis and Valeant, acted as a speaker for Merck and Valeant, and received a research grant from Novartis. Ms. Fortier-Brochu has indicated no financial conflicts of interest.
REFERENCES
- 1.Morin CM, LeBlanc M, Daley M, Gregoire JP, Merette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123–30. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Morin CM, LeBlanc M, Belanger L, Ivers H, Merette C, Savard J. Prevalence of insomnia and its treatment in Canada. Can J Psychiatry. 2011;56:540–8. doi: 10.1177/070674371105600905. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 5.Leger D, Stal V, Guilleminault C, Raffray T, Dib M, Paillard M. Les consequences diurnes de l'insomnie: impact sur la qualite de vie. Rev Neurol (Paris) 2001;157:1270–8. [PubMed] [Google Scholar]
- 6.Buysse DJ, Thompson W, Scott J, et al. Daytime symptoms in primary insomnia: a prospective analysis using ecological momentary assessment. Sleep Med. 2007;8:198–208. doi: 10.1016/j.sleep.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riedel BW, Lichstein KL. Insomnia and daytime functioning. Sleep Med Rev. 2000;4:277–98. doi: 10.1053/smrv.1999.0074. [DOI] [PubMed] [Google Scholar]
- 8.Shekelton JA, Rogers NL, Rajaratnam SMW. Searching for the daytime impairments of primary insomnia. Sleep Med Rev. 2010;14:47–60. doi: 10.1016/j.smrv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Fulda S, Schulz H. Cognitive dysfunction in sleep disorders. Sleep Med Rev. 2001;5:423–45. doi: 10.1053/smrv.2001.0157. [DOI] [PubMed] [Google Scholar]
- 10.Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. 2012;16:83–94. doi: 10.1016/j.smrv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Drummond SP, Walker M, Almklov E, Campos M, Anderson DE, Straus LD. Neural correlates of working memory performance in primary insomnia. Sleep. 2013;36:1307–16. doi: 10.5665/sleep.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoffers D, Altena E, van der Werf YD, et al. The caudate: a key node in the neuronal network imbalance of insomnia? Brain. 2014;137:610–20. doi: 10.1093/brain/awt329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altena E, Van Der Werf YD, Sanz-Arigita EJ, et al. Prefrontal hypoactivation and recovery in insomnia. Sleep. 2008;31:1271–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 15.Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Relations between sleep, fatigue, and health-related quality of life in individuals with insomnia. J Psychosom Res. 2010;69:475–83. doi: 10.1016/j.jpsychores.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-Mendoza J, Calhoun S, Bixler EO, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33:459–65. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edinger JD, Means MK, Krystal A. Does physiological hyperarousal enhance error rates among insomnia sufferers? Sleep. 2013;36:1179–86. doi: 10.5665/sleep.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vignola A, Lamoureux C, Bastien CH, Morin CM. Effects of chronic insomnia and use of benzodiazepines on daytime performance in older adults. J Gerontol B Psychol Sci Soc Sci. 2000;55:P54–62. doi: 10.1093/geronb/55.1.p54. [DOI] [PubMed] [Google Scholar]
- 19.Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–29. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 20.Feige B, Baglioni C, Spiegelhalder K, Hirscher V, Nissen C, Riemann D. The microstructure of sleep in primary insomnia: an overview and extension. Int J Psychophysiol. 2013;89:171–80. doi: 10.1016/j.ijpsycho.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Van Der Werf YD, Altena E, Schoonheim MM, et al. Sleep benefits subsequent hippocampal functioning. Nat Neurosci. 2009;12:122–3. doi: 10.1038/nn.2253. [DOI] [PubMed] [Google Scholar]
- 22.Smallwood J, Fitzgerald A, Miles LK, Phillips LH. Shifting moods, wandering minds: negative moods lead the mind to wander. Emotion. 2009;9:271–6. doi: 10.1037/a0014855. [DOI] [PubMed] [Google Scholar]
- 23.Scheibe S, Blanchard-Fields F. Effects of regulating emotions on cognitive performance: what is costly for young adults is not so costly for older adults. Psychol Aging. 2009;24:217–23. doi: 10.1037/a0013807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7:336–53. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 25.Van der Linden D, Frese M, Meijman TF. Mental fatigue and the control of cognitive processes: effects on perseveration and planning. Acta Psychologica. 2003;113:45–65. doi: 10.1016/s0001-6918(02)00150-6. [DOI] [PubMed] [Google Scholar]
- 26.van der Linden D, Frese M, Sonnentag S. The impact of mental fatigue on exploration in a complex computer task: rigidity and loss of systematic strategies. Hum Factors. 2003;45:483–94. doi: 10.1518/hfes.45.3.483.27256. [DOI] [PubMed] [Google Scholar]
- 27.Hart RP, Morin CM, Best AM. Neuropsychological performance in elderly insomnia patients. Aging and Cognition. 1995;2:268–78. [Google Scholar]
- 28.Bastien CH, Fortier-Brochu E, Rioux I, LeBlanc M, Daley M, Morin CM. Cognitive performance and sleep quality in the elderly suffering from chronic insomnia. Relationship between objective and subjective measures. J Psychosom Res. 2003;54:39–49. doi: 10.1016/s0022-3999(02)00544-5. [DOI] [PubMed] [Google Scholar]
- 29.Crenshaw MC, Edinger JD. Slow-wave sleep and waking cognitive performance among older adults with and without insomnia complaints. Physiol Behav. 1999;66:485–92. doi: 10.1016/s0031-9384(98)00316-3. [DOI] [PubMed] [Google Scholar]
- 30.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 31.Edinger JD, Kirby AC, Lineberg MD, Loiselle MM, Wohlgemuth WK, Means MK. Duke Structured Interview Schedule for DSM-IV-TR and ICSD-2 Sleep Disorders Diagnoses. Durham, NC: Veterans Affairs and Duke University Medical Centers; 2004. [Google Scholar]
- 32.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 33.American Academy of Sleep Medicine. AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2012. Version 2.0. www.aasmnet.org. [Google Scholar]
- 34.Brunner DP, Vasko RC, Detka CS, Monahan JP, Reynolds CF, 3rd, Kupfer DJ. Muscle artifacts in the sleep EEG: automated detection and effect on all-night EEG power spectra. J Sleep Res. 1996;5:155–64. doi: 10.1046/j.1365-2869.1996.00009.x. [DOI] [PubMed] [Google Scholar]
- 35.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 36.Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and neuropsychological functioning: a meta-analysis. J Sleep Res. 2008;17:14. [Google Scholar]
- 37.Wechsler D. WMS-III Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 38.Gronwall D, Wrightsman P. Delayed recovery of intellectual function after minor head injury. Lancet. 1974;2:604–9. doi: 10.1016/s0140-6736(74)91939-4. [DOI] [PubMed] [Google Scholar]
- 39.Delis DC, Kaplan E, Kramer J. Delis-Kaplan Executive Function System (D-KEFS) Examiner's Manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 40.Conners CK. Conners Continuous Performance Test II, Version 5 for Windows (CPT II): Psychological Assessment Resources. 2000 [Google Scholar]
- 41.Delis DC, Kramer J, Kaplan E, Ober BA. California Verbal Learning Test–II. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 42.Troyer AK, Rich JB. Psychometric properties of a new metamemory questionnaire for older adults. J Gerontol B Psychol Sci Soc Sci. 2002;57:P19–27. doi: 10.1093/geronb/57.1.p19. [DOI] [PubMed] [Google Scholar]
- 43.Fort I, Adoul L, Holl D, Kaddour J, Gana K. Psychometric properties of the French version of the Multifactorial Memory Questionnaire for adults and the elderly. Can J Aging. 2004;23:347–57. doi: 10.1353/cja.2005.0020. [DOI] [PubMed] [Google Scholar]
- 44.Broadbent DE, Cooper PF, Fitzgerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21:1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 45.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 46.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 47.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6:143–52. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 48.Fillion L, Gelinas C, Simard S, Savard J, Gagnon P. Validation evidence for the French Canadian adaptation of the Multidimensional Fatigue Inventory as a measure of cancer-related fatigue. Cancer Nurs. 2003;26:143–54. doi: 10.1097/00002820-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Coren S, Mah KB. Prediction of physiological arousability: a validation of the Arousal Predisposition Scale. Behav Res Ther. 1993;31:215–9. doi: 10.1016/0005-7967(93)90076-7. [DOI] [PubMed] [Google Scholar]
- 50.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 51.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th ed. Boston, MA: Allyn and Bacon; 2007. [Google Scholar]
- 52.Altena E, Van Der Werf YD, Strijers RL, Van Someren EJ. Sleep loss affects vigilance: effects of chronic insomnia and sleep therapy. J Sleep Res. 2008;17:335–43. doi: 10.1111/j.1365-2869.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- 53.Edinger JD, Means MK, Carney CE, Krystal AD. Psychomotor performance deficits and their relation to prior nights' sleep among individuals with primary insomnia. Sleep. 2008;31:599–607. doi: 10.1093/sleep/31.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Randazzo AC, Schweitzer PK, Stone KL, Compton JD, Walsh JK. Impaired cognitive function in insomniacs vs. normals. Sleep. 2000;23:A4. Abstract Suppl. [Google Scholar]
- 56.Szelenberger W, Niemcewicz S. Event-related current density in primary insomnia. Acta Neurobiol Exp (Wars) 2001;61:299–308. doi: 10.55782/ane-2001-1405. [DOI] [PubMed] [Google Scholar]
- 57.Broman JE, Lundh LG, Aleman K, Hetta J. Subjective and objective performance in patients with primary insomnia. Scandinavian Journal of Behaviour Therapy. 1992;21:115–26. [Google Scholar]
- 58.Hauri PJ. Cognitive deficits in insomnia patients. Acta Neurol Belg. 1997;97:113–7. [PubMed] [Google Scholar]
- 59.Mendelson WB, Garnett D, Gillin JC, Weingartner H. The experience of insomnia and daytime and nighttime functioning. Psychiatry Res. 1984;12:235–50. doi: 10.1016/0165-1781(84)90029-5. [DOI] [PubMed] [Google Scholar]
- 60.Pedrosi B, Roehrs T, Rosenthal LD, Fortier J, Roth T. Daytime function and benzodiazepine effects in insomniacs compared to normals. Sleep Res. 1995;24:48. [Google Scholar]
- 61.Nissen C, Kloepfer C, Nofzinger EA, Feige B, Voderholzer U, Riemann D. Impaired sleep-related memory consolidation in primary insomnia--a pilot study. Sleep. 2006;29:1068–73. doi: 10.1093/sleep/29.8.1068. [DOI] [PubMed] [Google Scholar]
- 62.Griessenberger H, Heib DP, Lechinger J, et al. Susceptibility to declarative memory interference is pronounced in primary insomnia. PLoS One. 2013;8:e57394. doi: 10.1371/journal.pone.0057394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kazdin AE. The meanings and measurements of clinical significance. J Consult Clin Psychol. 1999;67:332–9. doi: 10.1037//0022-006x.67.3.332. [DOI] [PubMed] [Google Scholar]
- 64.Orff HJ, Drummond SP, Nowakowski S, Perils ML. Discrepancy between subjective symptomatology and objective neuropsychological performance in insomnia. Sleep. 2007;30:1205–11. doi: 10.1093/sleep/30.9.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shahly V, Berglund PA, Coulouvrat C, et al. The associations of insomnia with costly workplace accidents and errors: results from the America Insomnia Survey. Arch Gen Psychiatry. 2012;69:1054–63. doi: 10.1001/archgenpsychiatry.2011.2188. [DOI] [PubMed] [Google Scholar]
- 66.Kucharczyk ER, Morgan K, Hall AP. The occupational impact of sleep quality and insomnia symptoms. Sleep Med Rev. 2012;16:547–59. doi: 10.1016/j.smrv.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Lovato N, Lack L, Wright H, Cant M, Humphreys J. Working memory performance of older adults with insomnia. J Sleep Res. 2013;22:251–7. doi: 10.1111/jsr.12010. [DOI] [PubMed] [Google Scholar]
- 68.Oosterman JM, van Someren EJ, Vogels RL, Van Harten B, Scherder EJ. Fragmentation of the rest-activity rhythm correlates with age-related cognitive deficits. J Sleep Res. 2009;18:129–35. doi: 10.1111/j.1365-2869.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- 69.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 70.Espie CA, Broomfield NM, MacMahon KM, Macphee LM, Taylor LM. The attention-intention-effort pathway in the development of psychophysiologic insomnia: a theoretical review. Sleep Med Rev. 2006;10:215–45. doi: 10.1016/j.smrv.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 71.Zimmerman ME, Bigal ME, Katz MJ, Brickman AM, Lipton RB. Sleep onset/maintenance difficulties and cognitive function in nondemented older adults: the role of cognitive reserve. J Int Neuropsychol Soc. 2012;18:461–70. doi: 10.1017/S1355617711001901. [DOI] [PMC free article] [PubMed] [Google Scholar]


