Abstract
Aims
It is a dogma of cardiovascular pathophysiology that the increased cardiac mass in response to increased workload is produced by the hypertrophy of the pre-existing myocytes. The role, if any, of adult-resident endogenous cardiac stem/progenitor cells (eCSCs) and new cardiomyocyte formation in physiological cardiac remodelling remains unexplored.
Methods and results
In response to regular, intensity-controlled exercise training, adult rats respond with hypertrophy of the pre-existing myocytes. In addition, a significant number (∼7%) of smaller newly formed BrdU-positive cardiomyocytes are produced by the exercised animals. Capillary density significantly increased in exercised animals, balancing cardiomyogenesis with neo-angiogenesis. c-kitpos eCSCs increased their number and activated state in exercising vs. sedentary animals. c-kitpos eCSCs in exercised hearts showed an increased expression of transcription factors, indicative of their commitment to either the cardiomyocyte (Nkx2.5pos) or capillary (Ets-1pos) lineages. These adaptations were dependent on exercise duration and intensity. Insulin-like growth factor-1, transforming growth factor-β1, neuregulin-1, bone morphogenetic protein-10, and periostin were significantly up-regulated in cardiomyocytes of exercised vs. sedentary animals. These factors differentially stimulated c-kitpos eCSC proliferation and commitment in vitro, pointing to a similar role in vivo.
Conclusion
Intensity-controlled exercise training initiates myocardial remodelling through increased cardiomyocyte growth factor expression leading to cardiomyocyte hypertrophy and to activation and ensuing differentiation of c-kitpos eCSCs. This leads to the generation of new myocardial cells. These findings highlight the endogenous regenerative capacity of the adult heart, represented by the eCSCs, and the fact that the physiological cardiac adaptation to exercise stress is a combination of cardiomyocyte hypertrophy and hyperplasia (cardiomyocytes and capillaries).
Keywords: Myocyte hyperplasia, Growth factors, Cardiac stem cells, Cardiomyogenesis, Exercise, Physiological remodelling
Introduction
Traditionally, the adult mammalian heart has been viewed as a post-mitotic organ with no, or very low, regenerative capacity. Since it is known that cardiomyocytes withdraw from the cell cycle shortly after birth, it follows that all cardiomyocytes have to be as old as the individual.1,2 This static view of the adult mammalian heart has been challenged by findings of myocyte replacement in the mammalian adult heart, under normal, pathological, and increased workload conditions,2–4 including the identification of human cardiomyocytes that are significantly younger than the individual.5
It has been generally accepted that any physiological or pathological increase in the myocardial mass had to be due solely to hypertrophy of existing cardiomyocytes.6 However, despite the contribution of cardiomyocyte hypertrophy as a compensatory mechanism, in the absence of neo-myogenesis the rate at which cardiomyocytes are lost would result in the atrophy and ultimate disappearance of the heart during the lifespan of any long-lived mammalian organism.2 Thus, cardiomyocyte renewal within the adult mammalian heart is not only possible, but it appears to be required for normal organ homoeostasis.7
The discovery of resident endogenous cardiac stem-progenitor cells (eCSCs) in the adult heart has contributed greatly to a new view of cardiac biology. A variety of markers have been proposed to identify eCSCs in different species;8 yet it remains to be determined whether these markers identify distinct populations of eCSCs or different developmental stages of a single cell type.9
Despite the significant regenerative potential of the c-kitposeCSCs,8 the role of c-kitposeCSCs in cardiac physiological remodelling induced by exercise training remains to be determined. Recent data have advanced the hypothesis that the ‘physiological hypertrophy’ produced by exercise training, at odds with pathological hypertrophy, is accompanied also by adult cardiomyocyte division in vivo.10 Here, we show that treadmill exercise training, which can be intensity controlled according to individual VO2 max, stimulates the activation of c-kitpos eCSCs and induces new cardiomyocyte and capillary formation, thereby contributing to the physiological remodelling of the heart. We also identified a group of exercise-induced growth factors involved in these adaptive processes.
Methods
For a full description of all methods used, see Supplementary material online, Methods. Briefly, male Wistar rats (∼230 g) were exercised on motorized treadmills for 30 min/day, 4 days/week for up to 4 weeks at either a low (55–60% of individual VO2 max) or high (85–90% of VO2 max) intensity. After 4 weeks of exercise training, VO2 max, myocardial mass, and cardiac function were assessed and compared with age-matched sedentary CTRLs. Cardiomyocyte hypertrophy, new cardiomyocyte and capillary formation, c-kitpos eCSC number, and their committed progeny were quantified by immunohistochemistry. qRT–PCR and western blot were performed on ventricular myocytes to determine the expression levels of an array of growth factors. Growth factors identified as up-regulated by exercise training were tested on c-kitpos, CD45neg eCSCs in vitro.
Results
New cardiomyocyte and capillary formation contribute to cardiac remodelling induced by exercise training in direct correlation to exercise intensity
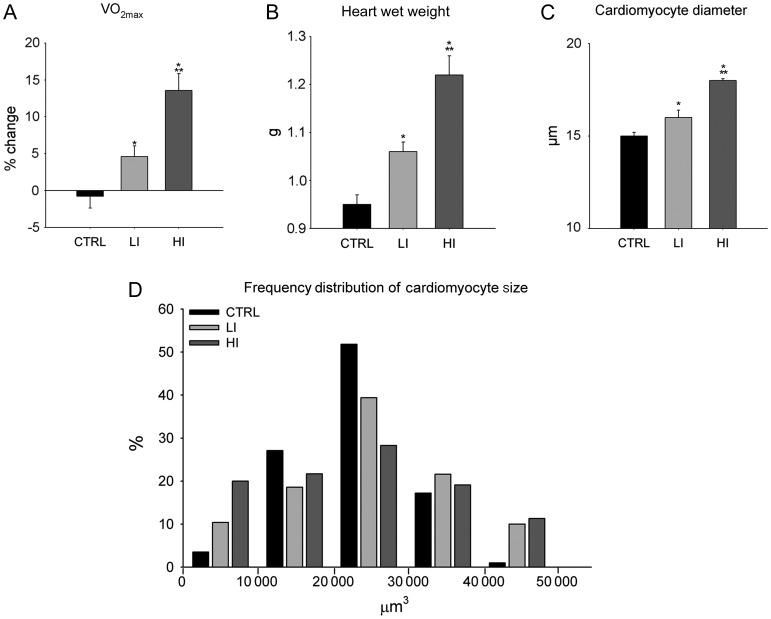
Both low intensity- (LI, 55–60% of VO2 max) and high intensity (HI, 85–90% of VO2 max)-controlled treadmill exercise training for up to 4 weeks (see Supplementary material online) improved aerobic exercise capacity as measured by changes in VO2 max (Figure 1A) and increased myocardial mass (Figure 1B). As expected, the HI exercise-trained group showed the greatest increases. Echocardiography revealed that the HI exercise actually produced positive anatomical and physiological cardiac remodelling as demonstrated by a thickening of the inter-ventricular septum and the left ventricle (LV) posterior wall, with an increased diastolic and reduced systolic internal LV diameter. These anatomical changes were accompanied by a 9% increase in both the ejection fraction and fractional shortening, compared with CTRL (Table 1).
Figure 1.
Treadmill exercise training improved VO2 max, myocardial mass and induced cardiomyocyte hypertrophy. (A–C) VO2 max (A), heart weight (B), and left ventricle cardiomyocyte diameter (C) following 4 weeks of low intensity and high intensity exercise training, compared with sedentary CTRL animals (data are mean ± SEM, *P < 0.05 vs. CTRL, **P < 0.05 vs. LI; n = 6/group). (D) The distribution plot of the ventricular cardiomyocyte volume (µm3) in exercise-trained and CTRL rats at 2 weeks (n = 6/group).
Table 1.
Echocardiographic data from high intensity exercised animals after 4 weeks of training compared with age-matched sedentary CTRLs
| Criteria | CTRL | HI | Significance |
|---|---|---|---|
| SWTD (mm) | 1.94 ± 0.05 | 2.26 ± 0.08 | P = 0.004 |
| SWTS (mm) | 3.06 ± 0.09 | 3.55 ± 0.07 | P = 0.002 |
| LVEDD (mm) | 6.43 ± 0.15 | 6.65 ± 0.11 | P = 0.262 |
| LVESD (mm) | 3.43 ± 0.07 | 2.92 ± 0.07 | P = 0.001 |
| PWTD (mm) | 1.88 ± 0.10 | 2.14 ± 0.09 | P = 0.044 |
| PWTS (mm) | 2.84 ± 0.17 | 3.25 ± 0.13 | P = 0.048 |
| Fractional shortening (%) | 46.66 ± 0.93 | 56.04 ± 0.48 | P = 0.000 |
| Ejection fraction (%) | 71.51 ± 0.98 | 80.66 ± 0.42 | P = 0.000 |
| Body weight (g) | 388.68 ± 13.30 | 387.72 ± 3.66 | P = 0.941 |
| LV mass (mg) | 848.00 ± 44.89 | 1097.30 ± 44.46 | P = 0.002 |
| LV/body weight ratio (mg/g) | 2.18 ± 0.10 | 2.83 ± 0.12 | P = 0.002 |
SWTD, septal wall thickness in diastole; SWTS, septal wall thickness in systole; LVEDD, left ventricle end-diastolic diameter; LVESD, left ventricle end-systolic diameter; PWTD, posterior wall thickness in diastole; PWTS, posterior wall thickness in systole (data are mean ± SEM, n = 6 for all).
The increase in the myocardial mass occurred at least in part through cardiomyocyte hypertrophy as the average LV cardiomyocyte diameter was significantly greater in exercising animals than CTRL (Figure 1C). However, when the myocyte size was measured, a distribution plot of the cell volume showed not only the presence of larger, hypertrophied (>40 000 µm3) myocytes, but intriguingly also a population of smaller (<10 000 µm3) ventricular cardiomyocytes in exercising animals, compared with the CTRLs (Figure 1D). The appearance of these smaller cardiomyocytes strongly suggested that in addition to the hypertrophic response, new myocyte generation participated in the myocardial adaptation to exercise training.
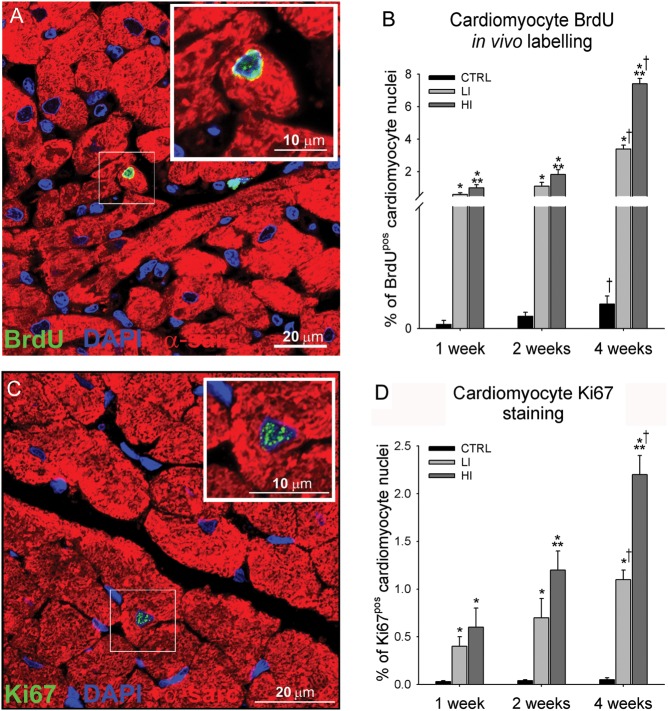
To directly monitor new myocyte formation, we injected BrdU to sedentary and exercising rats for the 4-week study. Double staining for BrdU/α-sarcomeric actin revealed the presence of a significant number of small newly formed, mononucleated, BrdUpos cardiomyocytes in the LV of exercising animals (Figure 2A; Supplementary material online, Figure S1). Some new myocyte formation was also detected in sedentary CTRL animals but their number was significantly lower than in exercised rats (Figure 2B). The number of BrdUpos cardiomyocytes progressively increased (P < 0.05) through the 4-week exercise-training programme in both LI and HI animals (Figure 2B). However, the intensity of neo-myogenesis was directly correlated with the intensity and duration of training, with the greatest number of newly formed cardiomyocytes detected in HI animals after 4 weeks (7.4 ± 0.3%; Figure 2B). The immunohistochemistry data were confirmed by detecting rod-shaped small, mononucleated BrdUpos myocytes in myocyte-enriched cell preparations isolated from HI hearts (Supplementary material online, Figure S1). No bi-nucleated myocytes were detected in this population (Supplementary material online, Figures S1 and S2). New cardiomyocyte formation was further confirmed by the presence of a subpopulation of cycling (Ki67pos) cardiomyocytes in LI and HI animals (Figure 2C–D). Endogenous cardiac stem-progenitor cell-derived myocyte precursors can cycle two or three times before undergoing terminal differentiation with permanent withdrawal from the cell cycle.
Figure 2.
Intensity-controlled treadmill exercise induced cardiac myogenesis (A) Representative image of a small newly formed BrdUpos (green) cardiomyocyte (α-sarcomeric actin; red) in the LV of a high intensity exercising animal. (Inset is ×2 zoom of boxed area). Nuclei detected by DAPI in blue. (B) The % number of BrdUpos cardiomyocytes formed was dependent on the exercise duration and intensity. (C) Representative image of a small newly formed Ki67pos (green) cardiomyocyte in the left ventricle of a high intensity exercising animal. (D) The % number of Ki67pos cardiomyocytes was dependent on the exercise duration and intensity (data are mean ± SEM, *P < 0.05 vs. CTRL, **P < 0.05 vs. LI, †P < 0.05 vs. 1 and 2 weeks; n = 6 for All).
BrdU labelling in vivo provided an accumulative measure of new myocyte formation over the 4-week exercise protocol while the Ki67pos myocytes are those that were still or had recently been in the cell cycle just prior to sacrifice. Therefore, it is not surprising that the number of BrdUpos myocytes is significantly higher than that of Ki67pos myocytes (Figure 2C and D). This interpretation of the data is strengthened by the fact that all the identified newly formed myocytes were still mononucleated and there was a progression of their size after their formation. The average diameter of Ki67pos myocytes, presumably formed shortly before sacrifice, was smaller than that of the BrdUpos myocyte cohort, which included myocytes born throughout the 4 weeks of exercise (Supplementary material online, Figure S3). Nevertheless, the latter were still significantly smaller than BrdUneg myocytes, representing those already present at the start of the exercise (Supplementary material online, Figure S3). The latter data show that it takes >4 weeks for the new myocytes to reach the full mature size. The intensity of BrdU labelling further rejects the hypothesis that BrdU incorporation is due to DNA repair or DNA synthesis in a process of bi-nucleation because the only labelled cells were those mononucleated and of the small size. Furthermore, the diploid DNA content of these small myocytes (data not shown) rejects polyploidization as a cause of DNA synthesis. All these features support the conclusion that the labelled cells are younger and have been generated after the start of the exercise programme. Tissue cell homoeostasis is generally maintained through a balance between cell loss and cell replacement. The very significant spurt of new myocyte formation in response to exercise could be the consequence of myocyte loss triggered by the workload. Thus, cardiomyocyte apoptosis was assessed in CTRL, LI, and HI animals after 2 and 4 weeks. Of the 25 animals examined, a single caspase-3-positive apoptotic cardiomyocyte nucleus was detected in the LV apex of one HI exercised animal after 2 weeks (Supplementary material online, Figure S4). This argues against a role for myocyte death as a trigger of new myocyte generation during exercise training.
The increase in the number of newly formed BrdUpos cardiomyocytes without significant cardiomyocyte loss infers an increase in cardiomyocyte number with exercise training. Indeed, the total number of ventricular cardiomyocytes increased with exercise training, amounting to a 4 and 7% increase following 4 weeks of LI and HI exercise training, respectively (Supplementary material online, Figure S5). These data agree with the percentage increase in the number of newly formed BrdUpos cardiomyocytes with LI and HI exercise training (Figure 2B).
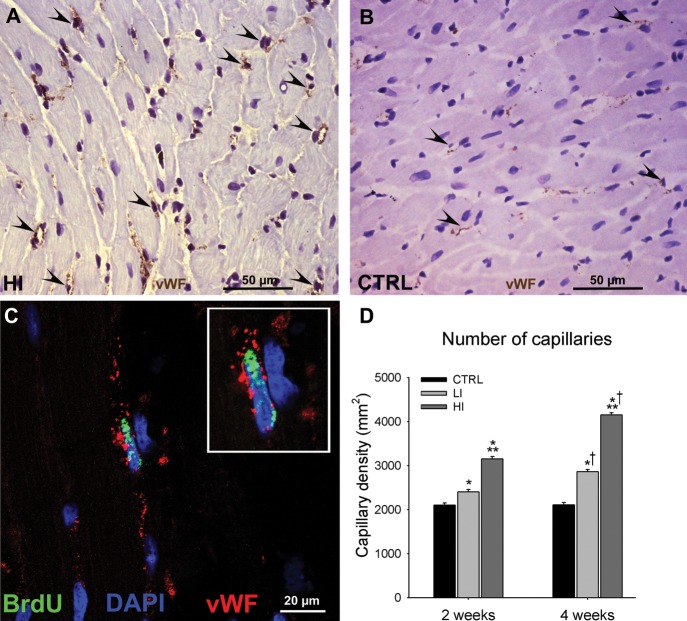
Finally, exercise training produced a balanced myocardial growth that encompassed myogenesis and angiogenesis. Indeed, capillary density was significantly greater in exercising animals compared with CTRL and this parameter too correlated with exercise intensity (Figure 3A–D). The presence of BrdUpos capillary cells in the exercised hearts confirmed their formation after the start of training (Figure 3C).
Figure 3.
Intensity-controlled treadmill exercise induced angiogenesis. (A and B), Representative images of vWF (brown) capillaries (arrowheads) identified in the left ventricle of a high intensity exercised (A) and CTRL (B) animal. (C) Representative image of a BrdUpos (green) capillary (vWF; red) from the left ventricle of a high intensity exercised animal (Inset is ×2 zoom). (D) The capillary density in the left ventricle of exercising animals was dependent on the exercise intensity and duration (data are mean ± SEM, *P < 0.05 vs. CTRL, **P < 0.05 vs. LI, †P < 0.05 vs. 2 weeks; n = 6 for all).
Multiplication and differentiation of c-kitpos endogenous cardiac stem-progenitor cells in response to exercised-induced increased workload contribute to physiological cardiac remodelling
To identify the source of the newly formed cardiomyocytes and capillaries shown above, we assessed the effect of the exercise training on the activation and differentiation of the resident c-kitpos eCSCs. Because new myocardial cell and capillary formation was higher in the HI protocol, we focused our assessment to HI exercised animals, compared with CTRLs.
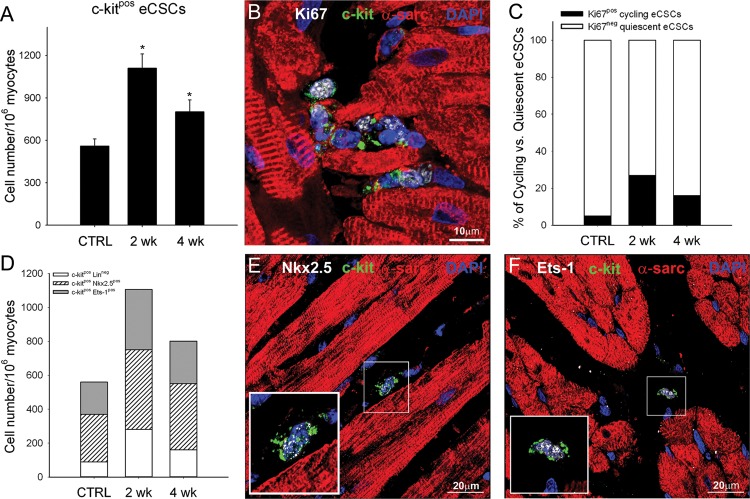
The frequency of c-kitpos eCSCs in the healthy myocardium of several mammalian species, including human, mouse, rat, and pig, is approximately one per every 1000–2000 myocytes, depending on age.8 As shown in Figure 4A, there was ∼1 c-kitpos eCSC per 1500 myocytes in the sedentary CTRL animals. These cells were ∼95% (95 ± 3) quiescent, that is, non-cycling, in these animals, as determined by their absence of the expression of Ki67 and no uptake of BrdU (Figure 4B and C). High intensity exercise training increased the c-kitpos eCSC number two-fold above that of CTRLs at 2 weeks (P < 0.05) and, although it subsequently decreased, it remained higher up to 4 weeks of HI training (Figure 4A–C). This early eCSC expansion was likely a consequence of the activation of a large fraction of them, so that 27 ± 4% and 16 ± 4% were Ki67pos at 2 and 4 weeks, respectively (Figure 4B and C), and >60% had cycled during the exercise protocol and incorporated BrdU compared with ∼5% in the CTRLs. The late decline in c-kitpos eCSC numbers appears due to a combination of their differentiation and loss of c-kit expression together with the decrease in the stimulus for their replication, which accompanies the fall in wall stress as the heart adapts its mass to the workload.11
Figure 4.
Activation and ensuing differentiation of c-kitpos endogenous cardiac stem-progenitor cells in hearts of exercising animals. (A) c-kitpos endogenous cardiac stem-progenitor cell number in the left ventricle of CTRL and following 2 or 4 weeks of high intensity exercise training. (B) Representative image showing c-kitpos (green), Ki67pos (white) endogenous cardiac stem-progenitor cells from the left ventricle (α-sarcomeric actin; red) of a 2-week HI exercised animal. Nuclei detected by DAPI in blue. (C and D) % number of c-kitposKi67pos (cycling) and c-kitposKi67neg (quiescent) cells (C) and number of uncommitted c-kitposLinneg, c-kitposNkx2.5pos, or c-kitposEts-1pos progenitor cell (D) in the left ventricle of CTRL animals and following 2 or 4 weeks of HI exercise training. (data are mean ± SEM, *P < 0.05 vs. CTRL; n = 6 for all). (E and F) c-kitpos (green) endogenous cardiac stem-progenitor cells express the transcription factors Nkx2.5 (white dots; E) or Ets-1 (white dots; F) indicating their commitment to the cardiomyocyte and endothelial lineage, respectively (inset is ×2 zoom of boxed area).
Interestingly, in the control healthy myocardium, ∼85% of the c-kitpos eCSCs were already committed to either the myocyte (Nkx2.5pos) or vascular (Ets-1pos) cell lineage (Figure 4D–F) and, therefore, could be considered as early progenitors/precursors, likely to have already lost their multipotency and being committed to one of the cardiac cell lineages. Therefore, the frequency of uncommitted, multi-potent c-kitpos eCSCs without the expression of cardiogenic lineage genes (linneg) in the non-stressed healthy myocardium is only ∼90 per106 myocytes (Figure 4D), that is, ∼15% of the total c-kitpos eCSC population.
The distribution of the different types of eCSCs expressing cardiac lineage markers remained constant throughout the whole study, suggesting that the level of activation, leading to multiplication and differentiation, is similar for all these committed cells (Figure 4D–F). On the other hand, there appeared to be a preferential (greater than three-fold) expansion of the ‘primitive’ c-kitpos linneg eCSCs subgroup (Figure 4D). This significant eCSC expansion affecting all subtypes coincided temporally and quantitatively with the progressive increase of newly formed myocytes and capillaries from 2 to 4 weeks (Figures 2 and 3). Taken together, these data are strongly supportive of a precursor–product relationship between the c-kitpos eCSCs and the newly formed, differentiated cardiac cell progeny.
Increased cardiomyocyte-specific growth factor expression in intensity-controlled exercise-trained hearts
Because myocytes constitute ∼80% of the myocardial mass, we hypothesized that the myocyte workload and consequential increase in wall stress produced by exercise training could induce an auto/paracrine response of the myocytes. This response, in addition to protecting the mature myocytes from apoptosis and stimulating their hypertrophy, could also have provided the stimulus for c-kitpos eCSC activation. Thus, to identify the source of putative eCSC-activating factors, pure populations of rod-shaped left ventricular cardiomyocytes isolated from the HI-group and CTRL hearts at different times points were analysed for the expression of a battery of growth factors and cytokines.
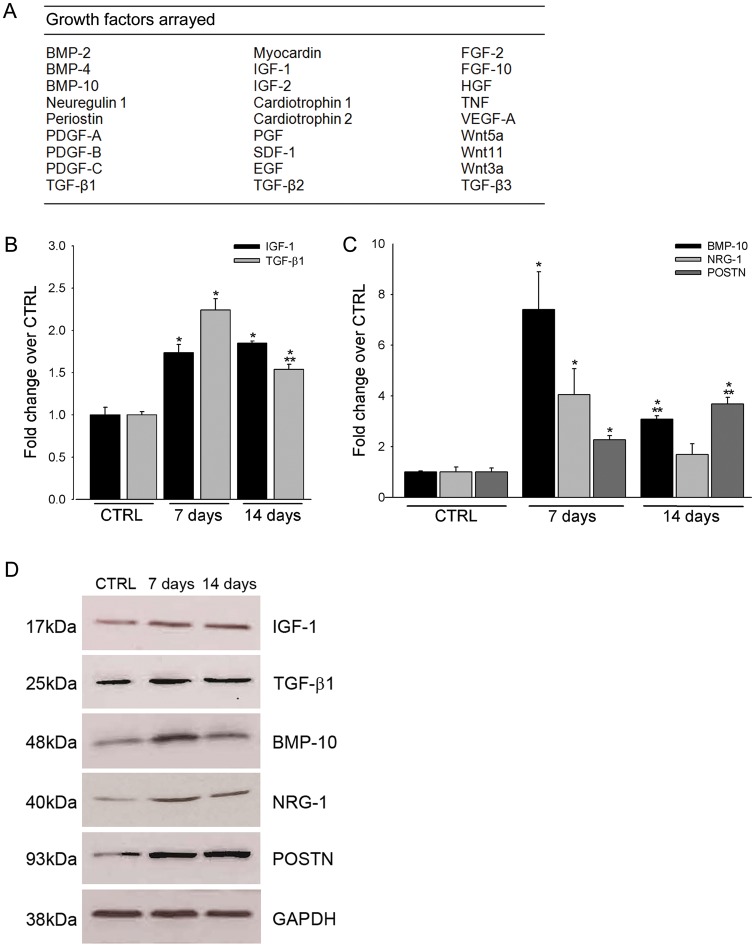
qRT–PCR analysis of 26 growth factors (Figure 5A) identified a subset which were significantly up-regulated in cardiomyocytes following intensity-controlled HI exercise training. Indeed, their insulin-like growth factor-1 (IGF-1) and transforming growth factor-β1 (TGF-β1) expression were significantly increased (P < 0.05) above those of CTRLs at 7 and 14 days after exercise training (Figure 5B). It is well known that IGF-1 is up-regulated following exercise and has been implicated in myocardial hypertrophy.12 Intriguingly, IGF-1 myocardial expression modulates eCSC survival and function, preventing eCSC ageing and cardiac dysfunction in aged mice.13 Furthermore, the TGF-β1 superfamily, including activins and bone morphogenetic proteins, stimulates cardiomyocyte differentiation of embryonic as well as adult cardiac progenitors.14 However, the greatest changes were observed on the levels of neuregulin-1 (NRG-1), periostin (POSTN), and BMP-10 (Figure 5C). POSTN and NRG-1 have been implicated in regulating cardiac hypertrophy following pressure overload15,16 and have also been implicated in myocyte replacement following cardiac injury.17,18 BMP-9, which shares homology with BMP-10, has been shown to stimulate angiogenesis in vivo.19 Up-regulated mRNA levels of the growth factors after exercise training determined by qRT–PCR were confirmed at the protein level by western blot (Figure 5D).
Figure 5.
Exercise-induced changes in myocardial growth factor expression. (A) The array of 26 growth factors that were analysed for transcript expression in cardiomyocytes following 4 weeks of high intensity exercise training. (B–C) qRT–PCR array analysis revealed up-regulation of insulin-like growth factor-1, transforming growth factor-β1 (B) bone morphogenetic protein-10, NRG-1, and POSTN (C) in cardiomyocytes isolated from high intensity animals after 7 and 14 days, compared with CTRL. (D) Western blot analysis confirmed up-regulation of insulin-like growth factor-1, transforming growth factor-β1, bone morphogenetic protein-10, NRG-1, and POSTN at the protein level (data are mean ± SEM, *P < 0.05 vs. CTRL, **P < 0.05 vs. 7 days; n = 3 for all).
Up-regulated exercise-induced myocardial growth factors differentially govern the fate of endogenous cardiac stem-progenitor cells
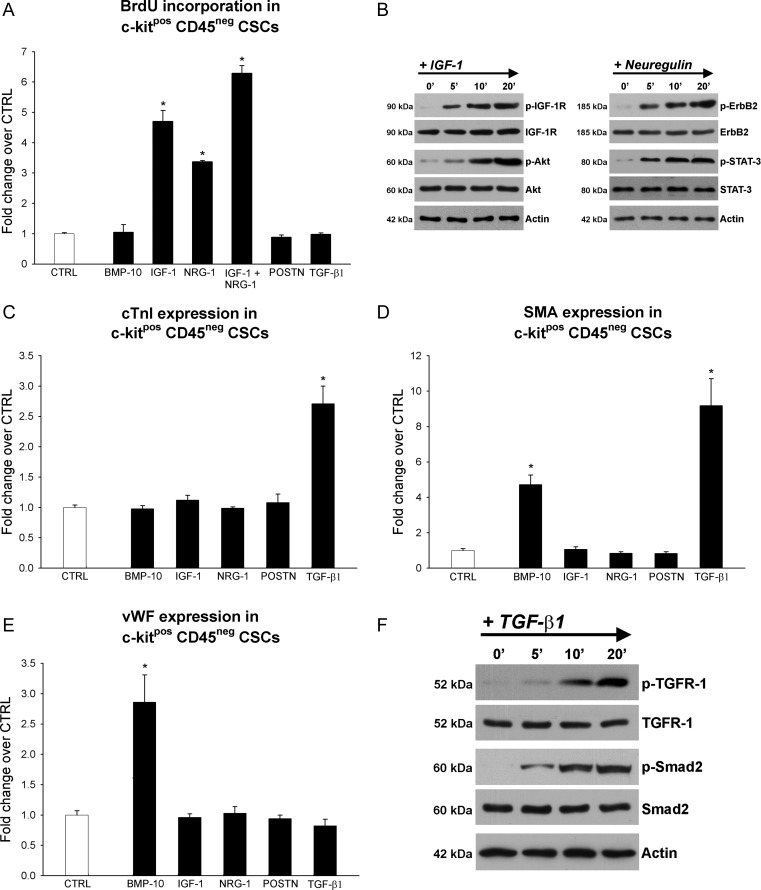
To ascertain whether the different up-regulated myocyte growth factors could be responsible for the activation of the c-kitpos eCSCs in the exercised animals, we tested their effects on c-kitpos eCSC proliferation and differentiation potential in vitro. Dose–response curves were constructed to determine the optimal concentration for each growth factor (Supplementary material online, Figure S6). Addition of IGF-1 and NRG-1 to the culture media enhanced eCSC BrdU incorporation by five- and three-fold, respectively, compared with CTRL (Figure 6A). Importantly, when IGF-1 and NRG-1 were added simultaneously to eCSC cultures, they showed an additive effect, suggesting that they act through separate molecular pathways (Figure 6A). The other growth factors tested did not have any detectable effect on eCSC proliferation. Accordingly, IGF-1 and NRG-1 stimulation was associated with the activation of their respective receptors and physiological molecular signalling targets, Akt and STAT-3, respectively (Figure 6B). The latter have been shown to play a fundamental role in the self-renewal of embryonic and adult stem cells,20,21 generating the hypothesis that these molecular cascades play a similar role in the fate of eCSCs.
Figure 6.
Exercise-induced cardiac growth factors govern c-kitpos endogenous cardiac stem-progenitor cell growth and differentiation in vitro. (A–F) Histogram plots and western blots showing the effect of bone morphogenetic protein-10 (10 ng/mL), insulin-like growth factor-1 (100 ng/mL), NRG-1 (100 ng/mL), POSTN (500 ng/mL), or transforming growth factor-β1 (5 ng/mL) supplementation on endogenous cardiac stem-progenitor cell proliferation, and cardiomyocyte, smooth muscle, and endothelial differentiation. Differences are expressed as the fold change over un-supplemented CTRL cells (data are mean ± SEM, *P < 0.05 vs. CTRL; n = 3 for all).
On the other hand, BMP-10 and TGF-β1 stimulated differentiation of the eCSCs into the three main cardiac lineages (Figure 6C–E). Specifically, BMP-10 supplementation led to a three-fold increase in cells positive for vWF, identifying endothelial cells (Figure 6E), and also produced a five-fold increase in smooth muscle actin (SMA)-positive cells (Figure 6D). BMP-10 did not increase the number of cells expressing cTnI, a marker of CSC specification towards the myocyte lineage. TGF-β1 supplementation resulted in a three-fold increase in myocyte-committed cTnI-positive cells (Figure 6C) and nine-fold increase in SMA-positive cells (Figure 6D). TGF-β1 administration was associated with the activation of its receptor followed by Smad2 phosphorylation (Figure 6F).
Recently, NRG-1 and POSTN have been reported to induce cardiomyocytes to re-enter the cell cycle and proliferate17,18 a phenomenon that was previously thought not to be possible. However, these findings have not been reproduced by others.22,23 Furthermore, NRG-1 enhances differentiation of ES cells into cardiomyocytes.24 In the present study, when rat ventricular cardiomyocytes were supplemented with either IGF-1, NRG-1, POSTN, BMP-10, or TGF-β1, individually or in different combinations, for 3, 7, and 14 days, neither DNA synthesis within cardiomyocytes, as assessed by BrdU incorporation, nor any myocyte mitosis (Ki67 or phospho-Histone H3) was detected.
Discussion
The results presented here challenge the prevalent view of cardiac growth from the early post-natal period and throughout life, which is based on the hypertrophy of existing myocytes without any significant contribution of new myocyte formation. Indeed, we show that the physiological adaptation of the adult heart to an increased cardiac workload, induced by the exercise programme, has three main components: (i) physiological hypertrophy of the existing myocytes, accompanied by their increased production of specific growth factors which through auto- and paracrine loops foster their own survival and hypertrophy as well as activating the c-kitpos eCSCs; (ii) the activation of c-kitpos eCSCs, which increase in number and undergo a process of cell specification and differentiation towards the myocyte and vascular lineages; and (iii) the accumulation of new myocardial cells, namely new myocytes and microvasculature. These cellular modifications are dependent on exercise duration and intensity and result in an increased contractile muscle mass and enhanced cardiac function, which reduces the wall stress.
Exercise exerts its beneficial effects not just through reducing the burden of classical cardiovascular risk factors, but also directly affecting the cellular and molecular structure and function of the heart.25 Here, we show that this response includes both neo-myogenesis and neo-angiogenesis, the latter likely resulting from a combination of replication of existing vascular cells and de novo differentiation of the eCSCs. The data presented here also provides insight in the molecular mechanisms involved in the hypertrophic and regenerative response. The mature myocytes respond to the increased workload by up-regulating a cohort of secreted growth factors and cytokines. These exercise up-regulated factors differentially govern c-kitposeCSC fate in vitro, pointing to a similar effect in vivo. Thus, eCSCs from the adult heart exhibit a response to many growth factors that recapitulates, at least in part, embryonic cardiac progenitor activation and lineage commitment.26 Furthermore, these data on c-kitpos eCSCs and growth factors in vitro generate the hypothesis that these growth factors could be effective in vivo to improve cardiac regeneration and repair after myocardial damage and dysfunction. Accordingly, we have recently demonstrated in a porcine model of myocardial infarction that it is possible to boost cardiac repair and regeneration through the intracoronary injection of small doses of IGF-1 and HGF.27 New experiments in small and large animals implementing NRG-1 and TGF-β1 to the above reparative/regenerative growth factor combination are ongoing.
A recent intriguing paper has reported that down-regulation of the transcription factor C/EBPβ in swim exercise-trained mice appears to be a central mediator of physiological myocyte hypertrophy and proliferation, which accounts for ∼6% of new myocyte formation.10 The number of proliferating myocytes closely match the number of newly formed myocytes generated following HI treadmill exercise training shown here. However, Boström et al.10 proposed that the new myocyte formation in exercised animals resulted from adult mature, cardiomyocyte division, even though they failed to show direct evidence of this phenomenon either in vivo or in vitro. Our results failed to demonstrate that the growth factor's up-regulated with exercise training induced mature, adult cardiomyocytes to divide, in vitro, and pointed to a precursor–product relationship between c-kitpos eCSCs and BrdUpos cardiomyocytes as the source of new cardiomyocyte formation. However, our results cannot rule out the occasional replication of a small number of mature cardiomyocytes with exercise training. Myocyte-restricted lineage tracing experiments are needed to definitively unravel this issue. Although we specifically characterized the role of c-kitpos eCSCs, these findings do not rule out the participation of other cardiac stem cell-like populations, such as those of pro-epicardial origin28,29 and the Sca1pos cells described in the mouse,30 in the physiological adaptation of the heart to exercise training.
The fact that a few weeks of vigorous exercise can significantly increase the myocyte count and mass indicates that this phenomenon is not just a biological curiosity but an important component of cardiac physiology and homoeostasis. The myocardial response described is not dependent on the type of exercise applied, but rather the intensity at which the exercise is performed. What remains to be determined is the cardiac response to continuously increased workload such as that which is experienced by endurance elite athletes. How much hyperplasia can the myocardium generate? How much can it tolerate before adversely affecting cardiac function? What happens to the cohort of new myocytes during de-training? Work to address these questions is now in progress.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by grants from the British Heart Foundation (PG/06/045; PG 08/085), Marie Curie FP7 (PIRG02-GA-2007-224853 to G.M.E), CARE-MI FP7-HEALTH-2009 (242038), Endostem FP7-HEALTH-2009 (241440), FIRB-Futuro-in-Ricerca (RBFR081CCS to D.T), Italian Ministry of Health (GR-2008-1142673 to D.T).
Conflict of interest: none declared.
Acknowledgements
We acknowledge the technical support of Roy Williams and Stephen Broadfoot.
References
- 1.Tam SK, Gu W, Mahdavi V, Nadal-Ginard B. Cardiac myocyte terminal differentiation. Potential for cardiac regeneration. Ann N Y Acad Sci. 1995;752:72–79. doi: 10.1111/j.1749-6632.1995.tb17407.x. [DOI] [PubMed] [Google Scholar]
- 2.Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res. 2003;92:139–150. doi: 10.1161/01.res.0000053618.86362.df. [DOI] [PubMed] [Google Scholar]
- 3.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 4.Anversa P, Nadal-Ginard B. Myocyte renewal and ventricular remodelling. Nature. 2002;415:240–243. doi: 10.1038/415240a. [DOI] [PubMed] [Google Scholar]
- 5.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 7.Ellison GM, Torella D, Karakikes I, Nadal-Ginard B. Myocyte death and renewal: modern concepts of cardiac cellular homeostasis. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl. 1):S52–S59. doi: 10.1038/ncpcardio0773. [DOI] [PubMed] [Google Scholar]
- 8.Torella D, Ellison GM, Karakikes L, Nadal-Ginard B. Resident cardiac stem cells. Cell Mol Life Sci. 2007;64:661–673. doi: 10.1007/s00018-007-6519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellison GM, Galuppo V, Vicinanza C, Aquila I, Waring CD, Leone A, Indolfi C, Torella D. Cardiac stem and progenitor cell identification: different markers for the same cell? Front Biosci (Schol Ed) 2010;2:641–652. doi: 10.2741/s91. [DOI] [PubMed] [Google Scholar]
- 10.Boström P, Mann N, Wu J, Quintero PA, Plovie ER, Panáková D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, Spiegelman BM. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellison GM, Torella D, Karakikes I, Purushothaman S, Curcio A, Gasparri C, Indolfi C, Cable NT, Goldspink DF, Nadal-Ginard B. Acute beta-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells. JBC. 2007;282:11397–11409. doi: 10.1074/jbc.M607391200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catalucci D, Latronico MV, Ellingsen O, Condorelli G. Physiological myocardial hypertrophy: how and why? Front Biosci. 2008;13:312–324. doi: 10.2741/2681. [DOI] [PubMed] [Google Scholar]
- 13.Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, Leri A. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 14.Goumans MJ, de Boer TP, Smits AM, van Laake LW, van Vliet P, Metz CH, Korfage TH, Kats KP, Hochstenbach R, Pasterkamp G, Verhaar MC, van der Heyden MA, de Kleijn D, Mummery CL, van Veen TA, Sluijter JP, Doevendans PA. TGF-beta1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res. 2007;1:138–149. doi: 10.1016/j.scr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, II, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–321. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 17.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/erbb4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y, Ohga N, Morishita Y, Hida K, Miyazono K, Watabe T. Bmp-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J Cell Sci. 2010;123:1684–1692. doi: 10.1242/jcs.061556. [DOI] [PubMed] [Google Scholar]
- 20.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paling NR, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem. 2004;279:48063–48070. doi: 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- 22.Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang L, Wakimoto H, Nayor M, Konno T, Gorham JM, Wolf CM, Kim JB, Schmitt JP, Molkentin JD, Norris RA, Tager AM, Hoffman SR, Markwald RR, Seidman CE, Seidman JG. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires tgf-beta. J Clin Invest. 2010;120:3520–3529. doi: 10.1172/JCI42028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorts A, Schwanekamp JA, Elrod JW, Sargent MA, Molkentin JD. Genetic manipulation of periostin expression in the heart does not affect myocyte content, cell cycle activity, or cardiac repair. Circ Res. 2009;104:e1–e7. doi: 10.1161/CIRCRESAHA.108.188649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Xu G, Wu Y, Guan Y, Cui L, Lei X, Zhang J, Mou L, Sun B, Dai Q. Neuregulin-1 enhances differentiation of cardiomyocytes from embryonic stem cells. Med Biol Eng Comput. 2009;47:41–48. doi: 10.1007/s11517-008-0383-2. [DOI] [PubMed] [Google Scholar]
- 25.Ellison GM, Waring CD, Vicinanza C, Torella D. Physiological cardiac remodelling in response to endurance exercise training: cellular and molecular mechanisms. Heart. 2012;98:5–10. doi: 10.1136/heartjnl-2011-300639. [DOI] [PubMed] [Google Scholar]
- 26.Noseda M, Peterkin T, Simões FC, Patient R, Schneider MD. Cardiopoietic factors: extracellular signals for cardiac lineage commitment. Circ Res. 2011;108:129–152. doi: 10.1161/CIRCRESAHA.110.223792. [DOI] [PubMed] [Google Scholar]
- 27.Ellison GM, Torella D, Dellegrottaglie S, Perez-Martinez C, Perez de Prado A, Vicinanza C, Purushothaman S, Galuppo V, Iaconetti C, Waring CD, Smith A, Torella M, Cuellas Ramon C, Gonzalo-Orden JM, Agosti V, Indolfi C, Galiñanes M, Fernandez-Vazquez F, Nadal-Ginard B. Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J Am Coll Cardiol. 2011;58:977–986. doi: 10.1016/j.jacc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Chong JJ, Chandrakanthan V, Xaymardan M, Asli NS, Li J, Ahmed I, Heffernan C, Menon MK, Scarlett CJ, Rashidianfar A, Biben C, Zoellner H, Colvin EK, Pimanda JE, Biankin AV, Zhou B, Pu WT, Prall OW, Harvey RP. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell. 2011;9:527–540. doi: 10.1016/j.stem.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smart N, Bollini S, Dubé KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, Pu W, Riley PR. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]