Abstract
Treatment of diabetics with metformin is associated with decreased breast cancer risk in observational studies, but it remains unclear if this drug has clinical antineoplastic activity. In a recent presurgical trial, we found a heterogeneous effect of metformin on breast cancer proliferation (ki-67) depending upon insulin resistance (HOMA index). Here, we determined the associations of additional serum biomarkers of insulin resistance, tumor subtype, and drug concentration with ki-67 response to metformin. Two-hundred non-diabetic women were randomly allocated to metformin (850 mg/bid) or placebo for 4 weeks prior to breast cancer surgery. The ki-67 response to metformin was assessed comparing data obtained from baseline biopsy (ki-67 and tumor subtype) and serum markers (HOMA index, C-peptide, IGF-I, IGFBP-1, IGFBP-3, free IGF-I, hs-CRP, adiponectin) with the same measurements at definitive surgery. For patients with a blood sample taken within 24 h from last drug intake, metformin level was measured. Compared with placebo, metformin significantly decreased ki-67 in women with HOMA > 2.8, those in the lowest IGFBP-1 quintile, those in the highest IGFBP-3 quartile, those with low free IGF-I, those in the top hs-CRP tertile, and those with HER2-positive tumors. In women with HOMA index > 2.8, drug levels were positively correlated with the ki-67 decrease, whereas no trend was noted in women with HOMA < 2.8 (p-interaction = 0.07). At conventional antidiabetic doses, the effect of metformin on tumor ki-67 of non-diabetic breast cancer patients varies with host and tumor characteristics. These findings are relevant to design breast cancer prevention and treatment trials with metformin.
Electronic supplementary material
The online version of this article (doi:10.1007/s10549-014-3141-1) contains supplementary material, which is available to authorized users.
Keywords: Metformin, ki-67, Breast cancer, Clinical trial, Insulin resistance, HER2 breast cancer
Introduction
The hypothesis that metformin, widely used in the treatment of type II diabetes, is useful in breast cancer prevention and/or treatment is receiving considerable attention. Both pharmaco-epidemiologic and laboratory studies have suggested anti-neoplastic activity of this compound, and plausible mechanisms of action have been proposed [1–4] These include on one hand direct actions requiring exposure of target tissues to adequate drug concentrations, and on the other, effects on the endocrine environment at the whole organism level that result largely from actions of the drug on the liver, including reduction in gluconeogenesis and in circulating levels of insulin or cytokines. Metformin would be an appealing agent for use in oncology, as it is well tolerated and is available as a low-cost generic compound.
However, some of the pharmaco-epidemiologic data are negative [5] or controversial [6], and many laboratory studies demonstrating anti-neoplastic activity use drug concentrations higher than those achieved in serum with conventional metformin dosing. Furthermore, it is plausible that any effects of metformin on breast cancer risk or prognosis would be confined to subgroups of women, such as those with high BMI and/or high baseline levels of insulin, where metformin is known to have the largest effects on whole organism physiology [7]. Moreover, the effect of metformin on specific tumor molecular subtypes is unclear, although some studies have shown an effect on HER2 +ve tumors [8]. Therefore, pilot ‘window of opportunity’ presurgical studies with surrogate endpoints have been undertaken to generate clinical data to complement the epidemiologic and laboratory results. These studies involve sequential tissue sampling and biomarker measurements after relatively short-term exposure to metformin. Initial results of our randomized placebo-controlled study in 200 non-diabetic women with breast cancer [9] revealed that metformin exposure for 4 weeks was not associated with significant overall reduction in neither insulin levels nor tumor proliferation rate as estimated by ki-67 labeling index. However, we did detect a reduction of ki-67 by the metformin in subsets characterized by insulin resistance (HOMA index > 2.8) and high BMI (>27, the upper quartile), a finding consistent with the heterogeneous effect of metformin on diabetes development [10]. Similar studies conducted by other investigators involved smaller study populations. An early study of 47 patients documented a reduction in ki-67 level [11]. Data from a third study [12] of 39 patients without a control group revealed no significant effect on insulin levels, but weight loss, a reduction in ki-67 staining of 3 %, and a significant increase in apoptosis (TUNEL) staining following metformin administration. In our larger placebo-controlled study, we showed no effect of metformin on apoptosis overall, but there was heterogeneity according to insulin resistance estimated by HOMA index [13]. Finally, a fourth uncontrolled study in 35 overweight/obese women with breast cancer found no change in ki-67 but a reduction of BMI, cholesterol, and leptin [14].
Here, we report further exploratory analysis of our window-of-opportunity trial addressing the effects of metformin according to biomarkers of insulin resistance, including HOMA index, BMI, C-peptide, IGF-I, IGFBP-1, IGFBP-3, free IGF-I, C-reactive protein, adiponectin, and by tumor subtype.
Methods
Study design and subjects
A detailed description of the main study characteristics and initial results of the clinical trial has previously been published [9]. Briefly, we conducted a randomized, phase II, double-blind, placebo-controlled trial in women with stage I-IIa breast cancer candidate to elective surgery who received either metformin or placebo for 4 weeks prior to surgery. Baseline core biopsies of tumor tissue and blood samples were obtained at study entry and before surgery for pre/post-treatment comparisons. Patients were randomly assigned to metformin, 850 mg tablets or placebo once daily on day 1–3 to adapt to gastrointestinal symptoms, followed by two 850 mg tablets after dinner from day 4 to 28 to minimize gastrointestinal symptoms during the daytime and to attain higher blood levels during morning blood sample. Treatment was stopped at least 48 h prior to anesthesia, in keeping with FDA and AIFA guidelines [15, 16], to avoid the risk of lactic acidosis [17]. The median (IQR range) time (h) elapsed from last drug intake to blood drawing for circulating biomarkers was 36 (14-43) versus 36 (19–43), for metformin and placebo arms, respectively. The median (IQR range) time (h) elapsed from last drug intake to surgery was 67 (21–74) versus 67 (21–75) in the metformin and placebo arms, respectively. Notably, this time gap might, if anything, dilute the metformin effects on ki-67 measurement toward the null hypothesis. Eligibility criteria were: age ≥ 18 years; PS = 0; palpable, histologically confirmed breast cancer candidate to surgery and no prior treatment; signed informed consent.
Pathology
Pathological assessment included evaluation of histological type, grade, peritumoral vascular invasion, ER, progesterone receptor (PgR), HER2 and ki-67, as previously described [18]. Specifically, ki-67 was assessed by IHC according to recent international recommendations [19] using the Mib-1 monoclonal antibody (1:200 dilution; Dako, Denmark). Cut slides were stained using an automated Dako immunostainer. The percentage of cells showing definite nuclear immunoreactivity among 2,000 invasive neoplastic cells in randomly selected, high-power (40×) fields at the periphery of the tumor was recorded. If heterogeneity of ki-67 staining was present due to hot spots, the overall average score was adopted. In the core biopsy, all cells were counted regardless of their location in the tumor. Florescence in situ hybridization (FISH; PATHvision, Abbott, IL) was undertaken for tumors with a 2 + HER2 IHC score. Molecular tumor subtypes were classified by IHC in four categories according to 2011 St. Gallen criteria [20].
Circulating biomarkers of insulin resistance and serum drug concentrations
Morning fasting blood samples were collected between 8 and 10 am at baseline and before surgery. To obtain serum, blood was allowed to clot at room temperature for 30 min and then centrifuged at 1,500 g for 10 min. Serum aliquots were stored at −80 °C until assayed. Serum glucose and total cholesterol concentrations were measured on fresh samples, whereas all other analytes were determined on frozen samples. We applied the homeostasis model assessment (HOMA) as a surrogate index of insulin sensitivity, obtained by the formula [fasting insulinemia (mU/L) x glycaemia (mmol/L)]/22.5. We applied a cut-off of the HOMA index at 2.8 for insulin resistance based on a population study conducted in Northern Italy, as previously described [21]. Insulin was measured with an electro-chemo-luminescence immunoassay by the use of COBAS e411 (Roche Diagnostics, Mannheim, Germany). The analytical sensitivity was 0.20 uU/mL and intra- and inter-assay coefficients of variation 3.1 and 5.0 %, respectively for a control sample of 23.80 uU/ml. Serum concentrations of glucose and highly sensitive C-reactive protein (hs-CRP) were determined by the use of the automated instrument COBAS INTEGRA 800 (Roche Diagnostics, Mannheim, Germany). The sensitivity for the glucose was 0.24 mmol/L (4.32 mg/dL) and the intra- and inter-assay coefficients of variation never exceeded 1.5 %. The sensitivity for hs-CRP assay was 0.1 mg/L and intra- and inter-assay coefficients of variation were 4.1 and 6.4 % for hs-CRP (0.423 mg/L). Adiponectin was measured by an enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA). The minimum detectable dose was 0.25 ng/mL; the intra-assay CVs were 2.5 % (19.8 ng/mL) and the inter-assay precisions from 40 different assays of 3 samples of known concentration of 20.5 ng/mL was 6.8 %. Serum IGF-I and IGFBP-3 were measured by ELISA using reagents from Immunodiagnostic System, Ltd (Boldon, UK). IGFBP-1 was measured by ELISA (Abcam, Cambridge, UK). The sensitivity of the assay was 5 pg/ml, and intra and inter-assay coefficients of variation were 3.9 and 6.3 %, respectively. The ratio of IGF-I over IGFBP-1 + IGFBP-3 was calculated as an estimate of free IGF-I.
Serum drug concentrations were measured at the end of the 4-week intervention in a subgroup of 41 subjects who had an interval <24 h from last drug intake to blood draw by HPLC, as previously described [22]. All biomarker measurements, including drug concentration levels, were done blinded to the allocated arm.
Statistical analysis
Main descriptive statistics were median and interquartile range (IQR).Spearman’s rank correlation coefficients were used to test univariate correlations between continuous variables. The effect of treatment was tested using the standard approach for studies of biomarker changes, i.e., an analysis of covariance (ANCOVA) linear regression model with change in ki-67 as response variable, and baseline ki-67 and treatment arm as covariates [23]. Treatment effect modifications were statistically assessed testing the appropriate interaction term in the linear regression model described above, adjusting for age and BMI. We used the subpopulation treatment effect pattern plot (STEPP) methodology [24] to graphically explore the effect of the treatment on the change in ki-67 considering increasing values of the biomarkers considered, as previously described in the initial study finding [9]. While many comparisons may expose to false discovery, we did not apply any adjustment for multiple testing given the exploratory nature of the study, mainly based on secondary endpoints, aimed at generating further hypotheses. Median values and IQR of ki-67 changes according to identified thresholds and treatment arm are also presented. Except for HOMA and ER status, which were predefined in the study protocol, and HER2 status, defined by positivity, putative thresholds on other biomarkers were empirically anticipated looking at the STEPP. Linear regression model was used also to assess the association of ki-67 change with blood drug levels and to test the interaction with HOMA index. Post-treatment ki-67 was log-transformed (lnki-67) and the normal distribution of residuals of fully adjusted models was graphically checked. All analyses were conducted using STATA (version 11) and SAS (version 9.2). A two-tailed p value of 0.05 was considered as cut-off value to define the statistical significance.
Results
At baseline, there were significant correlations among markers of insulin resistance. For instance, BMI was positively related with hs-CRP (r = 0.44, p < 0.0001) and negatively related with IGFBP-1 (r = −0.28, p < 0.0001), and HOMA index was negatively related with IGFBP-1 (r = −0.45, p < 0.0001). Interestingly, baseline ki-67 levels exhibited a borderline significant positive correlation with HOMA index (r = 0.13, p = 0.07) and a negative correlation with IGFBP-1 (r = −0.13, p = 0.07) (all data not shown).
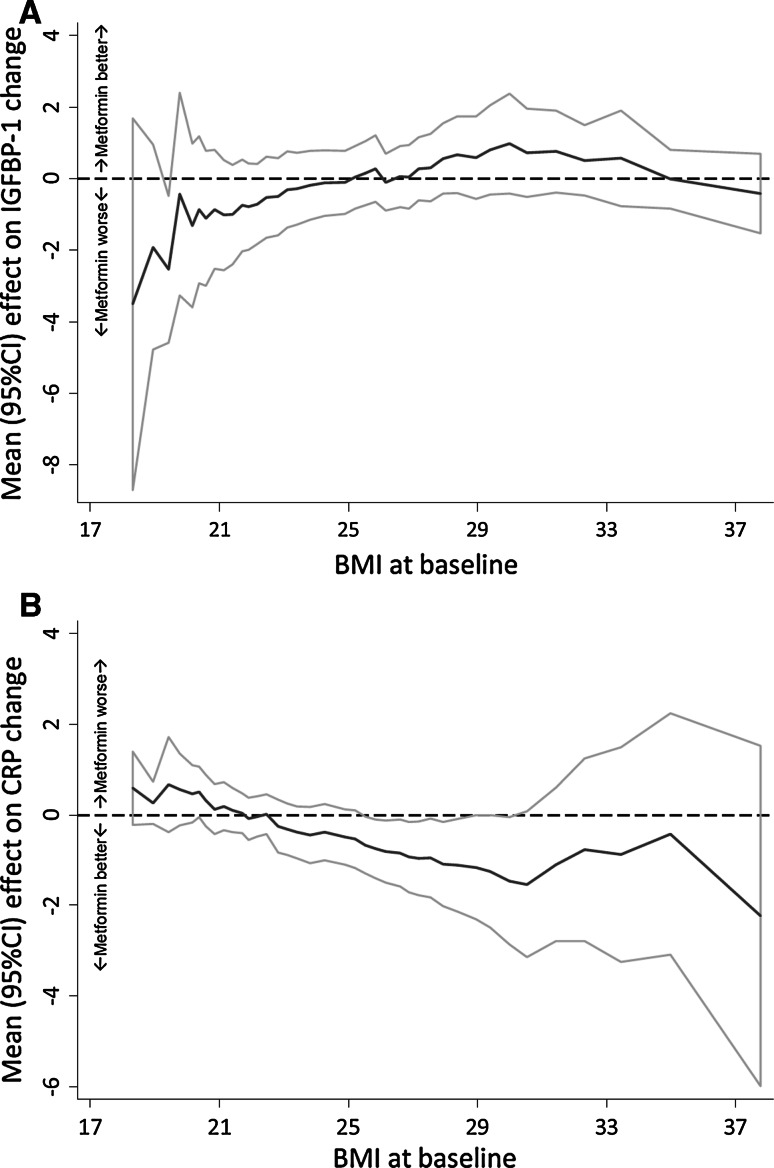
The effects of metformin and placebo on biomarkers of insulin resistance and ki-67 labeling index are illustrated in Table 1. Overall, there was no significant change compared with placebo on any biomarker except for adiponectin, which was decreased to a larger extent by metformin relative to placebo. However, the effect of metformin on IGFBP-1 and hs-CRP was significantly modified by BMI, since metformin increased IGFBP-1 and decreased hs-CRP levels only in women with BMI > 25 (Fig. 1).
Table 1.
Effect of metformin and placebo on biomarkers of insulin resistance and tumor proliferation (ki-67)
| Biomarker | Metformin | Placebo | Treatment effectc | p* | ||
|---|---|---|---|---|---|---|
| Pre-treatment N = 100 | Post-treatment N = 97 | Pre-treatment N = 100 | Post-treatment N = 99 | |||
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Mean (95 % CI) | ||
| Weight (kg) | 65(56; 73) | 65 (56; 74) | 62 (57; 71) | 63 (57; 71) | −0.09 (−0.85; 0.67) | 0.8 |
| BMI (kg/m2) | 24.1 (21.3; 27.4) | 24.1 (21.3; 27.5) | 23.9 (21.8; 27.3) | 24.1 (22.0; 27.3) | −0.05 (−0.35; 0.25) | 0.7 |
| Glucose (mmol/L) | 87 (83; 93) | 87 (81; 94) | 91 (87; 98) | 91 (83; 96) | −0.64 (−3.78; 2.49) | 0.7 |
| Insulin (mU/L) | 9.1 (5.7; 12.4) | 7.2 (5.6; 12.3) | 8.9 (6.6; 12.7) | 7.6 (5.2; 12.9) | −0.75 (−3.36; 1.85) | 0.6 |
| HOMA indexa | 1.9 (1.2; 2.8) | 1.6 (1.1; 2.8) | 2.0 (1.4; 3.0) | 1.7 (1.2; 3.1) | −0.27 (−1.19; 0.65) | 0.6 |
| C-peptide (ng/mL) | 1.5 (1.1; 2.2) | 1.5 (1.1; 2.0) | 1.6 (1.3; 2.0) | 1.5 (1.1; 2.2) | −0.04 (−0.25; 0.17) | 0.7 |
| hs-CRP (mg/L) | 1.4 (0.6; 3.0) | 1.0 (0.5; 2.1) | 1.0 (0.5; 2.1) | 1.2 (0.5; 2.1) | −0.47 (−1.06; 0.12) | 0.1 |
| IGFBP-1 (ng/mL) | 3.5 (2.1; 6.2) | 3.6 (2.0; 6.1) | 3.9 (2.5; 5.4) | 3.6 (2.4; 5.9) | −0.10 (−0.98; 0.78) | 0.8 |
| IGFBP-3 (μg/mL) | 4.1 (3.7; 4.6) | 4.0 (3.6; 4.5) | 4.3 (3.7; 4.7) | 4.2 (3.6; 4.6) | 0.03 (−0.10; 0.15) | 0.7 |
| IGF-I (ng/mL) | 136 (111; 164) | 134 (109; 164) | 150 (123; 177) | 147 (120; 184) | −3.1 (−10.0; 3.7) | 0.4 |
| Free IGF-Ib | 0.18 (0.15; 0.20) | 0.18 (0.16; 0.21) | 0.20 (0.17; 0.22) | 0.20 (0.16; 0.23) | −0.006 (−0.01; 0.002) | 0.1 |
| Adiponectin (ng/Ml) | 9.6 (6.2; 12.6) | 9.1 (5.9; 11.8) | 8.2 (6.0;13.6) | 8.4 (5.8; 12.6) | −0.64 (−1.17; −0.12) | 0.02 |
| ki-67 (%) | 19 (14; 33) | 21 (14; 32) | 18 (12; 29) | 20 (13; 31) | 0.30 (−1.93; 2.53) | 0.8 |
Data on HOMA index were available for 199 patients
IQR interquartile range, Δ post–pre treatment difference
* p-interaction between treatment and biomarker
aHomeostasis model assessment (HOMA) formula: fasting blood glucose (mmol/L) X insulin (mU/L)/22.5
bFree IGF-I = IGF-I/(IGFBP-1 + IGFBP-3)
cEffect of metformin relative to placebo on the change (difference surgery-baseline) calculated from the linear regression model (dependent variable: change of biomarker, adjusted for the biomarker level at baseline, BMI and age). Interpretation for treatment effect: a positive value stands for an increase in the metformin arm relative to the placebo arm, a negative value for a decrease
Fig. 1.
Subpopulation Treatment Effect Pattern Plots (STEPP) of the change (difference between post- and pre-treatment level) of IGFBP-1 (a) and hs-CRP (b) according to body mass index (BMI). For IGFBP-1, positive change, metformin better; negative change, metformin worse; for hs-CRP, positive change, metformin worse; negative change, metformin better. p value for interaction treatment * BMI (threshold 25 kg/m2) = 0.09 and 0.05, respectively (from the linear regression model, adjusting for the biomarker levels at baseline and age)
Figure 2 illustrates the STEPP analyses of the change of ki-67 (the difference between endpoint surgery and baseline biopsy) in the metformin arm relative to the placebo arm according to the continuous scale for the following markers of insulin resistance: C-peptide, IGFBP-3, IGFBP-1, and free IGF-I. Metformin had a heterogeneous effect depending on markers of insulin resistance, which manifested itself in a tendency to decrease ki-67 levels in women with insulin resistance (approximately a quarter), defined by high levels of HOMA, C-peptide, IGFBP-3, and by low levels of free IGF-I and IGFBP-1, whereas it showed a trend to an increase of ki-67 in the majority of the remaining women. Except for C-peptide (p-interaction = 0.3), all variables illustrated in Fig. 2 as well as hs-CRP significantly modified the effect of metformin on ki-67 at p < 0.1. Variables not exhibiting a significant interaction with metformin were: weight, BMI, glucose, insulin, adiponectin, and total IGF-I.
Fig. 2.
Subpopulation treatment effect pattern plots (STEPP) of the change (difference between endpoint surgery and baseline biopsy) of ki-67 according to the following covariates: C-peptide (panel A), free IGF-I (panel B), IGFBP-3 (panel C), IGFBP-1 (panel D). Positive change, metformin worse; negative change, metformin better. p values for interaction with treatment from the linear regression model (response variable: change of ki-67, adjusted for: ki-67 and BMI at baseline, age): C-peptide, p = 0.3 (threshold: median, 1.53 ng/mL); free IGF-I, p = 0.03 (continuous variable); IGFBP-3, p = 0.04 (median, 4.2 μg/mL); IGFBP-1, p = 0.016 (20th p.le, 1.91 ng/mL)
The differential effects of metformin on ki-67 changes according to the biomarker categories thresholds with p values for interactions are shown in Table 2. Approximately 25 % of our study population had biomarker levels of insulin resistance, whereas 11 % had HER2-positive tumors. In the placebo arm, a remarkable increase in median and IQR range of ki-67 between the baseline biopsy and the surgical sample was observed in women with HER2-positive tumors and in the highest hs-CRP tertile (>1.81 mg/L), and, to a lesser extent, in women in the lowest IGFBP-1 quintile (<2 ng/mL). A rise in ki-67 between the time of biopsy and definitive surgery in patients with HER2-positive tumors receiving placebo has been well documented [25], although the basis of this phenomenon is not clear. It may relate to a true biological increase, but the possibilities of technical factors (such as difference in tissue procurement [biopsy versus surgery]) have not been excluded. In any case, these increases were significantly blunted by metformin. Also, in women with no insulin resistance as identified by HOMA < 2.8 or IGFBP-3 below the upper quartile (4.6 μg/mL), metformin exhibited a trend to an increase in ki-67 compared to placebo, although the IQR range included negative values. There was no significant interaction between metformin and BMI categorized at either 25 or 27 (p = 0.22).
Table 2.
Median (IQR) ki-67 changes by treatment arm and biomarker cutoff points
| Risk biomarker threshold | N | Placebo | Metformin | p-interactiona |
|---|---|---|---|---|
| HOMA index > 2.8 | 53 | 0 (−2.0; 5.0) | 0 (−5.0; 2.5) | 0.03 |
| HOMA index ≤ 2.8 | 142 | 0 (−2.0; 4.0) | 1 (−2.0; 7.0) | |
| hs-CRP > 1.81 mg/L (3rdtertile) | 65 | 2.5 (0;7) | 0 (−3; 4) | 0.02 |
| hs-CRP ≤ 1.81 mg/L | 131 | 0 (−3;5) | 0.5 (−2; 8) | |
| IGFBP-3 > 4.6 μg/mL (4thquartile) | 50 | 0 (−5.0; 7.0) | 0 (−4.0; 2.0) | 0.04 |
| IGFBP-3 ≤ 4.6 μg/mL | 146 | 0 (−1.5; 4.0) | 1 (−3.0; 7.0) | |
| IGFBP-1 < 2 ng/mL (1st quintile) | 40 | 1 (−5.0; 14.0) | 0 (−4.0; 5.0) | 0.02 |
| IGFBP-1 ≥ 2 ng/mL | 156 | 0 (−2.0; 4.0) | 0.5 (−3.0; 7.0) | |
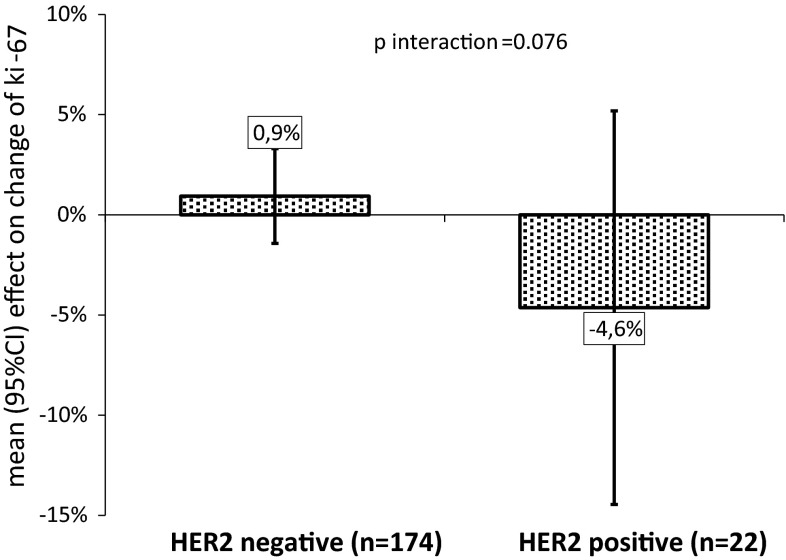
| HER2-positive | 22 | 3.5 (0; 14.0) | 0.5 (−4.0; 8.0) | 0.076 |
| HER2-negative | 174 | 0 (−3.0; 4.0) | 0 (−3.0; 7.0) |
IQR interquartile range
a p values for treatment, * covariate interaction on ki-67 change
The effect of metformin on ki-67 change in HER2-positive tumors is further illustrated in Fig. 3. Compared with placebo, metformin decreased ki-67 by a mean 5 % units in HER2-positive tumors, whereas there was a nearly 1 % mean unit increase under metformin in tumors not overexpressing HER2 (p-interaction = 0.076).
Fig. 3.
Effect of metformin on ki-67 change (difference between endpoint surgery and baseline biopsy) by HER2 tumor status
To further elucidate the effects of metformin on ki-67 according to HOMA index, serum drug concentrations were measured in a subgroup of 41 subjects in the metformin arm and in three random subjects in the placebo arm. Metformin was not detected in the placebo-treated patients. As expected, there was a significant negative correlation between serum drug levels and time (hours) since last drug intake (Spearman rho = −0.5, p = 0.001).However, time between drug intake was not associated with ki-67 change (coefficient = −0.27, p = 0.6, supplemental Table 1). Interestingly, there was a different association between serum drug levels and ki-67 change according to HOMA index (p-interaction = 0.07, Fig. 4). Specifically, in women with HOMA index > 2.8, higher drug concentrations were associated with a greater decrease of ki-67 (p = 0.04), whereas no association was noted in women with HOMA < 2.8 (p = 0.68). The association between drug levels and ki-67 change was independent of age, BMI, and interval from last drug intake. Likewise, no biomarker change was significantly affected by the time since last drug intake, including, blood glucose (Table 3).
Fig. 4.
Scatterplot of ki-67 change (difference between endpoint surgery and baseline biopsy) and blood levels of metformin in a selected subgroup of women (n = 40) according to HOMA index levels (≤2.8, n = 28, p = 0.6, and >2.8, n = 12, p = 0.04). p for treatment * HOMA index interaction = 0.07. Adjusted for age, BMI, and time from last drug intake. One subject was not assessable because ki-67 was missing at endpoint surgery
Table 3.
Association between time since last metformin intake and change in biomarker
| Biomarker | Coefficient | 95 % CI | p |
|---|---|---|---|
| ki-67 | −0.00202 | −0.01752 ÷ 0.01349 | 0.8 |
| Glucose | −0.00306 | −0.02319 ÷ 0.01706 | 0.8 |
| Insulin | −0.00801 | −0.02609 ÷ 0.01006 | 0.4 |
| HOMA index | −0.00231 | −0.00862 ÷ 0.00401 | 0.5 |
| C-peptide | −0.00046 | −0.00189 ÷ 0.00098 | 0.5 |
| hs-CRP | −0.00059 | −0.00478 ÷ 0.00360 | 0.8 |
| IGFBP-1 | −0.00104 | −0.00707 ÷ 0.00498 | 0.7 |
| IGFBP-3 | 0.65105 | −0.19487 ÷ 1.49696 | 0.1 |
| IGF-I | 0.00512 | −0.04094 ÷ 0.05118 | 0.8 |
| Free IGF-I | −0.00002 | −0.00007 ÷ 0.00004 | 0.6 |
| Adiponectin | −0.00016 | −0.00371 ÷ 0.00339 | 0.9 |
From linear regression models (one for each biomarker) setting biomarker change as the response variable and time since last metformin intake as explanatory variable, adjusting for age, treatment arm, and biomarker level at baseline. The coefficient is estimated for the variable “time since last metformin intake” from each regression model
Discussion
Our findings provide evidence for a complex heterogeneous effect of metformin on breast cancer proliferation in women without diabetes. Relative to placebo, metformin decreased ki-67 only in women with insulin resistance or HER2-positive tumors, whereas it showed a trend to an increase of ki-67 in the remaining women. In some subgroups, particularly in women with HER2-positive tumors and top hs-CRP levels, metformin significantly blunted the ki-67 rise observed in the placebo arm. This heterogeneous response to metformin also was influenced by serum drug measurements, inasmuch as high serum drug levels induced a greater ki-67 decrease in women with HOMA index > 2.8, whereas no effect was noted in women with HOMA < 2.8. All subgroup analyses, except for HOMA, are exploratory in nature and should, therefore, be considered hypothesis generating rather than definitive. However, given the epidemic of insulin resistance in western countries and the common association of insulin resistance and/or type II diabetes with breast cancer, our findings may have important clinical implications.
Our findings differ from those presented by Goodwin et al. in the first 500 patients enrolled in the NCIC CTG MA.32, a multicenter adjuvant trial in early-stage breast cancer, where favorable effects on metabolic markers were noted but no significant interaction between metformin and BMI or insulin was observed on insulin, glucose, leptin, and CRP at 6 months [26].However, comparison between the two studies is difficult since the two populations differ substantially in mean BMI (24 in our study versus 28 in MA.32) and because we measured insulin resistance biomarkers which were not explored in the MA.32 study.
Our complex results are partly attributable to the increase of ki-67 from baseline biopsy to endpoint surgery in the placebo arm in several subgroups, particularly women with HER2-positive tumors and hs-CRP > 1.8 mg/L. Increases of ki-67 in the placebo arms have been noted in studies of a similar design addressing hypotheses unrelated to metformin [25, 27, 28]. We favor the interpretation that these findings do reflect a genuine biopsy or surgery-induced wound healing effect that stimulates cancer proliferation in specific tumor subtypes and host-related characteristics. In this regard, a previous study [29] has shown that by comparing histological sections of primary breast cancers overexpressing HER2 with residual tumors found in re-excision specimens, ki-67 increased by 10 % after a mean interval of 5 weeks. Moreover, drainage fluids collected from breast cancer patients shortly after surgery were particularly active in stimulating HER2-positive cell lines, whereas wound-induced in vitro proliferation was blunted when these cell lines were treated with trastuzumab before drainage fluid was added. Our data show, with respect to this issue, that metformin is particularly effective in blunting the proliferative surge associated with HER2-positive tumors following surgery [30]. In vivo, metformin is able to induce downregulation of HER1 and HER2 and in vitro the drug inhibits self-renewal and proliferation of cancer stem cells in HER2 overexpressing breast cancer cell [31]. One implication of these data is that metformin administration may be of particular value if used in a neoadjuvant or perioperative context especially during the critical short period of metastasis formation which follows surgery, rather than postoperatively in an adjuvant fashion. A neoadjuvant clinical trial addressing the activity of metformin in HER2-positive breast tumors is underway [32].
We previously reported [9] metformin-associated reductions of ki-67 labeling index in an insulin–resistant subset of patients defined by HOMA index, BMI, or hs-CRP. We now shows that IGFBP-1 levels at baseline are particularly useful to identify a subpopulation in which metformin is associated with reduced ki-67 labeling. IGFBP-1 is strongly downregulated by insulin. Thus, low IGFBP-1 represents a consequence of hyperinsulinemia. The consequences of reduced IGFBP-1 would be expected to include increased bioactivity of IGF-I and IGF-II in microenvironments where IGF bioactivity is probably constrained by IGFBP-1. As expected, we observed that women with low IGFBP-1 tend to have high BMI and high insulin levels. Interestingly, baseline ki-67 levels exhibited a borderline significant positive correlation with HOMA index and a negative correlation with IGFBP-1, suggesting that hyperinsulinemia is associated with tumor proliferation. Also, women with high IGFPB-3 and consequently low levels of free IGF-I tend to respond to metformin with a ki-67 decrease.
The trend to an increased proliferation in insulin sensitive women, albeit non-significant, suggests a complex biological effect of metformin in these women. Animal studies have shown that metformin may have tumor suppressive effects where a metabolic phenotype of high caloric intake, metabolic syndrome, and diabetes exists, but no effect or even a growth promoting effect under normal energy intake [33, 34]. These data, together with laboratory studies that provide evidence that in certain contexts activation of AMPK may actually increase cell survival [35], and raise the possibility that metformin exposure may not be beneficial for all women in the context of breast cancer prevention and treatment.
It is clear that in breast cancer patients without diabetes, higher insulin or C-peptide levels are associated with poor prognosis [36, 37] a finding that extends also to colorectal and prostate cancer [38, 39]. However, it is not certain if insulin directly mediates this effect or acts as a surrogate for other effectors. The presence of insulin receptors in breast cancer tissue [40–42] certainly is in keeping with a direct mediating action of insulin, but recent data show that administration of insulin to diabetics (which leads to much higher circulating levels than are present in untreated type II diabetes) is not associated with an adverse effects on cancer endpoints [43, 44] emphasizing that caution is required in attempts to associate changes in insulin exposure with changes in breast cancer prognosis [45]. A prior phase III breast cancer adjuvant treatment trial used a somatostatin analog to lower insulin and IGF-I levels; statistically significant but small magnitude reductions in hormone levels were achieved, but treatment for two years was not associated with long-term clinical benefit [37].
Prior studies in different populations treated with metformin (for example [46] have not demonstrated the increase in adiponectin that would be expected with reduced insulin resistance. Indeed, we observed an unexpected modest decrease (0.64 ng/mL, 7 %) in adiponectin levels in women on metformin compared to those on placebo, but the magnitude of this change and its large statistical variability is of uncertain clinical significance.
Measurements of drug levels in a patient subgroup where blood draw was within 24 h of last drug intake reinforce the concept of heterogeneity of metformin effect on cancer proliferation depending upon insulin resistance. Although as expected the serum drug concentration was a function of the interval from the last drug intake, a positive relationship between drug levels and ki-67 response was evident only in women with HOMA > 2.8 and was independent of the interval from last drug intake. No relationship was noted between drug levels and ki-67 change in women without insulin resistance. These findings imply that drug levels achieved with conventional antidiabetic doses are sufficient to influence at least some tumors in patients with insulin resistance, likely by an “indirect” mechanism involving changes in the host hormonal milieu secondary to actions in the liver, where it is known that metformin at anti-diabetic doses has an impact on gluconeogenesis [1, 7]. “Direct” actions would be unlikely to vary with insulin resistance, while the hypothesized “indirect” mechanism would be expected to be more important when insulin resistance is high. Also in keeping with this interpretation is the fact that the measured serum concentration of metformin is lower than that used in most laboratory studies that demonstrate a “direct” effect. However, larger studies will be required to more formally evaluate the relationship between peak and through metformin levels and the influence of the drug on tumorki-67, or ultimately on clinical endpoints related to efficacy of breast cancer treatment. It is possible that phenformin or other biguanides may be more effective than metformin in achieving “direct” effects [1]. A potential limitation is the variation in the duration of the interval from last drug intake to blood drawing (median 36 h, IQR, 14–43) and surgery (median 67 h, IQR 21–74), which is longer than the blood half-life of metformin (approximately 18 h, ref 15). However, we observed no significant association between any biomarker changes and the interval from treatment cessation, including, quite unexpectedly, blood glucose levels, suggesting that metformin effects may persist for several days from drug cessation [47, 48]. For instance, studies in diabetics have shown that the activation of AMPK α2 by metformin persisted after an overnight withdrawal, when expected concentrations of the drug in blood would be low [49]. Finally, we gave metformin 1,700 mg once daily after dinner and not bis in die to minimize gastrointestinal symptoms during daytime and to attain higher blood levels in the subsequent morning blood sample. If anything, however, the wash-out time variability diluted our findings toward the null hypothesis without affecting overall conclusions.
In conclusion, our data describe effects of metformin on the hormonal/metabolic profile and tumor cell proliferation estimated by ki-67 labeling index in non-diabetic breast cancer patients, and show that there is considerable heterogeneity in these effects within the study population. We define novel biomarkers, such as HER2-positive tumors and serum IGFBP-1, which identify a subset of women for whom metformin given at conventional anti-diabetic doses reduces tumor cell proliferation. It is important to put the magnitude of effects of metformin on ki-67 into context relative to the effects of approved breast cancer drugs. Collectively, the metformin “window of opportunity trials”, including our data suggest an effect of metformin on ki-67-estimated proliferation in certain subgroups, but even within these subgroups the effect size is considerably less than that of tamoxifen [27] or aromatase inhibitors on ER-positive breast cancer [50].These findings suggest that benefits of metformin in breast cancer may be confined to specific patient subgroups. In view of low toxicity and cost, metformin deserves study in breast cancer treatment and prevention, particularly peri-operatively and in combination with other agents [51].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Supported by Lega Italiana per la Lotta contro i Tumori (Grant # 14/08), AIRC IG12072, Italian Ministry of Health RF-2009-1532226, and a Grant to MNP from the Canadian Cancer Society Research Institute. MS and UH have been supported by the Robert Bosch Stiftung, Germany.MS has been supported by and the Federal Ministry of Education and Research (BMBF, Berlin, Germany) Grant 03 IS 2061C. Metformin and placebo were donated by Laboratori Guidotti, Pisa, Italy.
Conflict of interest
The authors disclose no potential conflict of interest.
Footnotes
Bernardo Bonanni and Michael N. Pollak are Senior co-authors.
References
- 1.Pollak M. Potential applications for biguanides in oncology. J Clin Invest. 2013;123:3693–3700. doi: 10.1172/JCI67232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodwin PJ, Ligibel JA, Stambolic V. Metformin in breast cancer: time for action. J Clin Oncol. 2009;27:3271–3273. doi: 10.1200/JCO.2009.22.1630. [DOI] [PubMed] [Google Scholar]
- 3.Pollak M. Overcoming drug development bottlenecks with repurposing: repurposing biguanides to target energy metabolism for cancer treatment. Nat Med. 2014;20:591–593. doi: 10.1038/nm.3596. [DOI] [PubMed] [Google Scholar]
- 4.Gandini S, Puntoni M, Heckman-Stoddard BM, et al. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila) 2014;7:867–885. doi: 10.1158/1940-6207.CAPR-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lega IC, Austin PC, Gruneir A, et al. Association between metformin therapy and mortality after breast cancer: a population-based study. Diabetes Care. 2013;36:3018–3026. doi: 10.2337/dc12-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care. 2012;35:2665–2673. doi: 10.2337/dc12-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov. 2012;2:778–790. doi: 10.1158/2159-8290.CD-12-0263. [DOI] [PubMed] [Google Scholar]
- 8.He X, Esteva FJ, Ensor J, et al. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2 + breast cancer. Ann Oncol. 2012;23:1771–1780. doi: 10.1093/annonc/mdr534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonanni B, Puntoni M, Cazzaniga M, et al. Dual Effect of Metformin on Breast Cancer Proliferation in a Randomized Presurgical Trial. J Clin Oncol. 2012;30:2593–2600. doi: 10.1200/JCO.2011.39.3769. [DOI] [PubMed] [Google Scholar]
- 10.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadad S, Iwamoto T, Jordan L, et al. Evidence for biological effects of metformin in operable breast cancer: a pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res Treat. 2011;128:783–794. doi: 10.1007/s10549-011-1612-1. [DOI] [PubMed] [Google Scholar]
- 12.Niraula S, Dowling RJ, Ennis M, et al. Metformin in early breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res Treat. 2012;135:821–830. doi: 10.1007/s10549-012-2223-1. [DOI] [PubMed] [Google Scholar]
- 13.Cazzaniga M, Decensi A, Pruneri G, et al. The effect of metformin on apoptosis in a breast cancer presurgical trial. Br J Cancer. 2013;109:2792–2797. doi: 10.1038/bjc.2013.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalinsky K, Crew KD, Refice S, et al. Presurgical trial of metformin in overweight and obese patients with newly diagnosed breast cancer. Cancer Invest. 2014;32:150–157. doi: 10.3109/07357907.2014.889706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(GENERIC)Ref Type: Electronic Citation 2014.http://www.drugs.com/pro/metformin.html.
- 16.http://www.agenziafarmaco.gov.it/it/content/recommendations on the use of metformin (2011).08/04/201
- 17.Jones GC, Macklin JP, Alexander WD. Contraindications to the use of metformin. BMJ. 2003;326:4–5. doi: 10.1136/bmj.326.7379.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viale G, Giobbie-Hurder A, Regan MM, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26:5569–5575. doi: 10.1200/JCO.2008.17.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in Breast Cancer: recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst. 2011;103:1656–1664. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson H, Gandini S, Guerrieri-Gonzaga A, et al. Effect of fenretinide and low-dose tamoxifen on insulin sensitivity in premenopausal women at high risk for breast cancer. Cancer Res. 2008;68:9512–9518. doi: 10.1158/0008-5472.CAN-08-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fendt SM, Bell EL, Keibler MA, et al. Metformin decreases glucose oxidation and increases the dependency of prostate cancer cells on reductive glutamine metabolism. Cancer Res. 2013;73:4429–4438. doi: 10.1158/0008-5472.CAN-13-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senn S. Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Stat Med. 1994;13:197–198. doi: 10.1002/sim.4780130210. [DOI] [PubMed] [Google Scholar]
- 24.Bonetti M, Gelber RD. Patterns of treatment effects in subsets of patients in clinical trials. Biostatistics. 2004;5:465–481. doi: 10.1093/biostatistics/kxh002. [DOI] [PubMed] [Google Scholar]
- 25.Gandini S, Guerrieri-Gonzaga A, Pruneri G, et al. Association of molecular subtypes with Ki-67 changes in untreated breast cancer patients undergoing pre-surgical trials. Ann Oncol. 2013;25:618–623. doi: 10.1093/annonc/mdt528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodwin PJ, Parulekar W, Gelmon KA et al (2013). Effect of metformin versus placebo on weight and metabolic factors in initial patients enrolled onto NCIC CTG MA.32, a multicenter adjuvant randomized controlled trial in early-stage breast cancer (BC). J Clin Oncol 31 (suppl; abstr 1033)
- 27.Decensi A, Robertson C, Viale G, et al. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. 2003;95:779–790. doi: 10.1093/jnci/95.11.779. [DOI] [PubMed] [Google Scholar]
- 28.Decensi A, Puntoni M, Pruneri G, et al. Lapatinib activity in premalignant lesions and HER-2-positive cancer of the breast in a randomized, placebo-controlled presurgical trial. Cancer Prev Res (Phila) 2011;4:1181–1189. doi: 10.1158/1940-6207.CAPR-10-0337. [DOI] [PubMed] [Google Scholar]
- 29.Tagliabue E, Agresti R, Carcangiu ML, et al. Role of HER2 in wound-induced breast carcinoma proliferation. Lancet. 2003;362:527–533. doi: 10.1016/S0140-6736(03)14112-8. [DOI] [PubMed] [Google Scholar]
- 30.Spigel DR. HER2 and surgery: more questions to answer. Lancet. 2003;362:502–503. doi: 10.1016/S0140-6736(03)14147-5. [DOI] [PubMed] [Google Scholar]
- 31.Zhu P, Davis M, Blackwelder AJ, et al. Metformin selectively targets tumor-initiating cells in ErbB2-overexpressing breast cancer models. Cancer Prev Res (Phila) 2014;7:199–210. doi: 10.1158/1940-6207.CAPR-13-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin-Castillo B, Dorca J, Vazquez-Martin A, et al. Incorporating the antidiabetic drug metformin in HER2-positive breast cancer treated with neo-adjuvant chemotherapy and trastuzumab: an ongoing clinical-translational research experience at the Catalan Institute of Oncology. Ann Oncol. 2010;21:187–189. doi: 10.1093/annonc/mdp494. [DOI] [PubMed] [Google Scholar]
- 33.Phoenix KN, Vumbaca F, Fox MM, et al. Dietary energy availability affects primary and metastatic breast cancer and metformin efficacy. Breast Cancer Res Treat. 2010;123:333–344. doi: 10.1007/s10549-009-0647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Algire C, Amrein L, Bazile M, et al. Diet and tumor LKB1 expression interact to determine sensitivity to anti-neoplastic effects of metformin in vivo. Oncogene. 2011;30:1174–1182. doi: 10.1038/onc.2010.483. [DOI] [PubMed] [Google Scholar]
- 35.Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodwin PJ, Ennis M, Pritchard KI, et al. Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol. 2012;30:164–171. doi: 10.1200/JCO.2011.36.2723. [DOI] [PubMed] [Google Scholar]
- 37.Pritchard KI, Shepherd LE, Chapman JA, et al. Randomized trial of tamoxifen versus combined tamoxifen and octreotide LAR Therapy in the adjuvant treatment of early-stage breast cancer in postmenopausal women: NCIC CTG MA.14. J Clin Oncol. 2011;29:3869–3876. doi: 10.1200/JCO.2010.33.7006. [DOI] [PubMed] [Google Scholar]
- 38.Wolpin BM, Meyerhardt JA, Chan AT, et al. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol. 2009;27:176–185. doi: 10.1200/JCO.2008.17.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma J, Li H, Giovannucci E, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008;9:1039–1047. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belfiore A, Frasca F, Pandini G, et al. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 41.Law JH, Habibi G, Hu K, et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68:10238–10246. doi: 10.1158/0008-5472.CAN-08-2755. [DOI] [PubMed] [Google Scholar]
- 42.Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer. 2011;18:R125–R147. doi: 10.1530/ERC-11-0074. [DOI] [PubMed] [Google Scholar]
- 43.Carstensen B, Witte DR, Friis S. Cancer occurrence in Danish diabetic patients: duration and insulin effects. Diabetologia. 2012;55:948–958. doi: 10.1007/s00125-011-2381-4. [DOI] [PubMed] [Google Scholar]
- 44.Leow MK. Basal insulin and cardiovascular and other outcomes. N Engl J Med. 2012;367:1763–1764. doi: 10.1056/NEJMc1210553. [DOI] [PubMed] [Google Scholar]
- 45.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. doi: 10.1200/JCO.20.1.42. [DOI] [PubMed] [Google Scholar]
- 46.Doogue MP, Begg EJ, Moore MP, et al. Metformin increases plasma ghrelin in Type 2 diabetes. Br J Clin Pharmacol. 2009;68:875–882. doi: 10.1111/j.1365-2125.2009.03372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robert F, Fendri S, Hary L, Lacroix C, et al. Kinetics ofplasma and erythrocyte metformin after acute administration in healthy subjects. Diabetes Metab. 2003;29:279–283. doi: 10.1016/S1262-3636(07)70037-X. [DOI] [PubMed] [Google Scholar]
- 48.Graham GG, Punt J, Arora M, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 49.Musi N, Hirshman MF, Nygren J, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 50.Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. J Clin Oncol. 2011;29:2342–2348. doi: 10.1200/JCO.2010.31.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.