Abstract
Flies are frequently used for postmortem interval (PMI) estimations. These estimates are usually based on the age of larval or pupal specimens. However, the age defines only the minimum PMI. In order to move forensic entomology further, a method useful for the estimation of an interval preceding insect appearance on a corpse called the pre-appearance interval (PAI) is needed. Recently, it was demonstrated that the PAI of several carrion beetles is closely related to the temperature prevailing throughout this interval. Hence, it was postulated to estimate PAI from temperature. In order to check premises for using this approach with flies, a test of the relationship between adult or oviposition PAI and temperature was made for nine species of European flies. Data on PAI originated from pig carcasses decomposing under various temperatures. Adult PAI of Hydrotaea dentipes, Hydrotaea ignava, Hydrotaea similis, Phormia regina, and Stearibia nigriceps and oviposition PAI of S. nigriceps were exponentially related to temperature. Only S. nigriceps revealed a close relationship, demonstrating solid premises for PAI estimation from temperature alone. Adult and oviposition PAI of Calliphora vomitoria and adult PAI of Hydrotaea pilipes were not related to temperature. Adult and oviposition PAI of Lucilia sericata and Lucilia caesar responded similarly, with an abrupt and large increase in a narrow range of low temperatures and no response in a broad range of high temperatures. Probably, different mechanisms form the basis for the response of PAI to temperature in flies colonizing carcasses shortly after death and flies colonizing carcasses later in the decomposition process.
Keywords: Forensic entomology, Postmortem interval, Pre-appearance interval, Lucilia, Phormia, Calliphora, Hydrotaea, Stearibia
Introduction
Postmortem interval (PMI) may be estimated from the development or the succession of insects on corpses [1–5]. Entomological estimates are most frequently based on the age of larval or pupal specimens sampled from a corpse [1–5]. However, the age of an insect alone may define only the minimum PMI, as it gives information just about a development interval. Unfortunately, the age of an insect gives no information about an interval which precedes the appearance of a given specimen on a corpse which is named the pre-appearance interval (PAI) or the pre-colonization interval [6]. In the case of early arriving insects (e.g., blowflies), the minimum PMI is usually close to the actual PMI, but in the case of middle and late arriving insects, it defines actual PMI with the intrinsic and frequently very high inaccuracy. This is probably the main reason why middle and late arriving insects are rarely used for PMI estimation. However, even with the early arriving insects, the minimum PMI may be far from the actual PMI, as was exemplified by results of European, early spring carrion studies in which blowflies colonized carcasses with a delay of several days [7, 8]. Accordingly, in order to move forensic entomology further, a robust method for PAI estimation is needed.
In casework, an estimate of PAI may be reached through a qualitative evaluation of factors which delay colonization of carcasses by insects or through a reference to results of case-relevant pig carrion studies. Due to the lack of transparency and inapplicability to middle and late arriving insects, the first approach is inadequate for routine use. The second approach is, however, clearly impractical because it needs unattainable quantity of data from pig carrion studies. Recently, a novel approach was proposed. It was demonstrated that adult or larval PAI of some forensically important insects (particularly beetles) are closely related to the temperature prevailing in the corpse surrounding throughout the PAI [9–12]. Hence, it was postulated to estimate PAI from temperature, and several methods useful for that purpose were initially tested [9, 10]. This approach has several advantages. First, previous works demonstrated that models of the relationship between PAI and temperature give accurate estimates for cases from different habitats, seasons, and biogeographic areas [9, 10]. These results suggest that PAI may be estimated using case-specific temperature data and a species and stage-specific model for the relationship between PAI and temperature. Second, temperature is an elementary factor, and as such, it may be easily combined with other elementary factors (e.g., air humidity), if multifactor models for the relationship will be needed. Third, temperatures are the only case-specific data required by the method, and they may be gathered with well-defined and easily applicable protocols for measurement and retrospective correction [13–15].
In order to use the temperature approach to PAI estimation, the relationship between PAI and temperature should be close. It was found that several forensically important species of beetles meet this requirement [11]. However, no previous work tested the relationship in forensically important flies, insects which are most frequently used for the estimation of PMI [16, 17]. There are flies which colonize carcasses immediately after death, as for example Calliphoridae or Sarcophagidae, but there are also flies which colonize corpses later in the decomposition process, e.g., Muscidae, Fanniidae, or Piophilidae [8, 18–22]. A temperature method for the estimation of PAI would be particularly useful in the case of the latter group of flies. In order to calibrate the method for these flies, a much better insight into the relationship between PAI of carrion flies and temperature is necessary. Hence, in this article, the relationship is tested for several forensically important European species of flies.
Materials and methods
Experimental design
In order to collect PAI data from a broad range of temperatures, carcasses were separated in time and space. The study was divided into six placements: 18 April (four pigs), 15 June (six pigs), 4 July (six pigs), 21 July (four pigs), 16 August (four pigs), and 30 August (six pigs) of 2011. In each placement, half of carcasses were exposed in forest habitats (hornbeam–oak forest, alder forest, and birch forest) and half in open habitats (xerothermic grasslands, sparse clumps of young birches in grasslands, and edges of grasslands and pine–oak or birch forests). This design assumes that the effect of season or habitat on PAI reduces itself to the effect of temperature specific for that season or habitat.
The experiment was conducted in the Biedrusko Military Range (Western Poland, Europe). In order to decrease migrations of immature insects and joint attraction by carcasses, they were far away from each other by at least 50 m (the distance between particular pigs was, in most cases, much larger, e.g., “open” and “forest” carcasses by about 2–3 km).
Carcasses
In total, 30 domestic pig carcasses of similar mass (mean = 23 kg; range = 11.4–56.7 kg) were bought from a local pig farm. Pigs were killed at about 6 a.m. (a blow to the base of the skull) and, after 1 to 3 h, were exposed in the field. Carcasses were laid down on a metal grating and were protected with welded wire mesh.
Sampling of flies
Flies were sampled three times during the first day (after exposition, i.e., 1–3 h postmortem and then 4–6 and 10–11 h postmortem), two times a day for the following 5 days (between 10 a.m. and 1 p.m. and between 4 and 6 p.m.), and then once a day for the rest of the study (usually between 11 a.m. and 2 p.m.). The sampling was more frequent during the first days postmortem, as we wanted to increase the accuracy of PAI determinations for the early arriving flies. Two researchers performed the morning inspections, whereas the afternoon inspections were made by one person. The former lasted about 30 min and the latter about 15 min.
Samples included pitfall trap, aerial sweep net, manual and soil collections. Two traps (diameter = 16 cm, height = 17 cm, 50 % ethylene glycol) were buried dorsally and ventrally to the carcass. Adult flies were collected with a swatting technique, performed twice using large aerial sweep net (diameter = 55 cm). Manual sampling focused on the surface of a carcass and soil under and near the carcass. It lasted about 5 min. Trap, net, and manual collections were taken at every inspection. Samples were preserved in 70 % ethanol. Soil sampling started after termination of bloating and was made for the subsequent 2–3 weeks (every second day during the morning inspections). Samples (about 700 ml) were manually screened for larger insects and, afterwards, were put into the Tullgren funnels for 2 days.
Flies were determined using keys for identification [23–26] and collections of authors. In order to time oviposition, eggs were sampled for rearing purposes. Larvae were kept on pork in 20 °C until adults emerged. Rearing containers were filled with vermiculite (Calliphoridae) or sand (Piophilidae).
Temperature measurements
Because carcasses were exposed on the ground, ground-level temperature was measured. HOBO U23 Pro v2 2× External Temperature Data Loggers (Onset Computer Corporation, Bourne, MA, USA) were used with sensors positioned on the ground (one dorsally and one ventrally to the carcass). Sensors were not protected in any way (debris, plants, etc. were carefully removed). Logging started at 6 a.m. of the first day and 5-min logging intervals were used. Temperatures relevant for every PAI were averaged separately for each sensor and then a single mean was calculated.
Data analyses
The relationship between PAI and temperature was tested separately for the adult PAI and the oviposition PAI. The adult PAI is an interval from the moment of death until the arrival of first adult insect of a given species. The oviposition PAI is an interval from the moment of death until the appearance of the first eggs of a given species. The relationship between the oviposition PAI and temperature was analyzed for Calliphora vomitoria, Lucilia caesar, Lucilia sericata (Calliphoridae), and Stearibia nigriceps (Piophilidae). As for the other species, there were only singular observations which precluded any robust analyses.
The relationship was modeled from at least 14 observations (Table 1). Data sets usually covered a broad range of temperatures (Table 1). In order to check for colinearity, scatter plots were made. As expected, for most species, the relationship was exponential with a clear representation for the minimum PAI. Consequently, the exponential model with asymptote displaced from zero was fitted (, where c is “the minimum PAI,” e is “the base of the natural logarithm,” b 0 and b 1 are parameters responsible for the shape of the curve). Because the estimate of c regularly differed from the observed minimum PAI, models with c fixed a priori were also tested. Graphs are given for models with estimated c, equations for both types of models. Parameters were approximated with the Levenberg–Marquardt procedure.
Table 1.
Ranges in PAI and temperature for particular species
| Family | Species | Stage | N | PAI range [days] | Temperature range [°C] |
|---|---|---|---|---|---|
| Calliphoridae | Calliphora vomitoria (Linnaeus, 1758) | A | 25 | 0.2–9.4 | 13.0–23.3 |
| E | 19 | 0.2–26.3 | 13.3–21.6 | ||
| Lucilia caesar (Linnaeus, 1758) | A | 26 | 0.2–3.2 | 14.0–33.7 | |
| E | 26 | 0.2–3.4 | 13.5–33.7 | ||
| Lucilia sericata (Meigen, 1826) | A | 15 | 0.2–5.2 | 14.0–25.1 | |
| E | 14 | 0.4–12.2 | 15.4–25.1 | ||
| Phormia regina (Meigen, 1826) | A | 25 | 1.2–8.4 | 14.8–25.5 | |
| Muscidae | Hydrotaea dentipes (Fabricius, 1805) | A | 15 | 0.3–11.0 | 13.2–24.4 |
| Hydrotaea ignava (Harris, 1780) | A | 16 | 2.2–24.3 | 12.5–23.8 | |
| Hydrotaea pilipes Stein, 1903 | A | 21 | 2.3–12.3 | 13.8–20.6 | |
| Hydrotaea similis Meade, 1887 | A | 20 | 0.3–11.0 | 12.8–19.5 | |
| Piophilidae | Stearibia nigriceps (Meigen, 1826) | A | 23 | 3.4–23.3 | 12.3–23.5 |
| E | 22 | 8.3–37.3 | 13.3–22.4 |
A adult stage, E egg stage
For species which revealed no relationship, only scatter plots are given. For species with statistically insignificant relationship, scatter plots and models with estimated c are presented. For the other species, both types of models and lower temperature (LT) thresholds are given. LT thresholds were determined using the method of the least coefficient of variation in accumulation of degree-days [11]. For all analyses, the 5 % level of significance was accepted. Calculations were made using Statistica 9.1 (StatSoft, Inc., 2010).
Results
Adult and oviposition PAI of C. vomitoria and adult PAI of Hydrotaea pilipes showed no relation to temperature at all (Fig. 1). Adult PAI of Hydrotaea dentipes, Hydrotaea ignava, Hydrotaea similis, and Phormia regina were exponentially related to temperature with a clear representation of the minimum PAI, however, with no statistical significance (nonlinear regression, t test for b 1, P = 0.47, 0.11, 0.19, and 0.3 respectively; Fig. 2). Adult and oviposition PAI of S. nigriceps were exponentially and significantly associated to temperature with evident representation of the minimum PAI (nonlinear regression, t test for b 1, P < 0.001; Fig. 3). LT thresholds for S. nigriceps were 11.3 °C for the adult PAI and 11.4 °C for the oviposition PAI (Table 2).
Fig. 1.
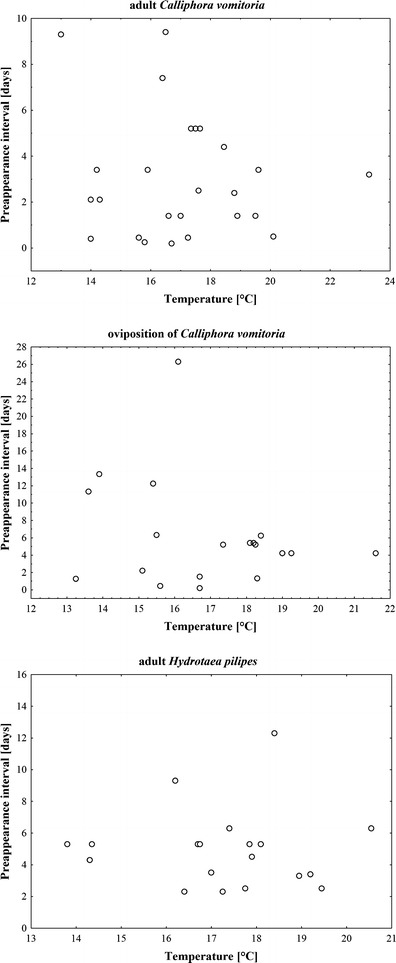
The PAI and ground-level temperatures averaged for the duration of PAI for C. vomitoria and H. pilipes
Fig. 2.
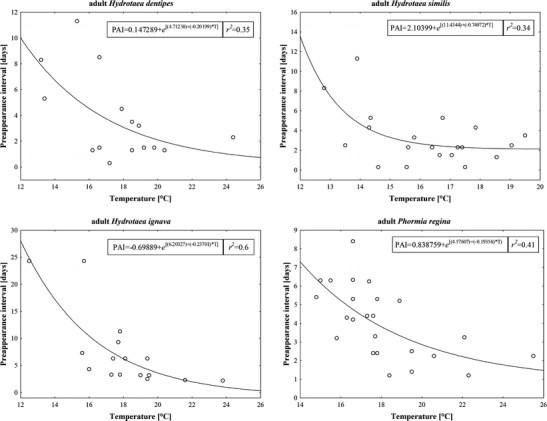
The relationship between the PAI of selected Muscidae and Calliphoridae and ground-level temperatures averaged for the duration of PAI (T)
Fig. 3.
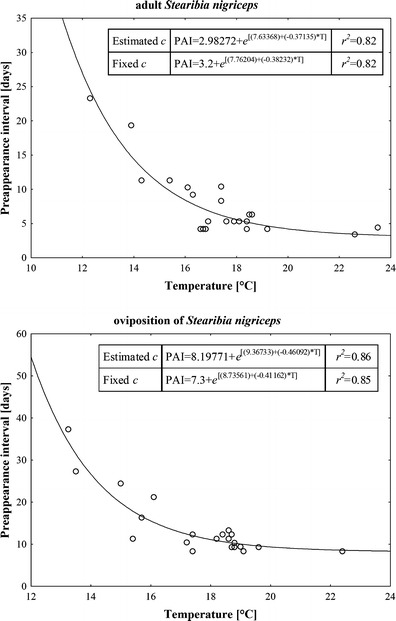
The relationship between the PAI of S. nigriceps and ground-level temperatures averaged for the duration of PAI (T). Graphs represent models with estimated c
Table 2.
LT thresholds and accumulations of degree-days (ADD) for the adult and oviposition PAI of S. nigriceps
| PAI | LT [°C] | ADD | Coefficient of variation in ADD [%] | |||
|---|---|---|---|---|---|---|
| Mean | N | Standard error | Range | |||
| Adult | 11.3 | 39.29 | 23 | 2.32 | 23.2–64.3 | 28.32 |
| Oviposition | 11.4 | 75.57 | 22 | 3.07 | 45.1–109.3 | 19.04 |
Adult and oviposition PAI of L. caesar and L. sericata responded similarly to temperature (Fig. 4). In a high temperature range, PAI was more or less constant and unrelated to temperature (Fig. 4). Under daily average temperatures above 17 °C, the oviposition of L. caesar was always recorded within the first 12 h postmortem, whereas in the case of L. sericata, it started, in each case, with temperatures above 18 °C within the first 35 h after death (Fig. 4). In a low temperature range, adult and oviposition PAI of L. caesar responded erratically to the temperature with some cases near the minimum PAI and some cases largely above the minimum PAI (Fig. 4). In the case of L. sericata, the response of PAI to low temperature was similar, although less erratic (Fig. 4). For both species and both kinds of PAI, the low temperature change in PAI may be represented by a near-vertical line (Fig. 4).
Fig. 4.
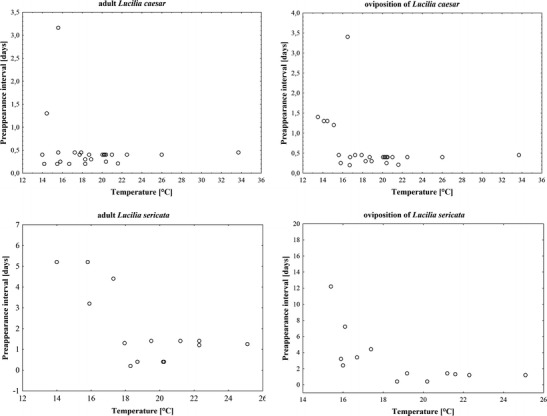
The PAI and ground-level temperatures averaged for the duration of PAI for L. caesar and L. sericata
Discussion
Previous results indicate that an exponential decrease in PAI with an increase in temperature is a general model for the relationship between PAI and temperature in carrion insects [11]. Current results partly support this proposition. For most species of flies, PAI decreased exponentially with an increase in average daily temperatures. A clear exception was only adult and oviposition PAI of L. caesar and L. sericata, in which case lack of response in a high temperature range was followed by an abrupt and large increase in PAI in a narrow range of low temperatures. In order to understand this unusual response, one should look at factors governing the attraction of insects to carcasses.
Carrion insects respond to volatile organic compounds emitted from a decomposing carcass [27–32]. For example L. sericata and L. caesar are attracted by oligosulfides, in particular dimethyl disulfide [27, 29], which is formed early in carrion decomposition from the sulfur-containing amino acids [33, 34]. These results indicate that the emission of relevant attractants is the most important precondition for the appearance of insects on a carcass. Consequently, it was suggested that processes in which attractants are generated depend on temperature, and as a result, PAI for most carrion insects is also temperature-dependent [9, 10]. Previous [9–11] and current models for S. nigriceps, P. regina, and species of Hydrotaea support this hypothesis. However, the unusual pattern of PAI response to temperature, as recorded for both species of Lucilia, indicates that the processes-oriented hypothesis may not be valid for all carrion insects.
Apart from the presence of VOCs, there are two classes of factors which may potentially affect PAI: factors related to the release of attractants and factors related to the activity of insects (for a broad discussion of factors affecting colonization of carcasses by flies, see [35]). The first group is composed of barriers of any kind for attractants. However, they are irrelevant for the current results; hence, they will not be discussed here. The second class are abiotic mediators of insect activity, e.g., air temperature or humidity [36, 37], repellents [38–40], or the time of the day [41–45] and biotic mediators of insect activity, e.g., group oviposition effects [46] or ovarian development status. From this point of view, air temperature seems to be particularly important for the current results. It was found that ambient air temperature is a positive predictor for visitation on non-odor sticky traps by adult Lucilia flies [47] and for blowfly oviposition on a beef liver bait [37]. These results directly demonstrate that forensically relevant aspects of blowfly activity are mediated by temperature. Species of Lucilia have high temperature thresholds for the activity, as was recently exemplified by Richards et al. [36] for South African L. sericata, which revealed the onset of coordinated muscle activity (i.e., standing) at a temperature of about 21 °C. It seems that European Lucilia flies also have high thresholds for the activity. As for oviposition, it was suggested that L. sericata normally needs carcass surface temperatures above 30 °C to start this behavior [48]. It was also demonstrated that the oviposition rate of L. sericata increases with average daily temperature, and temperature at which this rate is 0 is about 11 °C [49]. These results indicate that adult Lucilia flies have high temperature threshold for the activity (flying, locating a corpse, etc.), probably about 20 °C, and even higher threshold for oviposition. Temperatures below these thresholds will result in longer adult or oviposition PAI due to the lack of relevant activity of flies. Hence, we hypothesize that abrupt and erratic increase in PAI, as recorded in a low temperature range for L. sericata and L. caesar, resulted simply from ambient air temperatures being below the threshold for the relevant activity. In order to check whether this was true, the raw temperature data were inspected. Interestingly, a similar pattern of temperature change was present in a majority of “high PAI” carcasses, i.e., an interval of low temperatures (approximately equal in length to PAI) followed by a substantial temperature increase before the first sampling of a given species and stage. Summarizing, current results corroborate the view that the PAI response to temperature in those insects which visit carcasses early in the decomposition process is better explained by the activity-oriented hypothesis than the processes-oriented hypothesis. The latter hypothesis is superior in the case of middle and late arrivers.
The strength of the relationship was generally weaker than expected. Apart from S. nigriceps, all the other species revealed at most weak premises for the estimation of PAI from temperature. When one compares current models with those for beetles [11], it is evident that the temperature-based estimation of PAI is more promising for beetles than flies. Differences between these models resulted, to some extent, from the methods used in this study. The resolution of sampling must have resulted in higher inaccuracy of PAIs in the early arrivers (flies) than in the late arrivers (beetles). Moreover, techniques of sampling, as being less effective for flies than beetles, must have given further inaccuracies of the PAI for flies. However, these methodical factors may not explain all the differences. It is suggested that factors related to the biology of flies and beetles or the nature of processes in which relevant attractants are produced may be more important. Anyway, further studies are needed.
Summarizing, only S. nigriceps revealed solid premises for estimating adult or oviposition PAI from temperature alone. In the case of the other species, temperature alone is a poor predictor for PAI, which suggests that models useful for the estimation of PAI in most carrion flies will be more complex. Therefore, future works should focus on other abiotic or biotic factors which may affect PAI in forensically useful flies.
Acknowledgments
We would like to thank Captain R. Matera, Commander of the Biedrusko Military Range, and Z. Szeląg, Forest Inspector of the Łopuchówko Forest Inspectorate, for granting access to the Biedrusko Military Range. Thanks are also extended to M. Jarmusz (Poznań, Poland) for the assistance in the field and laboratory work and K. Szpila (Toruń, Poland) for the identification of adult and larval Piophilidae. The experiment was funded by the Ministry of Science and Higher Education (grants nos. IP2010025170 and IP2011034671).
References
- 1.Wells JD, LaMotte LR. Estimating the postmortem interval. In: Byrd JH, Castner JL, editors. Forensic entomology. The utility of arthropods in legal investigations. Boca Raton: CRC; 2010. pp. 367–388. [Google Scholar]
- 2.Amendt J, Campobasso CP, Gaudry E, Reiter C, LeBlanc H, Hall M. Best practice in forensic entomology. Int J Legal Med. 2007;121:90–104. doi: 10.1007/s00414-006-0086-x. [DOI] [PubMed] [Google Scholar]
- 3.Villet M, Amendt J (2009) Advances in entomological methods for death time estimation. In: Turk EE (ed) Forensic pathology reviews, vol. 6. Springer, Berlin, pp 213–237
- 4.Amendt J, Richards CS, Campobasso CP, Zehner R, Hall M. Forensic entomology: applications and limitations. Forensic Sci Med Pathol. 2011;7:379–392. doi: 10.1007/s12024-010-9209-2. [DOI] [PubMed] [Google Scholar]
- 5.Villet MH, Richards CS, Midgley JM. Contemporary precision, bias and accuracy of minimum post-mortem intervals estimated using development of carrion-feeding insects. In: Amendt J, Campobasso CP, Goff ML, Grassberger M, editors. Current concepts in forensic entomology. Dordrecht: Springer; 2010. pp. 109–138. [Google Scholar]
- 6.Tomberlin JK, Mohr R, Benbow ME, Tarone AM, VanLaerhoven S. A roadmap for bridging basic and applied research in forensic entomology. Ann Rev Entomol. 2011;56:401–421. doi: 10.1146/annurev-ento-051710-103143. [DOI] [PubMed] [Google Scholar]
- 7.Bourel B, Martin-Bouyer L, Hedouin V, Cailliez JC, Derout D, Gosset D. Necrophilous insect succession on rabbit carrion in sand dune habitats in Northern France. J Med Entomol. 1999;36:420–425. doi: 10.1093/jmedent/36.4.420. [DOI] [PubMed] [Google Scholar]
- 8.Matuszewski S, Bajerlein D, Konwerski S, Szpila K. Insect succession and carrion decomposition in selected forests of Central Europe. Part 3: succession of carrion fauna. Forensic Sci Int. 2011;207:150–163. doi: 10.1016/j.forsciint.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 9.Matuszewski S. Estimating the pre-appearance interval from temperature in Necrodes littoralis L. (Coleoptera: Silphidae) Forensic Sci Int. 2011;212:180–188. doi: 10.1016/j.forsciint.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Matuszewski S. Estimating the pre-appearance interval from temperature in Creophilus maxillosus L. (Coleoptera: Staphylinidae) J Forensic Sci. 2012;57:136–145. doi: 10.1111/j.1556-4029.2011.01958.x. [DOI] [PubMed] [Google Scholar]
- 11.Matuszewski S, Szafałowicz M. Temperature-dependent appearance of forensically useful beetles on carcasses. Forensic Sci Int. 2013;229:92–99. doi: 10.1016/j.forsciint.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Michaud JP, Moreau G. Predicting the visitation of carcasses by carrion-related insects under different rates of degree-day accumulation. Forensic Sci Int. 2009;185:78–83. doi: 10.1016/j.forsciint.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Archer MS. The effect of time after body discovery on the accuracy of retrospective weather station ambient temperature corrections in forensic entomology. J Forensic Sci. 2004;49:553–559. doi: 10.1520/JFS2003258. [DOI] [PubMed] [Google Scholar]
- 14.Scala JR, Wallace JR. Forensic meteorology: the application of weather and climate. In: Byrd JH, Castner JL, editors. Forensic entomology. The utility of arthropods in legal investigations. Boca Raton: CRC; 2010. pp. 519–538. [Google Scholar]
- 15.Johnson AP, Wallman JF, Archer MS. Experimental and casework validation of ambient temperature corrections in forensic entomology. J Forensic Sci. 2012;57:215–221. doi: 10.1111/j.1556-4029.2011.01900.x. [DOI] [PubMed] [Google Scholar]
- 16.Smith KG. A manual of forensic entomology. London: The Trustees of the British Museum; 1986. [Google Scholar]
- 17.Greenberg B, Kunich JC. Entomology and the law. Flies as forensic indicators. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- 18.Byrd JH, Castner JL. Insects of forensic importance. In: Byrd JH, Castner JL, editors. Forensic entomology. The utility of arthropods in legal investigations. Boca Raton: CRC; 2010. pp. 39–126. [Google Scholar]
- 19.Grassberger M, Frank C. Initial study of arthropod succession on pig carrion in a central European urban habitat. J Med Entomol. 2004;41:511–523. doi: 10.1603/0022-2585-41.3.511. [DOI] [PubMed] [Google Scholar]
- 20.Anton E, Niederegger S, Beutel RG. Beetles and flies collected on pig carrion in an experimental setting in Thuringia and their forensic implications. Med Vet Entomol. 2011;25:353–364. doi: 10.1111/j.1365-2915.2011.00975.x. [DOI] [PubMed] [Google Scholar]
- 21.Prado E, Castro C, Serrano A, da Silva Martins P, Garcia MD. Carrion flies of forensic interest: a study of seasonal community composition and succession in Lisbon, Portugal. Med Vet Entomol. 2012;26:417–431. doi: 10.1111/j.1365-2915.2012.01031.x. [DOI] [PubMed] [Google Scholar]
- 22.Benbow ME, Lewis AJ, Tomberlin JK, Pechal JL. Seasonal necrophagous insect community assembly during vertebrate carrion decomposition. J Med Entomol. 2013;50:440–450. doi: 10.1603/ME12194. [DOI] [PubMed] [Google Scholar]
- 23.Szpila K. Key for the identification of third instars of European blowflies (Diptera: Calliphoridae) of forensic importance. In: Amendt J, Campobasso CP, Goff ML, Grassberger M, editors. Current concepts in forensic entomology. Dordrecht: Springer; 2010. pp. 43–56. [Google Scholar]
- 24.Szpila K. Key for identification of European and Mediterranean blowflies (Diptera, Calliphoridae) of medical and veterinary importance—adult flies. In: Gennard D, editor. Forensic entomology. An introduction. Chichester: Willey-Blackwell; 2012. pp. 77–81. [Google Scholar]
- 25.Gregor F, Rozkošný R, Barták M, Vanhara J. The Muscidae (Diptera) of Central Europe. Brno: Folia Biologia; 2002. [Google Scholar]
- 26.McAlpine JF. A revised classification of the Piophilidae including ‘Neotophilidae’ and ‘Tyreophoridae’ (Diptera: Schizophora) Mem Entomol Soc Can. 1977;103:1–66. doi: 10.4039/entm109103fv. [DOI] [Google Scholar]
- 27.Stensmyr MC, Urru I, Collu I, Celander M, Hansson BS, Angioy AM. Rotting smell of dead-horse arum florets. Nature. 2002;420:625–626. doi: 10.1038/420625a. [DOI] [PubMed] [Google Scholar]
- 28.Kalinová B, Podskalská H, Růžička J, Hoskovec M. Irresistible bouquet of death—how are burying beetles (Coleoptera: Silphidae: Nicrophorus) attracted by carcasses. Naturwissenschaften. 2009;96:889–899. doi: 10.1007/s00114-009-0545-6. [DOI] [PubMed] [Google Scholar]
- 29.Frederickx C, Dekeirsschieter J, Verheggen FJ, Haubruge E. Responses of Lucilia sericata Meigen (Diptera: Calliphoridae) to cadaveric volatile organic compounds. J Forensic Sci. 2012;57:386–390. doi: 10.1111/j.1556-4029.2011.02010.x. [DOI] [PubMed] [Google Scholar]
- 30.Dekeirsschieter J, Frederickx C, Lognay G, Brostaux Y, Verheggen FJ, Haubruge E (2013) Electrophysiological and behavioral responses of Thanatophilus sinuatus Fabricius (Coleoptera: Silphidae) to selected cadaveric volatile organic compounds. J Forensic Sci 58:917–923 [DOI] [PubMed]
- 31.vonHoermann C, Ruther J, Reibe S, Madea B, Ayasse M. The importance of carcass volatiles as attractants for the hide beetle Dermestes maculatus (De Geer) Forensic Sci Int. 2011;212:173–179. doi: 10.1016/j.forsciint.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 32.vonHoermann C, Steiger S, Müller JK, Ayasse M. Too fresh is unattractive! The attraction of newly emerged Nicrophorus vespilloides females to odour bouquets of large cadavers at various stages of decomposition. PLoS ONE. 2013;8:e58524. doi: 10.1371/journal.pone.0058524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dekeirsschieter J, Verheggen FJ, Gohy M, Hubrecht F, Bourguignon L, Lognay G, Haubruge E. Cadaveric volatile organic compounds released by decaying pig carcasses (Sus domesticus L.) in different biotopes. Forensic Sci Int. 2009;189:46–53. doi: 10.1016/j.forsciint.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 34.Paczkowski S, Schütz S. Post-mortem volatiles of vertebrate tissue. Appl Microbiol Biotechnol. 2011;91:917–935. doi: 10.1007/s00253-011-3417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campobasso CP, Di Vella G, Introna F. Factors affecting decomposition and Diptera colonization. Forensic Sci Int. 2001;120:18–27. doi: 10.1016/S0379-0738(01)00411-X. [DOI] [PubMed] [Google Scholar]
- 36.Richards CS, Price BW, Villet MH. Thermal ecophysiology of seven carrion-feeding blowflies in Southern Africa. Entomol Exp Appl. 2009;131:11–19. doi: 10.1111/j.1570-7458.2009.00824.x. [DOI] [Google Scholar]
- 37.George KA, Archer MS, Toop T. Abiotic environmental factors influencing blowfly colonization patterns in the field. Forensic Sci Int. 2013;229:100–107. doi: 10.1016/j.forsciint.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 38.Marchenko MI. Medicolegal relevance of cadaver entomofauna for the determination of the time of death. Forensic Sci Int. 2001;120:89–109. doi: 10.1016/S0379-0738(01)00416-9. [DOI] [PubMed] [Google Scholar]
- 39.Charabidze D, Bourel B, Hedouin V, Gosset D. Repellent effect of some household products on fly attraction to cadavers. Forensic Sci Int. 2009;189:28–33. doi: 10.1016/j.forsciint.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Shelomi M, Matern LM, Dinstell JM, Harris DW, Kimsey RB. DEET (N,N-diethyl-meta-toluamid) induced delay of blowfly landing and oviposition rates on treated pig carrion (Sus scrofa L.) J Forensic Sci. 2012;57:1507–1511. doi: 10.1111/j.1556-4029.2012.02159.x. [DOI] [PubMed] [Google Scholar]
- 41.Amendt J, Zehner R, Reckel F. The nocturnal oviposition behavior of blowflies (Diptera: Calliphoridae) in Central Europe and its forensic implications. Forensic Sci Int. 2007;175:61–64. doi: 10.1016/j.forsciint.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Baldridge R, Wallace S, Kirkpatrick R. Investigation of nocturnal oviposition by necrophilous flies in Central Texas. J Forensic Sci. 2006;51:125–126. doi: 10.1111/j.1556-4029.2005.00022.x. [DOI] [PubMed] [Google Scholar]
- 43.Woodridge J, Scrace L, Wall R. Flight activity of the blowflies, Calliphora vomitoria and Lucilia sericata, in the dark. Forensic Sci Int. 2007;172:94–97. doi: 10.1016/j.forsciint.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Zurawski KN, Benbow ME, Miller JR, Merritt RW. Examination of nocturnal blow fly (Diptera: Calliphoridae) oviposition on pig carcasses in mid-Michigan. J Med Entomol. 2009;46:671–679. doi: 10.1603/033.046.0335. [DOI] [PubMed] [Google Scholar]
- 45.Berg MC, Benbow ME. Environmental factors associated with Phormia regina (Diptera: Calliphoridae) oviposition. J Med Entomol. 2013;50:451–457. doi: 10.1603/ME12188. [DOI] [PubMed] [Google Scholar]
- 46.Barton Browne L, Bartell RJ, Shorey HH. Pheromone-mediated behaviour leading to group oviposition in the blowfly Lucilia cuprina. J Insect Physiol. 1969;15:1003–1014. doi: 10.1016/0022-1910(69)90140-1. [DOI] [Google Scholar]
- 47.Cruickschank I, Wall R. Aggregation and habitat use in Lucilia blowflies (Diptera: Calliphoridae) in pasture. Bull Entomol Res. 2002;92:153–158. doi: 10.1079/BER2001149. [DOI] [PubMed] [Google Scholar]
- 48.Cragg JB. The olfactory behaviour of Lucilia species (Diptera) under natural conditions. Ann Appl Biol. 1956;44:467–477. doi: 10.1111/j.1744-7348.1956.tb02141.x. [DOI] [Google Scholar]
- 49.Pitts KM, Wall R. Adult mortality and oviposition rates in field and captive populations of the blowfly Lucilia sericata. Ecol Entomol. 2004;29:727–734. doi: 10.1111/j.0307-6946.2004.00653.x. [DOI] [Google Scholar]


