Abstract
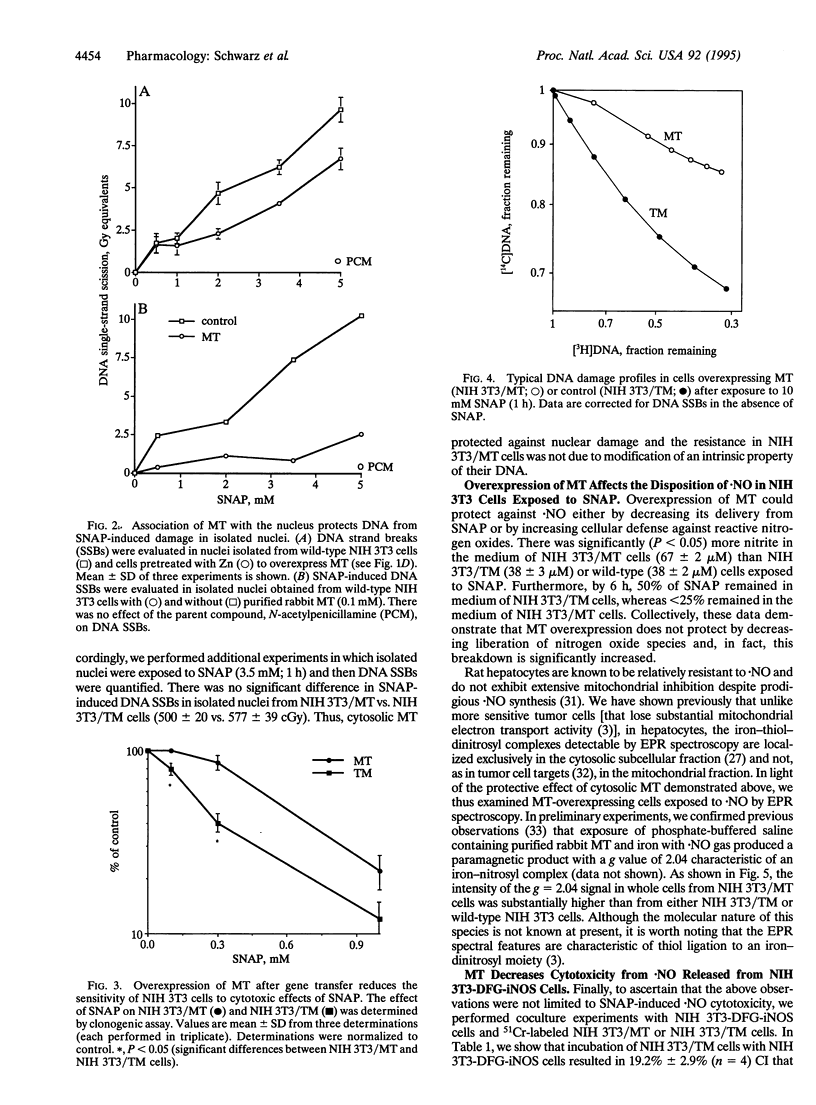
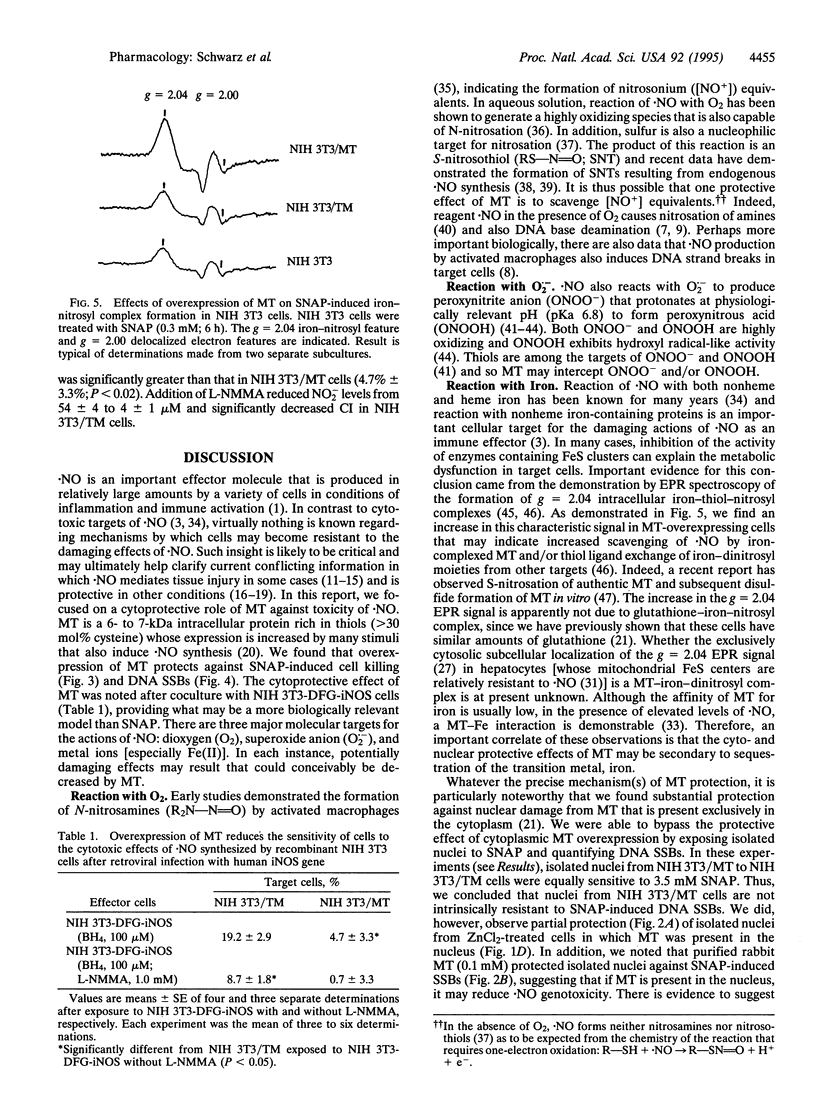
In inflammatory states, nitric oxide (.NO) may be synthesized from precursor L-arginine via inducible .NO synthase (iNOS) in large amounts for prolonged periods of time. When .NO acts as an effector molecule under these conditions, it may be toxic to cells by inhibition of iron-containing enzymes or initiation of DNA single-strand breaks. In contrast to molecular targets of .NO, considerably less is known regarding mechanisms by which cells become resistant to .NO. Metallothionein (MT), the major protein thiol induced in cells exposed to cytokines and bacterial products, is capable of forming iron-dinitrosyl thiolates in vitro. Therefore, we tested the hypothesis that overexpression of MT reduces the sensitivity of NIH 3T3 cells to the .NO donor, S-nitrosoacetylpenicillamine (SNAP), and to .NO released from cells (NIH 3T3-DFG-iNOS) after infection with a retroviral vector expressing human iNOS gene. There was a 4-fold increase in MT in cells transfected with the mouse MT-1 gene (NIH 3T3/MT) compared to cells transfected with the promoter-free inverted gene (NIH 3T3/TM). NIH 3T3/MT cells were more resistant than NIH 3T3/TM cells to the cytotoxic effects of SNAP (0.1-1.0 mM) or .NO released from NIH 3T3-DFG-iNOS cells. A brief (1 h) exposure to 10 mM SNAP caused DNA single-strand breaks that were 9-fold greater in NIH 3T3/TM compared to NIH 3T3/MT cells. Electron paramagnetic resonance spectroscopy of NIH 3T3 cells revealed a greater peak at g = 2.04 (e.g., iron-dinitrosyl complex) in NIH 3T3/MT than NIH 3T3/TM cells. These data are consistent with a role for cytoplasmic MT in interacting with .NO and reducing .NO-induced cyto- and nuclear toxicity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S., Crow J. P. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem Soc Trans. 1993 May;21(2):330–334. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- Bremner I., Beattie J. H. Metallothionein and the trace minerals. Annu Rev Nutr. 1990;10:63–83. doi: 10.1146/annurev.nu.10.070190.000431. [DOI] [PubMed] [Google Scholar]
- Cazevieille C., Muller A., Meynier F., Bonne C. Superoxide and nitric oxide cooperation in hypoxia/reoxygenation-induced neuron injury. Free Radic Biol Med. 1993 Apr;14(4):389–395. doi: 10.1016/0891-5849(93)90088-c. [DOI] [PubMed] [Google Scholar]
- Chubatsu L. S., Meneghini R. Metallothionein protects DNA from oxidative damage. Biochem J. 1993 Apr 1;291(Pt 1):193–198. doi: 10.1042/bj2910193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R. C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier J. C., Pellat C., Henry Y. Generation of EPR-detectable nitrosyl-iron complexes in tumor target cells cocultured with activated macrophages. J Biol Chem. 1991 Jun 5;266(16):10162–10167. [PubMed] [Google Scholar]
- Eaton D. L., Toal B. F. Evaluation of the Cd/hemoglobin affinity assay for the rapid determination of metallothionein in biological tissues. Toxicol Appl Pharmacol. 1982 Oct;66(1):134–142. doi: 10.1016/0041-008x(82)90068-0. [DOI] [PubMed] [Google Scholar]
- Fehsel K., Jalowy A., Qi S., Burkart V., Hartmann B., Kolb H. Islet cell DNA is a target of inflammatory attack by nitric oxide. Diabetes. 1993 Mar;42(3):496–500. doi: 10.2337/diab.42.3.496. [DOI] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989 May;83(5):1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbrecht B. G., Billiar T. R., Stadler J., Demetris A. J., Ochoa J., Curran R. D., Simmons R. L. Inhibition of nitric oxide synthesis during endotoxemia promotes intrahepatic thrombosis and an oxygen radical-mediated hepatic injury. J Leukoc Biol. 1992 Oct;52(4):390–394. doi: 10.1002/jlb.52.4.390. [DOI] [PubMed] [Google Scholar]
- Henry Y., Lepoivre M., Drapier J. C., Ducrocq C., Boucher J. L., Guissani A. EPR characterization of molecular targets for NO in mammalian cells and organelles. FASEB J. 1993 Sep;7(12):1124–1134. doi: 10.1096/fasebj.7.12.8397130. [DOI] [PubMed] [Google Scholar]
- Ioannidis I., de Groot H. Cytotoxicity of nitric oxide in Fu5 rat hepatoma cells: evidence for co-operative action with hydrogen peroxide. Biochem J. 1993 Dec 1;296(Pt 2):341–345. doi: 10.1042/bj2960341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X. B., Hollocher T. C. Mechanism for nitrosation of 2,3-diaminonaphthalene by Escherichia coli: enzymatic production of NO followed by O2-dependent chemical nitrosation. Appl Environ Microbiol. 1988 Jul;54(7):1791–1794. doi: 10.1128/aem.54.7.1791-1794.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G., 3rd, Tsao P. S., Lefer A. M. Cardioprotective effects of authentic nitric oxide in myocardial ischemia with reperfusion. Crit Care Med. 1991 Feb;19(2):244–252. doi: 10.1097/00003246-199102000-00021. [DOI] [PubMed] [Google Scholar]
- Kennedy M. C., Gan T., Antholine W. E., Petering D. H. Metallothionein reacts with Fe2+ and NO to form products with A g = 2.039 ESR signal. Biochem Biophys Res Commun. 1993 Oct 29;196(2):632–635. doi: 10.1006/bbrc.1993.2296. [DOI] [PubMed] [Google Scholar]
- Kohn K. W., Erickson L. C., Ewig R. A., Friedman C. A. Fractionation of DNA from mammalian cells by alkaline elution. Biochemistry. 1976 Oct 19;15(21):4629–4637. doi: 10.1021/bi00666a013. [DOI] [PubMed] [Google Scholar]
- Koppenol W. H., Moreno J. J., Pryor W. A., Ischiropoulos H., Beckman J. S. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol. 1992 Nov-Dec;5(6):834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- Kröncke K. D., Fehsel K., Schmidt T., Zenke F. T., Dasting I., Wesener J. R., Bettermann H., Breunig K. D., Kolb-Bachofen V. Nitric oxide destroys zinc-sulfur clusters inducing zinc release from metallothionein and inhibition of the zinc finger-type yeast transcription activator LAC9. Biochem Biophys Res Commun. 1994 Apr 29;200(2):1105–1110. doi: 10.1006/bbrc.1994.1564. [DOI] [PubMed] [Google Scholar]
- Kubes P. Ischemia-reperfusion in feline small intestine: a role for nitric oxide. Am J Physiol. 1993 Jan;264(1 Pt 1):G143–G149. doi: 10.1152/ajpgi.1993.264.1.G143. [DOI] [PubMed] [Google Scholar]
- Lancaster J. R., Jr Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheis G., Sherman M. P., Buckberg G. D., Haybron D. M., Young H. H., Ignarro L. J. Role of L-arginine-nitric oxide pathway in myocardial reoxygenation injury. Am J Physiol. 1992 Feb;262(2 Pt 2):H616–H620. doi: 10.1152/ajpheart.1992.262.2.H616. [DOI] [PubMed] [Google Scholar]
- Miwa M., Stuehr D. J., Marletta M. A., Wishnok J. S., Tannenbaum S. R. Nitrosation of amines by stimulated macrophages. Carcinogenesis. 1987 Jul;8(7):955–958. doi: 10.1093/carcin/8.7.955. [DOI] [PubMed] [Google Scholar]
- Morton K. A., Jones B. J., Sohn M. H., Schaefer A. E., Phelps R. C., Datz F. L., Lynch R. E. Uptake of cadmium is diminished in transfected mouse NIH/3T3 cells enriched for metallothionein. J Biol Chem. 1992 Feb 15;267(5):2880–2883. [PubMed] [Google Scholar]
- Mulligan M. S., Hevel J. M., Marletta M. A., Ward P. A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6338–6342. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaki T., Nakayama M., Kato R. Inhibition by nitric oxide and nitric oxide-producing vasodilators of DNA synthesis in vascular smooth muscle cells. Eur J Pharmacol. 1990 Dec 15;189(6):347–353. doi: 10.1016/0922-4106(90)90031-r. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nguyen T., Brunson D., Crespi C. L., Penman B. W., Wishnok J. S., Tannenbaum S. R. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3030–3034. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussler A. K., Billiar T. R. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993 Aug;54(2):171–178. [PubMed] [Google Scholar]
- Pellat C., Henry Y., Drapier J. C. IFN-gamma-activated macrophages: detection by electron paramagnetic resonance of complexes between L-arginine-derived nitric oxide and non-heme iron proteins. Biochem Biophys Res Commun. 1990 Jan 15;166(1):119–125. doi: 10.1016/0006-291x(90)91919-j. [DOI] [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991 Mar 5;266(7):4244–4250. [PubMed] [Google Scholar]
- Sato M., Bremner I. Oxygen free radicals and metallothionein. Free Radic Biol Med. 1993 Mar;14(3):325–337. doi: 10.1016/0891-5849(93)90029-t. [DOI] [PubMed] [Google Scholar]
- Schwarz M. A., Lazo J. S., Yalowich J. C., Reynolds I., Kagan V. E., Tyurin V., Kim Y. M., Watkins S. C., Pitt B. R. Cytoplasmic metallothionein overexpression protects NIH 3T3 cells from tert-butyl hydroperoxide toxicity. J Biol Chem. 1994 May 27;269(21):15238–15243. [PubMed] [Google Scholar]
- Stadler J., Bergonia H. A., Di Silvio M., Sweetland M. A., Billiar T. R., Simmons R. L., Lancaster J. R., Jr Nonheme iron-nitrosyl complex formation in rat hepatocytes: detection by electron paramagnetic resonance spectroscopy. Arch Biochem Biophys. 1993 Apr;302(1):4–11. doi: 10.1006/abbi.1993.1173. [DOI] [PubMed] [Google Scholar]
- Stadler J., Curran R. D., Ochoa J. B., Harbrecht B. G., Hoffman R. A., Simmons R. L., Billiar T. R. Effect of endogenous nitric oxide on mitochondrial respiration of rat hepatocytes in vitro and in vivo. Arch Surg. 1991 Feb;126(2):186–191. doi: 10.1001/archsurg.1991.01410260074010. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Jaraki O., Osborne J., Simon D. I., Keaney J., Vita J., Singel D., Valeri C. R., Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Tamai K. T., Gralla E. B., Ellerby L. M., Valentine J. S., Thiele D. J. Yeast and mammalian metallothioneins functionally substitute for yeast copper-zinc superoxide dismutase. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8013–8017. doi: 10.1073/pnas.90.17.8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink D. A., Darbyshire J. F., Nims R. W., Saavedra J. E., Ford P. C. Reactions of the bioregulatory agent nitric oxide in oxygenated aqueous media: determination of the kinetics for oxidation and nitrosation by intermediates generated in the NO/O2 reaction. Chem Res Toxicol. 1993 Jan-Feb;6(1):23–27. doi: 10.1021/tx00031a003. [DOI] [PubMed] [Google Scholar]
- Wink D. A., Hanbauer I., Krishna M. C., DeGraff W., Gamson J., Mitchell J. B. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9813–9817. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink D. A., Kasprzak K. S., Maragos C. M., Elespuru R. K., Misra M., Dunams T. M., Cebula T. A., Koch W. H., Andrews A. W., Allen J. S. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science. 1991 Nov 15;254(5034):1001–1003. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- Zhang J., Dawson V. L., Dawson T. M., Snyder S. H. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994 Feb 4;263(5147):687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]




