Abstract
The Ndt80 protein of the yeast Saccharomyces cerevisiae is the founding member of a new sub-family of proteins in the Ig-fold superfamily of transcription factors. The crystal structure of Ndt80 bound to DNA shows that it makes contacts through several loops on one side of the protein that connect β-strands which form the β-sandwich fold common to proteins in this superfamily. However, the DNA-binding domain of Ndt80 is considerably larger than many other members of the Ig-fold superfamily and it appears to make a larger number of contacts with the DNA than these proteins. To determine the contribution of each of these contacts and to examine if the mechanism of Ndt80 DNA binding was similar to other members of the Ig-fold superfamily, amino acid substitutions were introduced at each residue that contacts the DNA and assayed for their effect on Ndt80 activity. Many of the mutations caused significant decreases in DNA-binding affinity and transcriptional activation. Several of these are in residues that are not found in other sub-families of Ig-fold proteins. These additional contacts are likely responsible for Ndt80’s ability to bind DNA as a monomer while most other members require additional domains or cofactors to recognize their sites.
INTRODUCTION
Transcription is regulated primarily through proteins that recognize specific DNA sequences in promoters to activate or repress RNA polymerase. These site-specific transcription factors are often modular in their design, containing domains that are involved in binding DNA and domains that interact with cofactor proteins to regulate transcription. The DNA-binding domains of different transcription factors often share considerable sequence and structural homology and can be grouped into families based on their mechanism of binding DNA (1). The sequence similarity among proteins within a family, along with the determination of what residues are important for their DNA-binding activity, can then be used to identify additional members of the family and assign a potential function to these genes.
Although many families of the DNA-binding proteins bind DNA through stable α-helical or β-sheet structures, one growing class of proteins, referred to as the Ig-fold superfamily, uses a series of flexible loops to contact the DNA (2). Proteins within this superfamily have been identified based on a common β-sandwich core, similar in structure to the s-type immunoglobulin fold, and use a combination of loops between the β-strands on one face of the protein to make contacts with the DNA. The sequence and structure of these loops are quite diverse among members of this superfamily, making it difficult to identify transcription factors containing an Ig-fold based on sequence data alone (2). However, the structure determinations of a number of members within this superfamily, such as p53, Rel homology domain, STAT and the Runt domain, have allowed the proteins to be further classified into sub-families based upon the mechanism they use to contact the DNA (3–7). Grouping Ig-fold proteins into different sub-families has been helpful in identifying additional proteins in this superfamily. Mutations in Ig-fold transcription factors have been associated with leukemia and have important roles in hematopoiesis and development of bone tissue in mammals (8,9). The identification of additional proteins in this superfamily may therefore uncover the regulatory mechanisms of other important cellular and developmental processes and diseases.
The Ndt80 protein from the yeast Saccharomyces cerevisiae appears to be a founding member of a new subfamily within the Ig-fold class of transcription factors and is most similar in its core structure to the Rel domain of NFκB (10,11). Expression of the Ndt80 protein is induced during the early stages of meiosis and as the levels of Ndt80 increase, it activates the expression of the genes at the middle stages of the meiosis and sporulation pathway (12,13). Ndt80 binds with varying affinities to sites called middle sporulation elements (MSEs) in the promoters of middle sporulation genes (14). Variations in the binding affinity by Ndt80 to different MSEs is thought to allow for differential timing and level of expression of genes at specific points in the pathway.
Ndt80 contains 627 amino acids and deletion analysis of the protein reveals two domains, an N-terminal DNA-binding domain and a C-terminal activation domain (10,11,15). Proteins with sequence similarity to the DNA-binding domain of Ndt80 have been found in other fungi, Caenorhabditis elegans, Drosophila and humans, suggesting that this DNA-binding motif is evolutionarily conserved (11). The crystal structure of the DNA-binding domain of Ndt80 has been solved, in both a DNA bound complex and in an unbound form (10,11). Like other members of the Ig-fold superfamily, the core of the DNA-binding domain is comprised of an elaborate β-sandwich with two antiparallel sheets. However, the DNA-binding domain of Ndt80 is significantly larger than most other members of this superfamily and contains additional structural features. The structure of the Ndt80–DNA complex showed that residues in several loops positioned along one surface of the protein make contacts in successive grooves of the DNA in a manner similar to other Ig-fold proteins (10) as shown in Figure 1B. Mutations in some of the residues in these loops affect Ndt80 DNA-binding affinity in vitro and transcriptional activation during sporulation in vivo (11). However, there are also several additional regions that contact DNA, which are not found in other members of the Ig-fold superfamily. To investigate the role of these additional contacts and the mechanism of DNA binding by Ndt80 we have examined the contribution to binding and transcriptional activation of each residue in Ndt80 that contacts DNA in the structure of the complex (10). Our results support the model for DNA binding derived from the crystal structures and show that although there are similarities, there are also significant differences in the mechanism of Ndt80 binding DNA compared to other members of the Ig-fold superfamily. The determination of residues that have an important role in Ndt80 may help in the identification of other members in this recently identified subfamily of Ig-fold transcription factors.
Figure 1.
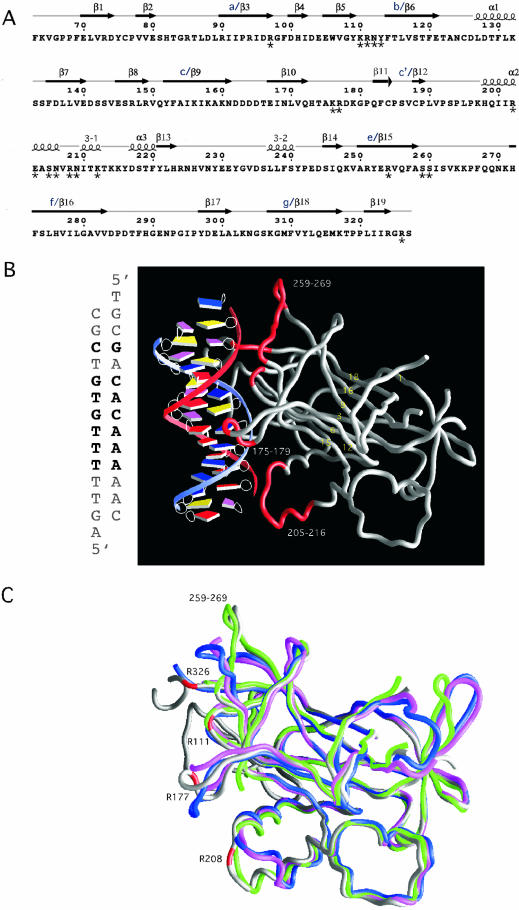
(A) The sequence of the DNA-binding domain of Ndt80 (1M6U) (11) with the indicated secondary structures that were derived from ESPript (26). Secondary structural elements are labeled as previously reported. For the sake of comparison, alternate names (a–g) for structural elements in the β-sandwich core have also been included (10). Residues interacting with the DNA that have been mutated are indicated with an asterisk below the amino acid. Additional residues that we have mutated (see Table 1) that are not included in this schematic include K50, K54, I55, P57 and R58. (B) The crystal structure of the DNA-binding domain of Ndt80 bound to MSE DNA. The sequence of the MSE DNA that is bound to Ndt80 in the crystal structure is shown on the left. The protein molecule is shown as a worm rendering and the DNA as a spline with boxes representing individual bases (25). The base pairs that are in common with the SMK1-MSE DNA used in our assays are in bold letters. The β-strands that are in the β-sandwich core are labeled in yellow. Residues in loop regions that interact directly with the MSE DNA are highlighted in red. (C) A comparison of the Ndt80 peptide backbone structures shown as worm renderings in the absence (PDB: 1M6U, blue; 1M7U, pink; 1MN4 green) and presence of DNA (PDB: 1MNN, gray). Representative residues that contact the DNA in the crystal structure of Ndt80 in complex with DNA are highlighted in red. Regions of the protein that differ significantly in the structures compared include residues 175–180, 205–216, 259–269 and the C-terminus of the protein including residue 326.
MATERIALS AND METHODS
Plasmids and strains
Site-directed mutagenesis of NDT80 was performed using the Stratagene QuikChange mutagenesis kit. Mutations were made in both an Escherichia coli expression vector, pIF68 (Ndt801–409–HisX6), as well as a yeast expression vector pMP331 (Ndt801–627) for the in vitro and in vivo studies, respectively (11). All clones were verified by sequence analysis.
To construct integrated, epitope-tagged versions of the ndt80 mutants expressed from a GAL1-10 promoter, site-directed amino acid substitutions were made in plasmid pMP268, a derivative of pSC193 (pGAL1-10-NDT80-HA, HIS3) (13). The mutant plasmids were linearized by NheI digestion within HIS3 and transformed into strain JXY25, a derivative of W303 with ndt80 and sum1 null mutations (MATα ade2-1 trp1-1 his3-11, 15 can1-100 ura3-1 leu2-3,112 ndt80::KanMX sum1::KanMX). Transformants were selected by plating on SD-His, and the integrations were confirmed by PCR using primers that hybridize within NDT80. To induce NDT80 expression, cells were grown in SD medium lacking Ura (SD-Ura) to mid log phase, diluted 1:100 into SRaf-Ura (2% raffinose) for 5 h, washed and resuspended in SGal-Ura (2% galactose) for 4 h. Transcriptional activation by Ndt80 was assayed by measuring β-galactosidase expression from pJX43, which contains the lacZ gene under the control of the SMK1 MSE (16).
Sporulation assays
To assay the ability of the site-directed Ndt80 mutants to complement an ndt80/ndt80 mutant for defects in sporulation, plasmid pMP331, containing wild-type NDT80 or its mutant derivatives, were transformed into the SK1-derived strain MPY44 (a/α ura3-SK1/ura3-SK1 leu2::hisG/leu2::hisG lys2/lys2 ho::LYS2/ho::LYS2 trp1-hisG/trp1-hisG ndt80::Kan/ndt80::Kan) (11). Transformants were grown to mid-log phase in YEPD [2% (w/v) peptone, 1% (w/v) yeast extract, 2% (w/v) dextrose], transferred to YEPA [2% (w/v) peptone, 1% (w/v) yeast extract, 2% KOAc] for 12 h, and then transferred to sporulation media (2% KOAc + 10 µg adenine/ml, 5 µg histidine/ml, 30 µg leucine/ml, 7.5 µg lysine/ml, 10 µg tryptophan/ml, 5 µg uracil/ml) for 24 h. After 24 h in sporulation media, cells were fixed in 95% ethanol at room temperature for 10 min, washed and resuspended in water containing 4,6′-diamidino-2-phenylindole (DAPI) at 0.5 µg/ml. The percent of cells entering meiosis (MI and MII) was determined by examining the cells under a fluorescence microscope and counting the number of cells with multiple DAPI stained foci, indicating cells that have progressed through at least one of the meiotic divisions. The formation of spore walls was monitored by the presence of dityrosine with UV light as previously described (17).
DNA-binding assays
The binding affinity of the Ndt80 mutants was measured in vitro by electrophoretic mobility shift assays (EMSAs) using purified fragments (residues 1–409) of the mutants with a 26 bp oligonucleotide probe encoding wild-type and mutant SMK1 MSEs (14). Wild-type and mutant Ndt801–409 proteins were purified from E.coli strain BL21+ (Invitrogen), by affinity chromatography using the Novagen His-bind- purification kit as described previously (11).
Western blot analysis
Cells expressing wild-type and mutant forms of NDT80 were induced by growth in SGal-Ura media for 4 h and lysed by glass bead breakage. Equal quantities of protein from these extracts were separated on a 10% SDS–PAGE gel, transferred to nitrocellulose membrane, probed using antibodies specific to the HA epitope, and detected using ECL western blot detection (Amersham Pharmacia Biotech).
Computational analysis
The structures of Ndt80 with PDB accession codes 1M6U (A molecule), 1M7U (A molecule), 1MN4 and 1MNN have been superimposed by using the least-squares algorithm implemented in O (18) for either all Cα atoms or those in the β-sandwich core as detailed in the Results. Both 1M6U and 1M7U structures include two molecules in the asymmetric unit, the unique repeating unit of the crystal, only one of which (that designated as the A molecule) has been used for this comparative analysis. Hydrogen-bonding interactions have been defined for the Ndt80–DNA complex structure (coordinate file 1MNN.pdb) by using CONTACT (CCP4) and differ in detail from those originally reported for some residues (10). Criteria used for defining hydrogen bonds include appropriate donor–acceptor pairs, angle of bonding, as well as distance (2.4–3.4 Å).
RESULTS
Structural changes of Ndt80 upon DNA binding
It is often informative to compare the structure of a DNA-binding protein in the presence and absence of DNA to determine which regions of the protein adopt a different structure upon binding DNA and may thereby contribute to the ability of the protein to recognize a specific DNA sequence. Of the many examples of transcription factor–DNA complexes for which structures have been reported, the protein is most often found to adopt a conformation favorable for interaction with a specific DNA sequence in a B-like conformation (1). This is in contrast to the few examples in which interaction with the protein results in a significant change in the structure of the DNA such as bending, for example. For Ndt80, there are crystal structures available of the DNA-binding domain of the protein alone as well as in a complex with an MSE-containing oligonucleotide, thus allowing us to analyze conformational differences that may occur upon DNA binding. In this case, the structure of the DNA is overall B-form and thus not significantly distorted upon interaction with the protein (10). However, two conserved YpG steps in the MSE site allow Ndt80 to take advantage of their inherent flexibility in specific recognition of the MSE sequence (19).
We have compared the crystal structures of the Ndt80 DNA-binding domain bound to MSE DNA with the three reported structures for the unbound Ndt80 (10,11). The structural model of Ndt80 in complex with DNA (PDB accession code: 1MNN) includes residues 33–335, with residues missing for the 140–145 and 287–293 loop regions. The models of the unbound Ndt80 include the structures with PDB accession codes 1M6U, 1M7U and 1MN4. All of the unbound structures lack the N-terminal 30 residues found in the Ndt80 molecule in the complex with the DNA. The 1M6U structure includes the only complete model of the protein for residues 63–327. The 1M7U structure includes residues 65–325, with residues missing for loop regions 176–178, 264–269 and 289–291. The 1MN4 structure includes 63–325, with residues missing for loop regions 175–184 and 287–293. The superpositioning of 252 Cα atoms that are common in 1MNN (Ndt80–DNA complex) and 1M6U structures gives an r.m.s.d. of 2.67 Å. Superpositioning of their respective β-sandwich cores including 79 Cα atoms of residues 69–75, 85–97, 113–121, 153–161, 187–191, 250–259, 271–283 and 306–318 gives an r.m.s.d. of 0.62 Å. Similarly, r.m.s.d. of 0.75 and 0.34 Å were obtained upon superpositioning of the same core residues in 1M7U and 1MN4 with 1MNN. These results indicate that overall the structures are invariant in the core of the protein. However, there are significant differences in other regions.
Based on the superpositioning of the invariant core domains, a comparison of the four structures reveals structural differences in some of the regions that interact with DNA. For example, Cα positions on and around residues R177, R208, S259 and R326 are different in the Ndt80–DNA and unbound Ndt80 structures (Fig. 1C). The β10β11 loop [cc′ loop defined as an insertion of the Ig-fold (10)] containing residues 175–180 adopts a conformation that allows for H-bonding of R177 to the guanine base (G10, see Fig. 2) in the major groove of the DNA. In the structures without DNA bound, this same loop points in the opposite direction in 1M6U and is disordered in 1M7U and 1MN4 structures. Main chain interactions of R208 and N209 (in the α2α3 or c′e loop) to the phosphate backbone and a water-mediated interaction of the N209 side chain to the sugar backbone appear to stabilize a conformation for residues 205–216 in the Ndt80–DNA structure. However, this same conformation for residues 205–216 is found in the unbound Ndt80 1MN4 structure but not in the 1M6U and 1M7U structures. These residues are part of the floret region of the structure (residues 192–248). The loop containing residues 259–269 adopts a slightly different conformation in the unbound 1MN4 structure than in the Ndt80–DNA complex, but is rotated almost 90° relative to its conformation in the 1M6U and 1M7U structures. The conformation of this loop in the Ndt80–DNA complex is nearly parallel to the DNA trajectory and appears to be stabilized by a side chain interaction of S259 (in β15 or the ef loop) to the phosphate backbone of the DNA. Near the C-terminus of the DNA-binding domain, the polypeptide around R326 adopts different conformations in the 1MNN and 1M6U structures pointing in nearly opposite directions. This region is disordered in the 1MN4 and 1M7U structures. Thus, of the regions that differ most significantly in the four structures, only the β10β11 loop (cc′ loop) including residue R177 and the C-terminal region including R326 appear to have structural differences that correlate with the bound versus unbound state of Ndt80. It is also interesting that the R177 loop and C-terminal R326 region are disordered in two of the three unbound structures.
Figure 2.
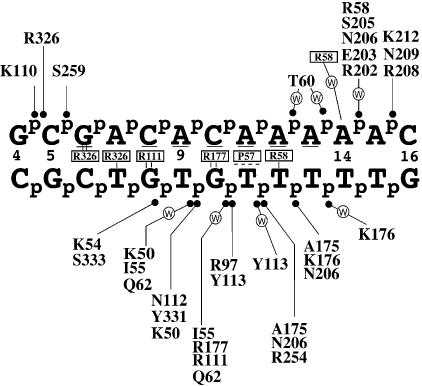
A schematic of the contacts by Ndt80 to the DNA that were derived from the Ndt80–DNA structure (10). Residues in boxes indicate base-specific contacts with the number of lines indicating the number of H-bonds with the base. The dashes for P57 indicate van der Waals interactions. Residues that make contacts with the DNA phosphate backbone are indicated with a line and circle. A ‘W’ indicates a water-mediated hydrogen bond between the protein and DNA. Bases that are underlined are identical between the MSEs used in the structural analysis and this study.
Although many of the DNA-binding residues are in different positions, other key DNA-binding residues do not show significant structural differences in the unbound versus bound structures. Most of these residues are found in structural elements that form part of the core of the protein. For example, R97 is in a β-turn between the β3 and β4 strands (ab loop) that are part of the β-sandwich core of the protein, and it is nearly in an identical position in both the 1M6U and 1MNN structures (10,11). Likewise, residues R111, N112 and Y113, which form a β-hairpin between β5 and β6 (ab loop), and R254, which is part of a β15 (e β-strand) in the core of the protein, do not show significant differences in the different structures indicating that these loops are ordered in the absence of DNA. As noted previously, it is not possible to compare the N-terminal regions of Ndt80 as these residues are not present in any of the unbound structures. Thus, the comparative structural analysis suggests a complex set of interactions between Ndt80 and the MSE with regions of the protein that adopt different conformations in bound and unbound states and regions that do not change structure upon complex formation. The complexity of the DNA-interacting surface of the protein may be important for its function in regulating expression of middle meiosis genes.
Effects of alanine substitutions on DNA binding
As previously reported, there are numerous amino acid main chain, side chain and water-mediated interactions to the DNA in the structure of the Ndt80–DNA complex (Fig. 2) (10). Hydrogen-bonding interactions of protein, DNA and water atoms in the Ndt80–DNA complex structure have been analyzed using CONTACT (CCP4) (see Materials and Methods). The side chain atoms of residues R58, R111, R177 and R326 are involved in direct base-specific hydrogen-bonding interactions with the MSE. Side chain atoms of residues K54, R97, K110, R111, N112, K176, R177, N206, K212, R254, S259, R326, Y331 and S333 and the main chain atoms of residues K50, Y113, R208, N209 and R326 interact with the phosphate backbone of the DNA. The R58 side chain also interacts with the sugar backbone. Some of the residues previously noted also make water-mediated interactions to the DNA. In addition, the side chains of Q62, S205, Y331, and the main chains of I55, T60, A175, R202 and E203, are involved in water-mediated interactions with the DNA.
To test the contributions of all of the interactions by Ndt80 with the DNA, alanine substitutions were introduced for each residue shown in Table 1 that potentially contacts the DNA in the Ndt80–DNA structure and the purified mutant proteins were assayed for binding affinity to the SMK1 MSE by EMSAs. An example of the mutant binding assays is shown in Figure 3, and a summary of all the mutants is in Table 1. As expected, mutations at several of the positions that make base-specific contacts, such as R58, R111 and R177 caused significant decreases in binding affinity. The R111 and R177 residues not only make bidentate direct H-bonds to the Gs at positions 8 and 10, respectively, but these residues also indirectly recognize the pyrimidine bases 5′ to these Gs (19). Interestingly, alanine substitution of P57, which makes a number of significant van der Waals interactions with the MSE, results in decreased DNA binding. A role in recognizing 5′-YpG-3′ steps has been proposed for P57 (19). It is also possible that P57 plays an important structural role by limiting the conformational flexibility of this N-terminal loop thereby enhancing its ability to interact with the MSE. Alanine substitution of S59, for which the closest atom to the DNA in the reported complex structure (PDB accession code: 1MNN) is 4.5 Å away, also results in decreased DNA binding. The S59 side chain OH forms a hydrogen bond with the main chain N of residue 61 stabilizing a turn in the polypeptide and may therefore play an important structural role rather than a role in directly interacting with the MSE DNA. Thus, substitution of either P57 or S59 residues may affect the structure in this region of the protein resulting in loss of DNA binding. In contrast, alanine substitution of R326, the only other residue that makes direct base-specific contacts in the Ndt80–DNA structure, had little effect on the binding affinity in vitro. Although R326 makes hydrogen bonds to G6 and T7, the residue is not coplanar with the G as are contacts by R111 and R177. The R326 contacts with the DNA may therefore not be optimal and may make a smaller contribution to the overall DNA-binding affinity.
Table 1. Ndt80 mutations and their effect in vitro and in vivo.
| Mutant | MSE DNA contacta | DNA-binding affinityb (%) | Meiosisc (%) | Dityrosined | Activitye (%) |
|---|---|---|---|---|---|
| WT | 100 | 70 | + | 100 | |
| K50A | T9 bb | 30 | 41 | +/– | 88 |
| K54A | G8 bb | 50 | 24 | +/– | 100 |
| I55A | T9,G10 bb | 100 | 54 | +/– | ND |
| P57A | T11 vdw | 35 | <1 | – | 28 |
| R58A | T12 A14 bs /A15 bb | 35 | <1 | – | 25 |
| S59A | LR | 14 | <1 | – | 80 |
| T60A | A13 A14 bb | 100 | 20 | + | ND |
| R97A | G10 bb | 33 | <1 | – | 20 |
| K110A | C5 bb | 100 | 10 | – | ND |
| R111A | G8 bs / G10 bb | 5 | <1 | – | 20 |
| N112A | T9 bb | 100 | <1 | – | ND |
| Y113F | T11 G10 bb | 20 | <1 | – | ND |
| K176A | T12 T13 bb | 50 | 27 | + | ND |
| R177A | G10 bs / G10 bb | 4 | <1 | – | 15 |
| R202A | A15 bb | 100 | 5 | – | 128 |
| E203A | A15 bb | 100 | 52 | + | ND |
| S205A | A15 bb | 100 | 42 | + | ND |
| N206A | T11 T12 A15 bb | 100 | 5 | – | 70 |
| R208A | C16 bb | 50 | <1 | – | 27 |
| N209A | C16 bb | 100 | 47 | + | ND |
| K212A | C16 bb | 100 | 7 | – | 89 |
| R254A | T11 bb | 7 | <1 | – | ND |
| S259A | G6 bb | 100 | 44 | + | 79 |
| S260A | LR | 100 | 43 | + | ND |
| R326A | G6 T7 bs/C5 bb | 50 | <1 | – | 30 |
| Y331A | T9 bb | 100 | 38 | + | 172 |
aContacts with the MSE are given with the base number corresponding to the schematic in Figure 2. The symbols bs, bb, and vdw represent base-specific, backbone contacts and van der Waals contacts, respectively. The interactions designated by bs or bb are direct hydrogen bonds or water-mediated hydrogen bonds (refer to Fig. 2).
bDNA-binding affinity of the mutants in comparison to wild-type protein.
cPercentage of cells containing two or more DAPI-staining foci after 24 h in sporulation media.
dCells were monitored for the presence of dityrosine fluorescence after 24 h in sporulation media. ‘+’ wild-type levels of fluorescence, ‘+/–’ low but detectable levels of fluorescence, ‘–’ no fluorescence.
eTranscriptional activation of a MSE-regulated promoter in comparison to the wild-type protein. ND indicates that the specified mutant was not tested in this assay.
Figure 3.
DNA binding of Ndt80 mutants. Select mutants are shown binding to a wild-type SMK1 MSE. (A) WT (lanes 2–7), T60A (lanes 8–13), R97A (lanes 14–19), N112A (lanes 20–26). Lanes 2, 8, 14, 20 and 26 contain the same amount of purified protein and 5-fold serial dilutions of each mutant protein were used. (B) WT (lanes 1–5), K50A (lanes 6–10), K54A (lanes 11–15), NP57A (lanes 16–20), R58A (lanes 21–25) and S59A (lanes 26–30). Lanes 1, 6, 11, 16, 21 and 26 contain the same amount of purified protein and 3-fold serial dilutions of each mutant protein were used.
Protein residues that make contacts with the phosphate backbone of the DNA often make significant contributions to the DNA-binding specificity and affinity of the protein (20,21). We therefore expected mutation of residues that make contacts with the phosphate backbone of DNA to have an effect on the DNA-binding affinity of the protein. Surprisingly, among the mutants examined only R97A, Y113F and R254A, which are all involved in backbone contacts with T11, had significant decreases in DNA-binding affinity. It has been proposed that this base forms a 5′-YpG-3′ dinucletotide step with G10 that plays an important role in recognition by the protein (19). This step causes a change in the base stacking and a localized shift in the backbone conformation. The backbone contacts by these residues in Ndt80, which are located in invariant regions of the structure, may help stabilize this conformation.
In contrast to mutations at R97, Y113 and R254, alanine substitutions at other residues involved in phosphate-backbone contacts, such as K54, K110, N112, K176, N206, R208, N209, K212 and S259, had little or no effect on binding. Furthermore, substitution of most of the residues that make water-mediated contacts with the DNA, I55, T60, R202, E203, S205 and Y331, had little or no effect on the affinity of the complex. Only alanine substitution of K50, in which the peptide backbone makes a water-mediated contact with the DNA, showed a decrease in binding affinity. It is possible that this substitution had an effect on the proper folding of the protein causing a loss of the peptide backbone contact with the DNA. Alternatively, this side chain could be involved in an electrostatic interaction with the DNA that contributes to the DNA-binding affinity.
Although many of the mutants tested above did not have an apparent effect on DNA binding, it seemed unlikely that they did not play some role in Ndt80 function. We therefore constructed substitutions at multiple residues that make DNA contacts and examined the effect of these mutants on DNA binding. Residues K110 and S259 make phosphate backbone contacts on one side of the MSE (10). We therefore anticipated that the K110A S259A double mutant might show a decrease in binding affinity. However, the double mutant has wild-type binding affinity in the EMSA (data not shown). Likewise, the combination of the S205A and N206A mutants, which together make phosphate backbone contacts on the other side of the MSE, had no effect on DNA-binding affinity. Therefore, these residues only weakly contribute to the binding affinity.
Effects of alanine substitutions on Ndt80 function in vivo
We next tested the ability of each mutant to complement an ndt80/ndt80 null mutant to enter into meiosis by measuring the number of cells that had multiple nuclear DAPI-stained foci (Table 1). We also measured the ability of the mutants to induce processes later in the sporulation pathway, such as spore formation, by monitoring the presence of dityrosine, a component of the spore wall. In general, mutants that caused greater than a 2-fold decrease in binding affinity showed a complete failure to enter the meiotic divisions and form spore walls. Interestingly, many of the mutants that showed only modest defects in binding failed to undergo the meiotic divisions and synthesize dityrosine. These findings indicate that sporulation is either very sensitive to relatively small decreases in Ndt80 DNA-binding affinity or that these mutations are affecting another component of Ndt80- dependent transcriptional activation.
Possible explanations for the observed differences in the in vitro and in vivo phenotypes of some of the mutants include improper expression of NDT80 or stability (i.e. susceptibility to proteolysis) of the protein. Furthermore, the NDT80 promoter contains two MSEs and it has been shown that Ndt80 binds to these sites and activates its own expression (22). Mutations that cause even a slight decrease in binding affinity may not activate NDT80 expression to wild-type levels, causing lower levels of the protein to be expressed. In order to examine the autoregulation and stability of the mutant proteins, we ectopically expressed the mutants in mitotic cells from a heterologous promoter and performed western blot analysis to monitor the level of the protein in the cell. All of the mutants were present at roughly wild-type levels, indicating that the alanine substitutions did not significantly affect the stability of the protein (data not shown).
Many of the mutants showed decreased levels of meiosis and formation of the spore wall. These processes require the activation of many middle sporulation genes, each with MSEs that vary in affinity for the wild-type protein (14). Sporulation may therefore be blocked by mutations in Ndt80 that cause it to fail to activate genes with MSEs with weak binding affinity for the wild-type protein.
To quantify if the mutants had a large or small effect on Ndt80 binding in vivo, we measured the ability of the mutants to activate expression of a lacZ reporter that was under the transcriptional control of the SMK1 MSE (Table 1). In general, there was a very good correlation between the level of activation of the MSE-regulated reporter promoter and the ability to sporulate. Many of the mutants that had significant decreases in DNA-binding affinity in vitro caused large decreases in the ability to activate the reporter.
The effects of the alanine substitutions on DNA-binding affinity and sporulation are summarized in Figure 4. The residues are divided into three categories. Mutants with decreased DNA-binding affinity compared to the wild-type protein and <1% sporulation include K54A, P57A, R58A, S59A, R97A, R111A, Y113A, R177A, R254A and R326A (red). As noted above, although the DNA-binding affinity of the R326A mutant is reduced only 2-fold compared to the wild-type protein, it does not sporulate. Mutants with near wild-type DNA-binding affinity but decreased sporulation (15–50% of wild-type values) include N112A, T60A, K110A, K176A, R202A, N206A, R208A and K212A (blue). And, finally mutants with near wild-type sporulation and DNA-binding affinity include K50A, I55A, E203A, S205A, N209A, S259A, S260A and Y331A (green). The surface renderings are shown in the absence (Fig. 4A) and presence (Fig. 4B) of the MSE DNA with functionally important residues highlighted in red and blue. Residues highlighted in green based on our analysis do not play an important role in interactions between Ndt80 and the MSE.
Figure 4.
Differential effects of alanine substitutions for surface residues of Ndt80 that interact with the MSE DNA. (A) A molecular surface rendering of the DNA-binding domain of Ndt80 is shown (27). The coordinate file with PDB accession code 1MNN was used to generate the molecular surface. The effects of the alanine substitutions on DNA-binding affinity and sporulation are summarized. Residues shown in red include K54, P57, R58, S59, R97, R111, Y113, R177, R254 and R326. Alanine substitution of these residues results in mutants with decreased DNA-binding affinity that fail to sporulate. Residues shown in blue include N112, T60, K110, K176, R202, N206, R208 and K212. Alanine mutants for the blue residues have near wild-type DNA- binding affinity but sporulate poorly. And, finally residues shown in green include K50, I55, E203, S205, N209, S259, S260 and Y331. Alanine mutants at these positions retain wild-type DNA binding affinity and sporulate. (B) The same molecular surface rendering as in (A) is shown with the MSE DNA.
Relaxed binding specificity by Ndt80 mutants
The crystal structure of the Ndt80–DNA complex has provided detailed information regarding specific interactions of amino acid residues in the protein with specific bases within the Ndt80 MSE, one of several MSE promoter elements recognized by Ndt80 (10). To further characterize the sequence specificity of these interactions, we assayed the ability of the Ala substitution mutants to bind to different mutant MSEs. R111 makes direct base-specific contacts with a G in position 8 of the MSE. Although the R111A mutant showed a significant reduction in binding to the wild-type MSE, it was able to bind with approximately the same affinity to a MSE with a mutation at position 8 as it did to the wild-type site (Fig. 5B). This result suggests that the R111A mutant is unable to discriminate among different bases at position 8 and that R111 therefore likely makes a base-specific contact at this position.
Figure 5.
Relaxed DNA-binding specificity of Ndt80 mutants. EMSAs performed using WT (lanes 1–4), G6C (lanes 5–8), C8G (lanes 9–12) and C10G (lanes 13–16) MSEs in combination with mutant Ndt80 constructs. Serial 3-fold titrations of Ndt80 are shown binding to the wild-type and mutant MSEs. The starting concentrations of the WT (A), R111 (B) and R326 (D) were the same in lanes 1, 5, 9 and 13. Due to the poor DNA-binding affinity of the protein, the starting concentration for R177A (C) was 7.5-fold higher.
R177 makes direct base-specific contacts to the G at position 10 of the MSE (10). An alanine substitution at this residue caused the most severe effect on DNA binding of all the mutants tested. The R177A mutation also showed a significant loss in binding to the wild-type, G6C and C8G MSEs (Fig. 5C). Interestingly, the C10G mutation in the MSE did not cause a further reduction in the binding affinity of the R177A mutant. In fact, the R177A mutant shows better binding to the C10G MSE than the wild-type protein. This indicates that the alanine substitution for R177 relaxes the base-pair specificity at this position in the MSE, supporting the model that this residue contacts this position.
Finally, R326 makes a specific contact to base G6 of the MSE. R326A bound with similar affinity to a wild-type MSE and a MSE containing a G6C substitution. In contrast, the C8G and C10G mutations caused a further decrease in binding by this mutant protein (Fig. 5D). These results suggest that the R326A mutant is unable to discriminate among sites with different bases at position G6 and supports the model derived from the Ndt80–DNA structure in which residue R326 is contacting this position.
DISCUSSION
The yeast Ndt80 protein appears to be the founding member of a sub-family of proteins in the Ig-fold class of transcription factors (10). Although the DNA-binding domain of Ndt80 shares a similar β-sandwich core with other members of the Ig-fold superfamily, it is significantly larger than many of these proteins and makes a number of additional contacts with the DNA (10,11). Interestingly, the structure of the Ndt80–DNA complex predicts that relatively few of these contacts are base-specific and a large number of the remaining contacts are water-mediated bonds to the phosphate backbone. As expected, mutations that had the strongest effect on the binding affinity, such as R111A and R177A, are at positions that make multiple direct and indirect base-specific H-bonds with the DNA (19). The bases contacted by these residues are strongly conserved among Ndt80-regulated MSEs, and mutations at these positions in the site have a significant effect on DNA-binding activity in vitro and transcriptional activation in vivo (10,14). Interestingly, a substitution of alanine for R326, which also makes a base-specific contact, had little effect on DNA-binding affinity. Although the R326A mutation did relax the base-pair specificity at this position in the MSE, this position was not as strongly conserved among natural MSEs as other positions. Mutational analysis of this base pair also indicated that there are more relaxed sequence requirements at this position for Ndt80 binding and activation than at other base-specific contacts in the site (14). However, the base-pair specificity at this position in the MSE is critical for binding by Sum1, a transcription factor that represses a subset of Ndt80-activated middle sporulation genes during vegetative growth and early meiosis (14,23). Genome-wide analysis has shown that while some MSEs only function as Ndt80 activator binding sites, and others only as Sum1 repressor binding sites, there is also a third class that function as both Ndt80 and Sum1 binding sites (14). The different classes of MSEs may be important in fine tuning expression of genes during the middle stages of meiosis. The relaxed specificity of the bases contacted by R326 may allow Ndt80 to bind to sites that function as both Sum1 target sites and those that are not bound by Sum1.
Although many of the individual alanine mutations do not affect DNA binding in vitro, some of these residues are required for Ndt80 function in vivo. It is possible that mutations at these residues relax the DNA-binding specificity and allow the protein to bind to non-specific sites with a greater frequency. This would effectively titrate Ndt80 away from sites in the promoters of genes that it is required to activate. We have shown that several of the alanine substitutions relax the base-pair preferences at specific positions in the sites. Ndt80 binds with varying affinities to different MSEs found in the promoters of middle sporulation genes (14). The differential binding affinity may have an important biological role because some sites would be bound earlier in the sporulation pathway than others, allowing for the differential timing of expression of these genes as Ndt80 levels increase. A loss in DNA-binding specificity by the protein may therefore affect the timing at which specific target genes are expressed in the pathway, which in turn could affect events that are required at specific stages for progression through sporulation.
Members of the Ig-fold superfamily of transcription factors bind DNA through a series of loops between β-strands on one surface of the protein. Although the structure and exact contacts differ in each Ig-fold subfamily, most appear to use the loops between the A and B strands, E and F strands, and a C-terminal tail of the Ig-fold to contact the DNA (2). Based on the topology of the β-sandwich structure, these regions would roughly correspond to residues 110–113, 259–272 and 319–333 in Ndt80 (10). Each of these regions contact DNA in the crystal structure supporting the model that Ndt80 is a member of the Ig-fold superfamily. However, the contribution made by these regions in Ndt80 appears to be different than other Ig-fold proteins. For example, mutational analysis of DNA-binding residues in the Runt domain showed that all 10 DNA contacts make important contributions to the binding affinity (24). In contrast, our results show that while residues in Ndt80 corresponding to the A-B loop (110–113) make important contributions to binding, mutations in the other two regions that are common with Ig-fold proteins do not have a large effect on DNA-binding affinity. Perhaps most striking, while naturally occurring and site-directed mutations at residue R174 in the C-terminal tail of the Runt domain cause almost a 1000-fold decrease in binding affinity, the alanine substitution of R326, at the same relative position in the fold of Ndt80, has only a minor effect. These findings show that while Ndt80 may share a core β-sandwich structure with other members of the Ig-fold superfamily and use many of the same loops to contact the DNA, the contribution of those contacts are significantly different.
Another difference between Ndt80 and other Ig-fold proteins, is that while most members of the Ig-fold superfamily appear to bind DNA either as homodimers or cooperatively in complex with other cofactors (2), structural and mutational analysis of Ndt80 and its binding site suggest the DNA-binding domain of Ndt80 binds DNA as a monomer. Ndt80 is likely able to bind DNA as a monomer through the additional contacts made by the N-terminal region (residues 50–60) and the loops in residues 175–178 and 202–212, that are not present in many other Ig-fold transcription factors. In support of this model we have shown that many of these residues contribute to the binding affinity of the protein and are required for transcriptional activation in vivo. These data support the idea that Ndt80 is distinct from other previously identified proteins in the Ig-fold transcription factor superfamily. Interestingly, the 175–178 loop and R326 C-terminal regions are in significantly different conformations in the bound and unbound form of the protein. It is possible that this flexibility allows for a more complex interface with the DNA than is observed for other Ig-fold transcription factors.
Ndt80 has homologs in other fungal species, as well as higher organisms, suggesting that it is evolutionarily conserved and part of a family of DNA-binding proteins (11). The role of the genes in higher organisms such as flies, worms and humans is unknown, but it is possible that they are involved in developmental processes. The human homolog, C11orf9, has been shown to be expressed in several tumor cell lines (25). Although it was speculated that this may be a membrane bound protein, it shares significant sequence similarity with the DNA-binding domain of Ndt80 and is conserved at many of the residues that we have shown are important for DNA binding (11). Although we predict that C11orf9 binds DNA, we have been unable to detect DNA-binding activity in vitro of a fragment of the protein (residues 78–251) that contains homology to the Ndt80 DNA-binding domain (data not shown). Furthermore, a chimeric protein consisting of the DNA-binding domain of C11orf9 in the context of yeast Ndt80 was unable to complement an ndt80/ndt80 strain for defects in sporulation. However, the human protein is lacking regions similar to the N-terminus (amino acids 50–60) and the floret (amino acids 201–252) regions of Ndt80, which we have shown are important for DNA-binding as a monomer in vitro and activation in vivo. Although the lack of these regions in the human protein may prevent it from binding with strong affinity on its own, it is possible that it works with other cofactors to bind DNA as a complex with high affinity. It is also possible that the amino acid differences between Ndt80 and C11orf9 in residues that contact the DNA have altered the sequence specificity such that the S.cerevisiae MSE is not the optimal binding site for C11orf9.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Xie Jianxin Xie for strain JXY25 and Michael Pierce for plasmid pMP268. This work has been supported by NIH grants to M.M.G and A.K.V.
REFERENCES
- 1.Garvie C.W. and Wolberger,C. (2001) Recognition of specific DNA sequences. Mol. Cell, 8, 937–946. [DOI] [PubMed] [Google Scholar]
- 2.Rudolph M.J. and Gergen,J.P. (2001) DNA-binding by Ig-fold proteins. Nature Struct. Biol., 8, 384–386. [DOI] [PubMed] [Google Scholar]
- 3.Becker S., Groner,B. and Muller,C.W. (1998) Three-dimensional structure of the Stat3beta homodimer bound to DNA. Nature, 394, 145–151. [DOI] [PubMed] [Google Scholar]
- 4.Bravo J., Li,Z., Speck,N.A. and Warren,A.J. (2001) The leukemia-associated AML1 (Runx1)–CBF beta complex functions as a DNA-induced molecular clamp. Nature Struct. Biol., 8, 371–378. [DOI] [PubMed] [Google Scholar]
- 5.Cho Y., Gorina,S., Jeffrey,P.D. and Pavletich,N.P. (1994) Crystal structure of a p53 tumor suppressor–DNA complex: understanding tumorigenic mutations. Science, 265, 346–355. [DOI] [PubMed] [Google Scholar]
- 6.Chen X., Vinkemeier,U., Zhao,Y., Jeruzalmi,D., Darnell,J.E.,Jr and Kuriyan,J. (1998) Crystal structure of a tyrosine phosphorylated STAT-1 dimer bound to DNA. Cell, 93, 827–839. [DOI] [PubMed] [Google Scholar]
- 7.Tahirov T.H., Inoue-Bungo,T., Morii,H., Fujikawa,A., Sasaki,M., Kimura,K., Shiina,M., Sato,K., Kumasaka,T., Yamamoto,M. et al. (2001) Structural analyses of DNA recognition by the AML1/Runx-1 Runt domain and its allosteric control by CBFbeta. Cell, 104, 755–767. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q., Stacy,T., Binder,M., Marin-Padilla,M., Sharpe,A.H. and Speck,N.A. (1996) Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc. Natl Acad. Sci. USA, 93, 3444–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubnitz J.E. and Look,A.T. (1998) Molecular basis of leukemogenesis. Curr. Opin. Hematol., 5, 264–270. [DOI] [PubMed] [Google Scholar]
- 10.Lamoureux J.S., Stuart,D., Tsang,R., Wu,C. and Glover,J.N. (2002) Structure of the sporulation-specific transcription factor Ndt80 bound to DNA. EMBO J., 21, 5721–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montano S.P., Cote,M.L., Fingerman,I., Pierce,M., Vershon,A.K. and Georgiadis,M.M. (2002) Crystal structure of the DNA-binding domain from Ndt80, a transcriptional activator required for meiosis in yeast. Proc. Natl Acad. Sci. USA, 99, 14041–14046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu S. and Herskowitz,I. (1998) Gametogenesis in yeast is regulated by a transcriptional cascade dependent on Ndt80. Mol. Cell, 1, 685–696. [DOI] [PubMed] [Google Scholar]
- 13.Chu S., DeRisi,J., Eisen,M., Mulholland,J., Botstein,D., Brown,P.O. and Herskowitz,I. (1998) The transcriptional program of sporulation in budding yeast. Science, 282, 699–705. [DOI] [PubMed] [Google Scholar]
- 14.Pierce M., Benjamin,K.R., Montano,S.P., Georgiadis,M.M., Winter,E. and Vershon,A.K. (2003) Sum1 and Ndt80 proteins compete for binding to middle sporulation element sequences that control meiotic gene expression. Mol. Cell. Biol., 23, 4814–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sopko R., Raithatha,S. and Stuart,D. (2002) Phosphorylation and maximal activity of Saccharomyces cerevisiae meiosis-specific transcription factor Ndt80 is dependent on Ime2. Mol. Cell. Biol., 22, 7024–7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce M., Wagner,M., Xie,J., Gailus-Durner,V., Six,J., Vershon,A.K. and Winter,E. (1998) Transcriptional regulation of the SMK1 mitogen-activated protein kinase gene during meiotic development in Saccharomyces cerevisiae. Mol. Cell. Biol., 18, 5970–5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L., Ajimura,M., Padmore,R., Klein,C. and Kleckner,N. (1995) NDT80, a meiosis-specific gene required for exit from pachytene in Saccharomyces cerevisiae. Mol. Cell. Biol., 15, 6572–6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones T.A., Zou,J.Y., Cowan,S.W. and Kjeldgaard,M. (1991) Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crysallogr., A47, 110–119. [DOI] [PubMed] [Google Scholar]
- 19.Lamoureux J.S., Maynes,J.T. and Glover,J.N. (2004) Recognition of 5′-YpG-3′ sequences by coupled stacking/hydrogen bonding interactions with amino acid residues. J. Mol. Biol., 335, 399–408. [DOI] [PubMed] [Google Scholar]
- 20.Pabo C.O. and Sauer,R.T. (1992) Transcription factors: structural families and principles of DNA recognition. Annu. Rev. Biochem., 61, 1053–1095. [DOI] [PubMed] [Google Scholar]
- 21.Luscombe N.M. and Thornton,J.M. (2002) Protein–DNA interactions: amino acid conservation and the effects of mutations on binding specificity. J. Mol. Biol., 320, 991–1009. [DOI] [PubMed] [Google Scholar]
- 22.Pak J. and Segall,J. (2002) Regulation of the premiddle and middle phases of expression of the NDT80 gene during sporulation of Saccharomyces cerevisiae. Mol. Cell. Biol., 22, 6417–6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie J., Pierce,M., Gailus-Durner,V., Wagner,M., Winter,E. and Vershon,A.K. (1999) Sum1 and Hst1 repress middle sporulation-specific gene expression during mitosis in Saccharomyces cerevisiae. EMBO J., 18, 6448–6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z., Yan,J., Matheny,C.J., Corpora,T., Bravo,J., Warren,A.J., Bushweller,J.H. and Speck,N.A. (2003) Energetic contribution of residues in the Runx1 Runt domain to DNA binding. J. Biol. Chem., 278, 33088–33096. [DOI] [PubMed] [Google Scholar]
- 25.Stohr H., Marquardt,A., White,K. and Weber,B.H. (2000) cDNA cloning and genomic structure of a novel gene (C11orf9) localized to chromosome 11q12→q13.1 which encodes a highly conserved, potential membrane-associated protein. Cytogenet. Cell. Genet., 88, 211–216. [DOI] [PubMed] [Google Scholar]
- 26.Gouet P., Courcelle,E., Stuart,D.I. and Metoz,F. (1999) ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics, 15, 305–308. [DOI] [PubMed] [Google Scholar]
- 27.Nicholls A., Sharp,K.A. and Honig,B. (1991) Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins, 11, 281–296. [DOI] [PubMed] [Google Scholar]